Physicochemical Properties, Antioxidant Capacity, Prebiotic Activity and Anticancer Potential in Human Cells of Jackfruit (Artocarpus heterophyllus) Seed Flour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Jackfruit Seeds
2.2. Drying Kinetics of Jackfruit Seeds
2.3. Mathematical Modeling of Drying Curves
2.4. Analysis Performed in Jackfruit Seeds Flour
2.5. Chemical Composition
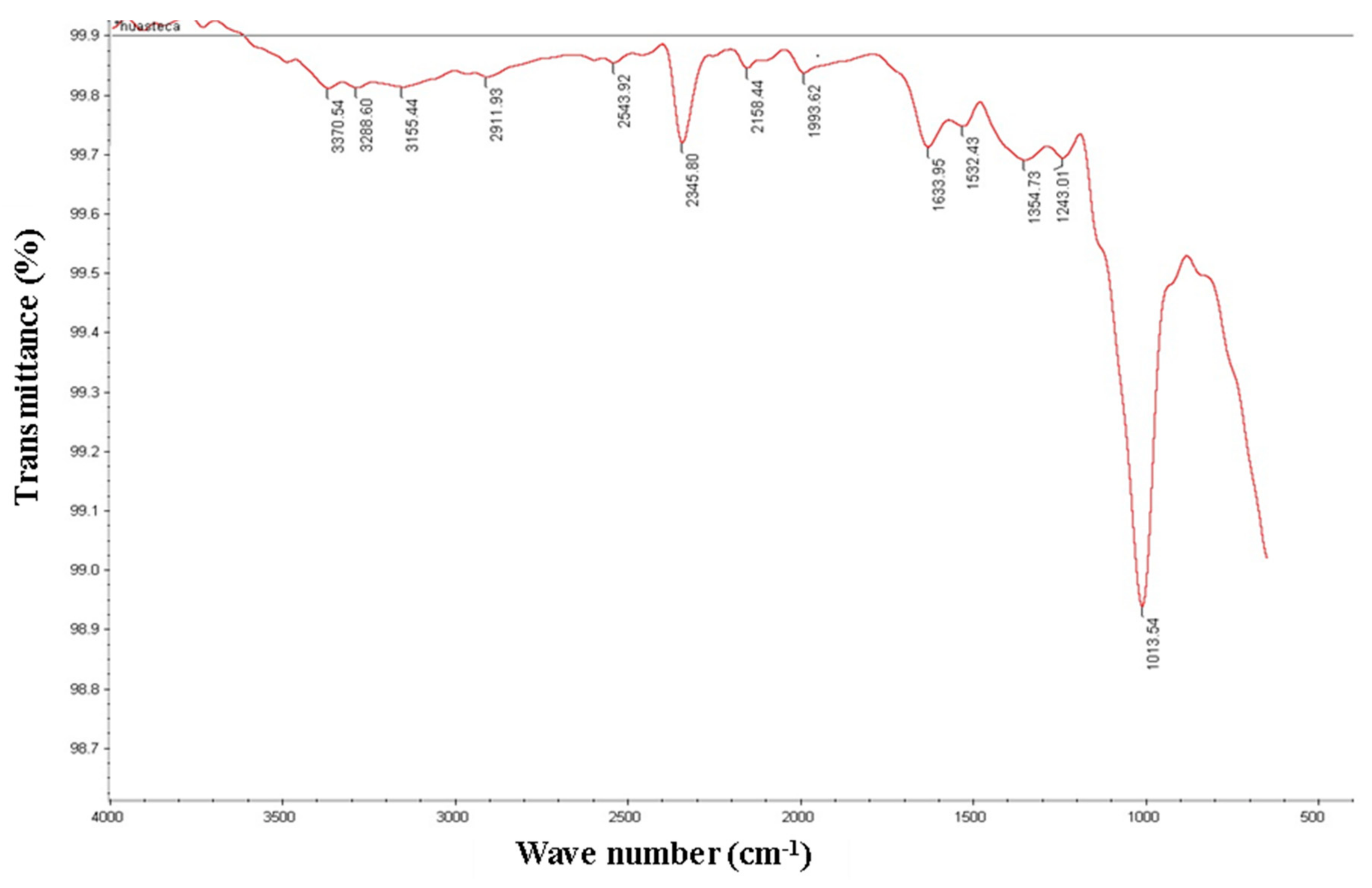
2.6. FTIR Infrared Spectroscopy
2.7. Carbohydrates Characterization by HPLC
2.8. Lipids Characterization
2.9. Sample Derivatization and Fatty Acid Profiles
2.10. Total Polyphenols Content and Antioxidant Capacity
2.11. Techno-Functional Properties
2.11.1. Water and Oil Absorption Capacity
2.11.2. Emulsifying Capacity
2.11.3. Swelling Power and Water Solubility Capacity
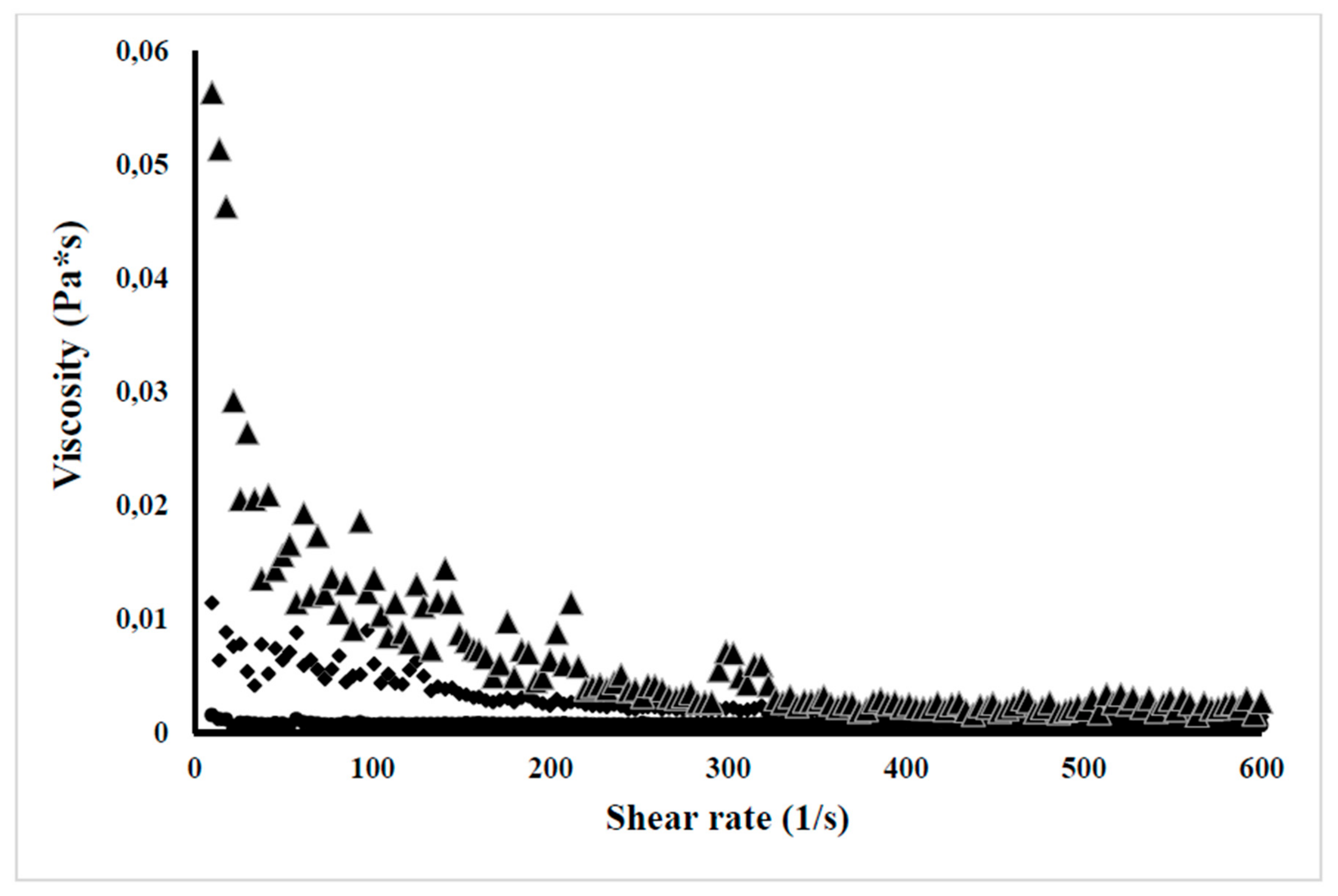
2.11.4. Viscosity Evaluation
2.12. Prebiotic Extracted from Jackfruit Seed
2.13. Prebiotic Effect of Jackfruits Seed Flour and Jackfruits Seeds Extracted
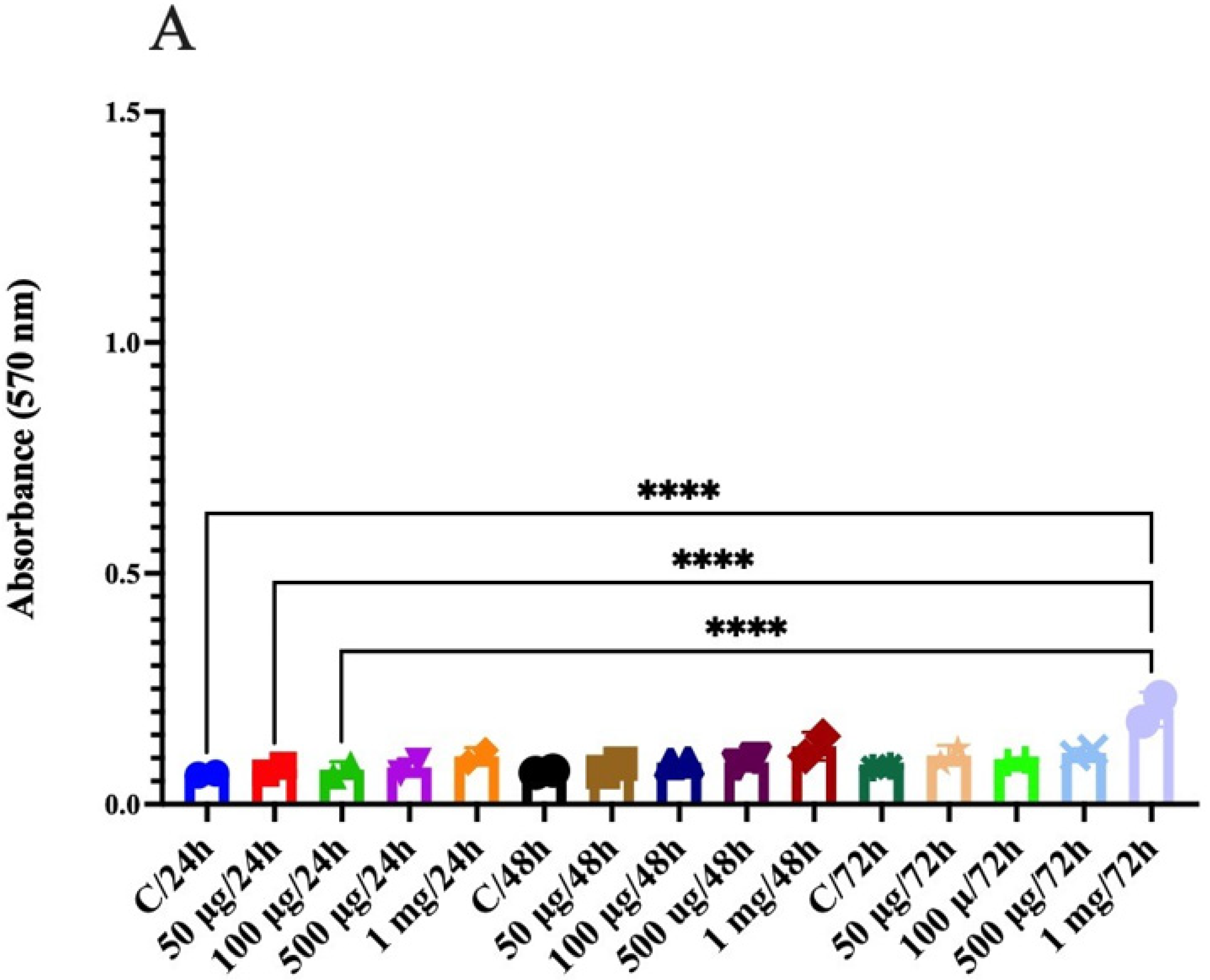
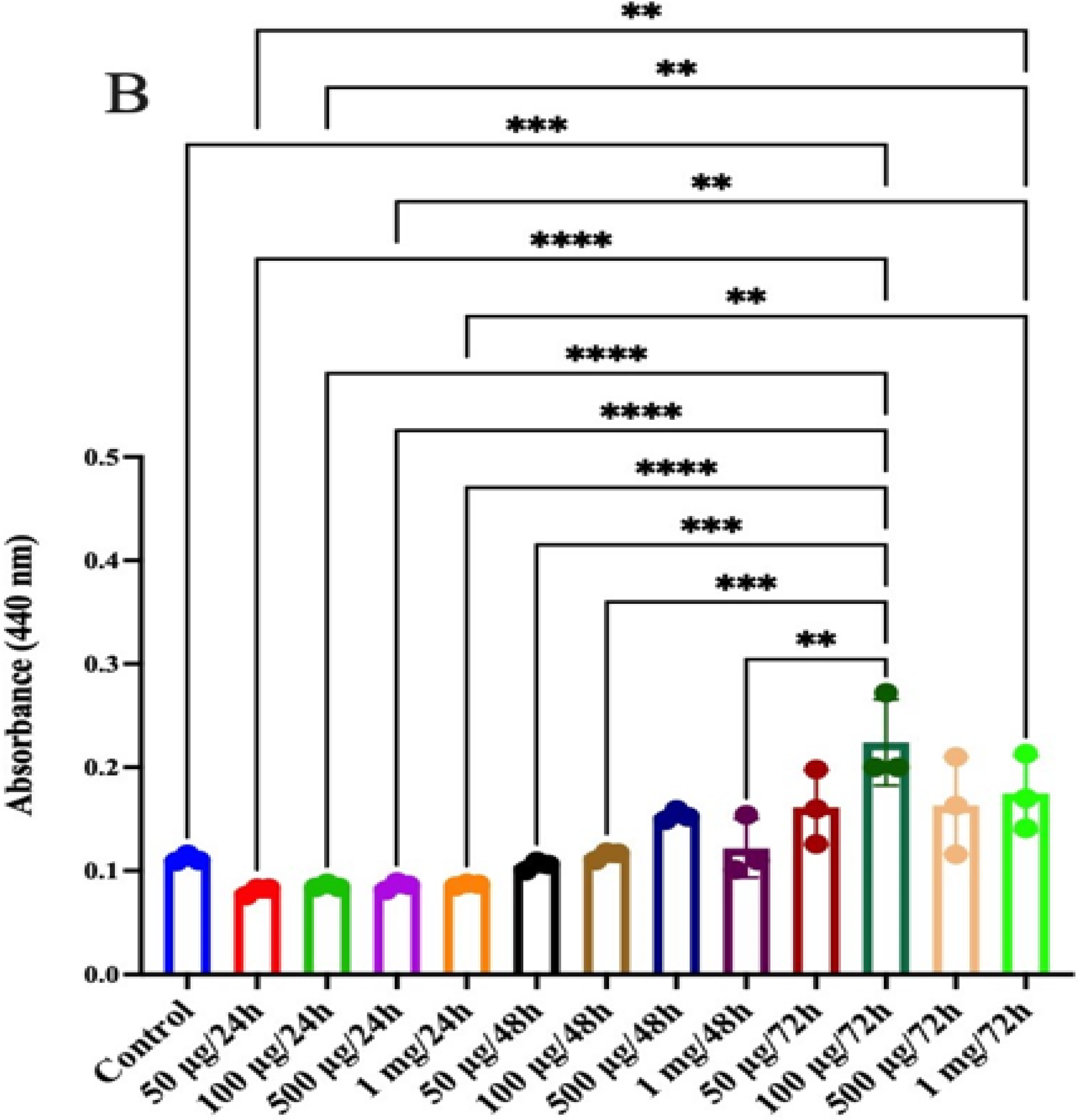
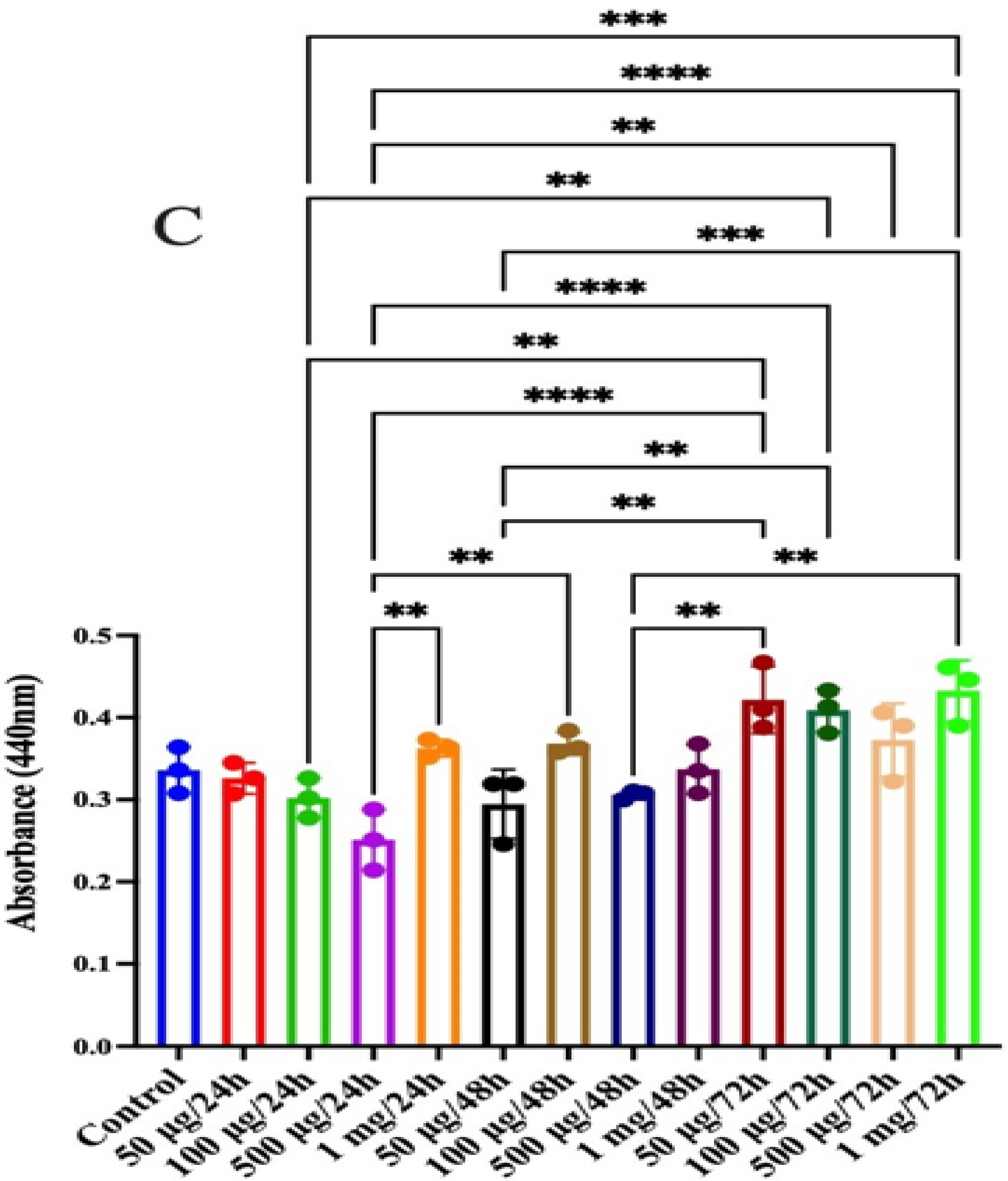
2.14. Anticancer Activity in Human Colon Epithelial Cells
2.14.1. Cell Cultures
2.14.2. Treatments
2.14.3. Cytotoxicity Assay
2.14.4. Antioxidant Assay
2.15. Statistical Analysis
3. Results and Discussion
3.1. Jackfruit Seeds
3.2. Drying Kinetics of Seeds
3.3. Chemical Composition
3.4. FTIR Analysis
3.5. Carbohydrates Characterization by HPLC
3.6. Lipids Characterization
3.7. Total Polyphenol Content and Antioxidant Capacity
3.8. Techno-Functional Properties of Flour
3.8.1. Water Absorption Capacity (WAC)
3.8.2. Oil Absorption Capacity (OAC)
3.8.3. Water Solubility Capacity (WSC) and Swelling Power
3.8.4. Dynamic Viscosity Evaluation
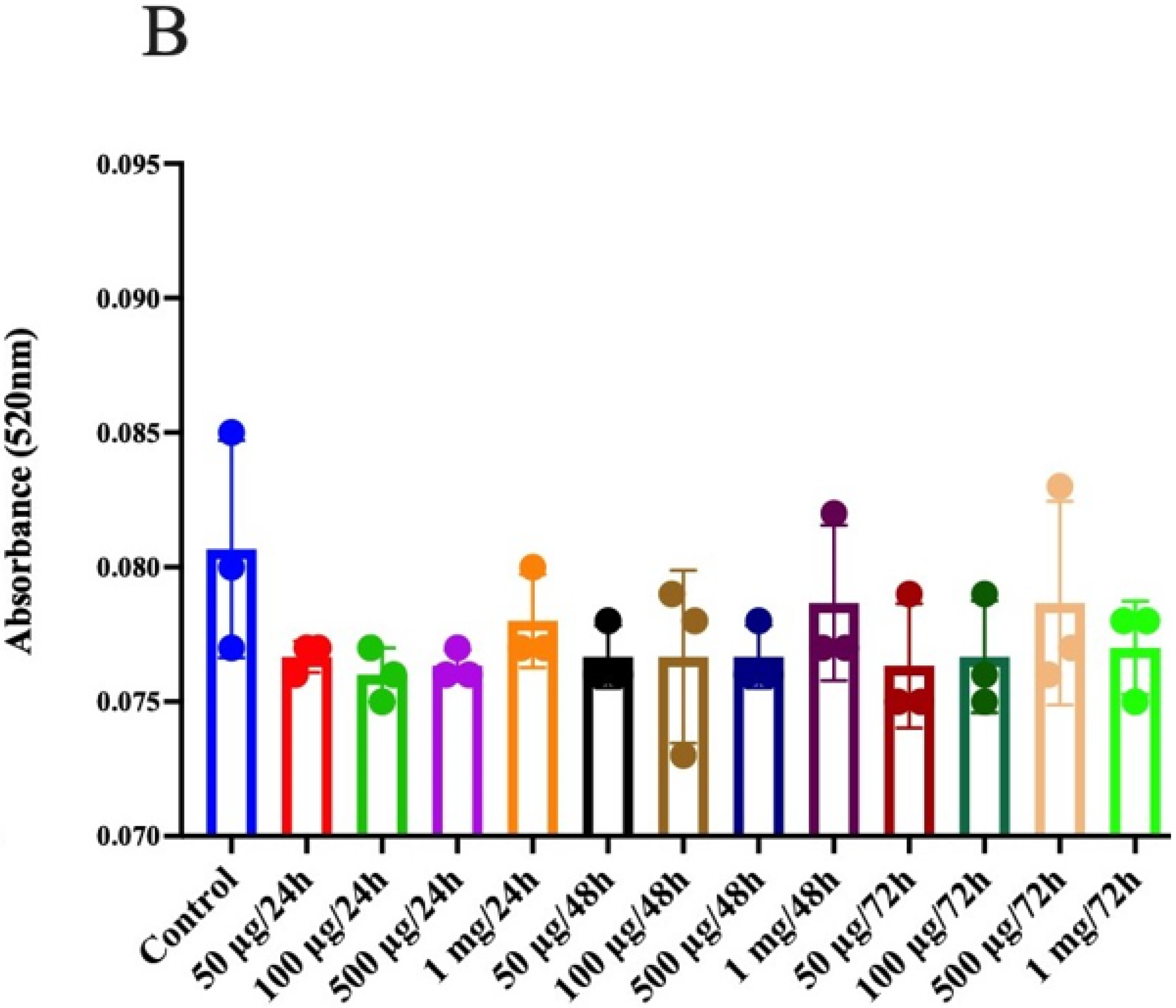
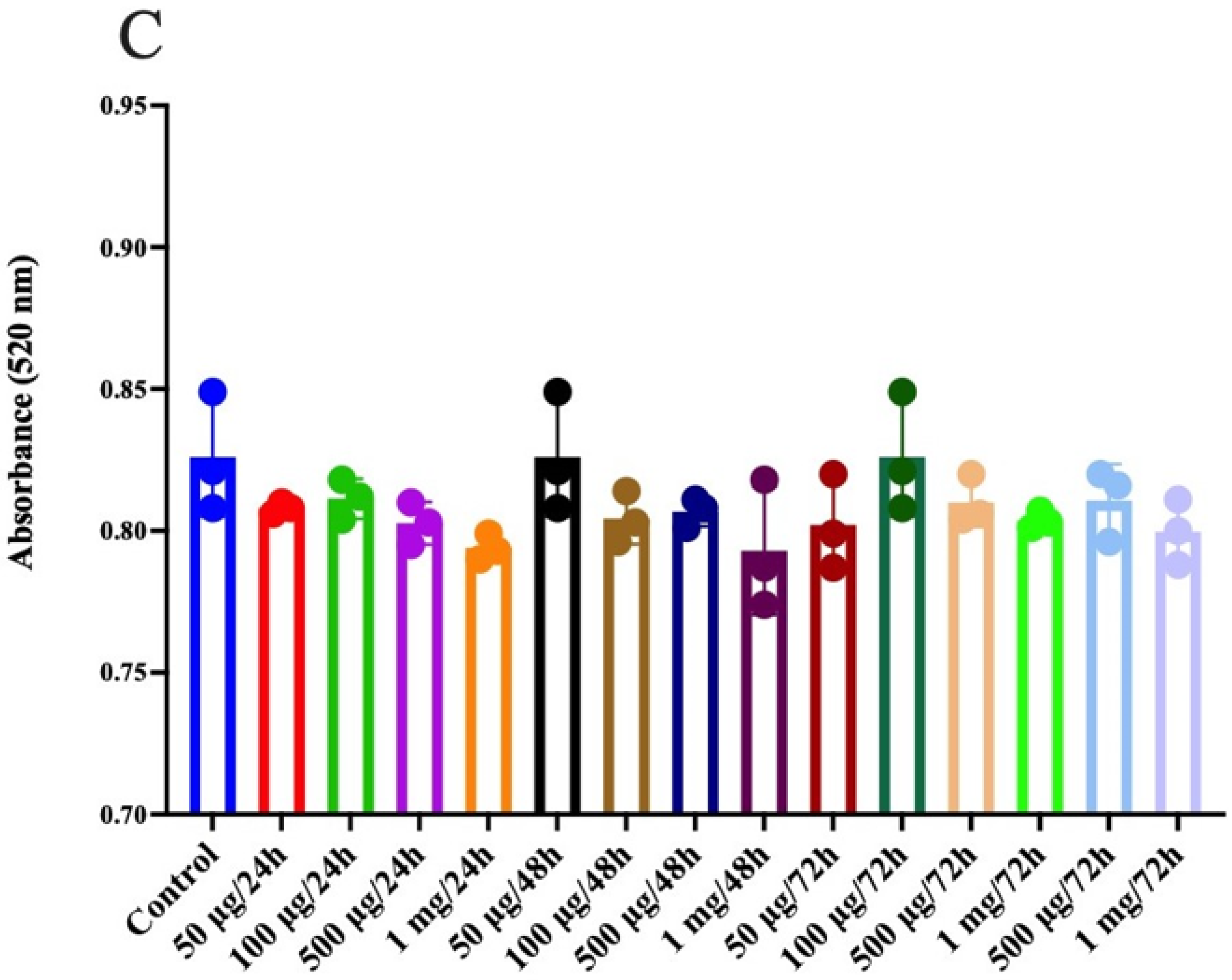
3.9. Prebiotic Effect of Jackfruit Seed Flour and Prebiotic Jackfruit Seed Extract
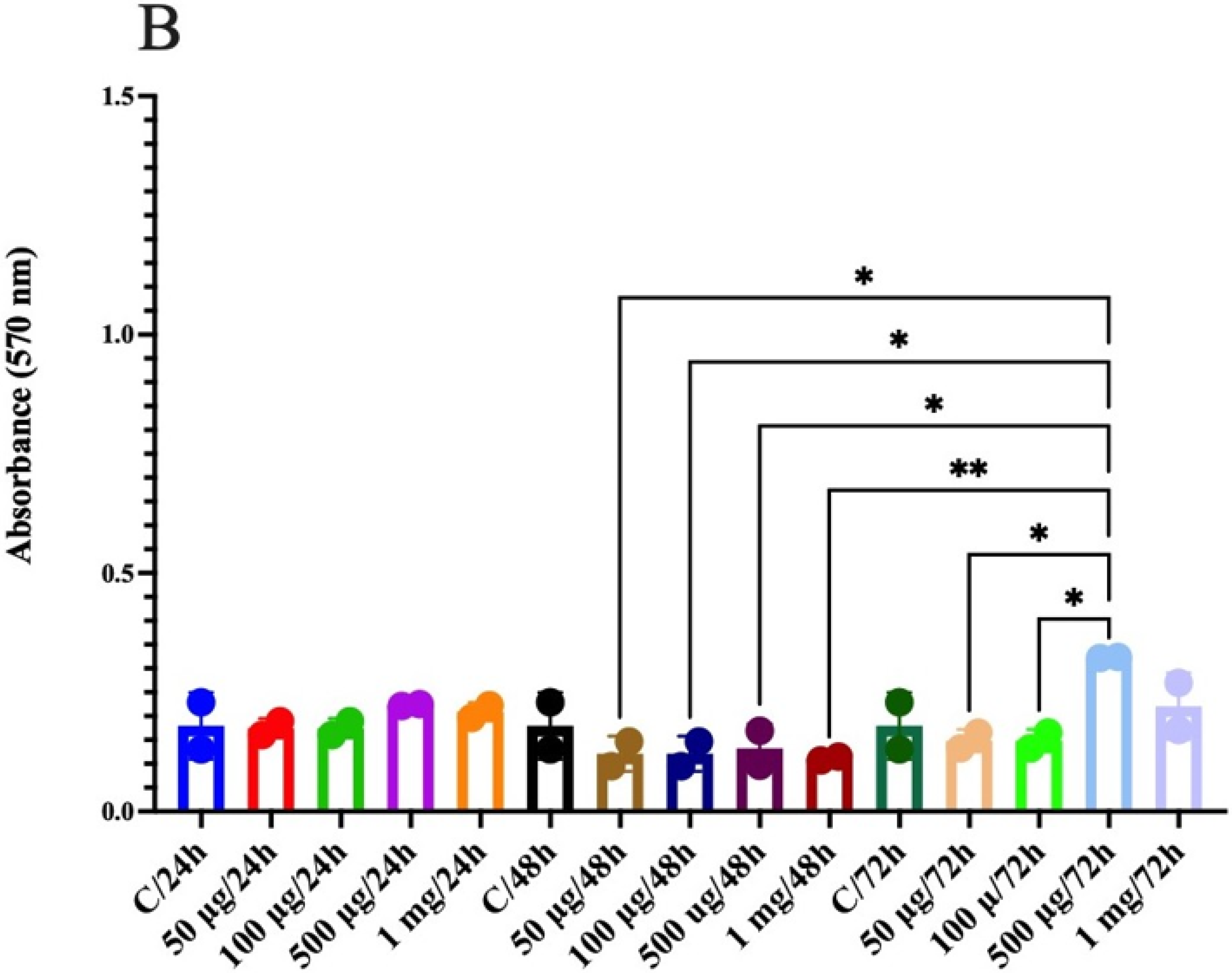
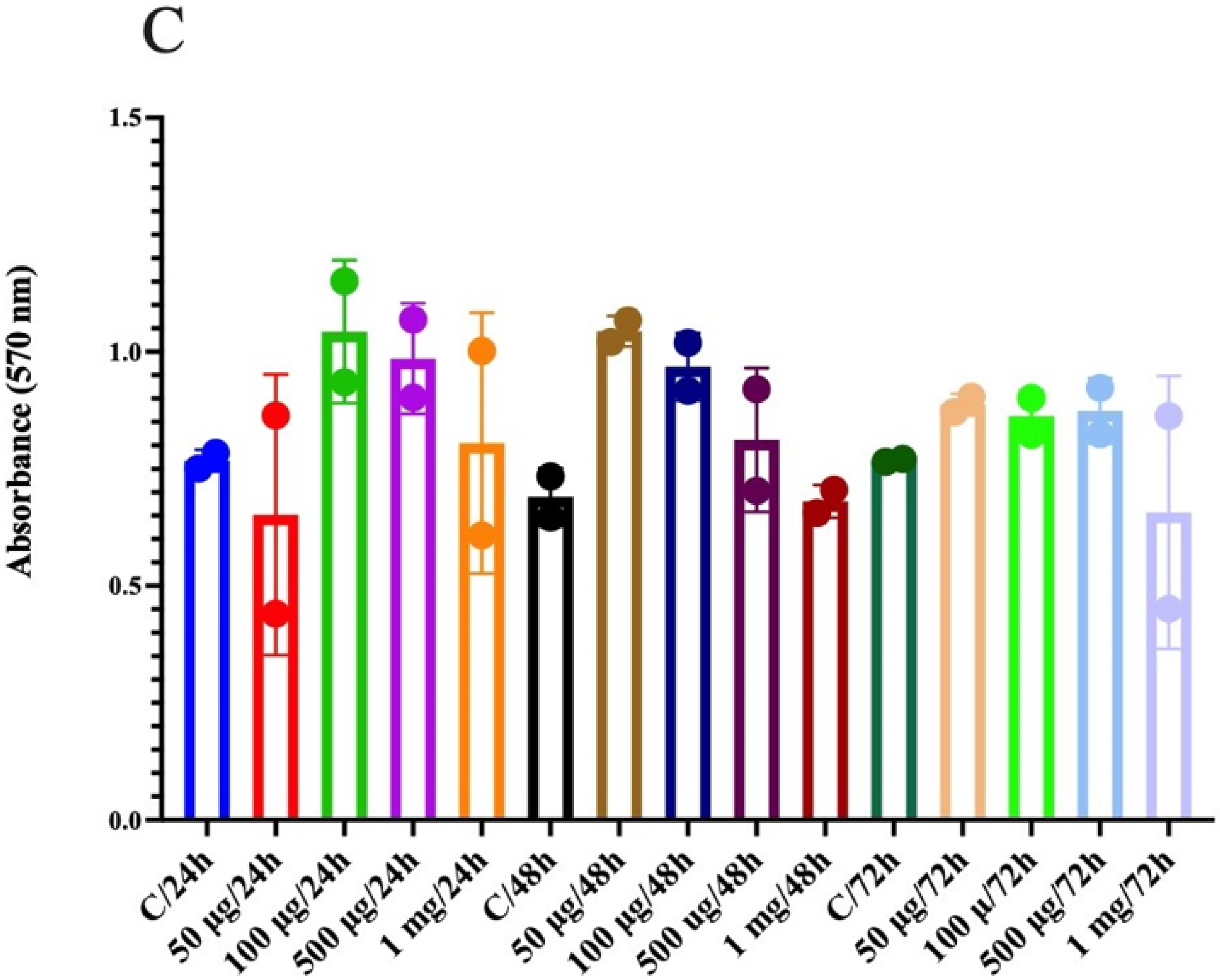
3.10. Anticancer Activity in Human Colon Epithelial Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lima, B.N.B.; Lima, F.F.; Tavares, M.I.B.; Costa, A.M.M.; Pierucci, A.P.T. Determination of the centesimal composition and characterization of flours from fruit seeds. Food Chem. 2014, 151, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Ocloo, F.C.K.; Bansa, D.; Boatin, R.; Adom, T.; Agbemavor, W.S. Physico-chemical, functional and pasting characteristics of flour produced from jackfruits (Artocarpus heterophyllus) seeds. Agric. Biol. J. N. Am. 2010, 1, 903–908. [Google Scholar] [CrossRef]
- Butool, S.; Butool, M. Nutritional quality on value addition to jack fruit seed, flour. Int. J. Sci. Res. 2015, 4, 2406–2411. [Google Scholar]
- Umesh, J.B.; Panaskar-Shrimant, N.; Bapat, V.A. Evaluation of antioxidant capacity and phenol content in jackfruit (Artocarpus heterophyllus) fruit pulp. Plant Foods Hum. Nutr. 2010, 65, 99–104. [Google Scholar]
- Madruga, M.S.; de Alburquerque, F.S.M.; Silva, I.R.A.; Do Amaral, D.S.; Magnani, M.; Neto, V.Q. Chemical morphological and functional properties of Brazilian jackfruit (Artocarpus heterophyllus L.) seeds starch. Food Chem. 2014, 143, 440–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reza, F.; Tajul-Aris, Y.; Wan-Nadiah, W.A.; Wahidu, Z. Effects of incorporation of jackfruit rind power on chemical and functional properties of bread. Trop. Life Sci. Res. 2018, 29, 113–126. [Google Scholar]
- Eke-Ejiofor, J.; Beleya, E.A.; Onyenorah, N.I. The effect of processing methods on the functional and compositional properties of jackfruit seed flour. Int. J. Nutr. Food Sci. 2014, 3, 166–173. [Google Scholar] [CrossRef]
- Islam, S.; Begum, R.; Khatun, M.; Dey, K.C. A study on nutritional and functional properties analysis of Jackfruit seed flour and value addition to biscuits. Int. J. Eng. Res. Technol. 2015, 4, 139–147. [Google Scholar]
- Prasertsit, K.; Thitipong, R.; Chetpattananondh, P. Possible prebiotic and gallic acid separation from jackfruit seed extract. Songklanakarin J. Sci. Technol. 2015, 37, 353–359. [Google Scholar]
- Faghfoori, Z.; Gargari, B.P.; Gharamaleki, A.S.; Bagherpour, H.; Khosroushahi, A.Y. Cellular and molecular mechanisms of probiotics effects on colorectal cancer. J. Funct. Foods 2015, 18, 463–472. [Google Scholar] [CrossRef]
- Franco-Robles, E.; López, M.G. Implication of fructans in health: Immunomodulatory and antioxidant mechanisms. Sci. World J. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, H.; Takemura, N.; Sonoyama, K.; Kawagishi, H.; Topping, D.L.; Conlon, M.A.; Morita, T. Degree of polymerization of inulin-type fructans differentially affects number of lactic acid bacteria, intestinal immune functions, and immunoglobulin A Secretion in the rat cecum. J. Agric. Food Chem. 2011, 59, 5771–5778. [Google Scholar] [CrossRef]
- Vogt, L.; Meyer, D.; Pullens, G.; Faas, M.; Smelt, M.; Venema, K.; Ramasamy, U.; Schols, H.A.; De Vos, P. Immunological properties of inulin-type fructans. Crit. Rev. Food Sci. Nutr. 2016, 55, 414–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOAC. Official Methods of Analysis of AOAC, 18th ed.; Association of Official Analytical Chemists: Gathersburg, MD, USA, 2005. [Google Scholar]
- González-Muñoz, A.; Montero, B.; Enrione, J.; Mariacevich, S. Rapid prediction of moisture content of quinoa (Chenopodium quinoa Willd.) flour by Fourier transform infrared (FTIR) spectroscopy. J. Cereal Sci. 2016, 71, 246–249. [Google Scholar] [CrossRef]
- Castillo-Andrade, A.I.; Rivera-Bautista, C.; Soria Guerra, R.E.; Ruiz-Cabrera, M.A.; Fuentes-Ahumada, C.; Garcia-Chávez, E.; Grajales-Lagunes, A. Agave fructans as gut health promoters: Prebiotic activity and inflammatory response in Wistar healthy rats. Int. J. Biol. Macromol. 2019, 136, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Soong, Y.; Barlow, P. Antioxidant activity and phenolic content of select fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Santos-Zea, L.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S. Comparative analyses of total phenols, antioxidant activity, and flavonol glycoside profile of cladode flours from different varieties of Opuntia spp. J. Agric. Food Chem. 2011, 59, 7054–7061. [Google Scholar] [CrossRef] [PubMed]
- Falade, K.O.; Okafor, C.A. Physical, functional, and pasting properties of flours from corms of two Cocoyam (Colocasia esculenta and Xanthosoma sagittifolium) cultivars. J. Food Sci. Technol. 2015, 52, 3440–3448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juárez-Barrientos, J.M.; Hernández-Santos, B.; Herman-Lara, E.; Martínez-Sánchez, C.E.; Torruco-Uco, G.J.; Ramírez-Rivera, E.J.; Pineda-Pineda, J.M.; Rodríguez-Miranda, J. Effects of boiling on the functional, thermal and compositional properties of the Mexican jackfruit (Artocarpus heterophyllus) seed jackfruit seed meal properties. Emir. J. Food Agric. 2017, 29, 1–9. [Google Scholar] [CrossRef]
- Moreno-Vilet, L.; García-Hernández, M.H.; Delgado-Portales, R.E.; Corral-Fernández, N.M.; Cortez-Espinoza, N.; Ruiz-Cabrera, M.A.; Portales-Pérez, D.P. In vitro assessment of Agave fructans (Agave salmiana) as prebiotics and immune system activators. Int. J. Biol. Macromol. 2014, 63, 181–187. [Google Scholar] [CrossRef] [PubMed]
- ICMSF (International Commission on Microbiological Specifications for Food of the International Union of Microbiological Societies). Microorganism in Foods 1: Their Significance and Methods and Enumeration Microorganism in Foods, 2nd ed.; University of Toronto Press: Toronto, ON, Canada, 1978. [Google Scholar]
- Amornrat, M.; Kamontip, S. Physico-chemical properties of flour and starch from jackfruit seeds (Artocarpus heterophyllus Lam.) compared with modified starches. Int. J. Food Sci. Technol. 2004, 39, 271–276. [Google Scholar]
- Hager, A.; Wolter, A.; Jacob, F.; Zannini, E.; Arendt, E. Nutritional properties and ultra-structure of commercial gluten free flours from different botanical sources compared to wheat flours. J. Cereal Sci. 2012, 56, 239–247. [Google Scholar] [CrossRef]
- Liu, H.; Chaudhary, D.; Shin-Ichi, Y.T.; Moses, O. Glycerol/starch/Na+ montmorillonite nanocomposites: An XRD, FTIR, DSC and 1H NMR study. Carbohydr. Polym. 2011, 83, 1591–1597. [Google Scholar] [CrossRef]
- Salunkhe, D.K.; Chavan, J.K.; Kadam, S.S.; Reddy, N.R. Pigeon pea as an important food source. Crit. Rev. Food Sci. Nutr. 1986, 23, 103–145. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Jalil-Masoumi, S.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanism and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirai, T.; Suzuki, Y.; Kamikado, K.; Koga, Y.; Aoki, R. Kestose, a prebiotic fructooligosaccharide, enhances intercelular tight junction recovery via a rho-associated knase-dependent mechanism in intestinal CaCo-2-Cells. Int. J. Probiotics Prebiotics 2013, 8, 53–60. [Google Scholar]
- Fernandes, F.; Ferreres, F.; Gil-Izquierdo, A.; Oliveira, A.; Valentão, P.; Andrade, P.B. Accumulation of primary and secondary metabolites in edible jackfruit seed tissues and scavenging of reactive nitrogen species. Food Chem. 2017, 233, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.S.; Park, Y. Effect of conjugated linoleic acid on bone formation and rheumatoid arthritis. Eur. J. Pharmacol. 2007, 568, 16–24. [Google Scholar]
- Granados, S.; Quiles, J.L.; Gil, A.; Ramírez-Tortosa, M.C. Lípidos de la dieta y cáncer. Nutr. Hosp. 2006, 21, 44–54. [Google Scholar]
- Simopoulos, A.P. Omega-6/omega-3 essential fatty acids: Biological effects. World Rev. Nutr. Diet. 2009, 99, 1–16. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary reference values for fat, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef] [Green Version]
- Kostic, A.Z.; Macucanovic-Jocic, M.P.; Spirovic-Trifunovic, B.D.; Vukasinovic, I.Z.; Pavlovic, V.B.; Pesic, M.V. Fatty acids of mize pollen—Quantification, nutritional and morphological evaluation. J. Cereal Sci. 2017, 77, 180–185. [Google Scholar] [CrossRef]
- Gupta, D.; Mann, S.; Sood, A.; Gupta, R.K. Phytochemical, nutritional and antioxidant activity evaluation of seeds of jackfruit (Artocarpus heterophyllus Lam.). Int. J. Pharm. Biol. Sci. 2011, 2, 336–345. [Google Scholar]
- Piga, A.; Del Caro, A.; Corda, G. From plums to prunes: Influence of drying parameters on polyphenols and antioxidant activity. J. Agric. Food Chem. 2003, 51, 3675–3681. [Google Scholar] [CrossRef] [PubMed]
- Que, F.; Mao, L.; Fang, X.; Wu, T. Comparison of hot air-drying and freeze-drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. Int. J. Food Sci. Technol. 2008, 43, 1195–1201. [Google Scholar] [CrossRef]
- Yadav, N.; Kaur, D.; Malaviya, R.; Singh, M.; Fatima, M.; Singh, L. Effect of thermal and non-thermal processing on antioxidant potential of cowpea seeds. Int. J. Food Prop. 2018, 21, 437–451. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, R.M.; Ida, E.I.; Falcão, H.G.; de Rezende, T.A.M.; de Santana Silva, J.; Fernandes Alves, C.C.; Pereira da Silva, M.A.; Buranelo-Egea, M. Evaluating technological quality of okara flours obtained by different drying processes. LWT Food Sci. Technol. 2020, 123, 1–28. [Google Scholar] [CrossRef]
- Vázquez-Ovando, A.; Rosado-Rubio, G.; Chel-Guerrero, L.; Betancur-Ancona, D. Physicochemical properties of a fibrous fraction from chia (Salvia hispanica L.). LWT Food Sci. Technol. 2009, 42, 168–173. [Google Scholar]
- Warechowska, M.; Warechowski, J.; Tyburski, J.; Siemianowska, E.; Nawrocka, A.; Mis, A.; Skrajda-Brdak, M. Evaluation of physicochemical properties, antioxidant potential and baking quality of grain and flour of primitive rye (Secale cereale var. Multicaule). J. Food Sci. Technol. 2019, 56, 3422–3430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Liu, F.; Xiao, Y.; Cao, J.; Wang, M.; Duan, X. Alterations in physicochemical and functional properties of buckwheat straw insoluble dietary fiber by alkaline hydrogen peroxide treatment. Food Chem. X 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Simpson, B.K.; Prasher, S.O.; Monpetit, D.; Malcolmson, L. Thermal processing effects on the functional properties and microstructure of lentil, chickpea, and pea flours. Food Res. Int. 2011, 44, 2534–2544. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y. Physicochemical and functional properties of coconut (Cocos nucifera L.) cake dietary fibres: Effects of cellulase hydrolysis, acid treatment and particle size distribution. Food Chem. 2018, 257, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, Q.; Wang, L.; Zha, S.; Zhang, L.; Zhao, B. Physicochemical and functional properties of dietary fiber from maca (Lepidium meyenii Walp.) liquor residue. Carbohydr. Polym. 2015, 132, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Kumuro, A.C.; Widiyanti, M.; Ratnawati, R.; Retnowati, D.S. Nutritional and functional properties changes during facultative submerged fermentation of gadung (Dioscorea hispida Dennst) tuber flour using Lactobacillus plantarum. Heliyon 2020, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kusumayanti, H.; Handayani, N.A.; Santosa, H. Swelling power and water solubility of cassava and sweet potatoes flour. Procedia Environ. Sci. 2015, 23, 164–167. [Google Scholar] [CrossRef] [Green Version]
- Motta-Romero, H.; Zhang, Y. Physicochemical properties and rheological behavior of flour and starches from four bean varieties for gluten-free pasta formulation. J. Agric. Food Res. 2019, 1, 100001. [Google Scholar] [CrossRef]
- Yu, J.; Ahmednay, M.; Goktepe, I. Peanut protein concentrate: Production and functional properties as affected by processing. Food Chem. 2007, 103, 121–129. [Google Scholar] [CrossRef]
- Regand, A.Z.; Chowdhury, S.M.; Tosh, T.M.; Wolever, S.; Wood, P. The molecular weight, solubility and viscosity of oat beta-glucan affect human glycemic response by modifying starch digestibility. Food Chem. 2011, 129, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Lima-Batista, V.; da Silva, T.F.; Lima de Jesús, L.C.; Coelho-Rocha, N.D.; Lima Barroso, F.A.; Tavares, L.M.; Azevedo, A.; Mancha-Agresti, M.; Martins-Drumond, M. Probiotics, prebiotic and synbiotic and paraprobiotics as a therapeutic alternative for intestinal mucositis. Front. Microbiol. 2020, 11, 544490. [Google Scholar] [CrossRef]
- Massa, L.N.M.; Menezesa, F.N.D.D.; de Albuquerquea, T.M.R.; de Oliveira, S.P.A.; dos Santos Lima, M.; Magnanic, M.; de Souzaa, E.L. Effects of digested jabuticaba (Myrciaria jaboticaba (Vell.) Berg) by-product on growth and metabolism of Lactobacillus and Bifidobacterium indicate prebiotic properties. LWT Food Sci. Technol. 2020, 131, 1–8. [Google Scholar]
- IARC, OMS. The Global Cancer Observatory: Colorectal Cancer Factsheet, 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/8-Colon-fact-sheet.pdf (accessed on 25 July 2021).
- Sun, G.; Zheng, Z.; Lee, M.H.; Xu, Y.; Kang, S.; Dong, Z.; Wang, M.; Gu, Z.; Li, H.; Chen, W. Chemoprevention of colorectal cancer by artocarpin, a dietary phytochemical from Artocarpus heterophyllus. J. Agric. Food Chem. 2017, 65, 3474–3480. [Google Scholar] [CrossRef] [PubMed]
- Wiater, A.; Paduch, R.; Trojnar, S.; Choma, A.; Pleszczy, M.; Adamczyk, P.; Pi, M.; Próchniak, K.; Szczodrak, J.; Strawa, J.; et al. The effect of water-soluble polysaccharide from jackfruit (Artocarpus heterophyllus Lam.) on human colon carcinoma cells cultured in vitro. Plants 2020, 9, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibovitz, A.; Stinson, J.C.; McCombs, W.B.; Ill McCoy, C.E.; Mazur, K.C.; Mabry, N.D. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976, 36, 4562–4569. [Google Scholar] [PubMed]
- Tomita, N.; Jiang, W.; Hibshoosh, H.; Warburton, D.; Kahn, S.M.; Weinstein, I.B. Isolation and characterization of a highly malignant variant of the SW480 human colon cancer cell line. Cancer Res. 1992, 52, 6840–6847. [Google Scholar] [PubMed]
- Basak, D.; Uddin, M.N.; Hancock, J. The role of oxidative stress and its counteractive utility in colorectal cancer (CRC). Cancers 2020, 12, 3336. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Aykin-Burns, N.; Ahmad, I.M.; Zhu, Y.; Oberley, L.W.; Spitz, D.R. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem. J. 2009, 418, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
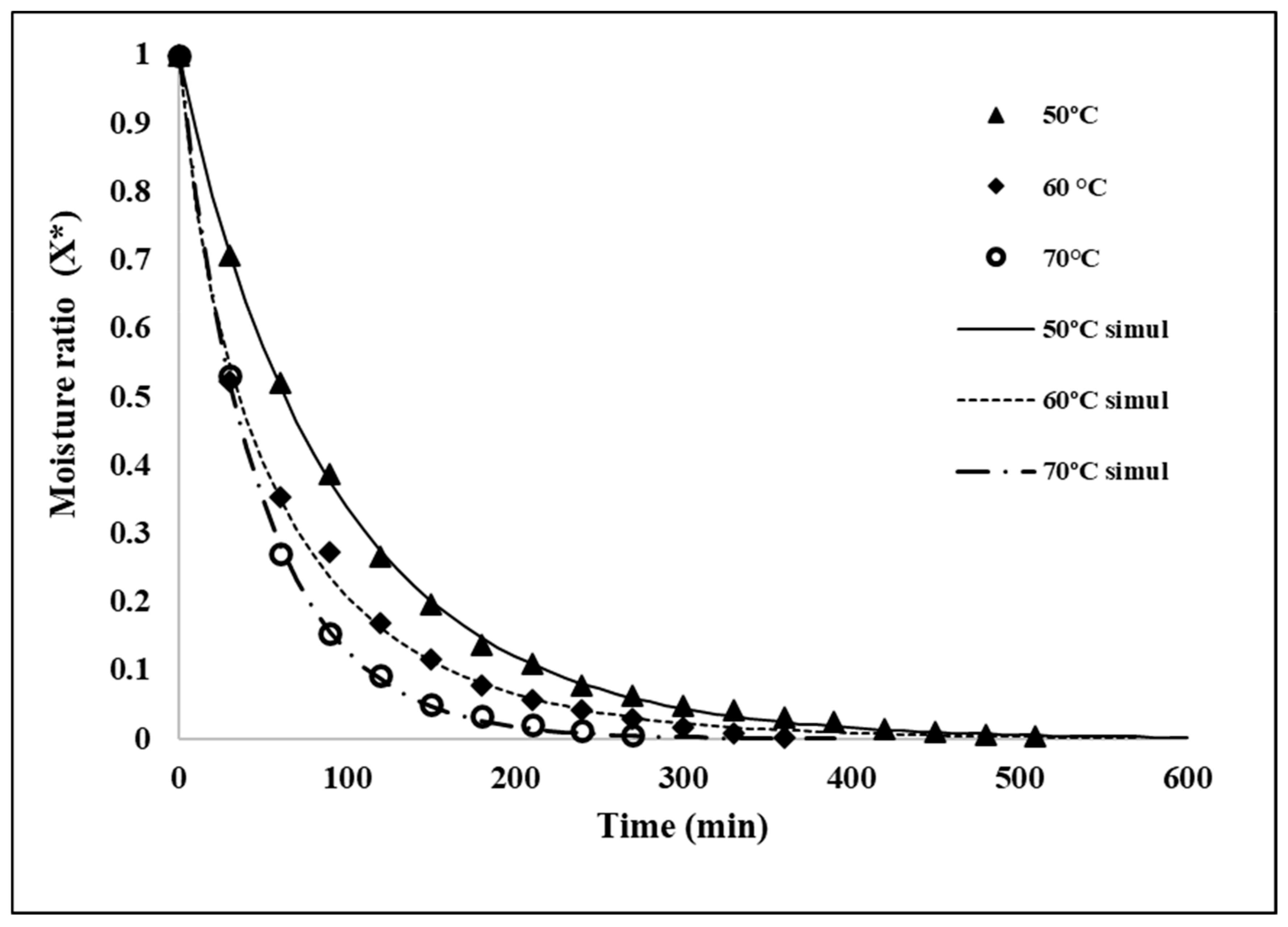



| Model | 50 °C | 60 °C | 70 °C | |||
|---|---|---|---|---|---|---|
| Page | Coefficients | r2 | Coefficients | r2 | Coefficients | r2 |
| k = 0.0129 | 0.999 | k = 0.0402 | 0.996 | k = 0.0248 | 0.999 | |
| n = 0.9618 | n = 0.7956 | n = 0.9593 | ||||
| Component g/100 g | Means ± Standard Deviation |
|---|---|
| Moisture | 6.54 ± 0.03 |
| Ash | 4.19 ± 0.13 |
| Total carbohydrates | 73.87 ± 1.06 |
| Reducing sugars | 6.11 ± 0.04 |
| Total dietary fiber | 31.59 ± 11.14 |
| Proteins ** | 14.07 ± 0.15 |
| Lipids | 1.3 ± 0.24 |
| Carbohydrate | Content (mg/g) |
|---|---|
| Compounds greater than 5 degrees of polymerization | 20.0 ± 1.3 |
| 1-Kestose | 3.8 ± 1.43 |
| Sucrose | 35.5 ± 5.4 |
| Glucose | 29.4 ± 2.7 |
| Fructose | 22.9 ± 6.2 |
| Fatty Acid | Content (g/100 g) |
|---|---|
| Palmitic acid (C16:0) | 36.20 ± 2.68 |
| Stearic acid (18:0) | 3.54 ± 0.50 |
| Tricosanoate acid (C23:0) | 9.39 ± 0.51 |
| Oleic acid (C18:1) | 4.15 ± 1.38 |
| Linoleic acid (C18:2) | 35.11 ± 0.59 |
| Linolenic acid (C18:3) | 2.82 ± 0.25 |
| Arachidonic acid (C20:4) | 3.82 ± 0.23 |
| Eicosapentaenoic acid (20:5 (n-3)) | 4.01 ± 0.65 |
| Docosadienoic acid (C22:2 (n-6)) | 0.97 ± 0.02 |
| Total saturated fatty acids | 49.13 ± 4.90 |
| Total monounsaturated fatty acids | 4.15 ± 2.30 |
| Total polyunsaturated fatty acids | 46.72 ± 2.12 |
| ω6/ω3 | 5:1 |
| Temperature (°C) | Polyphenol Content (mg of Gallic Acid/g of Sample) | DPPH Radical (µmol Trolox/100 g) | FRAP (µmol Trolox/100 g) |
|---|---|---|---|
| 50 | 2.65 ± 0.86 a | 1579.51 ± 1.91 a | 1717.20 ± 3.9 a |
| 60 | 2.42 ± 0.74 a | 1607.87 ± 2.14 b | 901.45 ± 5.84 b |
| 70 | 2.39 ± 0.92 a | 1617.48 ± 0.72 b | 489.77 ± 2.87 c |
| Fisher | 0.539 | 0.0211 | <0.0001 |
| Temperature (°C) | Water Solubility (%) | Swelling Power (g/g) |
|---|---|---|
| 25 | 0.087 ± 0.001 a | 4.062 ± 0.010 a |
| 40 | 0.1586 ± 0.0001 a | 4.779 ± 0.012 a |
| 50 | 0.1527 ± 0.007 a | 5.022 ± 0.045 a |
| 60 | 0.1580 ± 0.001 a | 5.163 ± 0.041 a |
| 70 | 0.1492 ± 0.004 a | 5.515 ± 0.088 a |
| Fisher | 0.069 | 0.112 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trejo Rodríguez, I.S.; Alcántara Quintana, L.E.; Algara Suarez, P.; Ruiz Cabrera, M.A.; Grajales Lagunes, A. Physicochemical Properties, Antioxidant Capacity, Prebiotic Activity and Anticancer Potential in Human Cells of Jackfruit (Artocarpus heterophyllus) Seed Flour. Molecules 2021, 26, 4854. https://doi.org/10.3390/molecules26164854
Trejo Rodríguez IS, Alcántara Quintana LE, Algara Suarez P, Ruiz Cabrera MA, Grajales Lagunes A. Physicochemical Properties, Antioxidant Capacity, Prebiotic Activity and Anticancer Potential in Human Cells of Jackfruit (Artocarpus heterophyllus) Seed Flour. Molecules. 2021; 26(16):4854. https://doi.org/10.3390/molecules26164854
Chicago/Turabian StyleTrejo Rodríguez, Ibna Suli, Luz Eugenia Alcántara Quintana, Paola Algara Suarez, Miguel Angel Ruiz Cabrera, and Alicia Grajales Lagunes. 2021. "Physicochemical Properties, Antioxidant Capacity, Prebiotic Activity and Anticancer Potential in Human Cells of Jackfruit (Artocarpus heterophyllus) Seed Flour" Molecules 26, no. 16: 4854. https://doi.org/10.3390/molecules26164854
APA StyleTrejo Rodríguez, I. S., Alcántara Quintana, L. E., Algara Suarez, P., Ruiz Cabrera, M. A., & Grajales Lagunes, A. (2021). Physicochemical Properties, Antioxidant Capacity, Prebiotic Activity and Anticancer Potential in Human Cells of Jackfruit (Artocarpus heterophyllus) Seed Flour. Molecules, 26(16), 4854. https://doi.org/10.3390/molecules26164854








