Bioactive Polyketide and Diketopiperazine Derivatives from the Mangrove-Sediment-Derived Fungus Aspergillus sp. SCSIO41407
Abstract
:1. Introduction
2. Results and Discussion
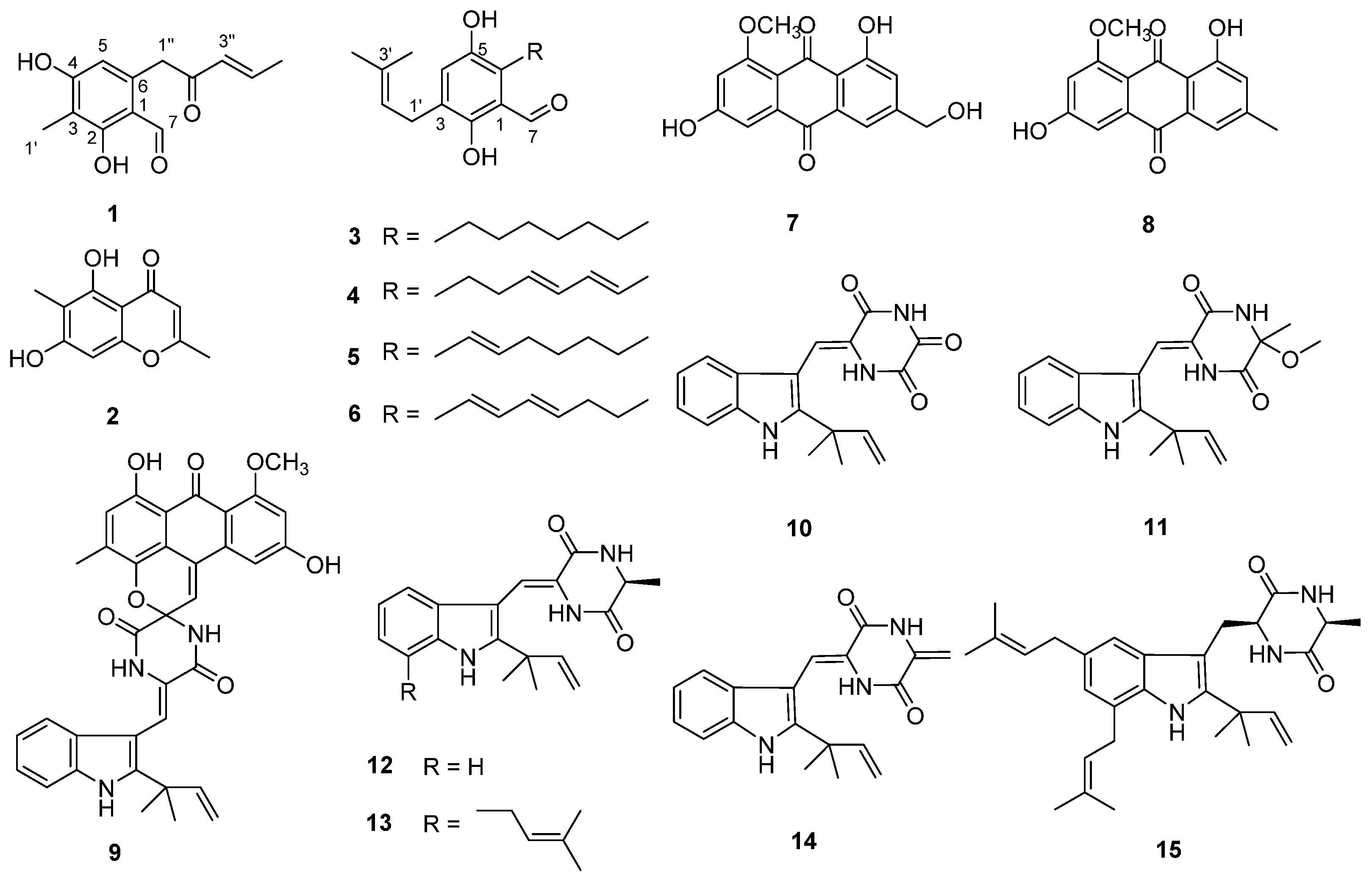
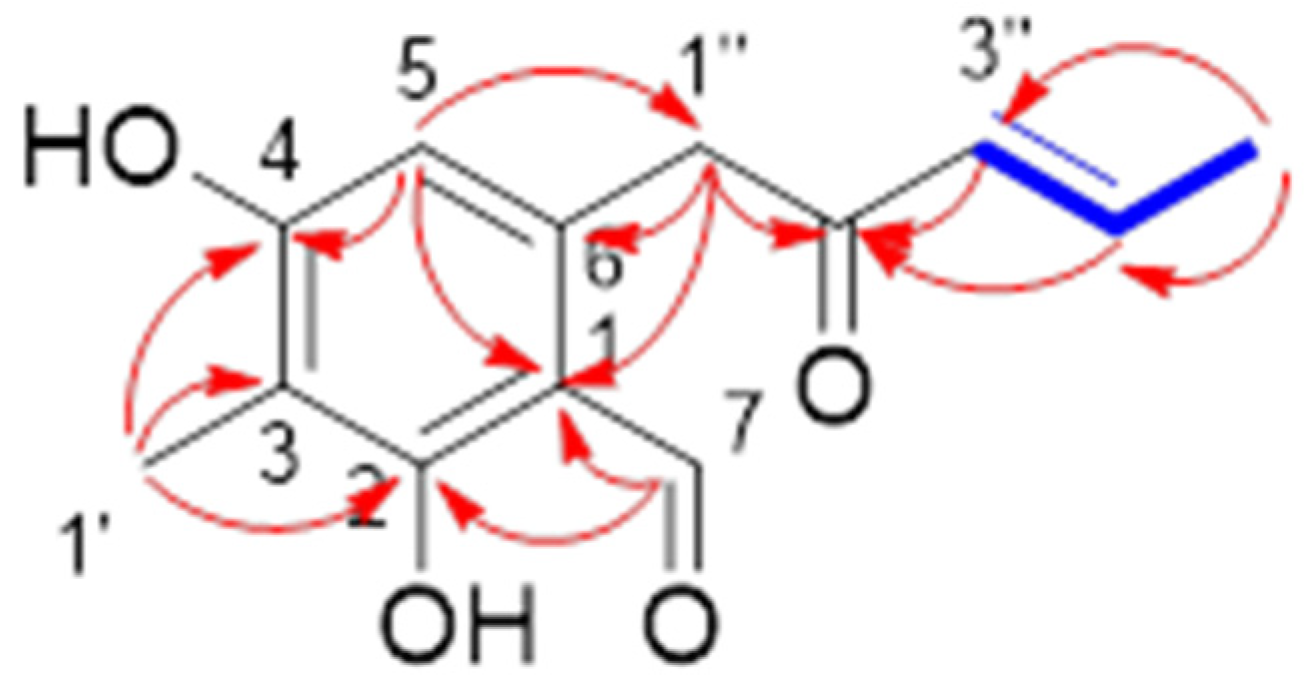
2.1. Structural Determination
2.2. Cytotoxic and Antibacterial Activities
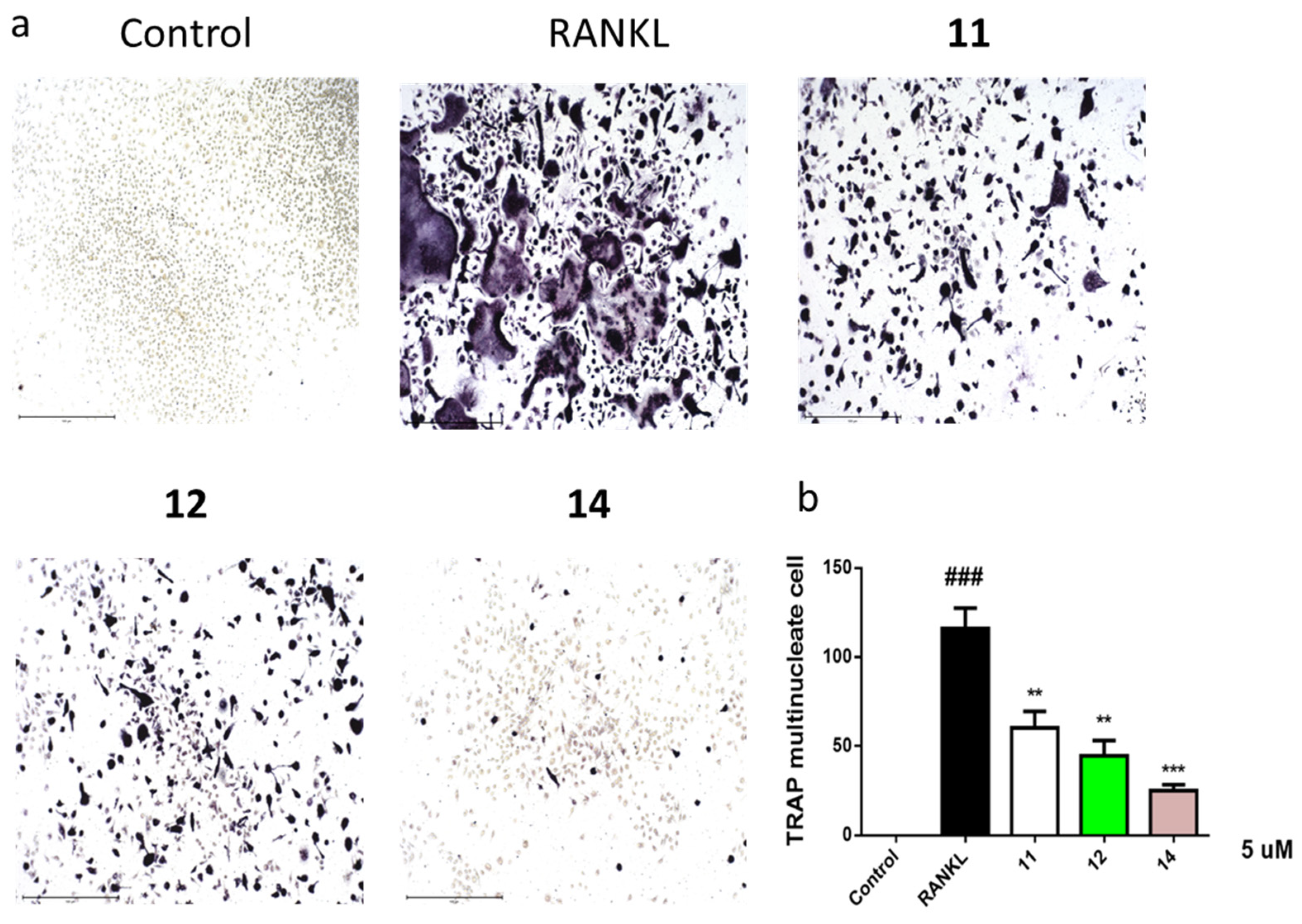
2.3. Acetylcholinesterase (AChE) Inhibitory Activities
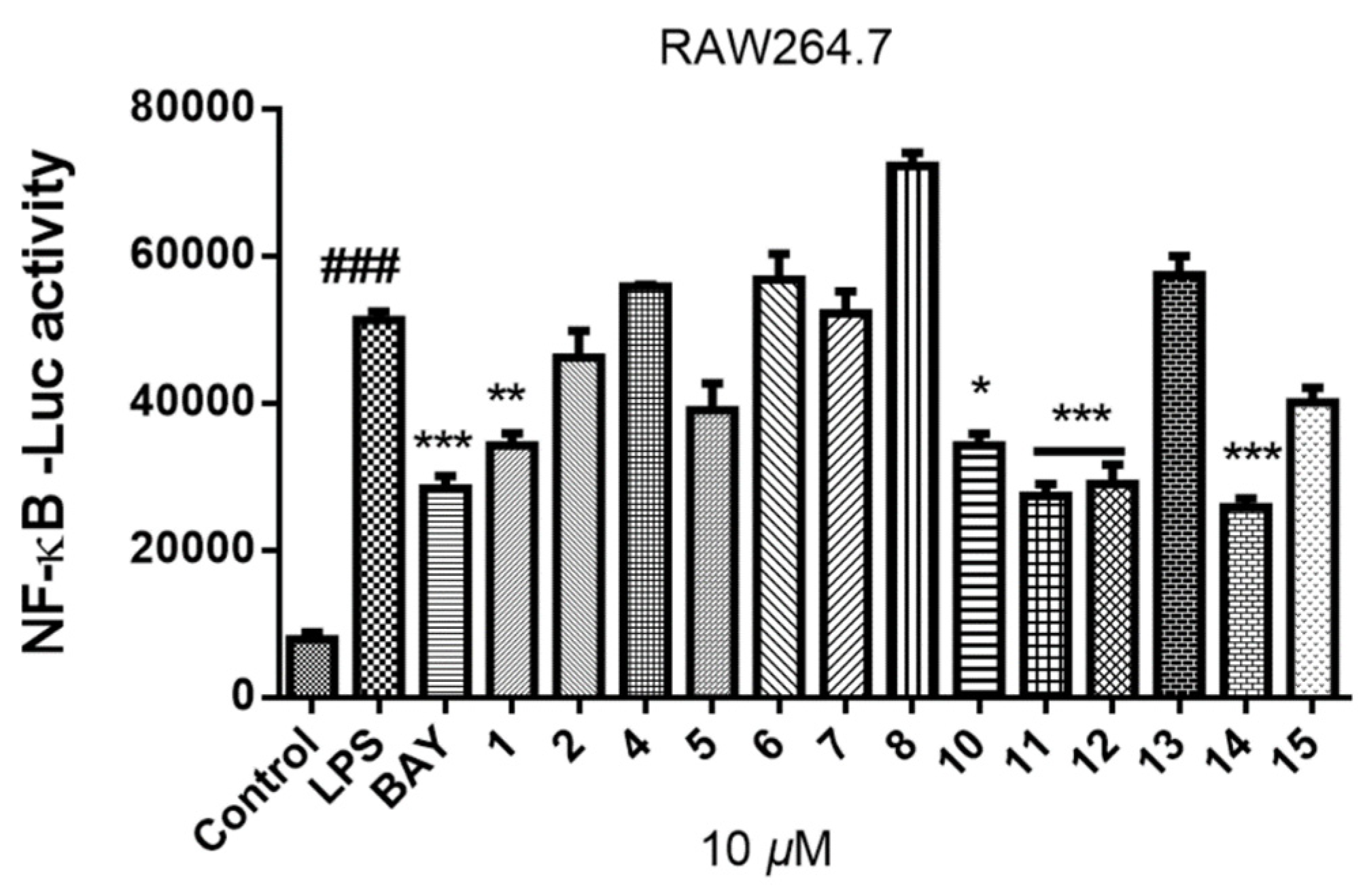
2.4. Inhibition of Lipopolysaccharide (LPS)-Induced Nuclear Factor-κB (NF-κB)
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction and Isolation
(E)-2,4-Dihydroxy-3-methyl-6-(2-oxopent-3-en-1-yl) benzaldehyde (1)
3.4. Bioactivity Assay
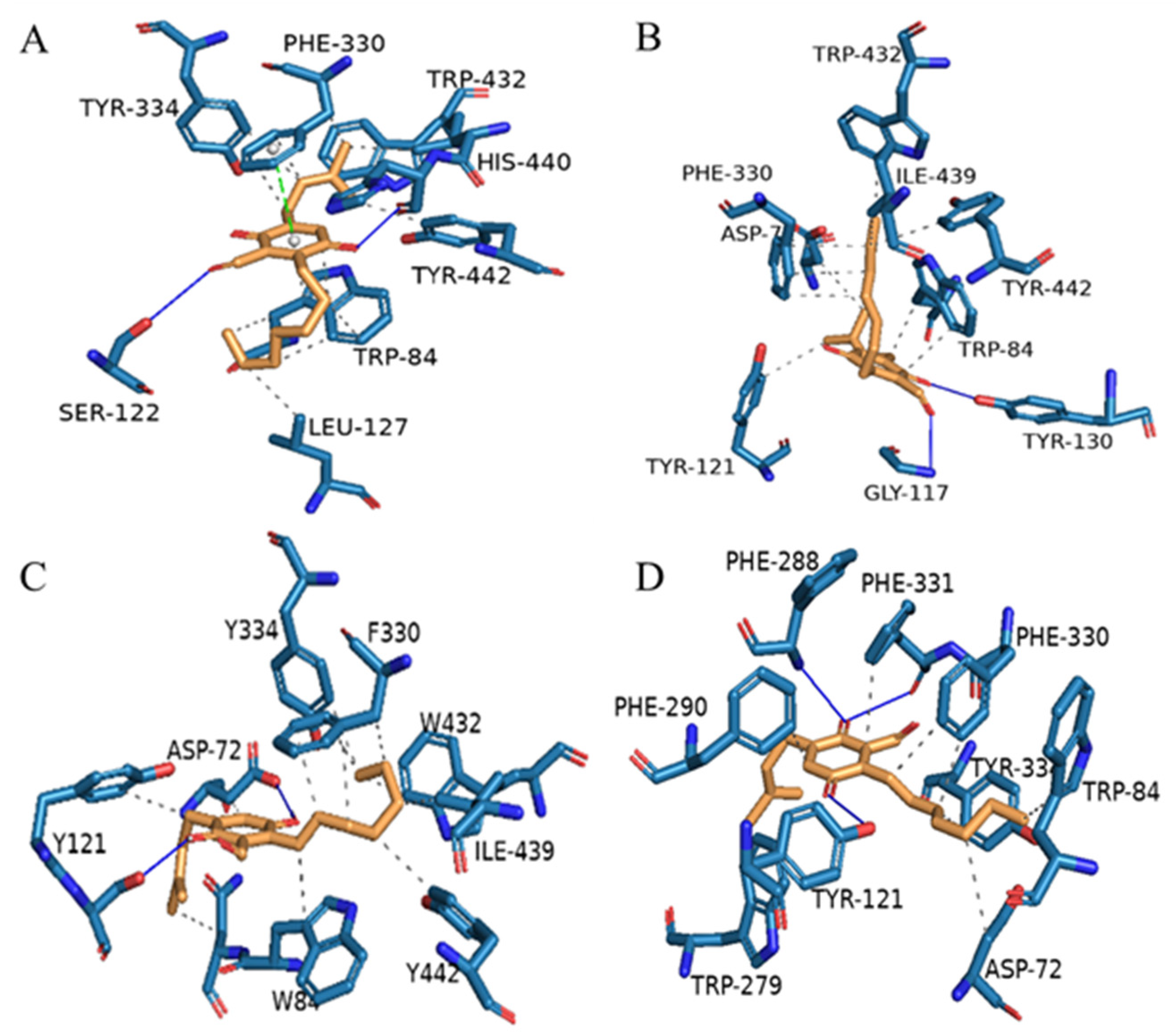
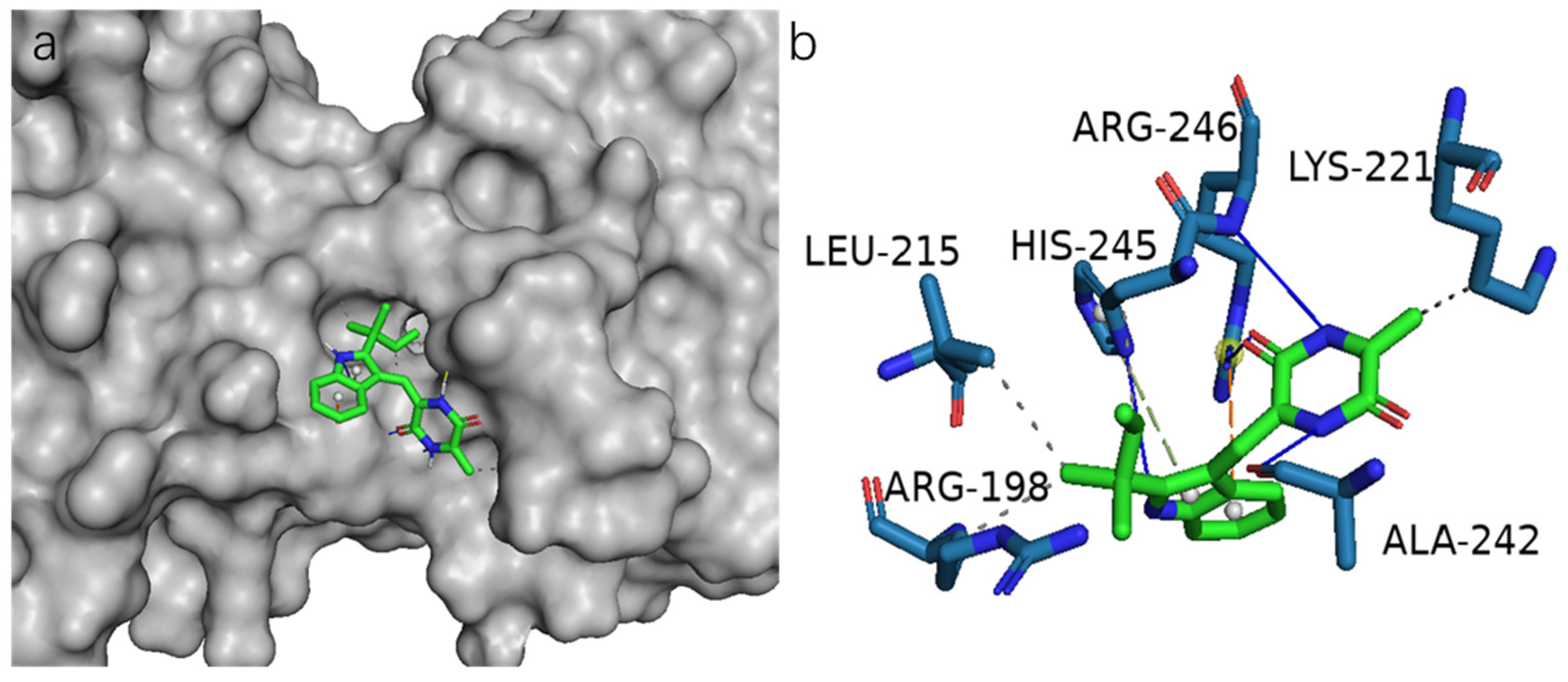
3.5. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- He, F.; Li, X.B.; Yu, J.H.; Zhang, X.Y.; Nong, X.H.; Chen, G.Y.; Zhu, K.K.; Wang, Y.Y.; Bao, J.; Zhang, H. Secondary metabolites from the mangrove sediment-derived fungus Penicillium pinophilum SCAU037. Fitoterapia 2019, 136, 104177. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.Q.; Kong, X.L.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Penipyrols A–B and peniamidones A–D from the mangrove derived Penicillium solitum GWQ-143. Arch. Pharm. Res. 2015, 38, 1449–1454. [Google Scholar] [CrossRef]
- Yang, B.; Tao, H.M.; Qin, X.C.; Wang, Z.; Dong, J.D.; Lin, X.P.; Zhou, X.F.; Li, J.L.; Tu, Z.C.; Liu, Y.H. Aspergone, a new chromanone derivative from fungus Aspergillus sp. SCSIO41002 derived of mangrove soil sample. J. Antibiot. 2017, 70. [Google Scholar] [CrossRef]
- Cai, S.X.; Sun, S.W.; Peng, J.X.; Kong, X.L.; Zhou, H.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Okaramines S–U, three new indole diketopiperazine alkaloids from Aspergillus taichungensis ZHN-7-07. Tetrahedron 2015, 71, 3715–3719. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Wang, Y.; Miao, C.D.; Liu, P.P.; Hong, K.; Zhu, W.M. Sesquiterpenoids and benzofuranoids from the marine-derived fungus Aspergillus ustus 094102. J. Nat. Prod. 2009, 72, 1761–1767. [Google Scholar] [CrossRef]
- Tao, H.M.; Li, Y.Q.; Lin, X.P.; Zhou, X.F.; Dong, J.D.; Liu, Y.H.; Yang, B. A new pentacyclic ergosteroid from fungus Aspergillus sp. SCSIO41211 derived of mangrove sediment sample. Nat. Prod. Commun. 2018, 13, 1629–1631. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, X.M.; Meng, L.H.; Wang, B.G. Polyketides from the marine mangrove-derived fungus Aspergillus ochraceus MA-15 and their activity against aquatic pathogenic bacteria. Phytochem. Lett. 2015, 12, 232–236. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Hirota, H.; Imachi, M.; Fujimuro, M.; Onuki, H.; Ohta, T.; Yokosawa, H. Himeic Acid A: A new ubiquitin-activating enzyme inhibitor isolated from a marine-derived fungus, Aspergillus sp. Bioorg. Med. Chem. Lett. 2005, 15, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Dong, Z.T.; Qiu, P.; Wang, Q.L.; Yan, J.J.; Lu, Y.J.; Wasu, P.; Hong, K.; She, Z.G. Two new bioactive steroids from a mangrove-derived fungus Aspergillus sp. Steroids 2018, 140, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.; Pombeiro-Sponchiado, S.R. Antioxidant activity of the melanin pigment extracted from Aspergillus nidulans. Biochem. Pharm. Bull. 2005, 28, 1129–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.Q.; Guo, W.Q.; Wang, Q.; Zhang, L.Q.; Zhu, M.L.; Zhu, T.J.; Gu, Q.Q.; Wang, W.; Li, D.H. Aspulvinones from a mangrove Rhizosphere soil-derived fungus Aspergillus terreus Gwq-48 with anti-influenza A viral (H1N1) activity. Bioorg. Med. Chem. Lett. 2013, 23, 1776–1778. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guo, W.Q.; Che, Q.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Versicones E-H and arugosin K produced by the mangrove-derived fungus Aspergillus versicolor HDN11-84. J. Antibiot. 2017, 70, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Pyser, J.B.; Dockrey, S.A.B.; Benítez, A.R.; Joyce, L.A.; Wiscons, R.A.; Smith, J.L.; Narayan, A.R.H. Stereodivergent, chemoenzymatic synthesis of azaphilone natural products. J. Am. Chem. Soc. 2019, 141, 18551–18559. [Google Scholar] [CrossRef]
- Bai, H.H.; Wu, L.W.; Yang, T.; Li, G.Y. Isolation and identification of secondary metabolites from fungus Chaetomium gracile and their antimicrobial activities. Chin. J. Appl. Environ. Biol. 2015, 21, 274–278. [Google Scholar]
- Wang, F.Z.; Huang, Z.; Shi, X.F.; Chen, Y.C.; Tian, X.P.; Li, J.; Zhang, W.M.; Zhang, S. Analysis of secondary metabolites produced by Eurotium sp. SCSIO F452 isolated from south china sea sediment. Chin. J. Mar. Drugs 2013, 32, 7–12. [Google Scholar]
- Sun, K.L.; Wang, Y.; Fu, P.; Liu, P.P.; Zhu, W.M. Studies on the secondary metabolites of Eurotium herbariorum HT-2 symbiotic with Enteromorpha prolifera. Chin. J. Mar. Drugs 2013, 32, 37–45. [Google Scholar]
- Miyake, Y.; Ito, C.; Itoigawa, M.; Osawa, T. Antioxidants produced by Eurotium herbariorum of filamentous fungi used for the manufacture of karebushi, dried bonito (Katsuobushi). Biosci. Biotechnol. Biochem. 2009, 73, 1323–1327. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Cao, F.; Guo, X.J.; Zhang, Y.R.; Zhu, H.J. Antibacterial indole alkaloids and anthraquinones from a sewage-derived fungus Eurotium sp. Chem. Nat. Compd. 2018, 54, 399–401. [Google Scholar] [CrossRef]
- Chen, G.D.; Bao, Y.R.; Huang, Y.F.; Hu, D.; Li, X.X.; Guo, L.D.; Li, J.; Yao, X.S.; Gao, H. Three pairs of variecolortide enantiomers from Eurotium sp. with caspase-3 inhibitory activity. Fitoterapia 2014, 92, 252–259. [Google Scholar] [CrossRef]
- Luo, M.H.; Huang, H.B.; Lu, C.L.; Ju, J.H. Diketopiperazine indole alkaloids from a marine-derived fungus Eurotium amstelodami SCSIO 151. Nat. Prod. Res. 2015, 27, 50–54. [Google Scholar]
- Wang, W.L.; Lu, Z.Y.; Tao, H.W.; Zhu, T.J.; Fang, Y.C.; Gu, Q.Q.; Zhu, W.M. Isoechinulin-type alkaloids, variecolorins A–L, from halotolerant Aspergillus wariecolor. J. Nat. Prod. 2007, 70, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Kang, M.-C.; Li, Y.; Kim, E.-A.; Kang, S.-M.; Jeon, Y.-J. Asperflavin, an anti-inflammatory compound produced by a marine-derived fungus, Eurotium amstelodami. Molecules 2017, 22, 1823. [Google Scholar] [CrossRef] [Green Version]
- Li, D.L.; Li, X.M.; Li, T.G.; Dang, H.Y.; Wang, B.G. Dioxopiperazine alkaloids produced by the marine mangrove derived endophytic fungus Eurotium rubrum. Helv. Chim. Acta 2008, 91, 1888–1893. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activitity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [PubMed]
- Tan, Y.H.; Dang, W.D.; Yang, Y.Y.; Ke, M.H.; Zou, B.H.; Luo, X.W.; Su, J.B.; Wang, Y.Y.; Xu, J.L.; Nandakumar, K.S.; et al. A marine fungus-derived nitrobenzoyl sesquiterpenoid suppresses RANKL-induced osteoclastogenesis and attenuates inflammatory bone destruction. Brit. J. Pharmacol. 2020, 177, 4242–4260. [Google Scholar]
- Luo, X.W.; Chen, C.M.; Tao, H.M.; Lin, X.P.; Yang, B.; Zhou, X.F.; Liu, Y.H. Structurally diverse diketopiperazine alkaloids from the marine-derived fungus Aspergillus Versicolor SCSIO 41016. Org. Chem. Front. 2019, 6, 736–740. [Google Scholar] [CrossRef]
- Pang, X.Y.; Lin, X.P.; Yang, J.; Zhou, X.F.; Yang, B.; Wang, J.F.; Liu, Y.H. Spiro-phthalides and isocoumarins isolated from the marine-sponge-derived fungus Setosphaeria sp. SCSIO41009. J. Nat. Prod. 2018, 81, 1860–1868. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. Software news and update autodock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar]
- Rydberg, E.H.; Brumshtein, B.; Greenblatt, H.M.; Wong, D.M.; Shaya, D.; Williams, L.D.; Carlier, P.R.; Pang, Y.P.; Silman, I.; Sussman, J.L. Complexes of alkylene-linked tacrine dimers with torpedo californica acetylcholinesterase: Binding of bis5-tacrine produces a dramatic rearrangement in the active-site gorge. J. Med. Chem. 2006, 49, 5491–5500. [Google Scholar]
| No. | δC | δH (J in Hz) | HMBC | COSY |
|---|---|---|---|---|
| 1 | 113.7 | |||
| 2 | 165.2 | |||
| 3 | 111.4 | |||
| 4 | 164.5 | |||
| 5 | 111.7 | 6.25 (CH, s) | C-1, C-1″, C-4 | |
| 6 | 139.5 | |||
| 7 | 194.8 | 9.80 (CH, s) | C-2, C-1, C-5 | |
| 1′ | 7.2 | 2.03 (CH3, s) | C-4, C-2, C-3 | |
| 1″ | 43.9 | 4.15 (CH2, s) | C-2″, C-6, C-1, C-5 | |
| 2″ | 198.9 | |||
| 3″ | 131.8 | 6.28 (CH, d, J = 14.6 Hz) | C-5″, 2″ | H-4″, H-5″ |
| 4″ | 146.3 | 7.09 (CH, m) | C-2″, C-5″ | H-5″, H-3″ |
| 5″ | 18.5 | 1.95 (CH3, d, J = 6.7 Hz) | C-4″, C-3″ | C-4″, C-3″ |
| Compounds | Cytotoxic Activities | Antibacterial Activities | |
|---|---|---|---|
| A549 (IC50, μM) | MRSA (MIC, μM) | * Other Bacterial Strains | |
| 2 | / | 485.4 μM | / |
| 3 | 22.2 μM | / | / |
| 1, 4–8, 10–15 | / | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Chen, C.; Tan, Y.; Chen, W.; Luo, X.; Luo, L.; Yang, B.; Liu, Y.; Zhou, X. Bioactive Polyketide and Diketopiperazine Derivatives from the Mangrove-Sediment-Derived Fungus Aspergillus sp. SCSIO41407. Molecules 2021, 26, 4851. https://doi.org/10.3390/molecules26164851
Cai J, Chen C, Tan Y, Chen W, Luo X, Luo L, Yang B, Liu Y, Zhou X. Bioactive Polyketide and Diketopiperazine Derivatives from the Mangrove-Sediment-Derived Fungus Aspergillus sp. SCSIO41407. Molecules. 2021; 26(16):4851. https://doi.org/10.3390/molecules26164851
Chicago/Turabian StyleCai, Jian, Chunmei Chen, Yanhui Tan, Weihao Chen, Xiaowei Luo, Lianxiang Luo, Bin Yang, Yonghong Liu, and Xuefeng Zhou. 2021. "Bioactive Polyketide and Diketopiperazine Derivatives from the Mangrove-Sediment-Derived Fungus Aspergillus sp. SCSIO41407" Molecules 26, no. 16: 4851. https://doi.org/10.3390/molecules26164851
APA StyleCai, J., Chen, C., Tan, Y., Chen, W., Luo, X., Luo, L., Yang, B., Liu, Y., & Zhou, X. (2021). Bioactive Polyketide and Diketopiperazine Derivatives from the Mangrove-Sediment-Derived Fungus Aspergillus sp. SCSIO41407. Molecules, 26(16), 4851. https://doi.org/10.3390/molecules26164851










