A Review on Preparation of Betulinic Acid and Its Biological Activities
Abstract
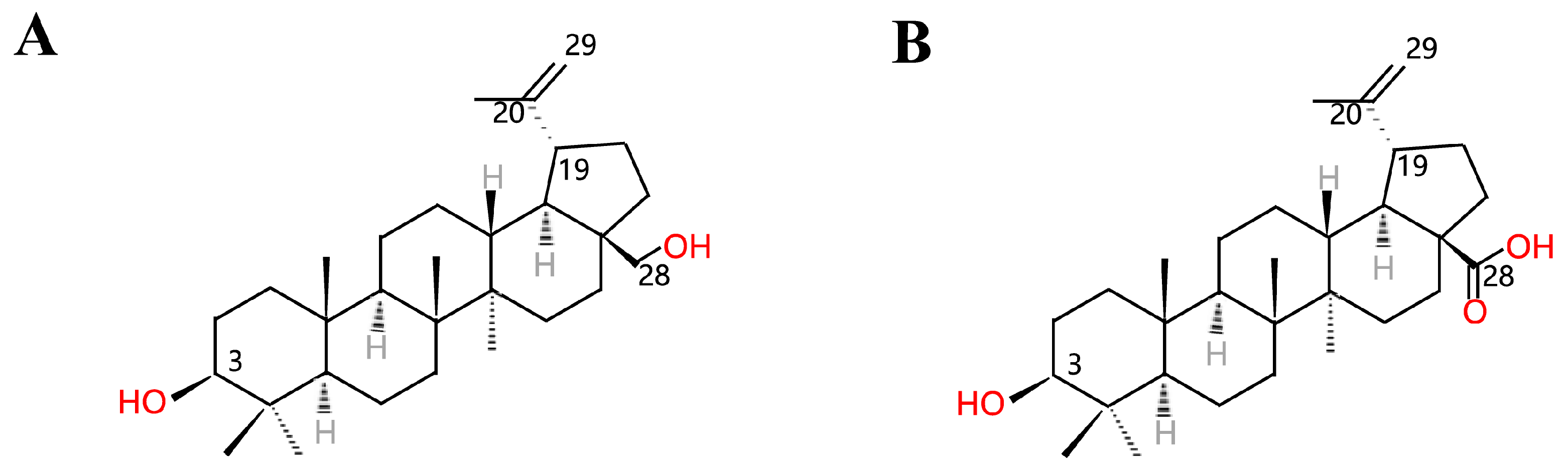
1. Introduction
2. Preparation of Betulinic Acid
2.1. Extract Betulinic Acid from Plants
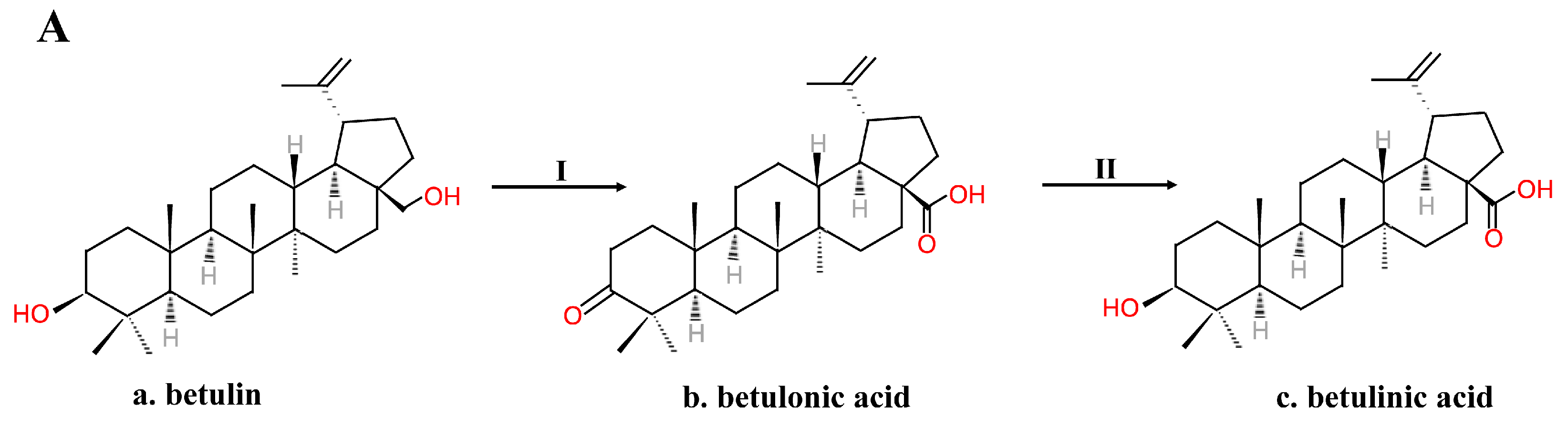
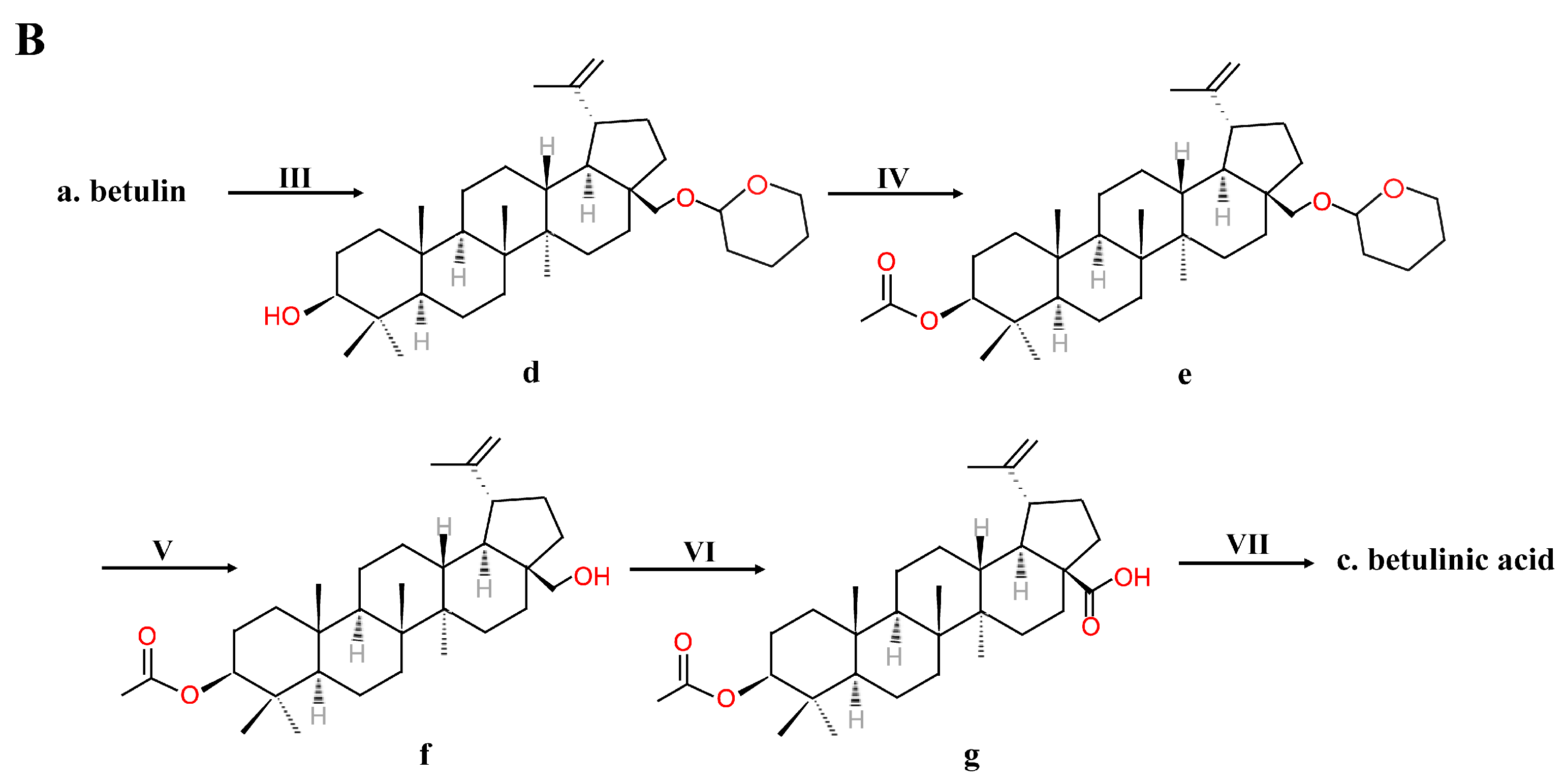
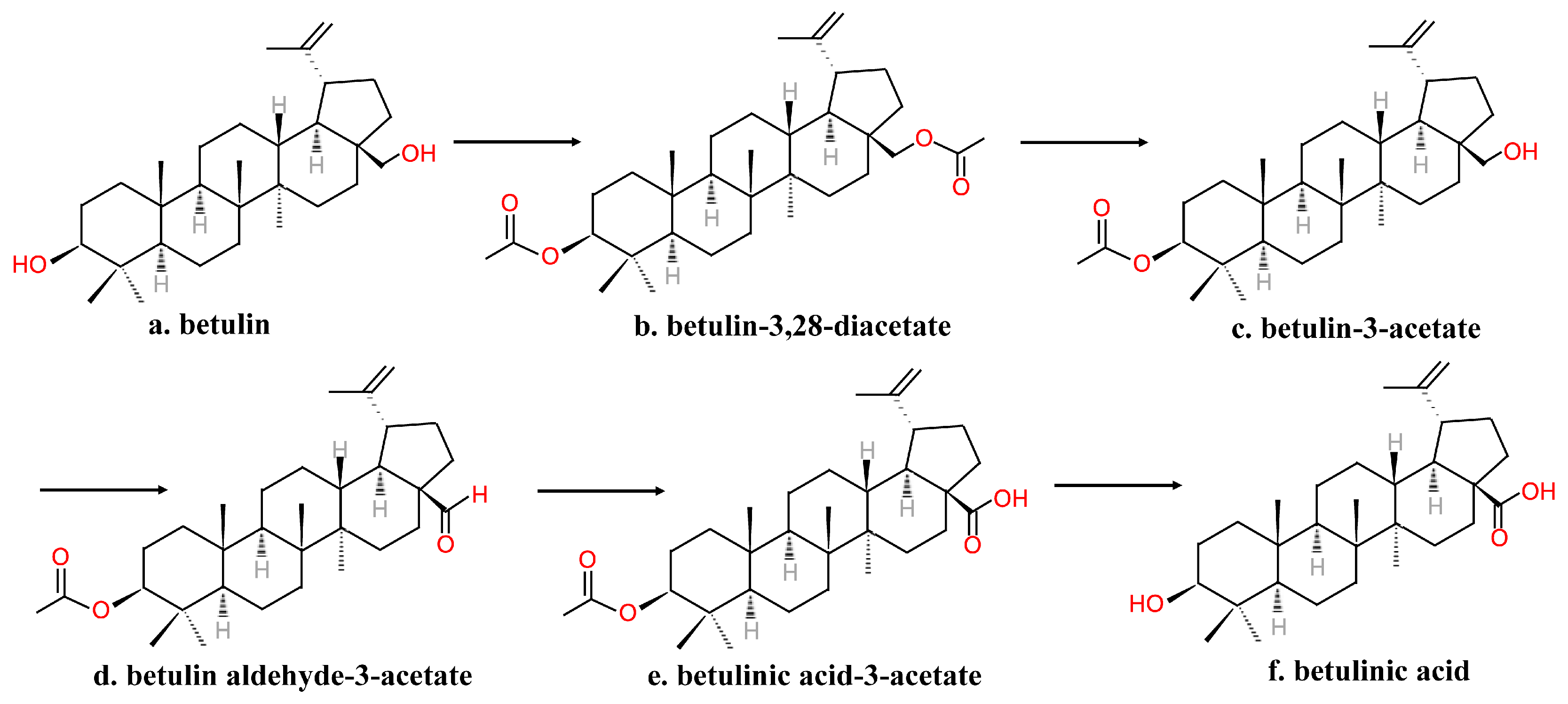
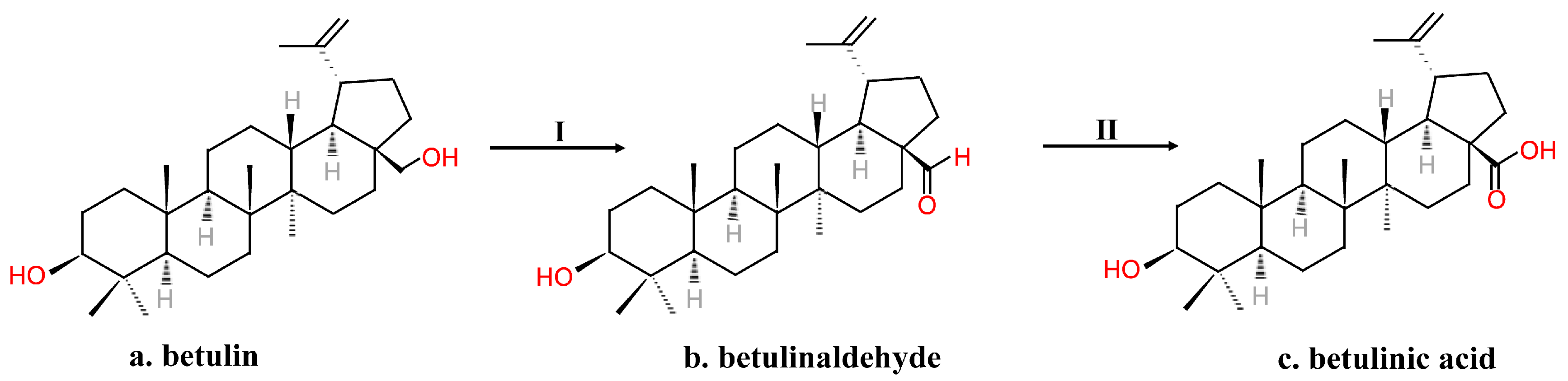
2.2. Chemical Synthesis
2.3. Biotransformation Process
2.3.1. Biotransformation by Fungi System
2.3.2. Tissue Culture
2.4. Gene Engineering
3. Biological Activities
3.1. Antitumor Activity
| Cancer Type | Cell Line/Animal | Dose | Reference |
|---|---|---|---|
| Leukemia | Cell line: HL-60 | IC50 = 5.7 μm (72 h) | [62] |
| Cell line: K-562 | IC50 = 21.26 μg/mL (24 h) | [66] | |
| Cell line: K-562 | IC50 = 12.5 μg/mL (48 h) | [75] | |
| Cell line: HL-60 | IC50 = 2.60 μg/mL (72 h) | [63] | |
| Cell line: WEHI-3B | IC50 = 2.10 μg/mL (72 h) | ||
| Cell line: HL-60 | IC50 = 8 μm (48 h) | [64] | |
| Colorectal carcinoma | Cell line: SW1463 | EC50 = 3.8 μg/mL (48 h) | [67] |
| Cell line: SW837 | EC50 = 11.3 μg/mL (48 h) | ||
| Cell line: RKO | EC50 = 9.5 μg/mL (48 h) | ||
| Cell line: CO115 | EC50 = 12.2 μg/mL (48 h) | ||
| Cell line: SW480 | EC50 = 15.1 μg/mL (48 h) | ||
| Cell line: T84 | EC50 = 11.3 μg/mL (48 h) | ||
| Cell line: HCT81 | EC50 = 16.4 μg/mL (48 h) | ||
| Cell line: LS180 | EC50 = 11.7 μg/mL (48 h) | ||
| Animals: female athymic nude Foxn1 mice xenografted with human colon cancer cell line SW480. | Intravenously injected 200 μL of BA-containing liposomes containing 5 mg/mL of BA three times a week. | [61] | |
| Lung carcinoma | Cell line: HCT116 | IC50 = 8.9 μg/mL (48h) | [70] |
| Cell line: A549 | IC50 = 8 μm (48h) | [64] | |
| Cell line: H460 | EC50 = 6.1 μg/mL (48 h) | [67] | |
| Cell line: A549 | EC50 = 8.3 μg/mL (48 h) | ||
| Cell line: H322 | EC50 = 12.3 μg/mL (48 h) | ||
| Cell line: GLC-2 | EC50 = 8.8 μg/mL (48 h) | ||
| Cell line: GLC-4 | EC50 = 10.0 μg/mL (48 h) | ||
| Cell line: GLC-36 | EC50 = 9.6 μg/mL (48 h) | ||
| Cell line: H187 | EC50 = 8.7 μg/mL (48 h) | ||
| Cell line: N417 | EC50 = 6.2 μg/mL (48 h) | ||
| Cell line: MBA9812 | EC50 = 7.6 μg/mL (48 h) | ||
| Animals: female athymic nude Foxn1 mice xenografted with human lung cancer cell line A549. | Intravenously injected 200 μL of BA-containing liposomes containing 5 mg/mL of BA three times a week. | [61] | |
| Prostate cancer | Cell line: PC-3 | IC50 = 7 μm (48 h) | [64] |
| Cell line: DU145 | EC50 = 11.6 μg/mL (48 h) | [67] | |
| Cell line: PC3 | EC50 = 12.3 μg/mL (48 h) | ||
| Cell line: 22Rv1 | EC50 = 10.1 μg/mL (48 h) | ||
| Cell line: LNCaP | EC50 = 11.9 μg/mL (48 h) | ||
| Pancreatic cancer | Cell line: MiaPaca-2 | IC50 = 7 μm (48 h) | [64] |
| Breast adenocarcinoma | Cell line: MCF-7 | IC50 =20.4 μg/mL (72 h) | [63] |
| Cell line: SKBR3 | EC50 = 16.2 μg/mL (48 h) | [67] | |
| Cell line: MDA231 | EC50 = 10.4 μg/mL (48 h) | ||
| Cell line: MDL13E | EC50 = 11.5 μg/mL (48 h) | ||
| Cell line: BT483 | EC50 = 12.8 μg/mL (48 h) | ||
| Cell line: BT474 | EC50 = 12.1 μg/mL (48 h) | ||
| Cell line: T47D | EC50 = 13.0 μg/mL (48 h) | ||
| Cell line: BT549 | EC50 = 5 μg/mL (48 h) | ||
| Brain tumor | Cell line: human glioblastoma DBTRG0.5MG | IC50 > 30.0 μg/mL (72 h) | [63] |
| Melanoma | Cell line: B16 | IC50 > 30.0 μg/mL (72 h) | [63] |
| Cervical carcinoma | Cell line: HeLa | IC50 = 2.5 μg/mL (72 h) | [63] |
| Cell line: CaSKi | EC50 = 9.6 μg/mL (48 h) | [67] | |
| Cell line: HeLa | EC50 = 14.3 μg/mL (48 h) | ||
| Cell line: SiHa | EC50 = 11.8 μg/mL (48 h) |
3.2. Anti-Inflammatory Activity
3.3. Anti-HIV Activity
3.4. Antidiabetic Activity
3.5. Antimalarial Activities
3.6. Other Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yogeeswari, P.; Sriram, D. Betulinic acid and its derivatives: A review on their biological properties. Curr. Med. Chem. 2005, 12, 657–666. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Formelli, F.J.C.L. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Fulda, S. Betulinic Acid for cancer treatment and prevention. Int. J. Mol. Sci. 2008, 9, 1096–1107. [Google Scholar] [CrossRef]
- Fulda, S. Betulinic acid: A natural product with anticancer activity. Mol. Nutr. Food Res. 2009, 53, 140–146. [Google Scholar] [CrossRef]
- Jager, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants - Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Csuk, R.; Schmuck, K.; Schafer, R. A practical synthesis of betulinic acid. Tetrahedron Lett. 2006, 47, 8769–8770. [Google Scholar] [CrossRef]
- Menard, H.; Cirtiu, C.M.; Lalancette, J.; Ruest, L.; Kaljaca, Z. Preparation of Betulinic Acid, Useful as e.g. Antitumor, Antiinflammatory Agent, Comprises Oxidizing Electrochemically Betulin to the Aldehyde; and Further Oxidizing to the Acid. U.S. Patent WO2006063464-A1, 2006. [Google Scholar]
- Roshchin, V.I.; Shabanova, N.Y.; Vedernikov, D.N. Synthesis of Betulinic Acid, Comprises Oxidation of Betuline with a Pyridine Dichromate Complex and Acetic Anhydride, Reduction of Betulonic Acid and Recrystallization. U.S. Patent RU2190622-C1, 2003. [Google Scholar]
- Liu, J.; Fu, M.L.; Chen, Q.H. Biotransformation optimization of betulin into betulinic acid production catalysed by cultured Armillaria luteo-virens Sacc ZJUQH100-6 cells. J. Appl. Microbiol. 2011, 110, 90–97. [Google Scholar] [CrossRef]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef]
- Ajikumar, P.K.; Xiao, W.H.; Tyo, K.E.J.; Wang, Y.; Simeon, F.; Leonard, E.; Mucha, O.; Phon, T.H.; Pfeifer, B.; Stephanopoulos, G. Isoprenoid Pathway Optimization for Taxol Precursor Overproduction in Escherichia coli. Science 2010, 330, 70–74. [Google Scholar] [CrossRef]
- Paddon, C.J.; Westfall, P.J.; Pitera, D.J.; Benjamin, K.; Fisher, K.; McPhee, D.; Leavell, M.D.; Tai, A.; Main, A.; Eng, D.; et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 2013, 496, 528. [Google Scholar] [CrossRef]
- Ro, D.K.; Paradise, E.M.; Ouellet, M.; Fisher, K.J.; Newman, K.L.; Ndungu, J.M.; Ho, K.A.; Eachus, R.A.; Ham, T.S.; Kirby, J.; et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 2006, 440, 940–943. [Google Scholar] [CrossRef]
- Wu, J.; Niu, Y.; Bakur, A.; Li, H.; Chen, Q. Cell-Free Production of Pentacyclic Triterpenoid Compound Betulinic Acid from Betulin by the Engineered Saccharomyces cerevisiae. Molecules 2017, 22, 1075. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Dubey, K.K. An efficient process for the transformation of betulin to betulinic acid by a strain of Bacillus megaterium. 3 Biotech 2017, 7, 157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mukherjee, D.; Kumar, N.S.; Khatua, T.; Mukherjee, P.K. Rapid Validated HPTLC Method for Estimation of Betulinic acid in Nelumbo nucifera (Nymphaeaceae) Rhizome Extract. Phytochem. Anal. 2010, 21, 556–560. [Google Scholar] [CrossRef]
- Kim, H.I.; Quan, F.S.; Kim, J.E.; Lee, N.R.; Kim, H.J.; Jo, S.J.; Lee, C.M.; Jang, D.S.; Inn, K.S. Inhibition of estrogen signaling through depletion of estrogen receptor alpha by ursolic acid and betulinic acid from Prunella vulgaris var. lilacina. Biochem. Biophys. Res. Commun. 2014, 451, 282–287. [Google Scholar] [CrossRef]
- Elusiyan, C.A.; Msagati, T.A.M.; Shode, F.O.; Mamba, B.B. Measurements of distribution coefficients and lipophilicity values for oleanolic acid and betulinic acid extracted from indigenous plants by hollow fibre supported liquid membrane. Bull. Chem. Soc. Ethiop. 2011, 25, 321–332. [Google Scholar] [CrossRef]
- Liu, J.; Chen, P.; Yao, W.J.; Wang, J.; Wang, L.Y.; Deng, L.H.; He, J.; Zhang, G.F.; Lei, J.D. Subcritical water extraction of betulinic acid from birch bark. Ind. Crop. Prod. 2015, 74, 557–565. [Google Scholar] [CrossRef]
- Bruckner, V.; Kovacs, J.; Koczka, I. Occurrence of betulinic acid in the bark of the plane tree. J. Chem. Soc. 1948, 948–951. [Google Scholar] [CrossRef]
- Galgon, T.; Hoke, D.; Drager, B. Identification and quantification of betulinic acid. Phytochem. Anal. 1999, 10, 187–190. [Google Scholar] [CrossRef]
- Pinilla, J.M.; Lopez-Padilla, A.; Vicente, G.; Fornari, T.; Quintela, J.C.; Reglero, G. Recovery of betulinic acid from plane tree (Platanus acerifolia L.). J. Supercrit. Fluids 2014, 95, 541–545. [Google Scholar] [CrossRef]
- Aisha, A.F.A.; Abu-Salah, K.M.; Alrokayan, S.A.; Siddiqui, M.J.; Ismail, Z.; Majid, A. Syzygium aromaticum extracts as good source of betulinic acid and potential anti-breast cancer. Rev. Bras. Farmacogn. Braz. J. Pharmacogn. 2012, 22, 335–343. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; de Melo, M.M.R.; Oliveira, E.L.G.; Neto, C.P.; Silvestre, A.J.D.; Silva, C.M. Optimization of the supercritical fluid extraction of triterpenic acids from Eucalyptus globulus bark using experimental design. J. Supercrit. Fluids 2013, 74, 105–114. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Sousa, G.D.A.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Eucalyptus globulus biomass residues from pulping industry as a source of high value triterpenic compounds. Ind. Crop. Prod. 2010, 31, 65–70. [Google Scholar] [CrossRef]
- Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Cavaleiro, J.A.S. Lipophilic extractives of the inner and outer barks of Eucalyptus globulus. Holzforschung 2002, 56, 372–379. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Sousa, G.D.A.; Silva, C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. High value triterpenic compounds from the outer barks of several Eucalyptus species cultivated in Brazil and in Portugal. Ind. Crop. Prod. 2011, 33, 158–164. [Google Scholar] [CrossRef]
- Tezuka, Y.; Stampoulis, P.; Banskota, A.H.; Awale, S.; Tran, K.Q.; Saiki, I.; Kadota, S. Constituents of the Vietnamese medicinal plant Orthosiphon stamineus. Chem. Pharm. Bull. 2000, 48, 1711–1719. [Google Scholar] [CrossRef]
- Machado, D.G.; Cunha, M.P.; Neis, V.B.; Balen, G.O.; Colla, A.; Bettio, L.E.B.; Oliveira, A.; Pazini, F.L.; Dalmarco, J.B.; Simionatto, E.L.; et al. Antidepressant-like effects of fractions, essential oil, carnosol and betulinic acid isolated from Rosmarinus officinalis L. Food Chem. 2013, 136, 999–1005. [Google Scholar] [CrossRef]
- Nyasse, B.; Nono, J.J.; Nganso, Y.; Ngantchou, I.; Schneider, B. Uapaca genus (Euphorbiaceae), a good source of betulinic acid. Fitoterapia 2009, 80, 32–34. [Google Scholar] [CrossRef]
- Sun, Y.F.; Song, C.K.; Viemstein, H.; Unger, F.; Liang, Z.S. Apoptosis of human breast cancer cells induced by microencapsulated betulinic acid from sour jujube fruits through the mitochondria transduction pathway. Food Chem. 2013, 138, 1998–2007. [Google Scholar] [CrossRef]
- Zhang, M.C.; Zhang, Y.Q.; Xie, J.B. Simultaneous determination of jujuboside A, B and betulinic acid in semen Ziziphi spinosae by high performance liquid chromatography-evaporative light scattering detection. J. Pharm. Biomed. Anal. 2008, 48, 1467–1470. [Google Scholar] [CrossRef]
- Adesanwo, J.K.; Makinde, O.O.; Obafemi, C.A.J.J.o.P.R. Phytochemical analysis and antioxidant activity of methanol extract and betulinic acid isolated from the roots of Tetracera potatoria. J. Pharm. Res. 2013, 6, 903–907. [Google Scholar] [CrossRef]
- Puniani, E.; Cayer, C.; Kent, P.; Mullally, M.; Sanchez-Vindas, P.; Alvarez, L.P.; Cal, V.; Merali, Z.; Arnason, J.T.; Durst, T. Ethnopharmacology of Souroubea sympetala and Souroubea gilgii (Marcgraviaceae) and identification of betulinic acid as an anxiolytic principle. Phytochemistry 2015, 113, 73–78. [Google Scholar] [CrossRef]
- Jeong, W.; Hong, S.S.; Kim, N.; Yang, Y.T.; Shin, Y.S.; Lee, C.; Hwang, B.Y.; Lee, D. Bioactive triterpenoids from Callistemon lanceolatus. Arch. Pharmacal Res. 2009, 32, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.K.; Tseng, C.K.; Chen, K.H.; Wu, S.H.; Liaw, C.C.; Lee, J.C. Betulinic acid exerts anti-hepatitis C virus activity via the suppression of NF-kappa B- and MAPK-ERK1/2-mediated COX-2 expression. Br. J. Pharmacol. 2015, 172, 4481–4492. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Chen, Z.D.; Nguyen, V.T.; Pezzuto, J.M.; Qiu, S.X.; Lu, Z.Z. A concise semi-synthetic approach to betulinic acid from betulin. Synth. Commun. 1997, 27, 1607–1612. [Google Scholar] [CrossRef]
- Kim, D.S.H.L.; Pezzuto, J.M. Manufacturing Beta Isomer of Betulinic Acid - Oxidising Betulin, Reducing the Product to Convert Keto Functionality to Secondary Alcohol and Separating and Purifying the Beta Isomer. U.S. Patent US5804575-A, 1998. [Google Scholar]
- Gaudet, D.; Pichette, A. Process for Preparing Natural Product Derivatives from Plants in a Single Step, Particularly for Preparing Derivatives of Betulin or Lupeol from Birch Bark. U.S. Patent WO200026174-A2, 2000. [Google Scholar]
- Krasutsky, P.A.; Carlson, R.M.; Nesterenko, V.V. Preparation of Betulin-3-Acetate Useful for Preparation of Betulinic acid Involves Acylating Betulin Followed by Alcoholyzing Obtained Betulin-3,28-Diacetate. U.S. Patent US2001007908-A1, 2001. [Google Scholar]
- Krasutsky, P.A.; Kolomitsyna, O.; Krasutsky, P. Preparation of 3-Esters of Betulinic Aldehyde Useful for Treating e.g. HIV, Herpes, Hepatitis, Cancer, Viral, Fungal and/or Bacterial Infection Involves Contacting Betulin Aldehyde with Keto Compound or Substituted Furan-2,5-Dione Compound. U.S. Patent WO2006105356-A2, 2007. [Google Scholar]
- Chen, Q.H.; Liu, J.; Zhang, H.F.; He, G.Q.; Fu, M.L. The betulinic acid production from betulin through biotransformation by fungi. Enzym. Microb. Technol. 2009, 45, 175–180. [Google Scholar] [CrossRef]
- Fu, M.L.; Liu, J.; Dong, Y.C.; Feng, Y.; Fang, R.S.; Chen, Q.H.; Liu, X.J. Effect of ionic liquid-containing system on betulinic acid production from betulin biotransformation by cultured Armillaria luteo-virens Sacc cells. Eur. Food Res. Technol. 2011, 233, 507–515. [Google Scholar] [CrossRef]
- Feng, Y.; Li, M.; Liu, J.; Xu, T.Y.; Fang, R.S.; Chen, Q.H.; He, G.Q. A novel one-step microbial transformation of betulin to betulinic acid catalysed by Cunninghamella blakesleeana. Food Chem. 2013, 136, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Qazi, M.S.; Sultana, N.; Kamal, M. Biotransformation of pentacyclic terpene isolated from Alstonia scholaris (R.BR.). Biocatal. Biotransformation 2013, 31, 148–152. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, Y.; Tan, Y.; Chen, H.; Mei, R.; Dong, X.; Wu, B. Triterpenoids of Inonotus obliquus. Chin. Tradit. Herb. Drugs 2015, 46, 2355–2360. [Google Scholar]
- Lou, H.; Li, H.; Wei, T.; Chen, Q. Stimulatory Effects of Oleci Acid and Fungal Elicitor on Betulinic Acid Production by Submerged Cultivation of Medicinal Mushroom Inonotus obliquus. J. Fungi 2021, 7, 266. [Google Scholar] [CrossRef]
- Tsiri, D.; Aligiannis, N.; Graikou, K.; Spyropoulos, C.; Chinou, I. Triterpenoids from Eucalyptus camaldulensis DEHNH. Tissue Cultures. Helv. Chim. Acta 2008, 91, 2110–2114. [Google Scholar] [CrossRef]
- Herrera, J.B.R.; Bartel, B.; Wilson, W.K.; Matsuda, S.P.T. Cloning and characterization of the Arabidopsis thaliana lupeol synthase gene. Phytochemistry 1998, 49, 1905–1911. [Google Scholar] [CrossRef]
- Morita, M.; Shibuya, M.; Kushiro, T.; Masuda, K.; Ebizuka, Y.J.F.J. Molecular cloning and functional expression of triterpene synthases from pea (Pisum sativum) new alpha-amyrin-producing enzyme is a multifunctional triterpene synthase. Eur. J. Biochem. 2010, 267, 3453–3460. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Achnine, L.; Xu, R.; Matsuda, S.P.T.; Dixon, R.A. A genomics approach to the early stages of triterpene saponin biosynthesis in Medicago truncatula. Plant J. 2002, 32, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Iturbe-Ormaetxe, I.; Haralampidis, K.; Papadopoulou, K.; Osbourn, A.E. Molecular cloning and characterization of triterpene synthases from Medicago truncatula and Lotus japonicus. Plant Mol. Biol. 2003, 51, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Kushiro, T.; Shibuya, M.; Ebizuka, Y. beta-Amyrin synthase-Cloning of oxidosqualene cyclase that catalyzes the formation of the most popular triterpene among higher plants. Eur. J. Biochem. 1998, 256, 238–244. [Google Scholar] [CrossRef]
- Fukushima, E.O.; Seki, H.; Ohyama, K.; Ono, E.; Umemoto, N.; Mizutani, M.; Saito, K.; Muranaka, T. CYP716A Subfamily Members are Multifunctional Oxidases in Triterpenoid Biosynthesis. Plant Cell Physiol. 2011, 52, 2050–2061. [Google Scholar] [CrossRef]
- Huang, L.L.; Li, J.; Ye, H.C.; Li, C.F.; Wang, H.; Liu, B.Y.; Zhang, Y.S. Molecular characterization of the pentacyclic triterpenoid biosynthetic pathway in Catharanthus roseus. Planta 2012, 236, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.S. Increase of betulinic acid production in Saccharomyces cerevisiae by balancing fatty acids and betulinic acid forming pathways. Appl. Microbiol. Biotechnol. 2014, 98, 3081–3089. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.S. Modulating betulinic acid production in Saccharomyces cerevisiae by managing the intracellular supplies of the co-factor NADPH and oxygen. J. Biosci. Bioeng. 2015, 119, 77–81. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.-S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.W.; Fong, H.H.S.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Y.Z.; Yu, Y.; Yin, P.H.; Xu, K.J.J.o.B.N. The Antitumor Activity of Betulinic Acid-Loaded Nanoliposomes Against Colorectal Cancer In Vitro and In Vivo via Glycolytic and Glutaminolytic Pathways. J. Biomed. Nanotechnol. 2020, 16, 235–251. [Google Scholar] [CrossRef]
- Mullauer, F.B.; van Bloois, L.; Daalhuisen, J.B.; Ten Brink, M.S.; Storm, G.; Medema, J.P.; Schiffelers, R.M.; Kessler, J.H. Betulinic acid delivered in liposomes reduces growth of human lung and colon cancers in mice without causing systemic toxicity. Anti-Cancer Drugs 2011, 22, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Poon, K.H.; Zhang, J.; Wang, C.; Tse, A.K.; Wan, C.K.; Fong, W.F. Betulinic acid enhances 1alpha,25-dihydroxyvitamin D3-induced differentiation in human HL-60 promyelocytic leukemia cells. Anti-Cancer Drugs 2004, 15, 619–624. [Google Scholar] [CrossRef]
- Faujan, N.H.; Alitheen, N.B.; Yeap, S.K.; Ali, A.M.; Muhajir, A.H.; Ahmad, F.B.H. Cytotoxic effect of betulinic acid and betulinic acid acetate isolated from Melaleuca cajuput on human myeloid leukemia (HL-60) cell line. Afr. J. Biotechnol. 2010, 9, 6387–6396. [Google Scholar]
- Khan, I.; Guru, S.K.; Rath, S.K.; Chinthakindi, P.K.; Singh, B.; Koul, S.; Bhushan, S.; Sangwan, P.L. A novel triazole derivative of betulinic acid induces extrinsic and intrinsic apoptosis in human leukemia HL-60 cells. Eur. J. Med. Chem. 2016, 108, 104–116. [Google Scholar] [CrossRef]
- Ehrhardt, H.; Fulda, S.; Fuhrer, M.; Debatin, K.M.; Jeremias, I. Betulinic acid-induced apoptosis in leukemia cells. Leukemia 2004, 18, 1406–1412. [Google Scholar] [CrossRef]
- Wu, Q.L.; He, J.; Fang, J.; Hong, M. Antitumor effect of betulinic acid on human acute leukemia K562 cells in vitro. J. Huazhong Univ. Sci. Technol. Med Sci. 2010, 30, 453–457. [Google Scholar] [CrossRef]
- Kessler, J.H.; Mullauer, F.B.; de Roo, G.M.; Medema, J.P. Broad in vitro efficacy of plant-derived betulinic acid against cell lines derived from the most prevalent human cancer types. Cancer Lett. 2007, 251, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Chintharlapalli, S.; Papineni, S.; Lei, P.; Pathi, S.; Safe, S. Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome-dependent and -independent downregulation of specificity proteins (Sp) transcription factors. Bmc Cancer 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.R.; Kim, K.J.; Choi, C.H.; Lee, T.B.; Han, S.I.; Han, H.K.; Lim, S.C. Effect of betulinic acid on anticancer drug-resistant colon cancer cells. Basic Clin. Pharmacol. Toxicol. 2007, 101, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Aisha, A.F.A.; Abu-Salah, K.M.; Ismail, Z.; Majid, A. alpha-Mangostin Enhances Betulinic Acid Cytotoxicity and Inhibits Cisplatin Cytotoxicity on HCT 116 Colorectal Carcinoma Cells. Molecules 2012, 17, 2939–2954. [Google Scholar] [CrossRef] [PubMed]
- Rabi, T.; Shukla, S.; Gupta, S. Betulinic Acid Suppresses Constitutive and TNF alpha-Induced NF-kappa B Activation and Induces Apoptosis in Human Prostate Carcinoma PC-3 Cells. Mol. Carcinog. 2008, 47, 964–973. [Google Scholar] [CrossRef]
- Reiner, T.; de Las Pozas, A.; Parrondo, R.; Palenzuela, D.; Cayuso, W.; Rai, P.; Perez-Stable, C. Mcl-1 protects prostate cancer cells from cell death mediated by chemotherapy-induced DNA damage. Oncoscience 2015, 2, 703–715. [Google Scholar] [CrossRef]
- Pandey, M.K.; Sung, B.; Aggarwal, B.B. Betulinic acid suppresses STAT3 activation pathway through induction of protein tyrosine phosphatase SHP-1 in human multiple myeloma cells. Int. J. Cancer 2010, 127, 282–292. [Google Scholar] [CrossRef]
- Potze, L.; Di Franco, S.; Grandela, C.; Pras-Raves, M.L.; Picavet, D.I.; van Veen, H.A.; van Lenthe, H.; Mullauer, F.B.; van der Wel, N.N.; Luyf, A.; et al. Betulinic acid induces a novel cell death pathway that depends on cardiolipin modification. Oncogene 2016, 35, 427–437. [Google Scholar] [CrossRef]
- Gopal, D.V.R.; Narkar, A.A.; Badrinath, Y.; Mishra, K.P.; Joshi, D.S. Betulinic acid induces apoptosis in human chronic myelogenous leukemia (CML) cell line K-562 without altering the levels of Bcr-Abl. Toxicol. Lett. 2005, 155, 343–351. [Google Scholar] [CrossRef]
- Costa, J.F.; Barbosa-Filho, J.M.; Maia, G.L.; Guimaraes, E.T.; Meira, C.S.; Ribeiro-dos-Santos, R.; de Carvalho, L.C.; Soares, M.B. Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. Int. Immunopharmacol. 2014, 23, 469–474. [Google Scholar] [CrossRef]
- Lingaraju, M.C.; Pathak, N.N.; Begum, J.; Balaganur, V.; Bhat, R.A.; Ramachandra, H.D.; Ayanur, A.; Ram, M.; Singh, V.; Kumar, D.; et al. Betulinic acid attenuates lung injury by modulation of inflammatory cytokine response in experimentally-induced polymicrobial sepsis in mice. Cytokine 2015, 71, 101–108. [Google Scholar] [CrossRef]
- Nader, M.A.; Baraka, H.N. Effect of betulinic acid on neutrophil recruitment and inflammatory mediator expression in lipopolysaccharide-induced lung inflammation in rats. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2012, 46, 106–113. [Google Scholar] [CrossRef]
- Armah, F.A.; Annan, K.; Mensah, A.Y.; Amponsah, I.K.; Tocher, D.A.; Habtemariam, S. Erythroivorensin: A novel anti-inflammatory diterpene from the root-bark of Erythrophleum ivorense (A Chev.). Fitoterapia 2015, 105, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Viji, V.; Helen, A.; Luxmi, V.R. Betulinic acid inhibits endotoxin-stimulated phosphorylation cascade and pro-inflammatory prostaglandin E2 production in human peripheral blood mononuclear cells. Br. J. Pharmacol. 2011, 162, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, C.A.; Liu, B.L.; Welch, C.J.; Perera, P.; Bohlin, L. Alphitol, a phenolic substance from Alphitonia zizyphoides which inhibits prostaglandin biosynthesis in vitro. Phytochemistry 1998, 48, 495–497. [Google Scholar] [CrossRef]
- Recio, M.D.; Giner, R.M.; Manez, S.; Gueho, J.; Julien, H.R.; Hostettmann, K.; Rios, J.L. Investigations on the steroidal anti-inflammatory activity of triterpenoids from Diospyros leucomelas. Planta Med. 1995, 61, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Manez, S.; Recio, M.C.; Giner, R.M.; Rios, J.L. Effect of selected triterpenoids on chronic dermal inflammation. Eur. J. Pharmacol. 1997, 334, 103–105. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Saha, K.; Das, J.; Pal, M.; Saha, B.P. Studies on the anti-inflammatory activity of rhizomes of Nelumbo nucifera. Planta Med. 1997, 63, 367–369. [Google Scholar] [CrossRef]
- Krogh, R.; Kroth, R.; Berti, C.; Madeira, A.O.; Souza, M.M.; Cechinel, V.; Delle-Monache, F.; Yunes, R.A. Isolation and identification of compounds with antinociceptive action from Ipomoea pes-caprae (L.) R-Br. Pharmazie 1999, 54, 464–466. [Google Scholar]
- Ryu, S.Y.; Oak, M.H.; Yoon, S.K.; Cho, D.I.; Yoo, G.S.; Kim, T.S.; Kim, K.M. Anti-allergic and anti-inflammatory triterpenes from the herb of Prunella vulgaris. Planta Med. 2000, 66, 358–360. [Google Scholar] [CrossRef]
- Huguet, A.I.; Recio, M.D.; Manez, S.; Giner, R.M.; Rios, J.L. Effect of triterpenoids on the inflammation induced by protein kinase C activators, neuronally acting irritants and other agents. Eur. J. Pharmacol. 2000, 410, 69–81. [Google Scholar] [CrossRef]
- Fujioka, T.; Kashiwada, Y.; Kilkuskie, R.E.; Cosentino, L.M.; Ballas, L.M.; Jiang, J.B.; Janzen, W.P.; Chen, I.S.; Lee, K.H. Anti-AIDS agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J. Nat. Prod. 1994, 57, 243–247. [Google Scholar] [CrossRef]
- Akihisa, T.; Ogihara, J.; Kato, J.; Yasukawa, K.; Ukiya, M.; Yamanouchi, S.; Oishi, K. Inhibitory effects of triterpenoids and sterols on human immunodeficiency virus-1 reverse transcriptase. Lipids 2001, 36, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Mayaux, J.F.; Bousseau, A.; Pauwels, R.; Huet, T.; Henin, Y.; Dereu, N.; Evers, M.; Soler, F.; Poujade, C.; Declercq, E.; et al. Triterpene derivatives that block entry of human immunodeficiency virus type 1 into cells. Proc. Natl. Acad. Sci. USA 1994, 91, 3564–3568. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Zeng, F.Q.; Wan, M.; Sim, K.Y. Anti-HIV triterpene acids from Geum japonicum. J. Nat. Prod. 1996, 59, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Quan, H.Y.; Jeong, K.J.; Kim, D.Y.; Kim, G.; Jo, H.K.; Chung, S.H. Beneficial effect of betulinic acid on hyperglycemia via suppression of hepatic glucose production. J. Agric. Food Chem. 2014, 62, 434–442. [Google Scholar] [CrossRef]
- Yoon, J.J.; Lee, Y.J.; Han, B.H.; Choi, E.S.; Kho, M.C.; Park, J.H.; Ahn, Y.M.; Kim, H.Y.; Kang, D.G.; Lee, H.S. Protective effect of betulinic acid on early atherosclerosis in diabetic apolipoprotein-E gene knockout mice. Eur. J. Pharm. 2017, 796, 224–232. [Google Scholar] [CrossRef]
- Xie, R.; Zhang, H.; Wang, X.Z.; Yang, X.Z.; Wu, S.N.; Wang, H.G.; Shen, P.; Ma, T.H. The protective effect of betulinic acid (BA) diabetic nephropathy on streptozotocin (STZ)-induced diabetic rats. Food Funct. 2017, 8, 299–306. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Shabani, R.; Mojaddami, S. Preventive effects of betulinic acid on streptozotocinnicotinamide induced diabetic nephropathy in male mouse. J. Nephropathol. 2016, 5, 128–133. [Google Scholar] [CrossRef][Green Version]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Arzani, G.; Afshari, G. Effects of Betulinic Acid on the Male Reproductive System of a Streptozotocin-Nicotinamide-Induced Diabetic Mouse Model. World J. Men’s Health 2016, 34, 209–216. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Z.; Xiong, F.; Chen, C.; Chao, X.; Huang, J.; Huang, H. Betulinic acid ameliorates experimental diabetic-induced renal inflammation and fibrosis via inhibiting the activation of NF-kappaB signaling pathway. Mol. Cell. Endocrinol. 2016, 434, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.C.P.; Warhurst, D.C.; Kirby, G.C.; Simmonds, M.S.J. In vitro andIn vivo evaluation of betulinic acid as an antimalarial. Phytother. Res. 1999, 13, 115–119. [Google Scholar] [CrossRef]
- de Sa, M.S.; Costa, J.F.O.; Krettli, A.U.; Zalis, M.G.; Maia, G.L.D.; Sette, I.M.F.; Camara, C.D.; Barbosa, J.M.; Giulietti-Harley, A.M.; dos Santos, R.R.; et al. Antimalarial activity of betulinic acid and derivatives in vitro against Plasmodium falciparum and in vivo in P-berghei-infected mice. Parasitol. Res. 2009, 105, 275–279. [Google Scholar] [CrossRef]
- Olanlokun, J.O.; Okoro, P.O.; Lawal, O.S.; Bodede, O.; Olotu, F.; Idowu, T.O.; Prinsloo, G.; Soliman, M.E.; Olorunsogo, O.O. Betulinic acid purified from Alstonia boonei inhibits folate biosynthesis in malarial Plasmodium, enhances mitochondrial pore opening and F1F0 ATPase in mice. J. Mol. Struct. 2021, 1239. [Google Scholar] [CrossRef]
- Apoptosis in liver during malaria: Role of oxidative stress and implication of mitochondrial pathway. FASEB J. 2006, 20, 1224–1226. [CrossRef]
- Karagoz, A.C.; Leidenberger, M.; Hahn, F.; Hampel, F.; Friedrich, O.; Marschall, M.; Kappes, B.; Tsogoeva, S.B. Synthesis of new betulinic acid/betulin-derived dimers and hybrids with potent antimalarial and antiviral activities. Bioorg. Med. Chem. 2019, 27, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Cargnin, S.T.; Staudt, A.F.; Medeiros, P.; de Medeiros Sol Sol, D.; de Azevedo Dos Santos, A.P.; Zanchi, F.B.; Gosmann, G.; Puyet, A.; Garcia Teles, C.B.; Gnoatto, S.B. Semisynthesis, cytotoxicity, antimalarial evaluation and structure-activity relationship of two series of triterpene derivatives. Bioorg. Med. Chem. Lett. 2018, 28, 265–272. [Google Scholar] [CrossRef]
- Szuster-Ciesielska, A.; Plewka, K.; Daniluk, J.; Kandefer-Szerszen, M. Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS), cytokine (TNF-alpha, TGF-beta) production and by influencing intracellular signaling. Toxicology 2011, 280, 152–163. [Google Scholar] [CrossRef]
- Jain, M.; Kapadia, R.; Jadeja, R.N.; Thounaojam, M.C.; Devkar, R.V.; Mishra, S.H. Hepatoprotective potential of Tecomella undulata stem bark is partially due to the presence of betulinic acid. J. Ethnopharmacol. 2012, 143, 194–200. [Google Scholar] [CrossRef]
- Yi, J.; Xia, W.; Wu, J.; Yuan, L.; Wu, J.; Tu, D.; Fang, J.; Tan, Z. Betulinic acid prevents alcohol-induced liver damage by improving the antioxidant system in mice. J. Vet. Sci. 2014, 15, 141. [Google Scholar] [CrossRef]
- Wan, Y.; Wu, Y.L.; Lian, L.H.; Xie, W.X.; Li, X.; Ouyang, B.Q.; Bai, T.; Li, Q.; Yang, N.; Nan, J.X. The anti-fibrotic effect of betulinic acid is mediated through the inhibition of NF-kappaB nuclear protein translocation. Chem. -Biol. Interact. 2012, 195, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Thakur, R.; Lingaraju, M.C.; Kumar, D.; Mathesh, K.; Telang, A.G.; Singh, T.U.; Kumar, D. Betulinic acid attenuates renal fibrosis in rat chronic kidney disease model. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 89, 796–804. [Google Scholar] [CrossRef]
- Xia, A.; Xue, Z.; Li, Y.; Wang, W.; Xia, J.; Wei, T.; Cao, J.; Zhou, W. Cardioprotective effect of betulinic Acid on myocardial ischemia reperfusion injury in rats. Evid. -Based Complementary Altern. Med. Ecam. 2014, 2014, 573745. [Google Scholar] [CrossRef]
- Lu, Q.; Xia, N.; Xu, H.; Guo, L.; Wenzel, P.; Daiber, A.; Munzel, T.; Forstermann, U.; Li, H. Betulinic acid protects against cerebral ischemia-reperfusion injury in mice by reducing oxidative and nitrosative stress. Nitric Oxide Biol. Chem. Off. J. Nitric Oxide Soc. 2011, 24, 132–138. [Google Scholar] [CrossRef]
- Afzal, M.; Kazmi, I.; Semwal, S.; Al-Abbasi, F.A.; Anwar, F. Therapeutic exploration of betulinic acid in chemically induced hypothyroidism. Mol. Cell. Biochem. 2014, 386, 27–34. [Google Scholar] [CrossRef]
- Jine, Y.; Lis, M.; Szczypka, M.; Obmińska-Mrukowicz, B. Influence of betulinic acid on lymphocyte subsets and humoral immune response in mice. Pol. J. Vet. Sci. 2012, 15. [Google Scholar] [CrossRef][Green Version]
- Kim, J.; Lee, Y.S.; Kim, C.S.; Kim, J.S. Betulinic acid has an inhibitory effect on pancreatic lipase and induces adipocyte lipolysis. Phytother. Res. PTR 2012, 26, 1103–1106. [Google Scholar] [CrossRef]
- Mullally, M.; Mimeault, C.; Otárola Rojas, M.; Sanchez Vindas, P.; Garcia, M.; Poveda Alvarez, L.; Moon, T.W.; Gilmour, K.M.; Trudeau, V.L.; Arnason, J.T. A botanical extract of Souroubea sympetala and its active principle, betulinic acid, attenuate the cortisol response to a stressor in rainbow trout, Oncorhynchus mykiss. Aquaculture 2017, 468, 26–31. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules 2021, 26, 5583. https://doi.org/10.3390/molecules26185583
Lou H, Li H, Zhang S, Lu H, Chen Q. A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules. 2021; 26(18):5583. https://doi.org/10.3390/molecules26185583
Chicago/Turabian StyleLou, Hanghang, Hao Li, Shengliang Zhang, Hongyun Lu, and Qihe Chen. 2021. "A Review on Preparation of Betulinic Acid and Its Biological Activities" Molecules 26, no. 18: 5583. https://doi.org/10.3390/molecules26185583
APA StyleLou, H., Li, H., Zhang, S., Lu, H., & Chen, Q. (2021). A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules, 26(18), 5583. https://doi.org/10.3390/molecules26185583







