In Situ Synchrotron X-ray Diffraction Studies of Hydrogen-Desorption Properties of 2LiBH4–Mg2FeH6 Composite
Abstract
:1. Introduction
| Name | Reaction | Equation |
| A | (1) | |
| B | (2) | |
| C | (3) |
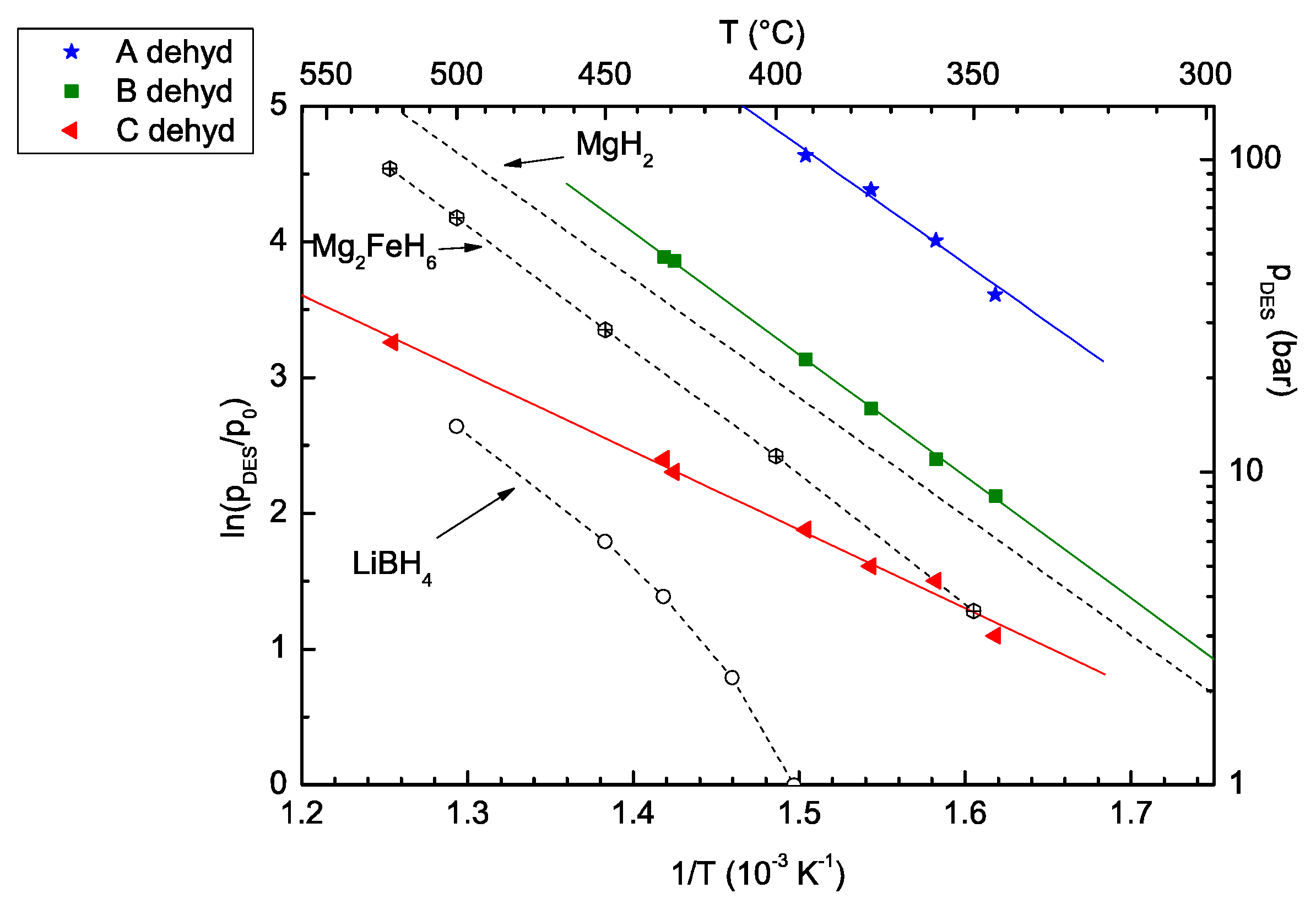
2. Results and Discussion
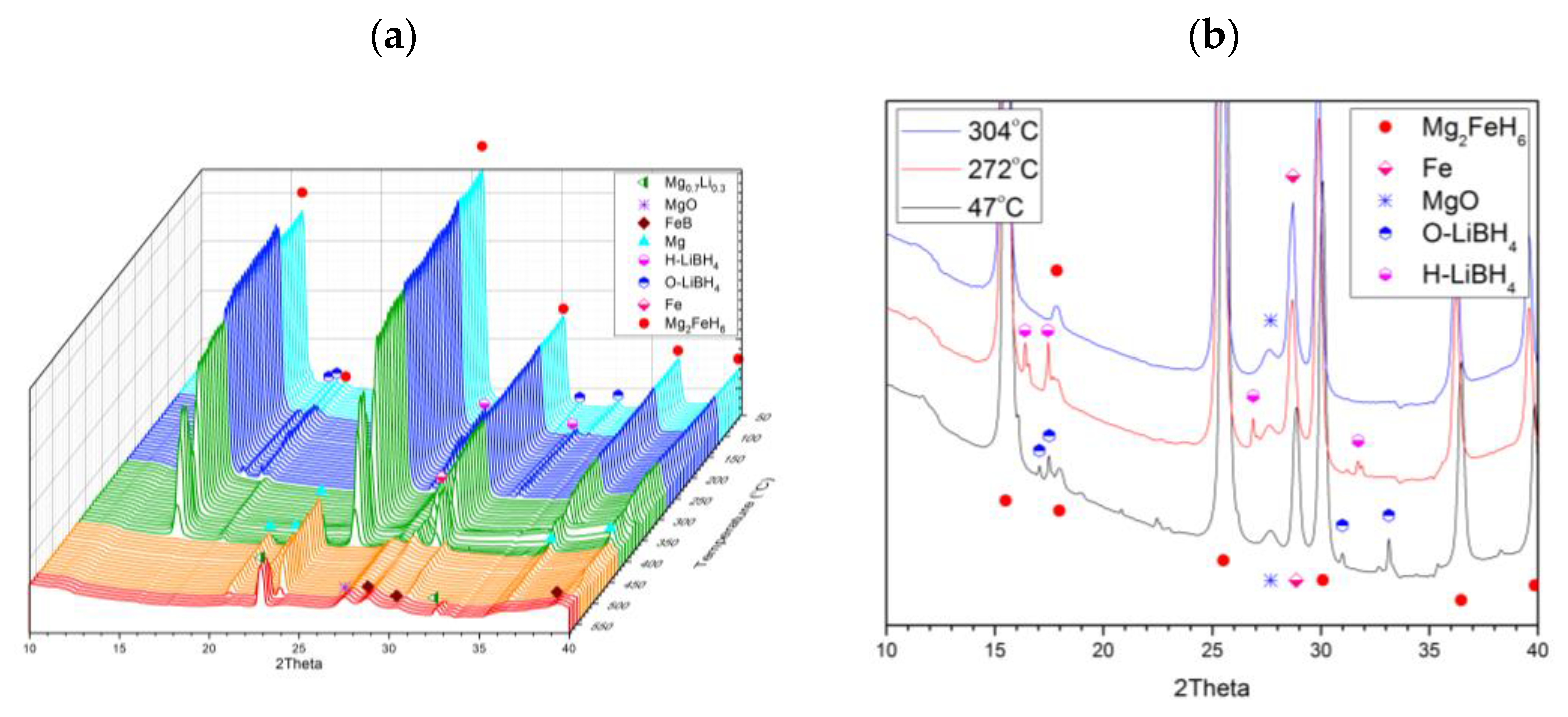
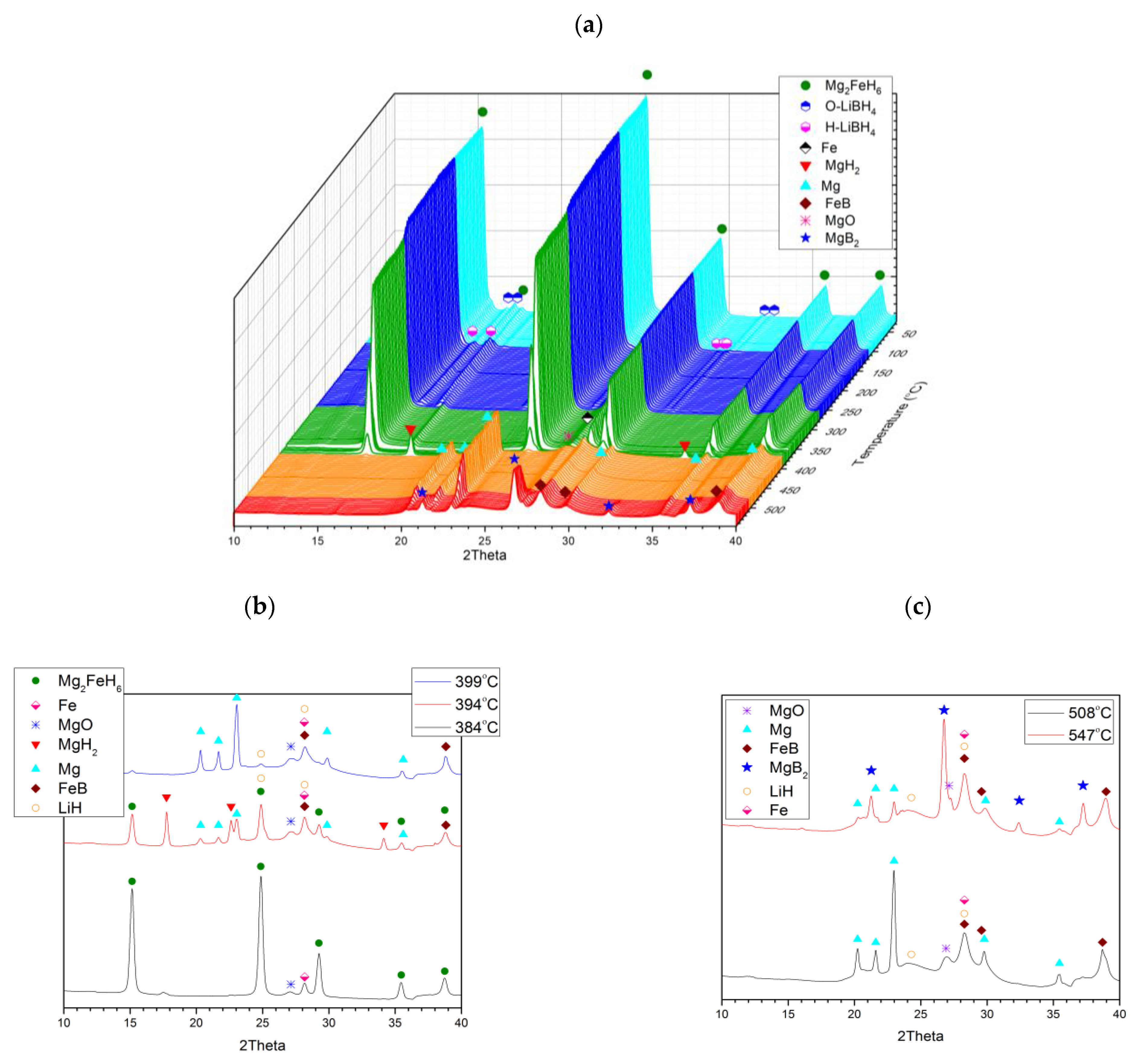
2.1. Purity of Mg2FeH6
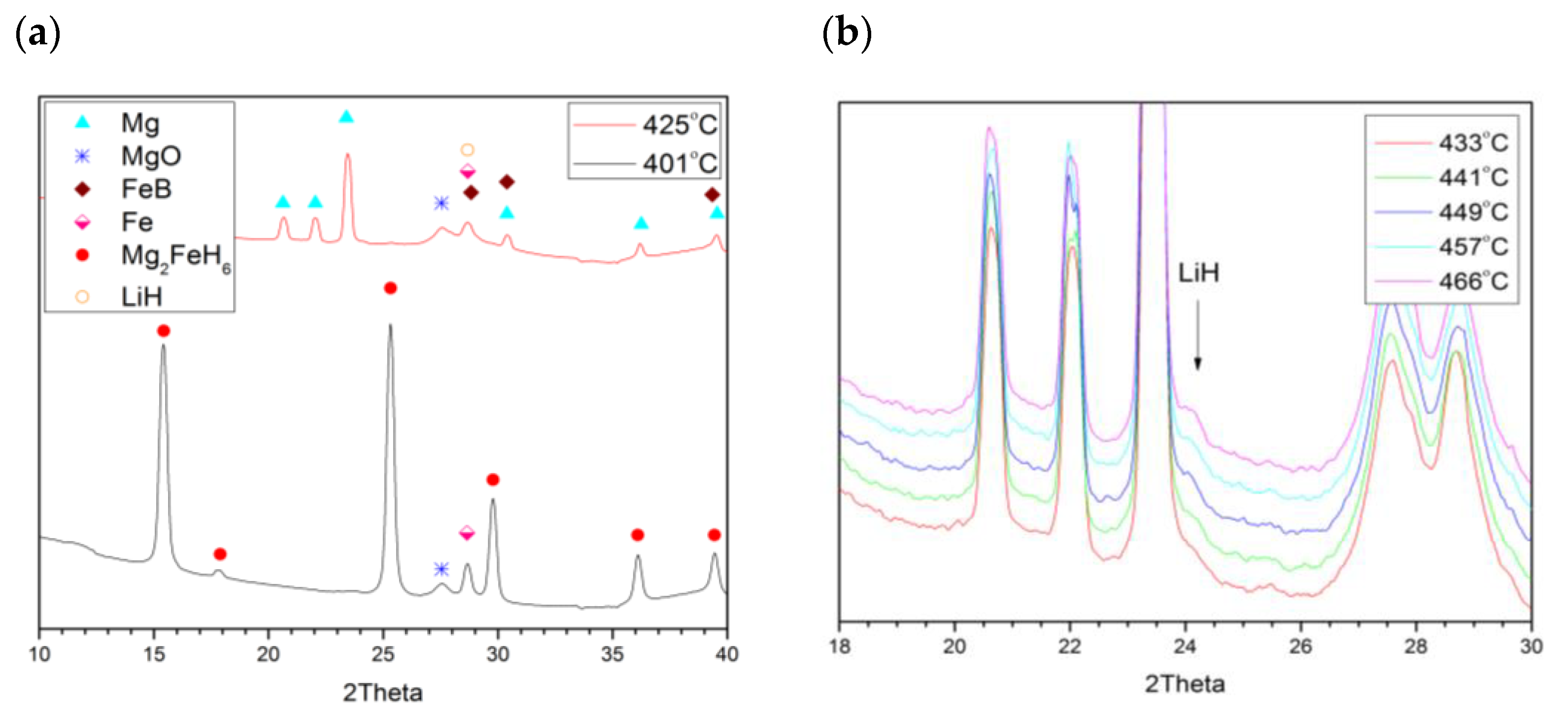
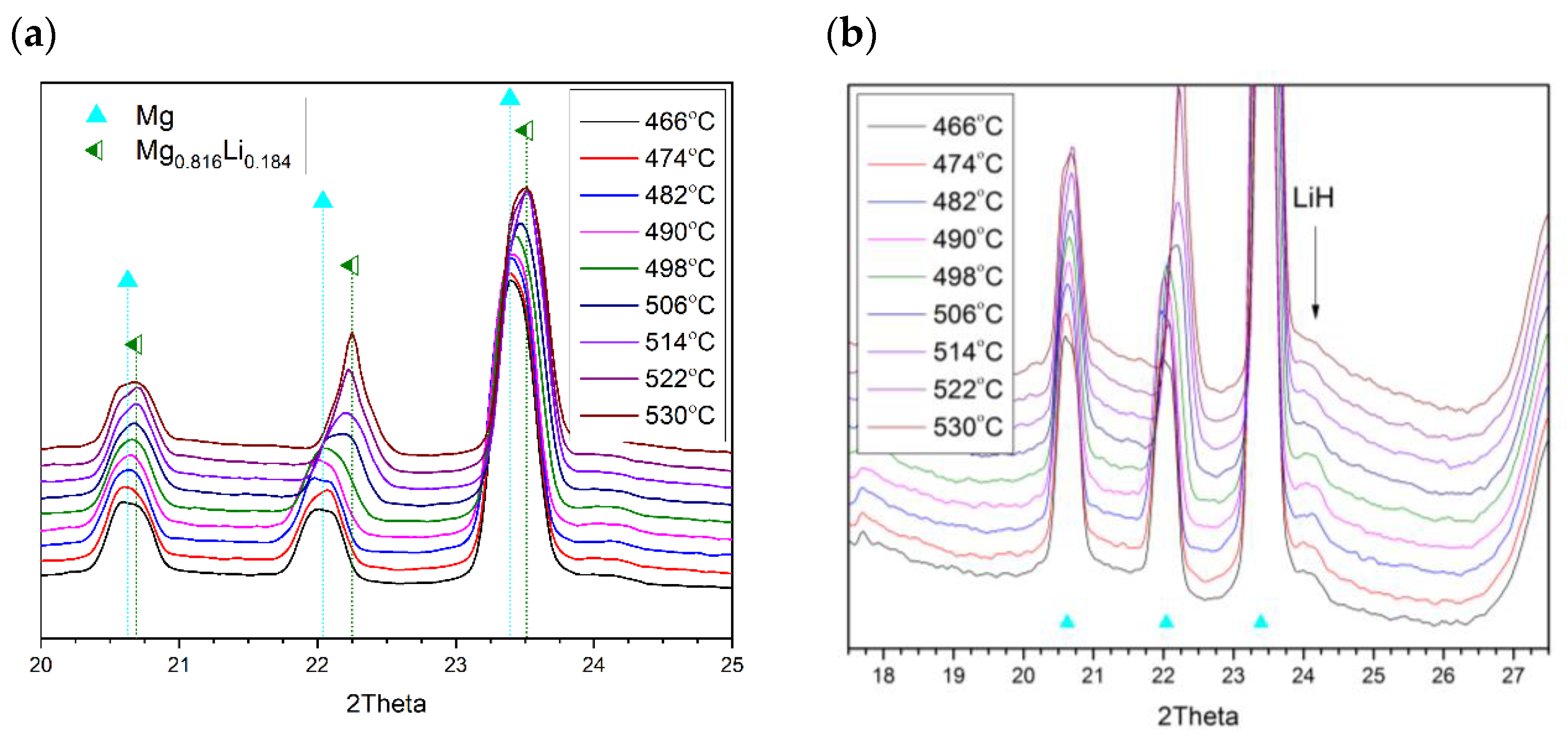
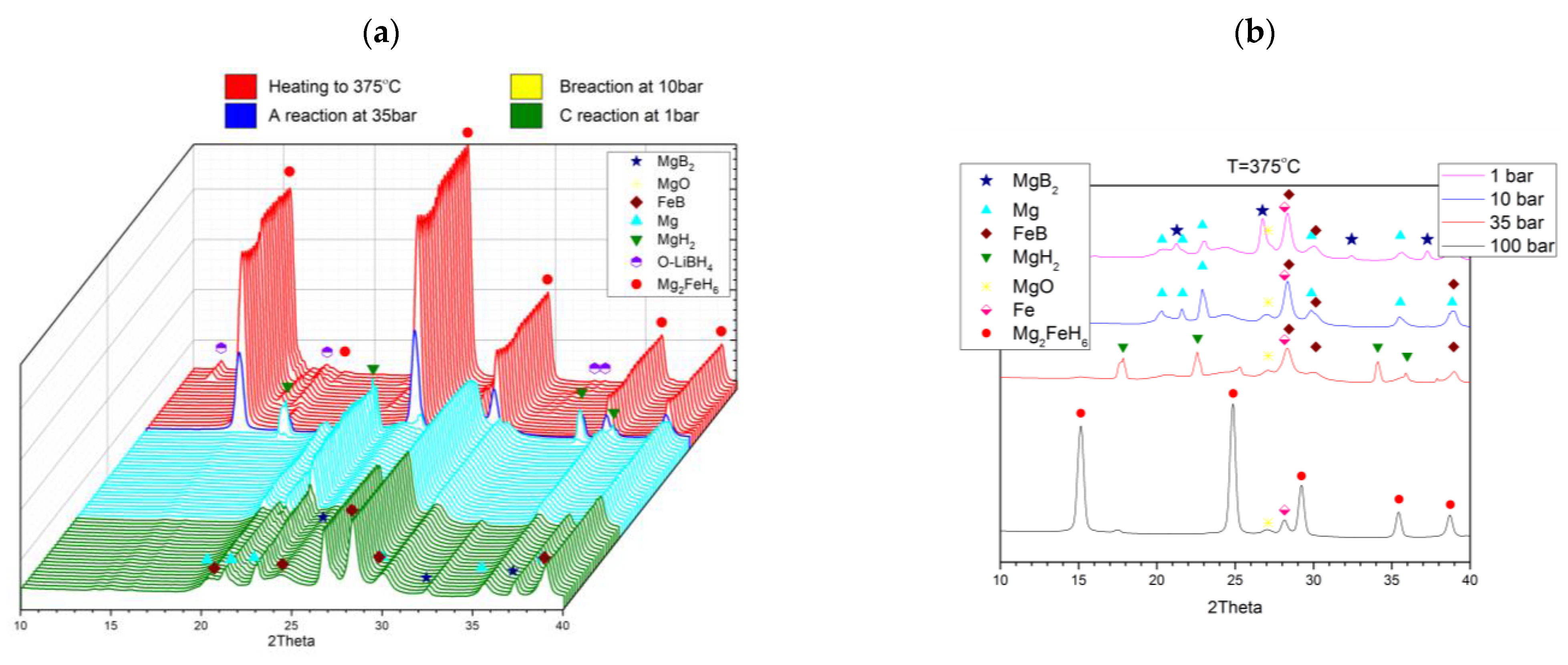
2.2. Joint Decomposition of 2LiBH4–Mg2FeH6
| Step | Reaction | Temperature (°C) | Equation |
| 1 | 425 | (4) | |
| 2 | 440 | (5) | |
| 3 | 500 | (6) | |
| 4 | + | 554 | (7) |
| (8) | |||
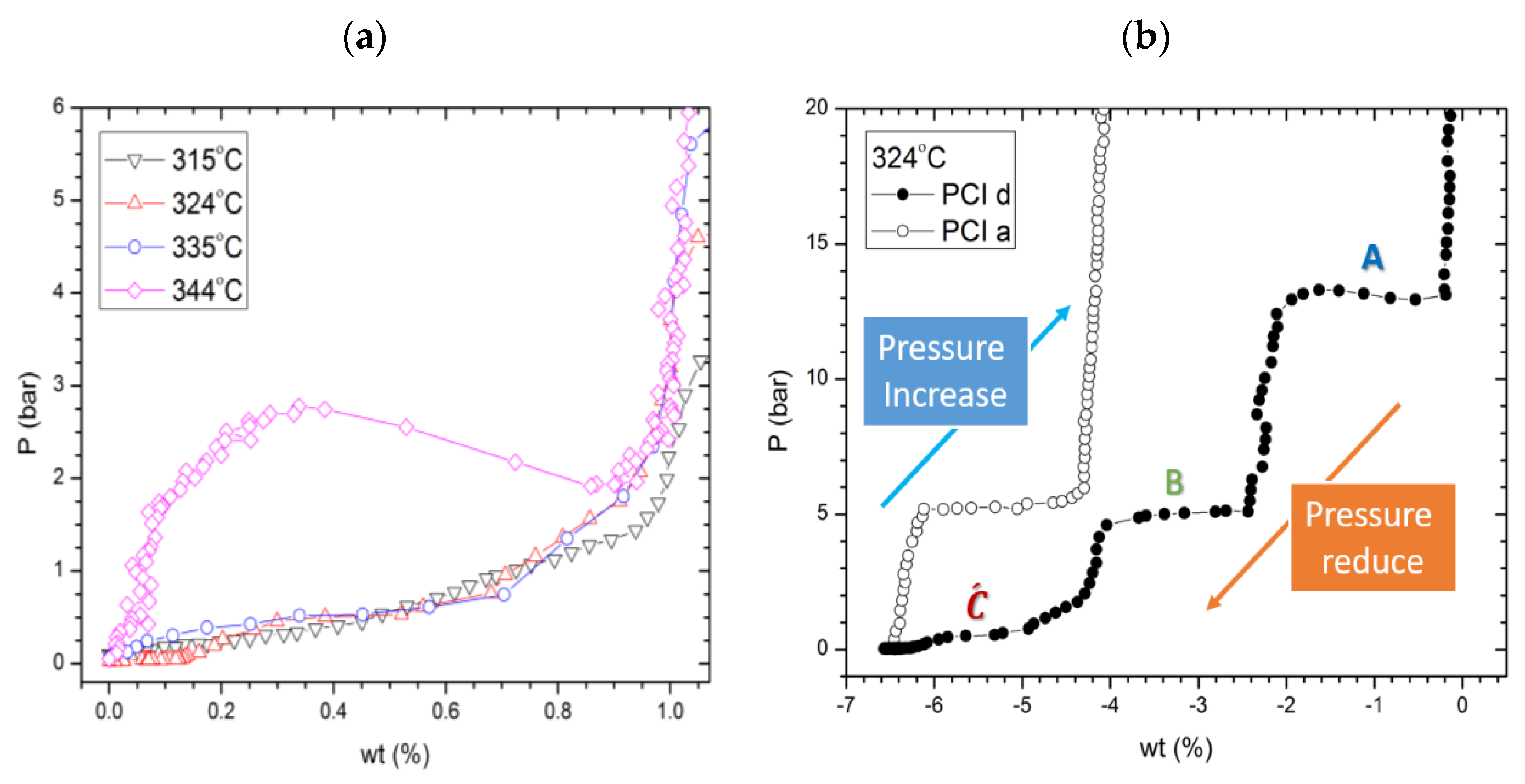
2.3. Reaction C’s Lower Temperature Limit
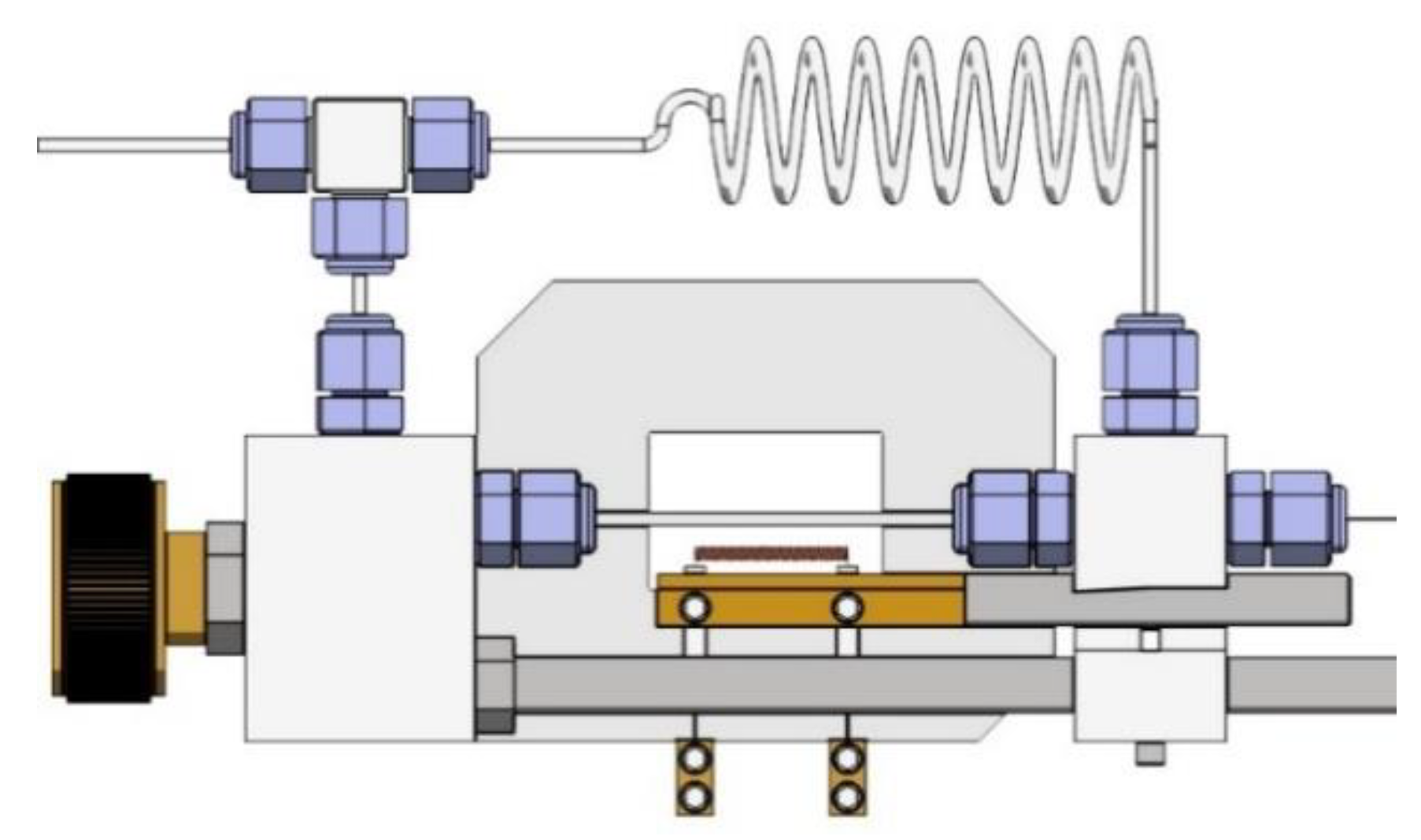
3. Experimental Methods
X-ray Diffraction
4. Conclusions
- -
- Longer incubation time required for reaction C;
- -
- Lower overheating needed for reaction C (slower reaction);
- -
- Lower underpressure (P-Peq) required for reaction C (slower reaction).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lin, H.-J.; Li, H.-W.; Shao, H.; Lu, Y.; Asano, K. In situ measurement technologies on solid-state hydrogen storage materials: A review. Mater. Today Energy 2020, 17, 100463. [Google Scholar] [CrossRef]
- Téliz, E.; Abboud, M.; Faccio, R.; Esteves, M.; Zinola, F.; Díaz, V. Hydrogen storage in AB2 hydride alloys: Diffusion processes analysis. J. Electroanal. Chem. 2020, 879, 114781. [Google Scholar] [CrossRef]
- Marinelli, M.; Santarelli, M. Hydrogen storage alloys for stationary applications. J. Energy Storage 2020, 32, 101864. [Google Scholar] [CrossRef]
- Züttel, A.; Borgschulte, A.; Schlapbach, L. Hydrogen as a Future Energy Carrier; Wiley: Hoboken, NJ, USA, 2011; ISBN 9783527622900. [Google Scholar]
- Bogdanović, B.; Reiser, A.; Schlichte, K.; Spliethoff, B.; Tesche, B. Thermodynamics and dynamics of the Mg–Fe–H system and its potential for thermochemical thermal energy storage. J. Alloys Compd. 2002, 345, 77–89. [Google Scholar] [CrossRef]
- Hirose, K. Handbook of Hydrogen Storage: New Materials for Future Energy Storage; John Wiley & Sons: Hoboken, NJ, USA, 2010; ISBN 3527322736. [Google Scholar]
- Vajo, J.J.; Olson, G.L. Hydrogen storage in destabilized chemical systems. Scr. Mater. 2007, 56, 829–834. [Google Scholar] [CrossRef]
- Chelbi, A.; Ait-Kadi, D. Generalized inspection strategy for randomly failing systems subjected to random shocks. Int. J. Prod. Econ. 2000, 64, 379–384. [Google Scholar] [CrossRef]
- Walker, G.S.; Grant, D.M.; Price, T.C.; Yu, X.; Legrand, V. High capacity multicomponent hydrogen storage materials: Investigation of the effect of stoichiometry and decomposition conditions on the cycling behaviour of LiBH4–MgH2. J. Power Sources 2009, 194, 1128–1134. [Google Scholar] [CrossRef] [Green Version]
- Siegel, D.J.; Wolverton, C.; Ozoliņš, V. Thermodynamic guidelines for the prediction of hydrogen storage reactions and their application to destabilized hydride mixtures. Phys. Rev. B 2007, 76, 134102. [Google Scholar] [CrossRef] [Green Version]
- Ghaani, M.R.; Catti, M.; Nale, A. Thermodynamics of dehydrogenation of the 2LiBH4-Mg 2FeH6 composite. J. Phys. Chem. C 2012, 116. [Google Scholar] [CrossRef] [Green Version]
- Catti, M.; Ghaani, M.R.; Pinus, I. Overpressure Role in Isothermal Kinetics of H2 Desorption–Absorption: The 2LiBH4–Mg2FeH6 System. J. Phys. Chem. C 2013, 117, 26460–26465. [Google Scholar] [CrossRef]
- Züttel, A.; Rentsch, S.; Fischer, P.; Wenger, P.; Sudan, P.; Mauron, P.; Emmenegger, C. Hydrogen storage properties of LiBH4. J. Alloys Compd. 2003, 356–357, 515–520. [Google Scholar] [CrossRef]
- Li, G.; Matsuo, M.; Deledda, S.; Sato, R.; Hauback, B.C.; Orimo, S. Dehydriding Property of LiBH4 Combined with Mg2FeH6. Mater. Trans. 2013, 54, 1532–1534. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Matsuo, M.; Aoki, K.; Ikeshoji, T.; Orimo, S. Dehydriding Process and Hydrogen–Deuterium Exchange of LiBH4–Mg2FeD6 Composites. Energies 2015, 8, 5459–5466. [Google Scholar] [CrossRef] [Green Version]
- Langmi, H.W.; McGrady, G.S.; Newhouse, R.; Rönnebro, E. Mg2FeH6–LiBH4 and Mg2FeH6–LiNH2 composite materials for hydrogen storage. Int. J. Hydrog. Energy 2012, 37, 6694–6699. [Google Scholar] [CrossRef]
- Deng, S.; Xiao, X.; Han, L.; Li, Y.; Li, S.; Ge, H.; Wang, Q.; Chen, L. Hydrogen storage performance of 5LiBH4 + Mg2FeH6 composite system. Int. J. Hydrog. Energy 2012, 37, 6733–6740. [Google Scholar] [CrossRef]
- Li, G.; Matsuo, M.; Takagi, S.; Chaudhary, A.-L.; Sato, T.; Dornheim, M.; Orimo, S. Thermodynamic Properties and Reversible Hydrogenation of LiBH4–Mg2FeH6 Composite Materials. Inorganics 2017, 5, 81. [Google Scholar] [CrossRef] [Green Version]
- Mauron, P.; Buchter, F.; Friedrichs, O.; Remhof, A.; Bielmann, M.; Zwicky, C.N.; Züttel, A. Stability and Reversibility of LiBH 4. J. Phys. Chem. B 2008, 112, 906–910. [Google Scholar] [CrossRef]
- Bogdanović, B.; Bohmhammel, K.; Christ, B.; Reiser, A.; Schlichte, K.; Vehlen, R.; Wolf, U. Thermodynamic investigation of the magnesium–hydrogen system. J. Alloys Compd. 1999, 282, 84–92. [Google Scholar] [CrossRef]
- Huot, J.; Boily, S.; Akiba, E.; Schulz, R. Direct synthesis of Mg2FeH6 by mechanical alloying. J. Alloys Compd. 1998, 280, 306–309. [Google Scholar] [CrossRef]
- Bassetti, A.; Bonetti, E.; Pasquini, L.; Montone, A.; Grbovic, J.; Vittori Antisari, M. Hydrogen desorption from ball milled MgH2 catalyzed with Fe. Eur. Phys. J. B 2005, 43, 19–27. [Google Scholar] [CrossRef]
- Castro, F.J.; Fuster, V.; Urretavizcaya, G. Hydrogen sorption properties of a MgH2–10wt.% graphite mixture. J. Alloys Compd. 2011, 509, S595–S598. [Google Scholar] [CrossRef]
- Haghparast, M.R.; Rajabi, M. Hydrogen Desorption Properties of MgH2-5 at% Ti-Cr-Mn-Fe-V Composite Via Combined Vacuum Arc Remelting and Mechanical Alloying. Procedia Mater. Sci. 2015, 11, 605–610. [Google Scholar] [CrossRef] [Green Version]
- Davis, W.D.; Mason, L.S.; Stegeman, G. The Heats of Formation of Sodium Borohydride, Lithium Borohydride and Lithium Aluminum Hydride. J. Am. Chem. Soc. 1949, 71, 2775–2781. [Google Scholar] [CrossRef]
- Catti, M.; Ghaani, M.R.; Nale, A. Reactions of the 2LiBH 4 –Mg 2 FeH 6 assemblage for hydrogen storage. Acta Crystallogr. Sect. A Found. Crystallogr. 2012, 68, S176. [Google Scholar] [CrossRef] [Green Version]
- Ghaani, M.R.; Catti, M. Study of New Materials and Their Functionality for Hydrogen Storage and Other Energy Applications. Ph.D. Thesis, University of Milano Bicocca Department, Milan, Italy, 2014. [Google Scholar]
- Zhang, X.; Yang, R.; Qu, J.; Zhao, W.; Xie, L.; Tian, W.; Li, X. The synthesis and hydrogen storage properties of pure nanostructured Mg 2 FeH 6. Nanotechnology 2010, 21, 095706. [Google Scholar] [CrossRef]
- Chaudhary, A.-L.; Dietzel, S.; Li, H.-W.; Akiba, E.; Bergemann, N.; Pistidda, C.; Klassen, T.; Dornheim, M. Synthesis of Mg2FeD6 under low pressure conditions for Mg2FeH6 hydrogen storage studies. Int. J. Hydrog. Energy 2017, 42, 11422–11428. [Google Scholar] [CrossRef]
- Cerenius, Y.; Ståhl, K.; Svensson, L.A.; Ursby, T.; Oskarsson, Å.; Albertsson, J.; Liljas, A. The crystallography beamline I711 at MAX II. J. Synchrotron Radiat. 2000, 7, 203–208. [Google Scholar] [CrossRef]
- Jensen, T.R.; Nielsen, T.K.; Filinchuk, Y.; Jørgensen, J.-E.; Cerenius, Y.; Gray, E.M.; Webb, C.J. Versatile in situ powder X-ray diffraction cells for solid–gas investigations. J. Appl. Crystallogr. 2010, 43, 1456–1463. [Google Scholar] [CrossRef] [Green Version]
- Hammersley, A.P.; Svensson, S.O.; Hanfland, M.; Fitch, A.N.; Hausermann, D. Two-dimensional detector software: From real detector to idealised image or two-theta scan. High Press. Res. 1996, 14, 235–248. [Google Scholar] [CrossRef]
- Kou, H.; Xiao, X.; Chen, L.; Li, S.; Wang, Q. Formation mechanism of MgB2 in 2LiBH4 + MgH2 system for reversible hydrogen storage. Trans. Nonferrous Met. Soc. China 2011, 21, 1040–1046. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaani, M.R.; Catti, M.; English, N.J. In Situ Synchrotron X-ray Diffraction Studies of Hydrogen-Desorption Properties of 2LiBH4–Mg2FeH6 Composite. Molecules 2021, 26, 4853. https://doi.org/10.3390/molecules26164853
Ghaani MR, Catti M, English NJ. In Situ Synchrotron X-ray Diffraction Studies of Hydrogen-Desorption Properties of 2LiBH4–Mg2FeH6 Composite. Molecules. 2021; 26(16):4853. https://doi.org/10.3390/molecules26164853
Chicago/Turabian StyleGhaani, Mohammad R., Michele Catti, and Niall J. English. 2021. "In Situ Synchrotron X-ray Diffraction Studies of Hydrogen-Desorption Properties of 2LiBH4–Mg2FeH6 Composite" Molecules 26, no. 16: 4853. https://doi.org/10.3390/molecules26164853
APA StyleGhaani, M. R., Catti, M., & English, N. J. (2021). In Situ Synchrotron X-ray Diffraction Studies of Hydrogen-Desorption Properties of 2LiBH4–Mg2FeH6 Composite. Molecules, 26(16), 4853. https://doi.org/10.3390/molecules26164853







