Phages and Enzybiotics in Food Biopreservation
Abstract
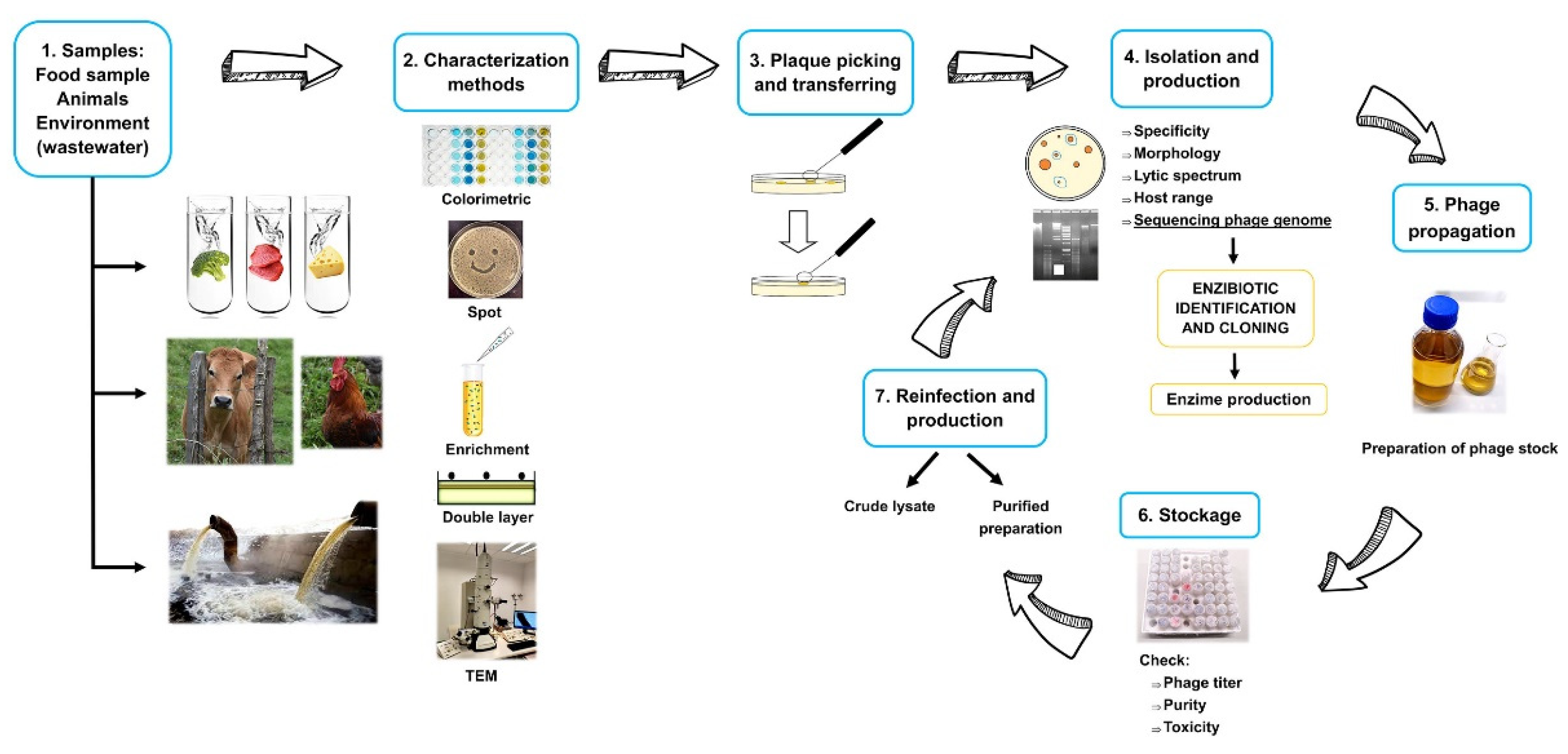
1. Introduction
2. Why Bacteriophages?
3. Spatial Distribution of Phages
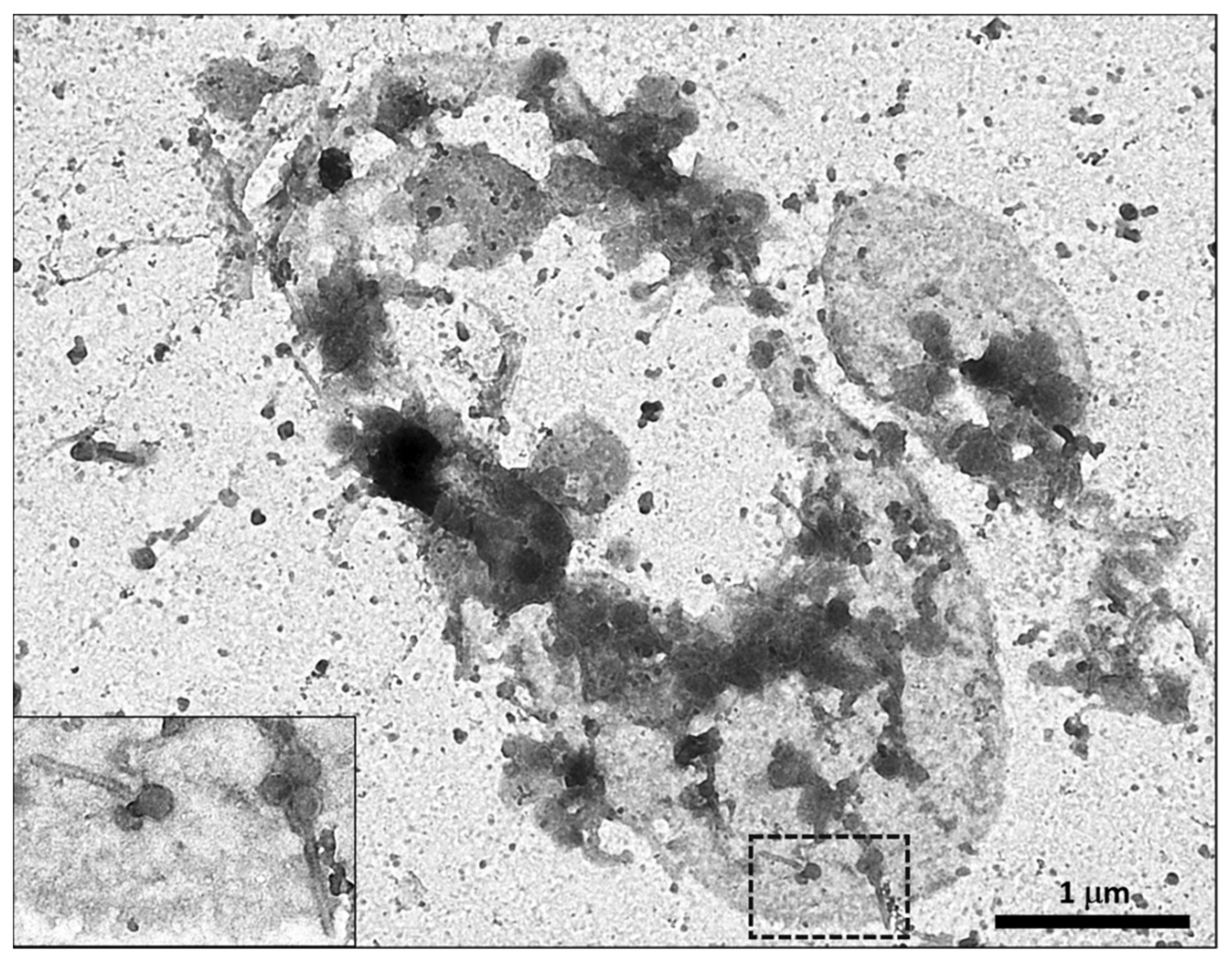
4. Morphology and Classification
5. Phage’s Life Cycle
6. Enzybiotics
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lundén, J.; Björkroth, J.; Korkeala, H. Contamination Routes and Analysis in Food Processing Environments. In Handbook of Hygiene Control in the Food Industry; Lelieved, H.L.M., Holah, M.A., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2005; pp. 539–555. [Google Scholar]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and Contamination Routes of Microbial Pathogens to Fresh Produce during Field Cultivation: A Review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Holley, R.A. Factors Influencing the Microbial Safety of Fresh Produce: A Review. Food Microbiol. 2012, 32, 1–19. [Google Scholar] [CrossRef]
- Kim, N.H.; Cho, T.J.; Rhee, M.S. Current Interventions for Controlling Pathogenic Escherichia coli. Adv. Appl. Microbiol. 2017, 100, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.; Shi, Z.; Ricke, S.C. Current Aspects of Salmonella Contamination in the US Poultry Production Chain and the Potential Application of Risk Strategies in Understanding Emerging Hazards. Crit. Rev. Microbiol. 2017, 43, 370–392. [Google Scholar] [CrossRef] [PubMed]
- Laroute, V.; Tormo, H.; Couderc, C.; Mercier-Bonin, M.; Le Bourgeois, P.; Cocaign-Bousquet, M.; Daveran-Mingot, M.-L. From Genome to Phenotype: An Integrative Approach to Evaluate the Biodiversity of Lactococcus Lactis. Microorganisms 2017, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Klaenhammer, T.R. Genetics of Bacteriocins Produced by Lactic Acid Bacteria. FEMS Microbiol. Rev. 1993, 12, 39–85. [Google Scholar] [CrossRef]
- Kang, M.-S.; Lee, D.-S.; Lee, S.-A.; Kim, M.-S.; Nam, S.-H. Effects of Probiotic Bacterium Weissella cibaria CMU on Periodontal Health and Microbiota: A Randomised, Double-Blind, Placebo-Controlled Trial. BMC Oral Health 2020, 20, 243. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Lee, C.Y.; Chung, D.K. Probiotic Lactic Acid Bacteria and Skin Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 2331–2337. [Google Scholar] [CrossRef] [PubMed]
- Esber, N.; Mauras, A.; Delannoy, J.; Labellie, C.; Mayeur, C.; Caillaud, M.-A.; Kashima, T.; Souchaud, L.; Nicolis, I.; Kapel, N.; et al. Three Candidate Probiotic Strains Impact Gut Microbiota and Induce Anergy in Mice with Cow’s Milk Allergy. Appl. Environ. Microbiol. 2020, 86, e01203-20. [Google Scholar] [CrossRef] [PubMed]
- Paparo, L.; Nocerino, R.; Di Scala, C.; Della Gatta, G.; Di Costanzo, M.; Buono, A.; Bruno, C.; Berni Canani, R. Targeting Food Allergy with Probiotics. Adv. Exp. Med. Biol. 2019, 1125, 57–68. [Google Scholar] [CrossRef]
- de Dapkevicius, M.L.E.; Sgardioli, B.; Câmara, S.P.A.; Poeta, P.; Malcata, F.X. Current Trends of Enterococci in Dairy Products: A Comprehensive Review of Their Multiple Roles. Foods 2021, 10, 821. [Google Scholar] [CrossRef]
- De Silva, L.a.D.S.; Wickramanayake, M.V.K.S.; Heo, G.-J. Virulence and Antimicrobial Resistance Potential of Aeromonas Spp. Associated with Shellfish. Lett. Appl. Microbiol. 2021, 73, 176–186. [Google Scholar] [CrossRef]
- Luque-Sastre, L.; Arroyo, C.; Fox, E.M.; McMahon, B.J.; Bai, L.; Li, F.; Fanning, S. Antimicrobial Resistance in Listeria Species. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Goodridge, L.; Abedon, S.T. Bacteriophage Biocontrol and Bioprocessing: Application of Phage Therapy to Industry. Soc. Ind. Microbiol. News 2003, 53, 254–262. [Google Scholar]
- Greer, G.G. Bacteriophage Control of Foodborne Bacteriat. J. Food Prot. 2005, 68, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, S.-M.; Guezenec, S.; Piot, M.; Foster, S.; Lortal, S. Mur-LH, the Broad-Spectrum Endolysin of Lactobacillus helveticus Temperate Bacteriophage Phi-0303. Appl. Environ. Microbiol. 2004, 70, 96–103. [Google Scholar] [CrossRef]
- Röhrig, C.; Huemer, M.; Lorgé, D.; Luterbacher, S.; Phothaworn, P.; Schefer, C.; Sobieraj, A.M.; Zinsli, L.V.; Mairpady Shambat, S.; Leimer, N.; et al. Targeting Hidden Pathogens: Cell-Penetrating Enzybiotics Eradicate Intracellular Drug-Resistant Staphylococcus aureus. mBio 2020, 11, e00209-20. [Google Scholar] [CrossRef]
- Dams, D.; Briers, Y. Enzybiotics: Enzyme-Based Antibacterials as Therapeutics. Adv. Exp. Med. Biol. 2019, 1148, 233–253. [Google Scholar] [CrossRef]
- Gerstmans, H.; Rodríguez-Rubio, L.; Lavigne, R.; Briers, Y. From Endolysins to Artilysin®s: Novel Enzyme-Based Approaches to Kill Drug-Resistant Bacteria. Biochem. Soc. Trans. 2016, 44, 123–128. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; García, P. Bacteriophages as Weapons Against Bacterial Biofilms in the Food Industry. Front. Microbiol. 2016, 7, 825. [Google Scholar] [CrossRef]
- Ganegama Arachchi, G.J.; Cridge, A.G.; Dias-Wanigasekera, B.M.; Cruz, C.D.; McIntyre, L.; Liu, R.; Flint, S.H.; Mutukumira, A.N. Effectiveness of Phages in the Decontamination of Listeria monocytogenes Adhered to Clean Stainless Steel, Stainless Steel Coated with Fish Protein, and as a Biofilm. J. Ind. Microbiol. Biotechnol. 2013, 40, 1105–1116. [Google Scholar] [CrossRef]
- Soni, K.A.; Nannapaneni, R. Removal of Listeria monocytogenes Biofilms with Bacteriophage P100. J. Food Prot. 2010, 73, 1519–1524. [Google Scholar] [CrossRef]
- Sillankorva, S.; Neubauer, P.; Azeredo, J. Phage Control of Dual Species Biofilms of Pseudomonas fluorescens and Staphylococcus lentus. Biofouling 2010, 26, 567–575. [Google Scholar] [CrossRef]
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to Conventional Antibiotics in the Era of Antimicrobial Resistance. Trends Microbiol. 2019, 27, 323–338. [Google Scholar] [CrossRef]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Effectiveness of Phage-Based Inhibition of Listeria monocytogenes in Food Products and Food Processing Environments. Microorganisms 2020, 8, 1764. [Google Scholar] [CrossRef]
- Zaburlin, D.; Quiberoni, A.; Mercanti, D. Changes in Environmental Conditions Modify Infection Kinetics of Dairy Phages. Food Environ. Virol. 2017, 9, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.J.; Phil, D.; Jansen, V.A. Phage Therapy: The Peculiar Kinetics of Self-Replicating Pharmaceuticals. Clin. Pharmacol. Ther. 2000, 68, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, I.-N. Bacteriophage Adsorption Rate and Optimal Lysis Time. Genetics 2008, 180, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Gáspár, S.; Rontó, G.; Müller, G. Determination of the Biological Parameters of Bacterium-Phage Complexes. Z. Allg. Mikrobiol. 1979, 19, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T. Kinetics of Phage-Mediated Biocontrol of Bacteria. Foodborne Pathog. Dis. 2009, 6, 807–815. [Google Scholar] [CrossRef]
- Abedon, S.T.; Katsaounis, T.I. Basic Phage Mathematics. Methods Mol. Biol. 2018, 1681, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.A.; Billington, C.; Carey-Smith, G.; Greening, G. Bacteriophages as Biocontrol Agents in Food. J. Food Prot. 2005, 68, 426–437. [Google Scholar] [CrossRef]
- Hudson, J.A.; Billington, C.; Wilson, T.; On, S.L.W. Effect of Phage and Host Concentration on the Inactivation of Escherichia coli O157:H7 on Cooked and Raw Beef. Food Sci. Technol. Int. 2015, 21, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Augustine, J.; Louis, L.; Varghese, S.M.; Bhat, S.G.; Kishore, A. Isolation and Partial Characterization of ΦSP-1, a Salmonella Specific Lytic Phage from Intestinal Content of Broiler Chicken. J. Basic Microbiol. 2013, 53, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Bigwood, T.; Hudson, J.A.; Billington, C. Influence of Host and Bacteriophage Concentrations on the Inactivation of Food-Borne Pathogenic Bacteria by Two Phages. FEMS Microbiol. Lett. 2009, 291, 59–64. [Google Scholar] [CrossRef]
- Sváb, D.; Falgenhauer, L.; Rohde, M.; Chakraborty, T.; Tóth, I. Identification and Characterization of New Broad Host-Range RV5-like Coliphages C203 and P206 Directed against Enterobacteria. Infect. Genet. Evol. 2018, 64, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Tarahovsky, Y.S.; Ivanitsky, G.R.; Khusainov, A.A. Lysis of Escherichia coli Cells Induced by Bacteriophage T4. FEMS Microbiol. Lett. 1994, 122, 195–199. [Google Scholar] [CrossRef]
- Delbrück, M. The growth of bacteriophage and lysis of the host. J. Gen. Physiol. 1940, 23, 643–660. [Google Scholar] [CrossRef]
- Sommer, J.; Trautner, C.; Witte, A.K.; Fister, S.; Schoder, D.; Rossmanith, P.; Mester, P.-J. Don’t Shut the Stable Door after the Phage Has Bolted-The Importance of Bacteriophage Inactivation in Food Environments. Viruses 2019, 11, 468. [Google Scholar] [CrossRef]
- Molina, F.; Simancas, A.; Ramírez, M.; Tabla, R.; Roa, I.; Rebollo, J.E. A New Pipeline for Designing Phage Cocktails Based on Phage-Bacteria Infection Networks. Front. Microbiol. 2021, 12, 564532. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Liang, L.; Yin, P.; Zhou, Y.; Sharoba, A.M.; Lu, Q.; Dong, X.; Liu, K.; Connerton, I.F.; Li, J. Application of a Novel Phage LPSEYT for Biological Control of Salmonella in Foods. Microorganisms 2020, 8, 400. [Google Scholar] [CrossRef]
- Zheng, X.-F.; Yang, Z.-Q.; Zhang, H.; Jin, W.-X.; Xu, C.-W.; Gao, L.; Rao, S.-Q.; Jiao, X.-A. Isolation of Virulent Phages Infecting Dominant Mesophilic Aerobic Bacteria in Cucumber Pickle Fermentation. Food Microbiol. 2020, 86, 103330. [Google Scholar] [CrossRef] [PubMed]
- Theuretzbacher, U.; Outterson, K.; Engel, A.; Karlén, A. The Global Preclinical Antibacterial Pipeline. Nat. Rev. Microbiol. 2020, 18, 275–285. [Google Scholar] [CrossRef]
- Duarte, J.; Pereira, C.; Costa, P.; Almeida, A. Bacteriophages with Potential to Inactivate Aeromonas hydrophila in Cockles: In Vitro and In Vivo Preliminary Studies. Antibiotics 2021, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Kim, M.; Ryu, S. Complete Genome Sequence of Bacillus cereus Bacteriophage PBC1. J. Virol. 2012, 86, 6379–6380. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Ryu, S. Bacteriophage PBC1 and Its Endolysin as an Antimicrobial Agent against Bacillus cereus. Appl. Environ. Microbiol. 2015, 81, 2274–2283. [Google Scholar] [CrossRef]
- Greer, G.G. Psychrotrophic Brocothrix thermosphacta Bacteriophages Isolated from Beef. Appl. Environ. Microbiol. 1983, 46, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Carey-Smith, G.V. The Use of Bacteriophages as a Biocontrol Mechanism for Campylobacter and Salmonella Contaminants of Food. Master’s Thesis, School of Biological Sciences, University of Canterbury, Christchurch, New Zealand, 2004. [Google Scholar]
- Atterbury, R.J.; Connerton, P.L.; Dodd, C.E.R.; Rees, C.E.D.; Connerton, I.F. Application of Host-Specific Bacteriophages to the Surface of Chicken Skin Leads to a Reduction in Recovery of Campylobacter jejuni. Appl. Environ. Microbiol. 2003, 69, 6302–6306. [Google Scholar] [CrossRef] [PubMed]
- Sails, A.D.; Wareing, D.R.; Bolton, F.J.; Fox, A.J.; Curry, A. Characterisation of 16 Campylobacter jejuni and C. coli Typing Bacteriophages. J. Med. Microbiol. 1998, 47, 123–128. [Google Scholar] [CrossRef]
- Siringan, P.; Connerton, P.L.; Payne, R.J.H.; Connerton, I.F. Bacteriophage-Mediated Dispersal of Campylobacter jejuni Biofilms. Appl. Environ. Microbiol. 2011, 77, 3320–3326. [Google Scholar] [CrossRef]
- Loc Carrillo, C.; Atterbury, R.J.; el-Shibiny, A.; Connerton, P.L.; Dillon, E.; Scott, A.; Connerton, I.F. Bacteriophage Therapy to Reduce Campylobacter jejuni Colonization of Broiler Chickens. Appl. Environ. Microbiol. 2005, 71, 6554–6563. [Google Scholar] [CrossRef] [PubMed]
- Goode, D.; Allen, V.M.; Barrow, P.A. Reduction of Experimental Salmonella and Campylobacter Contamination of Chicken Skin by Application of Lytic Bacteriophages. Appl. Environ. Microbiol. 2003, 69, 5032–5036. [Google Scholar] [CrossRef]
- Kropinski, A.M.; Arutyunov, D.; Foss, M.; Cunningham, A.; Ding, W.; Singh, A.; Pavlov, A.R.; Henry, M.; Evoy, S.; Kelly, J.; et al. Genome and Proteome of Campylobacter jejuni Bacteriophage NCTC 12673. Appl. Environ. Microbiol. 2011, 77, 8265–8271. [Google Scholar] [CrossRef]
- Mayer, M.J.; Payne, J.; Gasson, M.J.; Narbad, A. Genomic Sequence and Characterization of the Virulent Bacteriophage PhiCTP1 from Clostridium tyrobutyricum and Heterologous Expression of Its Endolysin. Appl. Environ. Microbiol. 2010, 76, 5415–5422. [Google Scholar] [CrossRef]
- Kim, K.-P.; Klumpp, J.; Loessner, M.J. Enterobacter sakazakii Bacteriophages Can Prevent Bacterial Growth in Reconstituted Infant Formula. Int. J. Food Microbiol. 2007, 115, 195–203. [Google Scholar] [CrossRef]
- Son, H.M.; Duc, H.M.; Masuda, Y.; Honjoh, K.-I.; Miyamoto, T. Application of Bacteriophages in Simultaneously Controlling Escherichia coli O157:H7 and Extended-Spectrum Beta-Lactamase Producing Escherichia coli. Appl. Microbiol. Biotechnol. 2018, 102, 10259–10271. [Google Scholar] [CrossRef] [PubMed]
- McLean, S.K.; Dunn, L.A.; Palombo, E.A. Phage Inhibition of Escherichia coli in Ultrahigh-Temperature-Treated and Raw Milk. Foodborne Pathog. Dis. 2013, 10, 956–962. [Google Scholar] [CrossRef]
- Liao, Y.-T.; Salvador, A.; Harden, L.A.; Liu, F.; Lavenburg, V.M.; Li, R.W.; Wu, V.C.H. Characterization of a Lytic Bacteriophage as an Antimicrobial Agent for Biocontrol of Shiga Toxin-Producing Escherichia coli O145 Strains. Antibiotics 2019, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Pan, Y.; Ebner, P.D. Meat Science and Muscle Biology Symposium: Development of Bacteriophage Treatments to Reduce Escherichia coli O157:H7 Contamination of Beef Products and Produce. J. Anim. Sci. 2014, 92, 1366–1377. [Google Scholar] [CrossRef]
- Hong, Y.; Pan, Y.; Harman, N.J.; Ebner, P.D. Complete Genome Sequences of Two Escherichia coli O157:H7 Phages Effective in Limiting Contamination of Food Products. Genome Announc. 2014, 2, e00519-14. [Google Scholar] [CrossRef]
- O’Flynn, G.; Ross, R.P.; Fitzgerald, G.F.; Coffey, A. Evaluation of a Cocktail of Three Bacteriophages for Biocontrol of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2004, 70, 3417–3424. [Google Scholar] [CrossRef]
- Akusobi, C.; Chan, B.K.; Williams, E.S.C.P.; Wertz, J.E.; Turner, P.E. Parallel Evolution of Host-Attachment Proteins in Phage PP01 Populations Adapting to Escherichia coli O157:H7. Pharmaceuticals 2018, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Tanji, Y.; Mizoguchi, K.; Akitsu, T.; Kijima, N.; Unno, H. Characterization of a Virulent Bacteriophage Specific for Escherichia coli O157:H7 and Analysis of Its Cellular Receptor and Two Tail Fiber Genes. FEMS Microbiol. Lett. 2002, 211, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.A.; Billington, C.; Cornelius, A.J.; Wilson, T.; On, S.L.W.; Premaratne, A.; King, N.J. Use of a Bacteriophage to Inactivate Escherichia coli O157:H7 on Beef. Food Microbiol. 2013, 36, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Kudva, I.T.; Jelacic, S.; Tarr, P.I.; Youderian, P.; Hovde, C.J. Biocontrol of Escherichia coli O157 with O157-Specific Bacteriophages. Appl. Environ. Microbiol. 1999, 65, 3767–3773. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Ryu, J.-H.; Beuchat, L.R. Inactivation of Escherichia coli O157:H7 in Biofilm on Stainless Steel by Treatment with an Alkaline Cleaner and a Bacteriophage. J. Appl. Microbiol. 2005, 99, 449–459. [Google Scholar] [CrossRef]
- Abuladze, T.; Li, M.; Menetrez, M.Y.; Dean, T.; Senecal, A.; Sulakvelidze, A. Bacteriophages Reduce Experimental Contamination of Hard Surfaces, Tomato, Spinach, Broccoli, and Ground Beef by Escherichia coli O157:H7. Appl. Environ. Microbiol. 2008, 74, 6230–6238. [Google Scholar] [CrossRef]
- Ferguson, S.; Roberts, C.; Handy, E.; Sharma, M. Lytic Bacteriophages Reduce Escherichia coli O157: H7 on Fresh Cut Lettuce Introduced through Cross-Contamination. Bacteriophage 2013, 3, e24323. [Google Scholar] [CrossRef]
- Tomat, D.; Mercanti, D.; Balagué, C.; Quiberoni, A. Phage Biocontrol of Enteropathogenic and Shiga Toxin-Producing Escherichia coli during Milk Fermentation. Lett. Appl. Microbiol. 2013, 57, 3–10. [Google Scholar] [CrossRef]
- Tomat, D.; Migliore, L.; Aquili, V.; Quiberoni, A.; Balagué, C. Phage Biocontrol of Enteropathogenic and Shiga Toxin-Producing Escherichia coli in Meat Products. Front. Cell Infect. Microbiol. 2013, 3, 20. [Google Scholar] [CrossRef]
- Snyder, A.B.; Perry, J.J.; Yousef, A.E. Developing and Optimizing Bacteriophage Treatment to Control Enterohemorrhagic Escherichia coli on Fresh Produce. Int. J. Food Microbiol. 2016, 236, 90–97. [Google Scholar] [CrossRef]
- Deasy, T.; Mahony, J.; Neve, H.; Heller, K.J.; van Sinderen, D. Isolation of a Virulent Lactobacillus brevis Phage and Its Application in the Control of Beer Spoilage. J. Food Prot. 2011, 74, 2157–2161. [Google Scholar] [CrossRef] [PubMed]
- Greer, G.G.; Dilts, B.D.; Ackermann, H.-W. Characterization of a Leuconostoc gelidum Bacteriophage from Pork. Int. J. Food Microbiol. 2007, 114, 370–375. [Google Scholar] [CrossRef]
- Hibma, A.M.; Jassim, S.A.; Griffiths, M.W. Infection and Removal of L-Forms of Listeria monocytogenes with Bred Bacteriophage. Int. J. Food Microbiol. 1997, 34, 197–207. [Google Scholar] [CrossRef]
- Klumpp, J.; Loessner, M.J. Listeria Phages: Genomes, Evolution, and Application. Bacteriophage 2013, 3, e26861. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Ackermann, H.W.; Pandian, S.; Picard, G.; Goulet, J. Biological Inactivation of Adhering Listeria monocytogenes by Listeriaphages and a Quaternary Ammonium Compound. Appl. Environ. Microbiol. 1993, 59, 2914–2917. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.W.; DuBow, M.S. Viruses of Prokaryotes: General Properties of Bacteriophages; CRC Press Inc.: Boca Raton, FL, USA, 1987; pp. 49–85. [Google Scholar]
- Zink, R.; Loessner, M.J. Classification of Virulent and Temperate Bacteriophages of Listeria Spp. on the Basis of Morphology and Protein Analysis. Appl. Environ. Microbiol. 1992, 58, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Loessner, M.J.; Goeppl, S.; Busse, M. Comparative Inducibility of Bacteriophage in Naturally Lysogenic and Lysogenized Strains of Listeria Spp. by u.v. Light and Mitomycin C. Lett. Appl. Microbiol. 1991, 12, 196–199. [Google Scholar] [CrossRef]
- Guenther, S.; Huwyler, D.; Richard, S.; Loessner, M.J. Virulent Bacteriophage for Efficient Biocontrol of Listeria monocytogenes in Ready-to-Eat Foods. Appl. Environ. Microbiol. 2009, 75, 93–100. [Google Scholar] [CrossRef]
- Klumpp, J.; Dorscht, J.; Lurz, R.; Bielmann, R.; Wieland, M.; Zimmer, M.; Calendar, R.; Loessner, M.J. The Terminally Redundant, Nonpermuted Genome of Listeria Bacteriophage A511: A Model for the SPO1-like Myoviruses of Gram-Positive Bacteria. J. Bacteriol. 2008, 190, 5753–5765. [Google Scholar] [CrossRef]
- Guenther, S.; Loessner, M.J. Bacteriophage Biocontrol of Listeria monocytogenes on Soft Ripened White Mold and Red-Smear Cheeses. Bacteriophage 2011, 1, 94–100. [Google Scholar] [CrossRef]
- Bigot, B.; Lee, W.-J.; McIntyre, L.; Wilson, T.; Hudson, J.A.; Billington, C.; Heinemann, J.A. Control of Listeria monocytogenes Growth in a Ready-to-Eat Poultry Product Using a Bacteriophage. Food Microbiol. 2011, 28, 1448–1452. [Google Scholar] [CrossRef] [PubMed]
- Scattolini, S.; D’Angelantonio, D.; Boni, A.; Mangone, I.; Marcacci, M.; Battistelli, N.; D’Agostino, K.; Pomilio, F.; Camma, C.; Migliorati, G.; et al. Characterization and In Vitro Efficacy against Listeria monocytogenes of a Newly Isolated Bacteriophage, ΦIZSAM-1. Microorganisms 2021, 9, 731. [Google Scholar] [CrossRef]
- Aprea, G.; D’Angelo, A.R.; Prencipe, V.A.; Migliorati, G. Bacteriophage Morphological Characterization by Using Transmission Electron Microscopy. J. Life Sci. 2015, 9, 214–220. [Google Scholar]
- Carlton, R.M.; Noordman, W.H.; Biswas, B.; de Meester, E.D.; Loessner, M.J. Bacteriophage P100 for Control of Listeria monocytogenes in Foods: Genome Sequence, Bioinformatic Analyses, Oral Toxicity Study, and Application. Regul. Toxicol. Pharmacol. 2005, 43, 301–312. [Google Scholar] [CrossRef]
- Oliveira, M.; Viñas, I.; Colàs, P.; Anguera, M.; Usall, J.; Abadias, M. Effectiveness of a Bacteriophage in Reducing Listeria monocytogenes on Fresh-Cut Fruits and Fruit Juices. Food Microbiol. 2014, 38, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Iacumin, L.; Manzano, M.; Comi, G. Phage Inactivation of Listeria monocytogenes on San Daniele Dry-Cured Ham and Elimination of Biofilms from Equipment and Working Environments. Microorganisms 2016, 4, 4. [Google Scholar] [CrossRef]
- Soni, K.A.; Nannapaneni, R. Bacteriophage Significantly Reduces Listeria monocytogenes on Raw Salmon Fillet Tissue. J. Food Prot. 2010, 73, 32–38. [Google Scholar] [CrossRef]
- Soni, K.A.; Nannapaneni, R.; Hagens, S. Reduction of Listeria monocytogenes on the Surface of Fresh Channel Catfish Fillets by Bacteriophage Listex P100. Foodborne Pathog. Dis. 2010, 7, 427–434. [Google Scholar] [CrossRef]
- Ellis, D.E.; Whitman, P.A.; Marshall, R.T. Effects of Homologous Bacteriophage on Growth of Pseudomonas fragi WY in Milk. Appl. Microbiol. 1973, 25, 24–25. [Google Scholar] [CrossRef]
- Whitman, P.A.; Marshall, R.T. Isolation of Psychrophilic Bacteriophage-Host Systems from Refrigerated Food Products. Appl. Microbiol. 1971, 22, 220–223. [Google Scholar] [CrossRef]
- Whitman, P.A.; Marshall, R.T. Characterization of Two Psychrophilic Pseudomonas Bacteriophages Isolated from Ground Beef. Appl. Microbiol. 1971, 22, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Greer, G.G. Homologous Bacteriophage Control of Pseudomonas Growth and Beef Spoilage 1, 2. J. Food Prot. 1986, 49, 104–109. [Google Scholar] [CrossRef]
- Greer, G.G. Psychrotrophic Bacteriophages for Beef Spoilage Pseudomonads 1. J. Food Prot. 1982, 45, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, C.; Yamada, K.; Takeuchi, H.; Inokuchi, Y.; Kashiwagi, A.; Toba, T. A Lytic Bacteriophage for Controlling Pseudomonas lactis in Raw Cow’s Milk. Appl. Environ. Microbiol. 2018, 84, e00111-18. [Google Scholar] [CrossRef] [PubMed]
- Sillankorva, S.; Neubauer, P.; Azeredo, J. Pseudomonas fluorescens Biofilms Subjected to Phage PhiIBB-PF7A. BMC Biotechnol. 2008, 8, 79. [Google Scholar] [CrossRef]
- Sillankorva, S.; Neubauer, P.; Azeredo, J. Isolation and Characterization of a T7-like Lytic Phage for Pseudomonas fluorescens. BMC Biotechnol. 2008, 8, 80. [Google Scholar] [CrossRef]
- Kang, H.-W.; Kim, J.-W.; Jung, T.-S.; Woo, G.-J. Wksl3, a New Biocontrol Agent for Salmonella Enterica Serovars Enteritidis and Typhimurium in Foods: Characterization, Application, Sequence Analysis, and Oral Acute Toxicity Study. Appl. Environ. Microbiol. 2013, 79, 1956–1968. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.J.; Jung, S.J.; Mizan, M.F.R.; Park, S.H.; Ha, S.-D. Characterization of Salmonella Spp.-Specific Bacteriophages and Their Biocontrol Application in Chicken Breast Meat. J. Food Sci. 2020, 85, 526–534. [Google Scholar] [CrossRef]
- Fiorentin, L.; Vieira, N.D.; Barioni, W. Oral Treatment with Bacteriophages Reduces the Concentration of Salmonella Enteritidis PT4 in Caecal Contents of Broilers. Avian Pathol. 2005, 34, 258–263. [Google Scholar] [CrossRef]
- Fiorentin, L.; Vieira, N.D.; Barioni, J.W.; Barros, S. In Vitro Characterization and in Vivo Properties of Salmonellae Lytic Bacteriophages Isolated from Free-Range Layers. Braz. J. Poult. Sci. 2004, 6, 121–128. [Google Scholar] [CrossRef][Green Version]
- Fiorentin, L.; Vieira, N.D.; Barioni, J.W. Use of Lytic Bacteriophages to Reduce Salmonella Enteritidis in Experimentally Contaminated Chicken Cuts. Braz. J. Poult. Sci. 2005, 7, 255–260. [Google Scholar] [CrossRef]
- Berchieri, A.; Lovell, M.A.; Barrow, P.A. The Activity in the Chicken Alimentary Tract of Bacteriophages Lytic for Salmonella Typhimurium. Res. Microbiol. 1991, 142, 541–549. [Google Scholar] [CrossRef]
- Sonalika, J.; Srujana, A.S.; Akhila, D.S.; Juliet, M.R.; Santhosh, K.S. Application of Bacteriophages to Control Salmonella Enteritidis in Raw Eggs. Iran J. Vet. Res. 2020, 21, 221–225. [Google Scholar] [PubMed]
- Islam, M.S.; Zhou, Y.; Liang, L.; Nime, I.; Liu, K.; Yan, T.; Wang, X.; Li, J. Application of a Phage Cocktail for Control of Salmonella in Foods and Reducing Biofilms. Viruses 2019, 11, 841. [Google Scholar] [CrossRef]
- Islam, M.S.; Zhou, Y.; Liang, L.; Nime, I.; Yan, T.; Willias, S.P.; Mia, M.Z.; Bei, W.; Connerton, I.F.; Fischetti, V.A.; et al. Application of a Broad Range Lytic Phage LPST94 for Biological Control of Salmonella in Foods. Microorganisms 2020, 8, 247. [Google Scholar] [CrossRef]
- Huang, C.; Virk, S.M.; Shi, J.; Zhou, Y.; Willias, S.P.; Morsy, M.K.; Abdelnabby, H.E.; Liu, J.; Wang, X.; Li, J. Isolation, Characterization, and Application of Bacteriophage LPSE1 Against Salmonella Enterica in Ready to Eat (RTE) Foods. Front. Microbiol. 2018, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Whichard, J.M.; Sriranganathan, N.; Pierson, F.W. Suppression of Salmonella Growth by Wild-Type and Large-Plaque Variants of Bacteriophage Felix O1 in Liquid Culture and on Chicken Frankfurters. J. Food Prot. 2003, 66, 220–225. [Google Scholar] [CrossRef]
- Whichard, J.M.; Weigt, L.A.; Borris, D.J.; Li, L.L.; Zhang, Q.; Kapur, V.; Pierson, F.W.; Lingohr, E.J.; She, Y.-M.; Kropinski, A.M.; et al. Complete Genomic Sequence of Bacteriophage Felix O1. Viruses 2010, 2, 710–730. [Google Scholar] [CrossRef] [PubMed]
- Felix, A.; Callow, B.R. Typing of Paratyphoid B Bacilli by Vi Bacteriophage. Br. Med. J. 1943, 2, 127–130. [Google Scholar] [CrossRef]
- Guenther, S.; Herzig, O.; Fieseler, L.; Klumpp, J.; Loessner, M.J. Biocontrol of Salmonella Typhimurium in RTE Foods with the Virulent Bacteriophage FO1-E2. Int. J. Food Microbiol. 2012, 154, 66–72. [Google Scholar] [CrossRef]
- Higgins, J.P.; Higgins, S.E.; Guenther, K.L.; Huff, W.; Donoghue, A.M.; Donoghue, D.J.; Hargis, B.M. Use of a Specific Bacteriophage Treatment to Reduce Salmonella in Poultry Products. Poult. Sci. 2005, 84, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Wall, S.K.; Zhang, J.; Rostagno, M.H.; Ebner, P.D. Phage Therapy to Reduce Preprocessing Salmonella Infections in Market-Weight Swine. Appl. Environ. Microbiol. 2010, 76, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hong, Y.; Harman, N.J.; Das, A.; Ebner, P.D. Genome Sequence of a Salmonella Phage Used to Control Salmonella Transmission in Swine. Genome Announc. 2014, 2, e00521-14. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Schmidt, K.; Marks, D.; Hatter, S.; Marshall, A.; Albino, L.; Ebner, P. Treatment of Salmonella-Contaminated Eggs and Pork with a Broad-Spectrum, Single Bacteriophage: Assessment of Efficacy and Resistance Development. Foodborne Pathog. Dis. 2016, 13, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, Y.; Li, W.; Zhu, W.; Wang, J.; Wang, X. Application of a Novel Lytic Podoviridae Phage Pu20 for Biological Control of Drug-Resistant Salmonella in Liquid Eggs. Pathogens 2021, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, W.; Li, W.; Ding, Y.; Zhang, Y.; Yang, Q.; Wang, J.; Wang, X. A Broad-Spectrum Phage Controls Multidrug-Resistant Salmonella in Liquid Eggs. Food Res. Int. 2020, 132, 109011. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, S.; Jung, L.-S.; Biswas, D. In Vitro Assessment of the Susceptibility of Planktonic and Attached Cells of Foodborne Pathogens to Bacteriophage P22-Mediated Salmonella Lysates. J. Food Prot. 2013, 76, 2057–2062. [Google Scholar] [CrossRef]
- Susskind, M.M.; Botstein, D. Molecular Genetics of Bacteriophage P22. Microbiol. Rev. 1978, 42, 385–413. [Google Scholar] [CrossRef]
- Zorn, G.A.; Gough, M. Morphology of Bacteriophage P22 as Seen in Thin Sections of Pelleted Phage. Virology 1976, 71, 434–443. [Google Scholar] [CrossRef]
- Zinno, P.; Devirgiliis, C.; Ercolini, D.; Ongeng, D.; Mauriello, G. Bacteriophage P22 to Challenge Salmonella in Foods. Int. J. Food Microbiol. 2014, 191, 69–74. [Google Scholar] [CrossRef]
- Islam, M.S.; Hu, Y.; Mizan, M.F.R.; Yan, T.; Nime, I.; Zhou, Y.; Li, J. Characterization of Salmonella Phage LPST153 That Effectively Targets Most Prevalent Salmonella Serovars. Microorganisms 2020, 8, 1089. [Google Scholar] [CrossRef]
- Spricigo, D.A.; Bardina, C.; Cortés, P.; Llagostera, M. Use of a Bacteriophage Cocktail to Control Salmonella in Food and the Food Industry. Int. J. Food Microbiol. 2013, 165, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Bardina, C.; Colom, J.; Spricigo, D.A.; Otero, J.; Sánchez-Osuna, M.; Cortés, P.; Llagostera, M. Genomics of Three New Bacteriophages Useful in the Biocontrol of Salmonella. Front. Microbiol. 2016, 7, 545. [Google Scholar] [CrossRef] [PubMed]
- Bardina, C.; Spricigo, D.A.; Cortés, P.; Llagostera, M. Significance of the Bacteriophage Treatment Schedule in Reducing Salmonella Colonization of Poultry. Appl. Environ. Microbiol. 2012, 78, 6600–6607. [Google Scholar] [CrossRef] [PubMed]
- Augustine, J.; Bhat, S.G. Biocontrol of Salmonella Enteritidis in Spiked Chicken Cuts by Lytic Bacteriophages ΦSP-1 and ΦSP-3. J. Basic Microbiol. 2015, 55, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Augustine, J.; Varghese, S.M.; Bhat, S.G. ΦSP-3, a Salmonella-Specific Lytic Phage Capable of Infecting Its Host under Nutrient-Deprived States. Ann. Microbiol. 2013, 63, 381–386. [Google Scholar] [CrossRef]
- Modi, R.; Hirvi, Y.; Hill, A.; Griffiths, M.W. Effect of Phage on Survival of Salmonella Enteritidis during Manufacture and Storage of Cheddar Cheese Made from Raw and Pasteurized Milk. J. Food Prot. 2001, 64, 927–933. [Google Scholar] [CrossRef]
- Bao, H.; Zhang, P.; Zhang, H.; Zhou, Y.; Zhang, L.; Wang, R. Bio-Control of Salmonella Enteritidis in Foods Using Bacteriophages. Viruses 2015, 7, 4836–4853. [Google Scholar] [CrossRef]
- Bao, H.; Zhou, Y.; Shahin, K.; Zhang, H.; Cao, F.; Pang, M.; Zhang, X.; Zhu, S.; Olaniran, A.; Schmidt, S.; et al. The Complete Genome of Lytic Salmonella Phage VB_SenM-PA13076 and Therapeutic Potency in the Treatment of Lethal Salmonella Enteritidis Infections in Mice. Microbiol. Res. 2020, 237, 126471. [Google Scholar] [CrossRef] [PubMed]
- Duc, H.M.; Son, H.M.; Yi, H.P.S.; Sato, J.; Ngan, P.H.; Masuda, Y.; Honjoh, K.-I.; Miyamoto, T. Isolation, Characterization and Application of a Polyvalent Phage Capable of Controlling Salmonella and Escherichia coli O157:H7 in Different Food Matrices. Food Res. Int. 2020, 131, 108977. [Google Scholar] [CrossRef]
- Kocharunchitt, C.; Ross, T.; McNeil, D.L. Use of Bacteriophages as Biocontrol Agents to Control Salmonella Associated with Seed Sprouts. Int. J. Food Microbiol. 2009, 128, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Pao, S.; Rolph, S.P.; Westbrook, E.W.; Shen, H. Use of Bacteriophages to Control Salmonella in Experimentally Contaminated Sprout Seeds. J. Food Sci. 2006, 69, 127–130. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Ding, Y.; Huang, C.; Zhang, Y.; Wang, J.; Wang, X. Characterization of a Novel Siphoviridae Salmonella Bacteriophage T156 and Its Microencapsulation Application in Food Matrix. Food Res. Int. 2021, 140, 110004. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; McAuliffe, O.; Ross, R.P.; Coffey, A. Prevention of Staphylococcus aureus Biofilm Formation and Reduction in Established Biofilm Density Using a Combination of Phage K and Modified Derivatives. Lett. Appl. Microbiol. 2012, 54, 286–291. [Google Scholar] [CrossRef]
- Gill, J.J. Revised Genome Sequence of Staphylococcus aureus Bacteriophage K. Genome Announc. 2014, 2, e01173-13. [Google Scholar] [CrossRef]
- Bueno, E.; García, P.; Martínez, B.; Rodríguez, A. Phage Inactivation of Staphylococcus aureus in Fresh and Hard-Type Cheeses. Int. J. Food Microbiol. 2012, 158, 23–27. [Google Scholar] [CrossRef]
- Garcia, P.; Madera, C.; Martinez, B.; Rodriguez, A. Biocontrol of Staphylococcus aureus in Curd Manufacturing Processes Using Bacteriophages. Int. Dairy J. 2007, 17, 1232–1239. [Google Scholar] [CrossRef]
- García, P.; Martínez, B.; Obeso, J.M.; Lavigne, R.; Lurz, R.; Rodríguez, A. Functional Genomic Analysis of Two Staphylococcus aureus Phages Isolated from the Dairy Environment. Appl. Environ. Microbiol. 2009, 75, 7663–7673. [Google Scholar] [CrossRef] [PubMed]
- Duc, H.M.; Son, H.M.; Ngan, P.H.; Sato, J.; Masuda, Y.; Honjoh, K.-I.; Miyamoto, T. Isolation and Application of Bacteriophages Alone or in Combination with Nisin against Planktonic and Biofilm Cells of Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2020, 104, 5145–5158. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Bai, J.; Lee, J.-H.; Ryu, S. Mutation of a Staphylococcus aureus Temperate Bacteriophage to a Virulent One and Evaluation of Its Application. Food Microbiol. 2019, 82, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-Q.; Tao, X.-Y.; Zhang, H.; Rao, S.-Q.; Gao, L.; Pan, Z.-M.; Jiao, X.-A. Isolation and Characterization of Virulent Phages Infecting Shewanella baltica and Shewanella putrefaciens, and Their Application for Biopreservation of Chilled Channel Catfish (Ictalurus Punctatus). Int. J. Food Microbiol. 2019, 292, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, R.; Bao, H. Phage Inactivation of Foodborne Shigella on Ready-to-Eat Spiced Chicken. Poult. Sci. 2013, 92, 211–217. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Z.; Zhou, Y.; Bao, H.; Wang, R.; Li, T.; Pang, M.; Sun, L.; Zhou, X. Application of a Phage in Decontaminating Vibrio parahaemolyticus in Oysters. Int. J. Food Microbiol. 2018, 275, 24–31. [Google Scholar] [CrossRef]
- Cooper, I.R. A Review of Current Methods Using Bacteriophages in Live Animals, Food and Animal Products Intended for Human Consumption. J. Microbiol. Methods 2016, 130, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.A.; Chandry, P.S.; Kaur, M.; Kocharunchitt, C.; Bowman, J.P.; Fox, E.M. Novel Biocontrol Methods for Listeria monocytogenes Biofilms in Food Production Facilities. Front. Microbiol. 2018, 9, 605. [Google Scholar] [CrossRef]
- Hsu, F.C.; Shieh, Y.S.C.; Sobsey, M.D. Enteric Bacteriophages as Potential Fecal Indicators in Ground Beef and Poultry Meat. J. Food Prot. 2002, 65, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wongsuntornpoj, S.; Moreno Switt, A.I.; Bergholz, P.; Wiedmann, M.; Chaturongakul, S. Salmonella Phages Isolated from Dairy Farms in Thailand Show Wider Host Range than a Comparable Set of Phages Isolated from U.S. Dairy Farms. Vet. Microbiol. 2014, 172, 345–352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muniesa, M.; Jofre, J. Abundance in Sewage of Bacteriophages Infecting Escherichia coli O157:H7. Methods Mol. Biol. 2004, 268, 79–88. [Google Scholar] [CrossRef]
- DePaola, A.; Motes, M.L.; Chan, A.M.; Suttle, C.A. Phages Infecting Vibrio vulnificus Are Abundant and Diverse in Oysters (Crassostrea virginica) Collected from the Gulf of Mexico. Appl. Environ. Microbiol. 1998, 64, 346–351. [Google Scholar] [CrossRef]
- Muniain-Mujika, I.; Calvo, M.; Lucena, F.; Girones, R. Comparative Analysis of Viral Pathogens and Potential Indicators in Shellfish. Int. J. Food Microbiol. 2003, 83, 75–85. [Google Scholar] [CrossRef]
- Leclerc, H.; Edberg, S.; Pierzo, V.; Delattre, J.M. Bacteriophages as Indicators of Enteric Viruses and Public Health Risk in Groundwaters. J. Appl. Microbiol. 2000, 88, 5–21. [Google Scholar] [CrossRef]
- Luo, E.; Aylward, F.O.; Mende, D.R.; DeLong, E.F. Bacteriophage Distributions and Temporal Variability in the Ocean’s Interior. mBio 2017, 8, e01903-17. [Google Scholar] [CrossRef]
- Suttle, C.A. Marine Viruses--Major Players in the Global Ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef]
- Kennedy, J.E.; Wei, C.I.; Oblinger, J.L. Distribution of Coliphages in Various Foods. J. Food Prot. 1986, 49, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.E.; Oblinger, J.L.; Bitton, G. Recovery of Coliphages from Chicken, Pork Sausage and Delicatessen Meats. J. Food Prot. 1984, 47, 623–626. [Google Scholar] [CrossRef]
- Kennedy, J.E.; Wei, C.I.; Oblinger, J.L. Methodology for Enumeration of Coliphages in Foods. Appl. Environ. Microbiol. 1986, 51, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Gautier, M.; Rouault, A.; Sommer, P.; Briandet, R. Occurrence of Propionibacterium freudenreichii Bacteriophages in Swiss Cheese. Appl. Environ. Microbiol. 1995, 61, 2572–2576. [Google Scholar] [CrossRef] [PubMed]
- Hagens, S.; Loessner, M.J. Bacteriophage for Biocontrol of Foodborne Pathogens: Calculations and Considerations. Curr. Pharm. Biotechnol. 2010, 11, 58–68. [Google Scholar] [CrossRef]
- Ackermann, H.-W. Sad State of Phage Electron Microscopy. Please Shoot the Messenger. Microorganisms 2013, 2, 1–10. [Google Scholar] [CrossRef]
- Available online: https://talk.ictvonline.org (accessed on 1 August 2021).
- Ackermann, H.W. Classification of Bacteriophages. In The Bacteriophages, 2nd ed.; Calendar, R., Abedon, S.T., Eds.; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Ackermann, H.-W. Phage Classification and Characterization. Methods Mol. Biol. 2009, 501, 127–140. [Google Scholar] [CrossRef]
- Ackermann, H.-W. 5500 Phages Examined in the Electron Microscope. Arch. Virol. 2007, 152, 227–243. [Google Scholar] [CrossRef]
- Adriaenssens, E.M.; Edwards, R.; Nash, J.H.E.; Mahadevan, P.; Seto, D.; Ackermann, H.-W.; Lavigne, R.; Kropinski, A.M. Integration of Genomic and Proteomic Analyses in the Classification of the Siphoviridae Family. Virology 2015, 477, 144–154. [Google Scholar] [CrossRef]
- Lavigne, R.; Darius, P.; Summer, E.J.; Seto, D.; Mahadevan, P.; Nilsson, A.S.; Ackermann, H.W.; Kropinski, A.M. Classification of Myoviridae Bacteriophages Using Protein Sequence Similarity. BMC Microbiol. 2009, 9, 224. [Google Scholar] [CrossRef]
- Lavigne, R.; Seto, D.; Mahadevan, P.; Ackermann, H.-W.; Kropinski, A.M. Unifying Classical and Molecular Taxonomic Classification: Analysis of the Podoviridae Using BLASTP-Based Tools. Res. Microbiol. 2008, 159, 406–414. [Google Scholar] [CrossRef]
- Dion, M.B.; Oechslin, F.; Moineau, S. Phage Diversity, Genomics and Phylogeny. Nat. Rev. Microbiol. 2020, 18, 125–138. [Google Scholar] [CrossRef]
- Fokine, A.; Rossmann, M.G. Molecular Architecture of Tailed Double-Stranded DNA Phages. Bacteriophage 2014, 4, e28281. [Google Scholar] [CrossRef] [PubMed]
- Casjens, S.R. Comparative Genomics and Evolution of the Tailed-Bacteriophages. Curr. Opin. Microbiol. 2005, 8, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Callewaert, L.; Michiels, C.W. Lysozymes in the Animal Kingdom. J. Biosci. 2010, 35, 127–160. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.; Joris, B.; Charlier, P.; Foster, S. Bacterial Peptidoglycan (Murein) Hydrolases. FEMS Microbiol. Rev. 2008, 32, 259–286. [Google Scholar] [CrossRef] [PubMed]
- Vermassen, A.; Leroy, S.; Talon, R.; Provot, C.; Popowska, M.; Desvaux, M. Cell Wall Hydrolases in Bacteria: Insight on the Diversity of Cell Wall Amidases, Glycosidases and Peptidases Toward Peptidoglycan. Front. Microbiol. 2019, 10, 331. [Google Scholar] [CrossRef]
- Young, I.; Wang, I.; Roof, W.D. Phages Will out: Strategies of Host Cell Lysis. Trends Microbiol. 2000, 8, 120–128. [Google Scholar] [CrossRef]
- Loessner, M.J. Bacteriophage Endolysins--Current State of Research and Applications. Curr. Opin. Microbiol. 2005, 8, 480–487. [Google Scholar] [CrossRef]
- Abedon, S.T. Lysis from Without. Bacteriophage 2011, 1, 46–49. [Google Scholar] [CrossRef]
- Catalão, M.J.; Gil, F.; Moniz-Pereira, J.; São-José, C.; Pimentel, M. Diversity in Bacterial Lysis Systems: Bacteriophages Show the Way. FEMS Microbiol. Rev. 2013, 37, 554–571. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Fernández, L.; Rodríguez, A.; García, P. Are Phage Lytic Proteins the Secret Weapon To Kill Staphylococcus aureus? mBio 2018, 9, e01923-17. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rubio, L.; Martínez, B.; Donovan, D.M.; Rodríguez, A.; García, P. Bacteriophage Virion-Associated Peptidoglycan Hydrolases: Potential New Enzybiotics. Crit. Rev. Microbiol. 2013, 39, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; São-José, C.; Azeredo, J. Phage-Derived Peptidoglycan Degrading Enzymes: Challenges and Future Prospects for In Vivo Therapy. Viruses 2018, 10, 292. [Google Scholar] [CrossRef]
- Latka, A.; Maciejewska, B.; Majkowska-Skrobek, G.; Briers, Y.; Drulis-Kawa, Z. Bacteriophage-Encoded Virion-Associated Enzymes to Overcome the Carbohydrate Barriers during the Infection Process. Appl. Microbiol. Biotechnol. 2017, 101, 3103–3119. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.-X.; Dao, F.-Y.; Lv, H.; Feng, P.-M.; Ding, H. Identifying Phage Virion Proteins by Using Two-Step Feature Selection Methods. Molecules 2018, 23, 2000. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.; Martínez, B.; Zhou, Y.; Rodríguez, A.; Donovan, D.M.; García, P. Lytic Activity of the Virion-Associated Peptidoglycan Hydrolase HydH5 of Staphylococcus aureus Bacteriophage VB_SauS-PhiIPLA88. BMC Microbiol. 2011, 11, 138. [Google Scholar] [CrossRef]
- Pires, D.P.; Oliveira, H.; Melo, L.D.R.; Sillankorva, S.; Azeredo, J. Bacteriophage-Encoded Depolymerases: Their Diversity and Biotechnological Applications. Appl. Microbiol. Biotechnol. 2016, 100, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Mao, J.; Mao, J.; Xie, J. Bacteriophage Polysaccharide Depolymerases and Biomedical Applications. BioDrugs 2014, 28, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Fenton, M.; Keary, R.; McAuliffe, O.; Ross, R.P.; O’Mahony, J.; Coffey, A. Bacteriophage-Derived Peptidase CHAP(K) Eliminates and Prevents Staphylococcal Biofilms. Int. J. Microbiol. 2013, 2013, 625341. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Ruas-Madiedo, P.; Martínez, B.; Rodríguez, A.; García, P. Effective Removal of Staphylococcal Biofilms by the Endolysin LysH5. PLoS ONE 2014, 9, e107307. [Google Scholar] [CrossRef]
- Sass, P.; Bierbaum, G. Lytic Activity of Recombinant Bacteriophage Phi11 and Phi12 Endolysins on Whole Cells and Biofilms of Staphylococcus aureus. Appl. Environ. Microbiol. 2007, 73, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Son, J.-S.; Lee, S.-J.; Jun, S.Y.; Yoon, S.J.; Kang, S.H.; Paik, H.R.; Kang, J.O.; Choi, Y.-J. Antibacterial and Biofilm Removal Activity of a Podoviridae Staphylococcus aureus Bacteriophage SAP-2 and a Derived Recombinant Cell-Wall-Degrading Enzyme. Appl. Microbiol. Biotechnol. 2010, 86, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Gerstmans, H.; Criel, B.; Briers, Y. Synthetic Biology of Modular Endolysins. Biotechnol. Adv. 2018, 36, 624–640. [Google Scholar] [CrossRef]
- Yang, H.; Yu, J.; Wei, H. Engineered Bacteriophage Lysins as Novel Anti-Infectives. Front. Microbiol. 2014, 5, 542. [Google Scholar] [CrossRef]
- Son, B.; Kong, M.; Cha, Y.; Bai, J.; Ryu, S. Simultaneous Control of Staphylococcus aureus and Bacillus cereus Using a Hybrid Endolysin LysB4EAD-LysSA11. Antibiotics 2020, 9, 906. [Google Scholar] [CrossRef]
- Son, B.; Yun, J.; Lim, J.-A.; Shin, H.; Heu, S.; Ryu, S. Characterization of LysB4, an Endolysin from the Bacillus cereus-Infecting Bacteriophage B4. BMC Microbiol. 2012, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Garde, S.; Calzada, J.; Sánchez, C.; Gaya, P.; Narbad, A.; Meijers, R.; Mayer, M.J.; Ávila, M. Effect of Lactococcus lactis Expressing Phage Endolysin on the Late Blowing Defect of Cheese Caused by Clostridium tyrobutyricum. Int. J. Food Microbiol. 2020, 329, 108686. [Google Scholar] [CrossRef]
- Mayer, M.J.; Gasson, M.J.; Narbad, A. Genomic Sequence of Bacteriophage ATCC 8074-B1 and Activity of Its Endolysin and Engineered Variants against Clostridium sporogenes. Appl. Environ. Microbiol. 2012, 78, 3685–3692. [Google Scholar] [CrossRef]
- Zimmer, M.; Vukov, N.; Scherer, S.; Loessner, M.J. The Murein Hydrolase of the Bacteriophage Phi3626 Dual Lysis System Is Active against All Tested Clostridium perfringens Strains. Appl. Environ. Microbiol. 2002, 68, 5311–5317. [Google Scholar] [CrossRef]
- Cho, J.-H.; Kwon, J.-G.; O’Sullivan, D.J.; Ryu, S.; Lee, J.-H. Development of an Endolysin Enzyme and Its Cell Wall-Binding Domain Protein and Their Applications for Biocontrol and Rapid Detection of Clostridium perfringens in Food. Food Chem. 2021, 345, 128562. [Google Scholar] [CrossRef] [PubMed]
- Loessner, M.J.; Wendlinger, G.; Scherer, S. Heterogeneous Endolysins in Listeria monocytogenes Bacteriophages: A New Class of Enzymes and Evidence for Conserved Holin Genes within the Siphoviral Lysis Cassettes. Mol. Microbiol. 1995, 16, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Gaeng, S.; Scherer, S.; Neve, H.; Loessner, M.J. Gene Cloning and Expression and Secretion of Listeria monocytogenes Bacteriophage-Lytic Enzymes in Lactococcus lactis. Appl. Environ. Microbiol. 2000, 66, 2951–2958. [Google Scholar] [CrossRef]
- Turner, M.S.; Waldherr, F.; Loessner, M.J.; Giffard, P.M. Antimicrobial Activity of Lysostaphin and a Listeria monocytogenes Bacteriophage Endolysin Produced and Secreted by Lactic Acid Bacteria. Syst. Appl. Microbiol. 2007, 30, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Campisi, E.; Li, J.; Fischetti, V.A. Decontamination of Escherichia coli O157:H7 on Fresh Romaine Lettuce Using a Novel Bacteriophage Lysin. Int. J. Food Microbiol. 2021, 341, 109068. [Google Scholar] [CrossRef] [PubMed]
- Ribelles, P.; Rodríguez, I.; Suárez, J.E. LysA2, the Lactobacillus casei Bacteriophage A2 Lysin Is an Endopeptidase Active on a Wide Spectrum of Lactic Acid Bacteria. Appl. Microbiol. Biotechnol. 2012, 94, 101–110. [Google Scholar] [CrossRef]
- Van Tassell, M.L.; Ibarra-Sánchez, L.A.; Hoepker, G.P.; Miller, M.J. Hot Topic: Antilisterial Activity by Endolysin PlyP100 in Fresh Cheese. J. Dairy Sci. 2017, 100, 2482–2487. [Google Scholar] [CrossRef]
- Zhang, H.; Bao, H.; Billington, C.; Hudson, J.A.; Wang, R. Isolation and Lytic Activity of the Listeria Bacteriophage Endolysin LysZ5 against Listeria monocytogenes in Soya Milk. Food Microbiol. 2012, 31, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Simmons, M.; Morales, C.A.; Oakley, B.B.; Seal, B.S. Recombinant Expression of a Putative Amidase Cloned from the Genome of Listeria monocytogenes That Lyses the Bacterium and Its Monolayer in Conjunction with a Protease. Probiotics Antimicrob. Proteins 2012, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Waldherr, F.; Loessner, M.J. Listeria Bacteriophage Peptidoglycan Hydrolases Feature High Thermoresistance and Reveal Increased Activity after Divalent Metal Cation Substitution. Appl. Microbiol. Biotechnol. 2012, 93, 633–643. [Google Scholar] [CrossRef]
- Dorscht, J.; Klumpp, J.; Bielmann, R.; Schmelcher, M.; Born, Y.; Zimmer, M.; Calendar, R.; Loessner, M.J. Comparative Genome Analysis of Listeria Bacteriophages Reveals Extensive Mosaicism, Programmed Translational Frameshifting, and a Novel Prophage Insertion Site. J. Bacteriol. 2009, 191, 7206–7215. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yang, R.; Yu, S.; Zhao, W. The Application of the Lytic Domain of Endolysin from Staphylococcus aureus Bacteriophage in Milk. J. Dairy Sci. 2021, 104, 2641–2653. [Google Scholar] [CrossRef]
- Gu, J.; Xu, W.; Lei, L.; Huang, J.; Feng, X.; Sun, C.; Du, C.; Zuo, J.; Li, Y.; Du, T.; et al. LysGH15, a Novel Bacteriophage Lysin, Protects a Murine Bacteremia Model Efficiently against Lethal Methicillin-Resistant Staphylococcus aureus Infection. J. Clin. Microbiol. 2011, 49, 111–117. [Google Scholar] [CrossRef]
- Chang, Y.; Kim, M.; Ryu, S. Characterization of a Novel Endolysin LysSA11 and Its Utility as a Potent Biocontrol Agent against Staphylococcus aureus on Food and Utensils. Food Microbiol. 2017, 68, 112–120. [Google Scholar] [CrossRef] [PubMed]
- García, P.; Martínez, B.; Rodríguez, L.; Rodríguez, A. Synergy between the Phage Endolysin LysH5 and Nisin to Kill Staphylococcus aureus in Pasteurized Milk. Int. J. Food Microbiol. 2010, 141, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.C.; Fernández, L.; De Maesschalck, V.; Gutiérrez, D.; Campelo, A.B.; Briers, Y.; Lavigne, R.; Rodríguez, A.; García, P. Synergistic Action of Phage PhiIPLA-RODI and Lytic Protein CHAPSH3b: A Combination Strategy to Target Staphylococcus aureus Biofilms. NPJ Biofilms Microbiomes 2021, 7, 39. [Google Scholar] [CrossRef]
- Rodríguez-Rubio, L.; Martínez, B.; Donovan, D.M.; García, P.; Rodríguez, A. Potential of the Virion-Associated Peptidoglycan Hydrolase HydH5 and Its Derivative Fusion Proteins in Milk Biopreservation. PLoS ONE 2013, 8, e54828. [Google Scholar] [CrossRef]
- Schmelcher, M.; Powell, A.M.; Camp, M.J.; Pohl, C.S.; Donovan, D.M. Synergistic Streptococcal Phage ΛSA2 and B30 Endolysins Kill Streptococci in Cow Milk and in a Mouse Model of Mastitis. Appl. Microbiol. Biotechnol. 2015, 99, 8475–8486. [Google Scholar] [CrossRef]
- Pritchard, D.G.; Dong, S.; Baker, J.R.; Engler, J.A. The Bifunctional Peptidoglycan Lysin of Streptococcus agalactiae Bacteriophage B30. Microbiology 2004, 150, 2079–2087. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.-H.; Duc, H.M.; Masuda, Y.; Honjoh, K.-I.; Miyamoto, T. Endolysin LysSTG2: Characterization and Application to Control Salmonella Typhimurium Biofilm Alone and in Combination with Slightly Acidic Hypochlorous Water. Food Microbiol. 2021, 98, 103791. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, Y.; Huang, C.; Wang, J.; Wang, X. An Endolysin LysSE24 by Bacteriophage LPSE1 Confers Specific Bactericidal Activity against Multidrug-Resistant Salmonella Strains. Microorganisms 2020, 8, 737. [Google Scholar] [CrossRef]
- Oliveira, H.; Thiagarajan, V.; Walmagh, M.; Sillankorva, S.; Lavigne, R.; Neves-Petersen, M.T.; Kluskens, L.D.; Azeredo, J. A Thermostable Salmonella Phage Endolysin, Lys68, with Broad Bactericidal Properties against Gram-Negative Pathogens in Presence of Weak Acids. PLoS ONE 2014, 9, e108376. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Lee, S.; Ryu, S. Identification and in Vitro Characterization of a Novel Phage Endolysin That Targets Gram-Negative Bacteria. Microorganisms 2020, 8, 447. [Google Scholar] [CrossRef]
- Skorynina, A.V.; Piligrimova, E.G.; Kazantseva, O.A.; Kulyabin, V.A.; Baicher, S.D.; Ryabova, N.A.; Shadrin, A.M. Bacillus-Infecting Bacteriophage Izhevsk Harbors Thermostable Endolysin with Broad Range Specificity. PLoS ONE 2020, 15, e0242657. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.; Son, B.; Ryu, S. Clostridium perfringens Virulent Bacteriophage CPS2 and Its Thermostable Endolysin LysCPS2. Viruses 2018, 10, 251. [Google Scholar] [CrossRef]
- Stentz, R.; Bongaerts, R.J.; Gunning, A.P.; Gasson, M.; Shearman, C. Controlled Release of Protein from Viable Lactococcus lactis Cells. Appl. Environ. Microbiol. 2010, 76, 3026–3031. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, B.K.; Tiwari, S.; Dubey, N.K. Essential Oils and Their Nanoformulations as Green Preservatives to Boost Food Safety against Mycotoxin Contamination of Food Commodities: A Review. J. Sci. Food Agric. 2021, 101, 4879–4890. [Google Scholar] [CrossRef]
- Tavakoli, S.; Regenstein, J.M.; Daneshvar, E.; Bhatnagar, A.; Luo, Y.; Hong, H. Recent Advances in the Application of Microalgae and Its Derivatives for Preservation, Quality Improvement, and Shelf-Life Extension of Seafood. Crit. Rev. Food Sci. Nutr. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Beya, M.M.; Netzel, M.E.; Sultanbawa, Y.; Smyth, H.; Hoffman, L.C. Plant-Based Phenolic Molecules as Natural Preservatives in Comminuted Meats: A Review. Antioxidants 2021, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.R.; Wohlgemuth, F.; Young, T.; Violet, J.; Dickinson, M.; Sanders, J.-W.; Vallieres, C.; Avery, S.V. Evolving Challenges and Strategies for Fungal Control in the Food Supply Chain. Fungal Biol. Rev. 2021, 36, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Ritota, M.; Manzi, P. Natural Preservatives from Plant in Cheese Making. Animals 2020, 10, 749. [Google Scholar] [CrossRef] [PubMed]
- Dykes, G.A.; Moorhead, S.M. Combined Antimicrobial Effect of Nisin and a Listeriophage against Listeria monocytogenes in Broth but Not in Buffer or on Raw Beef. Int. J. Food Microbiol. 2002, 73, 71–81. [Google Scholar] [CrossRef]
- Leverentz, B.; Conway, W.S.; Camp, M.J.; Janisiewicz, W.J.; Abuladze, T.; Yang, M.; Saftner, R.; Sulakvelidze, A. Biocontrol of Listeria monocytogenes on Fresh-Cut Produce by Treatment with Lytic Bacteriophages and a Bacteriocin. Appl. Environ. Microbiol. 2003, 69, 4519–4526. [Google Scholar] [CrossRef] [PubMed]
- Rohde, C.; Wittmann, J. Phage Diversity for Research and Application. Antibiotics 2020, 9, 734. [Google Scholar] [CrossRef]
- Sulakvelidze, A. Safety by Nature: Potential Bacteriophage Applications. Microbe 2011, 6, 122–126. [Google Scholar] [CrossRef]
- Naanwaab, C.; Yeboah, O.-A.; Ofori Kyei, F.; Sulakvelidze, A.; Goktepe, I. Evaluation of Consumers’ Perception and Willingness to Pay for Bacteriophage Treated Fresh Produce. Bacteriophage 2014, 4, e979662. [Google Scholar] [CrossRef]
- Dalmasso, M.; Hill, C.; Ross, R.P. Exploiting Gut Bacteriophages for Human Health. Trends Microbiol. 2014, 22, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage Applications for Food Production and Processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef] [PubMed]
| Target Bacteria | Phage/s | Source | Characterization Method | Genome Length | 2 Family | Food Application | Reference |
|---|---|---|---|---|---|---|---|
| Aeromonas hydrophila | AH-1 | Sewage samples | TEM | ND | Myoviridae | Depuration of artificially contaminated cockles | [45] |
| AH-4 | |||||||
| AH-5 | |||||||
| Bacillus cereus | PBC1 | Sewage sample | TEM, sequencing | 41,164 bp | Siphoviridae | Inhibition of B. cereus growth in boiled rice | [46,47] |
| Brochothrix thermosphacta | A3 | Spoiled retail rib steaks | TEM | ND | ND | Control of bacterial strains during refrigerated storage | [16,48] |
| Campylobacter jejuni | Cj6 | Unknown | - | ND | ND | Control of pathogens in liquid foods | [36,49] |
| C. jejuni | 2 | Unknown | 1 dsDNA | ~140 kb | Myoviridae | Reduction of C. jejuni contamination of retail poultry products | [50,51] |
| C. jejuni | CP8 CP30 | Poultry excreta | TEM, dsDNA | ~140 kb | Myoviridae | Reduction of food-borne bacteria and biofilms | [52,53] |
| C. jejuni | 12673 | NCTC (UK) | TEM, DNA sequencing | ~135 kb | Myoviridae | Reduction of bacterial contamination on chicken carcass surfaces | [54,55] |
| Clostridium tyrobutyricum and C. sporogenes | CTP1 | Landfill | TEM, DNA sequencing | 59,199 bp | Siphoviridae | Cheese manufacturing | [56] |
| Cronobacter (Enterobacter) sakazakii | ESP 1–3 | Sewage treatment plant | TEM, dsDNA | ND | Siphoviridae | Control of E. sakazakii in reconstituted infant formula | [57] |
| ESP 732–1 | Myoviridae | ||||||
| Escherichia coli | PE37 | Bovine intestine samples | TEM, DNA sequencing | 166,423 bp | Myoviridae | Biocontrol of E. coli STEC O157:H7 and ESBLEC. | [58] |
| E. coli | EC6 | Sewage | TEM, dsDNA | ND | Siphoviridae | Biocontrol against E. coli in UHT and raw bovine milk | [59] |
| EC9 | Myoviridae | ||||||
| EC11 | Podoviridae | ||||||
| E. coli (STEC) O145 | Ro145clw | Non-fecal compost samples | TEM, DNA sequencing | 42,031 bp | Siphoviridae | Control of foodborne STEC O145 | [60] |
| E. coli O157:H7 | vB_EcoS_FFH_1 | Wastewater treatment plants | TEM, sequencing | 108,483 bp 139,020 bp | Siphoviridae | Reduction of contamination in ground beef | [61,62] |
| _vB_EcoS_FFH_1 | Myoviridae | ||||||
| E. coli O157:H7 | e11/2 | Bovine farmyard Slurry samples Swine stool samples | TEM, dsDNA | ND ND ~140 kb | Myoviridae | Elimination or reduction of E. coli O157:H7 bacteria from meat carcasses | [63,64,65] |
| e4/1c | Siphoviridae | ||||||
| PP01 | Myoviridae | ||||||
| E. coli O157:H7 | FAHEc1 | Raw screened sewage | TEM, dsDNA | ~90 kb | Myoviridae | Inactivation of E. coli O157:H7 on beef | [34,66] |
| E. coli O157:H7 | KH1 | Cattle and sheep fecal samples | - | ND | ND | Elimination of O157:H7 from foods under refrigerated conditions. Reduction of E. coli on surfaces. | [67,68] |
| KH4 | |||||||
| KH5 | |||||||
| E. coli O157:H7 | ECML-4 | Fresh and salt water environments | TEM, DNA sequencing | 157,308 bp 66,854 bp 166,783 bp | Myoviridae | Reduction of contamination of hard surfaces and foods contaminated by E. coli O157:H7 | [69,70] |
| ECML-117 | |||||||
| ECML-134 | |||||||
| E. coli strains, Salmonella and Shigella spp. | C203 | Cottage cheese and from poultry liver | TEM, DNA sequencing | 138,073 bp | Myoviridae | Biocontrol agent against E. coli EHEC O157 | [37] |
| P206 | |||||||
| Shigatoxigenic E. coli Enteropathogenic E. coli | DT1 | Stool samples of patients with diarrhea | TEM | ND | Myoviridae | Control of pathogenic E.coli in meat products and during milk fermentation | [71,72] |
| DT5 | |||||||
| DT6 | |||||||
| E. coli strains including serotype O157:H7 | OSY-SP | Manure from cattle, sheep, and horse farms | Pulsed-field gel electrophoresis (PFGE) | ~150 Kb | Myoviridae | Reduction of E. coli in fresh produce type (cut green pepper or spinach leaves) | [73] |
| Lactobacillus brevis | SA-C12 | fresh silage | TEM | ND | Myoviridae | Control of L. brevis beer-spoilage | [74] |
| Leuconostoc gelidum | ggg | vacuum-packaged pork | TEM | ND | Siphoviridae | Inhibition of Leuconostoc in raw pork | [75] |
| Listeria monocytogenes | A500 ATCC® 23074-B1™ | L. monocytogenes isolated from Guinea pig | TEM | 38,867 bp | Siphoviridae | Control of L-forms of L. monocytogenes on surfaces | [76,77] |
| L. monocytogenes | H387 H387-A 2671 | - | TEM | ND | Siphoviridae | Disinfection of working surfaces of food processing plants | [78,79] |
| L. monocytogenes | LiMN4L | Seafood waste water treatment unit | ND | ND | ND | Control of L. monocytogenes on stainless steel in seafood processing environments | [22] |
| LiMN4p | |||||||
| LiMN17 | |||||||
| L. monocytogenes | A511 | Sewage from a sewage treatment plant | Phage typing, TEM, sequencing | 134,494 bp | Myoviridae | Ready-to-eat foods from plant and animal origin including cheeses | [80,81,82,83,84] |
| L. monocytogenes | FWLLm1 | Sheep feces | TEM, | ND | ND | Reduction of L. monocytogenes growth in ready-to-eat poultry products | [85] |
| L. monocytogenes | IZSAM-1 | Floor drain-water from an Italian blue cheese dairy factory | TEM, sequencing | ~50 kb | Siphoviridae | Biocontrol of L. monocytogenes within cheese industrial facilities | [86,87] |
| Listeria spp. | P100 | Sewage effluent from a dairy plant | TEM, sequencing | 131,384 bp | Myoviridae | Biocontrol of contaminated surfaces, the surface of soft cheeses, ready-to-eat foods, fresh-cut fruit, and fruit juices, raw fish fillets, | [88,89,90,91,92] |
| Pseudomonas fragi | Wy | Ground Beef | TEM, dsDNA | ND | - | Reduction of P. fragi in refrigerated raw milk | [93,94,95] |
| Pseudomonas sp. | C35 | spoiled retail beef | - | ND | - | Biological control of beef spoilage | [96,97] |
| Pseudomonas lactis | HU1 | sludge obtained after passing raw cow’s milk through a centrifugal clarifier | TEM, dsDNA | ~48 Kb | Podoviridae | Control of P. lactis in Raw Cow’s Milk | [98] |
| Pseudomonas fluorescens E. cloacae strains | PspYZU5415 | Sewage samples | TEM, sequencing | 39,636 bp | Siphoviridae Corticoviridae | Growth inhibition of E. cloacae and P. fluorescens in cucumber juice with different salt concentrations | [43] |
| EcpYZU01 | 39,767 bp | ||||||
| P. fluorescens | IBB-PF7A | Sewage treatment plant | TEM, dsDNA | ~42 kbp | Podoviridae | Biocontrol of P. fluorescens in dairy and other food industries | [99,100] |
| Salmonella Enteritidis, S. Typhimurium | wksl3 | Chicken by-product samples | TEM, sequencing | 42,766 bp | Siphoviridae | Control Salmonella on chicken skin. from broiler carcasses | [101] |
| Salmonella serovars | LPSEYT | Water samples | TEM, sequencing | 53,387 bp | Myoviridae | Biocontrol of Salmonella in food matrices including milk and lettuce | [42] |
| Salmonella Enteritidis | CAU-SEP-1 | River water samples | TEM | ND | Myoviridae and Siphoviridae | Control of S. Enteritidis in chicken breast meat | [102] |
| CAU-SEP-2 | |||||||
| CAU-SEP-3 | |||||||
| CAU-SEP-4 | |||||||
| Salmonella Enteritidis | CNPSA 1 CNPSA3 CNPSA4 | free-range chickens | TEM, dsDNA | ND | tailed dsDNA phages | Reduction of Salmonella Enteritidis in Contaminated Chicken Cuts | [103,104,105] |
| Salmonella Enteritidis | P29C | Raw human sewage | - | ND | Siphoviridae | Reduction of bacterial contamination on chicken carcass surfaces | [54,106] |
| Salmonella spp. | PSE5 | Poultry slaughterhouse wastewater | plaque morphology and RAPD analysis | ND | ND | Reduction of contamination in raw chicken eggs | [107] |
| Salmonella spp. | LPSTLL | Environmentally water samples | TEM | ND | Siphoviridae | Reduction of Salmonella counts in milk and chicken breast and on stainless still surfaces | [108,109] |
| LPST94 | Ackermannviridae | ||||||
| LPST153 | Podoviridae | ||||||
| Salmonella strains | LPSE1 | Environmental samples | TEM, dsDNA, sequencing | 41,854 bp | Siphoviridae | Control of Salmonella in ready-to-eat foods | [110] |
| Salmonella strains | Felix O1/Felix O1-E2 | Feces of paratyphoid B patients | TEM, Sequencing | 86,155 bp/~84 kb | Myoviridae | Suppression of Salmonella growth on chicken frankfurters, poultry products, and ready-to-eat foods | [111,112,113,114] |
| Salmonella strains | PHL4 | Wastewater treatment plant | - | ND | ND | Reduction of Salmonella growth poultry products | [115] |
| Salmonella strains | vB_SalS_SJ_3 | Wastewater | TEM, DNA sequencing | 162,910 bp | Siphoviridae | Biocontrol of Salmonella in contaminated Eggs and Pork | [116,117,118] |
| Salmonella strains | Pu20 | Sewage samples | TEM, sequencing | 59,435 bp | Podoviridae | Growth inhibition of Salmonella strains in liquid egg white and yolk | [119] |
| Salmonella strains | D1-2 | Environmental samples | TEM, sequencing | 86,878 bp | Myoviridae | Growth inhibition of Salmonella strains in liquid egg white and yolk | [120] |
| Salmonella Typhimurium | P22 [Argo4] | Salmonella enterica subsp. enterica serovar Typhimurium | TEM, sequencing. Reference strain ATCC® 97540™ | 41,724 bp | Podoviridae | Prevention of attachment to food surfaces and food matrices | [121,122,123,124] |
| Salmonella Typhimurium | P7 | Unknown | - | ND | ND | Control of pathogens in liquid foods | [36] |
| Salmonella serovars | LPST153 | Water samples | TEM, sequencing | 39,176 bp | Autographivirinae | Control of Salmonella in raw milk and raw beef sausages | [125] |
| S. enterica serovar Typhimurium S. enterica serovar Enteritidis | UAB_Phi 20 | Chicken | TEM, dsDNA, sequencing | 41,809 bp 44,110 bp 87,669 bp | Podoviridae Podoviridae Myoviridae | Reduction of Salmonella on foods and reduction of Salmonella Colonization of poultry | [126,127,128] |
| UAB_Phi78 | Chicken | ||||||
| UAB_Phi87 | pig | ||||||
| Salmonella Enteritidis | SP-1 | Intestinal content of broiler chickens | TEM, dsDNA, PCR amplification | ~86 kb | Podoviridae | Biocontrol of Salmonella in cooked chicken meat | [35,129,130] |
| SP-3 | ~88 kb | Siphoviridae | |||||
| Salmonella Enteritidis | SJ2 | Chicken egg | ND | ND | ND | Reduction of Salmonella counts in Cheddar cheese made from both raw and pasteurized milk, and in contaminated eggs and pork | [131] |
| Salmonella Enteritidis | vBSenM-PA13076 (PA13076) vBSenM-PC2184 (PC2184) | Chicken sewage | TEM | 52,474 bp ND | Myoviridae | Biocontrol of Salmonella in foods (chicken breast, pasteurized whole milk, Chinese cabbage) | [132,133] |
| Salmonella and E. coli O157:H7 | PS5 | Raw chicken products | TEM, sequencing | 158,400 bp | Myoviridae | Reduction of viable counts on solid and liquid foods | [134] |
| Salmonella Oranienburg | SSP5 SSP6 | Sewage samples | TEM | ND | Myoviridae Siphoviridae | Control of Salmonella Oranienburg on alfalfa seeds | [135] |
| S. Typhimurium S. Enteritidis S. Montevideo | A B | sewage treatment plant | TEM | ND | Myoviridae Siphoviridae | Control of Salmonella in mustard and broccoli seeds | [136] |
| Salmonella strains, including MDR Salmonella | T156 | Waste water | TEM, dsDNA, sequencing | 123,849 bp | Siphoviridae | Microencapsulated bacteriophage applied in skim milk and lettuce for biocontrol of Salmonella | [137] |
| Staphylococcus aureus | K | Deposited by EA Asheshov | ATCC® 19685-B1™ | 139,831 bp | Myoviridae | Removing S. aureus biofilms | [138,139] |
| S. aureus | H5 (phiPLA88) A72 (phiPLA35) | Raw milk | TEM, dsDNA, sequencing | 42,526 bp 45,344 bp | Siphoviridae | Curd manufacturing, fresh and hard-type cheeses | [140,141,142] |
| S. aureus | SA46-CTH2 | Food samples | TEM | 17,505 bp | Podoviridae | Inactivation of S. aureus planktonic cells in pasteurized milk and biofilms on stainless steel surfaces | [143] |
| S. aureus | SA13m | Temperate phage SA13 isolated from a goat fecal sample | TEM, sequencing | 42,652 bp | Siphoviridae | Biocontrol of S. aureus in pasteurized whole milk at refrigeration and ambient temperatures | [144] |
| Shewanella baltica and S. putrefaciens | SppYZU01 to SppYZU10 | Wastewater from freshwater and marine product marketplaces | TEM, sequencing | SppYZU01 (43,567 bp) SppYZU05 (54,319 bp) | Myoviridae Siphoviridae | Biopreservation of chilled channel catfish | [145] |
| Shigella spp. | SF-A2 | Spiced chicken | TEM | ND | Myoviridae | inactivation of foodborne Shigella on ready-to-eat chicken | [146] |
| SD-11 | Pig farm effluent | ||||||
| SS-92 | Pig farm effluent | ||||||
| Vibrio parahaemolyticus | vB_VpaS_OMN (designated as phage OMN) | Sea water | TEM, sequencing | 42,202 bp | Podoviridae | Inactivation of V. parahaemolyticus in oyster meat | [147] |
| Target Bacteria | Enzybiotic | Source | Food Application | Reference |
|---|---|---|---|---|
| Bacillus cereus, B. subtilis and L. monocytogenes | LysB4 | B. cereus phage B4 | antibacterial agent to control foodborne pathogens. | [196] |
| Clostridium tyrobutyricum and C. sporogenes | Ctp1L | Bacteriophage CTP1 isolated from landfill | Cheese manufacture, reduction of clostridial activity in cheese | [56,197] |
| C. tyrobutyricum C. acetobutylicum | CS74L | Lytic bacteriophage (ATCC® 8074-B1TM) of C. sporogenes | Biocontrol of clostridia strains in foods | [198] |
| C. perfringens | Ply3626 | C. perfringens ATCC 3626 | Control of anaerobic spore-formers | [199] |
| C. perfringens | LysCPAS15 | C. perfringens phage CPAS-15 | Inhibition of C. perfringens in sterilized milk | [200] |
| Bacillus subtilis B. megaterium L. monocytogenes | PLY118, PLY500 PLY 511 | Phages from Listeria monocytogenes | Production of airy starter cultures with biopreservation properties | [201,202,203] |
| E. coli O157:H7 | PlyEc2 | Phage from E. coli | Reduction of E. coli O157:H7 on contaminated lettuce | [204] |
| Lactococcus lactis, Pediococcus acidilactici and P. pentosaceus | LysA2 | L. casei bacteriophage A2 | Ripening of fermented products | [205] |
| Lactobacilli, lactococci, pediococci, B. Subtilis Brevibacterium linens Enterococcus faecium | Mur-LH | Phage 0303 from Lactobacillus helveticus CNRZ 303 | Preventing the growth of spoilage microbes | [17] |
| L. monocytogenes B. subtilis | PlyP100 | Phage from L. monocytogenes | Antimicrobial biopreservative in fresh cheese. | [206] |
| L. monocytogenes | LysZ5 | Phage from L. monocytogenes | Control pathogens in soya milk | [207] |
| L. monocytogenes | PlyLM | Phage from L. monocytogenes strain 4b | Proposed control of L. monocytogenes in food matrices and processing facilities | [208] |
| L. monocytogenes | HPL118 HPL500 HPL511 HPLP35 | Recombinant endolysins from L. monocytogenes phages | Reduction of L. monocytogenes viable counts in iceberg lettuce. Promising perspectives in production and packaging environments | [201,209,210] |
| Methicillin-resistant Staphylococcus aureus | LysGH15 | Phage isolated from Sewage samples | Biopreservative in whole and skim milk | [211,212] |
| methicillin-resistant S. aureus | LysSA11 | Staphylococcus aureus phage SA11 | Biocontrol of S. aureus on strain in pasteurized milk or ham and utensils | [213] |
| S. aureus Bacillus cereus | Hybrid LysB4EAD-LysSA11 | Phage SA11 from S. aureus phage B4 S from B. cereus | Biocontrol of S. aureus and B. cereus in boiled rice | [195] |
| S. aureus | LysH5 | Staphylococcal bacteriophage phi-SauS-IPLA88 | Disinfection process of industrial food facilities. Elimination of S. aureus in pasteurized milk | [190,214] |
| S. aureus | CHAPSH3b | Chimeric protein (CHAP domain from peptidoglycan hydrolase HydH5 and the SH3b cell wall-binding domain from lysostaphin) | S. aureus biofilm elimination | [215] |
| S. aureus | CHAPK | Truncated derivative of the phage lysin LysK from the staphylococcal bacteriophage K | Reduction of biofilm formation in processing systems | [189] |
| S. aureus | HydH5 HydH5Lyso HydH5SH3b CHAPSH3b and lysostaphin | S. aureus bacteriophage vB_SauS-phiIPLA88 | Biocontrol of S. aureus in dairy products | [216] |
| Streptococcus spp. | λSA2 | Streptococcus agalactiae (serotype III GBS strain 3330) bacteriophage B30 | Inactivation of Strepcococcus spp. in cow milk | [217,218] |
| S. Typhimurium | LysSTG2 | Salmonella-lytic bacteriophage STG2 | Combating S. Typhimurium biofilms in food industries | [219] |
| Salmonella strains | LysSE24 | Salmonella phage LPSE1 | Food Control of Salmonella strains | [220] |
| Several Gram-negative pathogens, particularly against Salmonella Typhimurium | Lys68 | Salmonella phage phi68 isolated from feces from a poultry farm | Combat Gram-negative pathogens in the food industry | [221] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Vivas, J.; Elexpuru-Zabaleta, M.; Samano, M.L.; Barrera, A.P.; Forbes-Hernández, T.Y.; Giampieri, F.; Battino, M. Phages and Enzybiotics in Food Biopreservation. Molecules 2021, 26, 5138. https://doi.org/10.3390/molecules26175138
Ramos-Vivas J, Elexpuru-Zabaleta M, Samano ML, Barrera AP, Forbes-Hernández TY, Giampieri F, Battino M. Phages and Enzybiotics in Food Biopreservation. Molecules. 2021; 26(17):5138. https://doi.org/10.3390/molecules26175138
Chicago/Turabian StyleRamos-Vivas, José, María Elexpuru-Zabaleta, María Luisa Samano, Alina Pascual Barrera, Tamara Y. Forbes-Hernández, Francesca Giampieri, and Maurizio Battino. 2021. "Phages and Enzybiotics in Food Biopreservation" Molecules 26, no. 17: 5138. https://doi.org/10.3390/molecules26175138
APA StyleRamos-Vivas, J., Elexpuru-Zabaleta, M., Samano, M. L., Barrera, A. P., Forbes-Hernández, T. Y., Giampieri, F., & Battino, M. (2021). Phages and Enzybiotics in Food Biopreservation. Molecules, 26(17), 5138. https://doi.org/10.3390/molecules26175138









