Unusual Lower Critical Solution Temperature Phase Behavior of Poly(benzyl methacrylate) in a Pyrrolidinium-Based Ionic Liquid
Abstract
1. Introduction
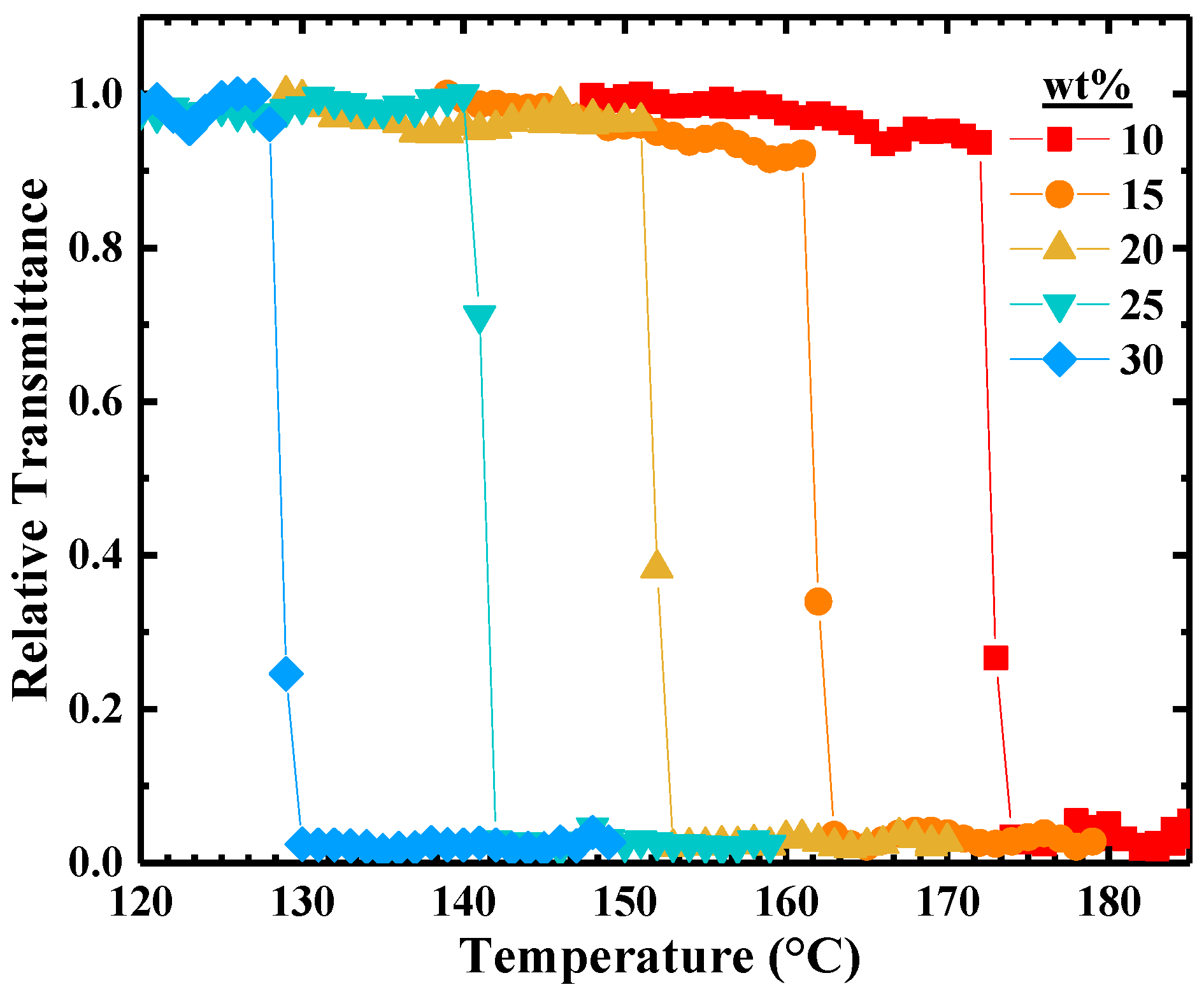
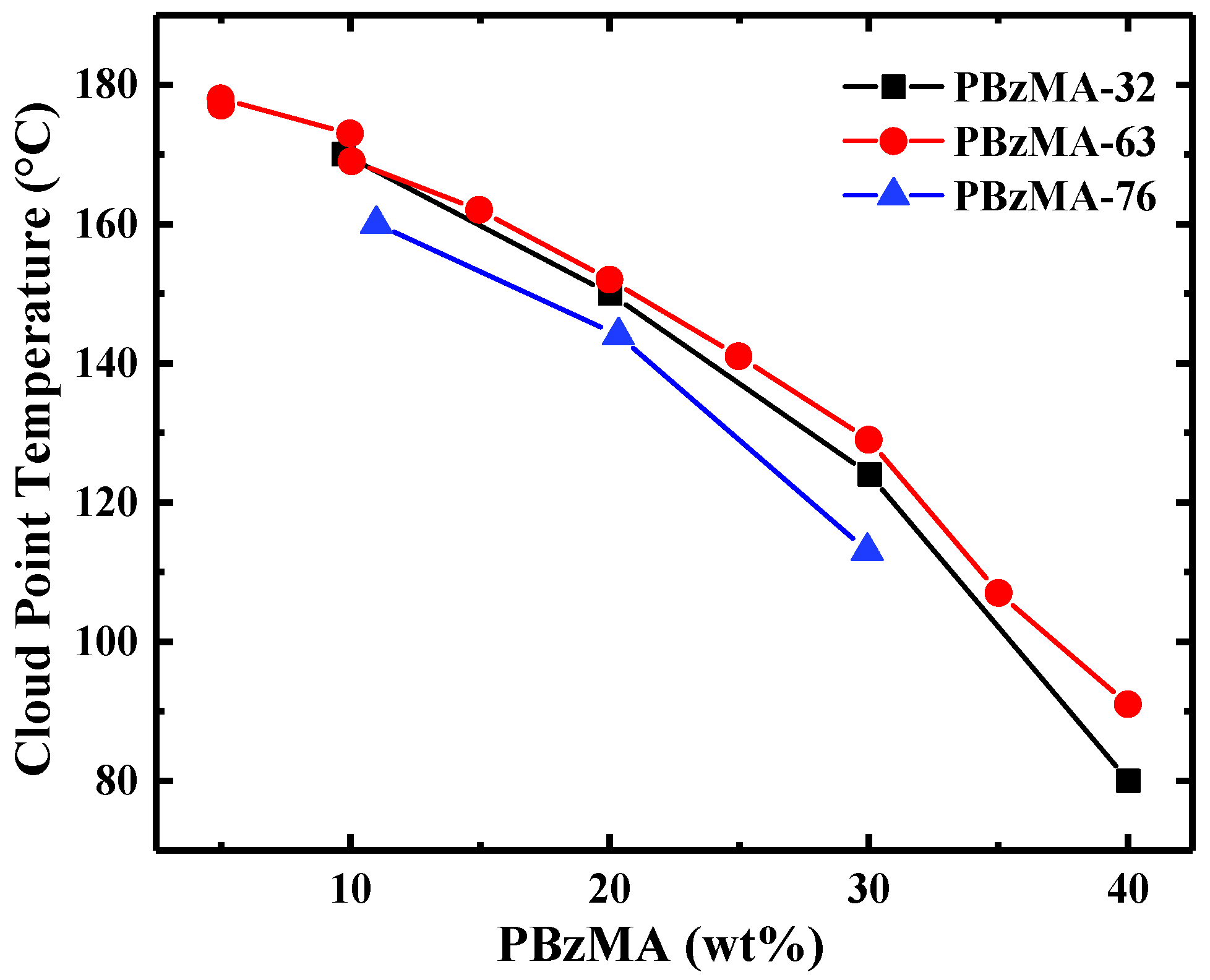
2. Results
3. Discussion
4. Materials and Methods
4.1. Polymer Synthesis
4.2. Solution Preparation
4.3. Cloud Point Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ueki, T.; Watanabe, M. Macromolecules in Ionic Liquids: Progress, Challenges, and Opportunities. Macromolecules 2008, 41, 3739–3749. [Google Scholar] [CrossRef]
- Ueki, T.; Watanabe, M. Polymers in Ionic Liquids: Dawn of Neoteric Solvents and Innovative Materials. Bull. Chem. Soc. Jpn. 2012, 85, 33–55. [Google Scholar] [CrossRef]
- Macfarlane, D.R.; Meakin, P.; Sun, J.; Amini, N.; Forsyth, M. Pyrrolidinium Imides: A New Family of Molten Salts and Conductive Plastic Crystal Phases. J. Phys. Chem. B 1999, 103, 4164–4170. [Google Scholar] [CrossRef]
- Tang, B.; White, S.P.; Daniel Frisbie, C.; Lodge, T.P. Synergistic Increase in Ionic Conductivity and Modulus of Triblock Copolymer Ion Gels. Macromolecules 2015, 48, 4942–4950. [Google Scholar] [CrossRef]
- Tokuda, H.; Ishii, K.; Abu Bin, M.; Susan, H.; Tsuzuki, S.; Hayamizu, K.; Watanabe, M. Physicochemical Properties and Structures of Room-Temperature Ionic Liquids. 3. Variation of Cationic Structures. J. Phys. Chem. B 2006, 110, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Ueki, T.; Yamaguchi, A.; Ito, N.; Kodama, K.; Sakamoto, J.; Ueno, K.; Kokubo, H.; Watanabe, M. Photoisomerization-Induced Tunable LCST Phase Separation of Azobenzene-Containing Polymers in an Ionic Liquid. J. Polym. Sci. Part A 2009, 25, 8845–8848. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Boswell, P.G.; Bu, P.; Lodge, T.P. Ion Gels by Self-Assembly of a Triblock Copolymer in an Ionic Liquid. J. Phys. Chem. B 2007, 111, 4645–4652. [Google Scholar] [CrossRef] [PubMed]
- Lodge, T.P.; Ueki, T. Mechanically Tunable, Readily Processable Ion Gels by Self-Assembly of Block Copolymers in Ionic Liquids. Acc. Chem. Res. 2016, 49, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Tomé, L.C.; Mecerreyes, D.; Freire, C.S.; Paulo Rebelo, L.N.; Marrucho, I.M. Pyrrolidinium-based polymeric ionic liquid materials: New perspectives for CO2 separation membranes. J. Membr. Sci. 2013, 428, 260–266. [Google Scholar] [CrossRef]
- Gu, Y.; Cussler, E.L.; Lodge, T.P. ABA-triblock copolymer ion gels for CO2 separation applications. J. Memb. Sci. 2012, 423–424, 20–26. [Google Scholar] [CrossRef]
- Lodge, T.P. A Unique Platform for Materials Design. Science 2008, 321, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Ueki, T. Stimuli-responsive polymers in ionic liquids. Polym. J. 2014, 46, 646–655. [Google Scholar] [CrossRef]
- Choi, J.H.; Xie, W.; Gu, Y.; Daniel Frisbie, C.; Lodge, T.P. Single Ion Conducting, Polymerized Ionic Liquid Triblock Copolymer Films: High Capacitance Electrolyte Gates for n-type Transistors. ACS Appl. Mater. Interfaces 2015, 7, 7294–7302. [Google Scholar] [CrossRef]
- Bai, J.; Lu, H.; Cao, Y.; Li, X.; Wang, J. A novel ionic liquid polymer electrolyte for quasi-solid state lithium air batteries. RSC Adv. 2017, 7, 30603–30609. [Google Scholar] [CrossRef]
- Ueki, T.; Usui, R.; Kitazawa, Y.; Lodge, T.P.; Watanabe, M. Thermally Reversible Ion Gels with Photohealing Properties Based on Triblock Copolymer Self-Assembly. Macromolecules 2015, 48, 5928–5933. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithica, NY, USA, 1953. [Google Scholar]
- Lodge, T.P.; Hiemenz, P.C. Polymer Chemistry, 3rd ed.; CRC Press: Boca Raton, NM, USA, 2020. [Google Scholar]
- Tsuda, R.; Kodama, K.; Ueki, T.; Kokubo, H.; Imabayashi, S.I.; Watanabe, M. LCST-type liquid-liquid phase separation behaviour of poly(ethylene oxide) derivatives in an ionic liquid. Chem. Commun. 2008, 4939–4941. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Tsuda, R.; Niitsuma, K.; Tamura, T.; Ueki, T.; Kokubo, H.; Watanabe, M. Structural effects of polyethers and ionic liquids in their binary mixtures on lower critical solution temperature liquid-liquid phase separation. Polym. J. 2011, 43, 242–248. [Google Scholar] [CrossRef]
- Lee, H.-N.; Lodge, T.P. Lower Critical Solution Temperature (LCST) Phase Behavior of Poly(ethylene oxide) in Ionic Liquids. J. Phys. Chem. Lett. 2010, 1, 1962–1966. [Google Scholar] [CrossRef]
- Lee, H.N.; Newell, N.; Bai, Z.; Lodge, T.P. Unusual Lower Critical Solution Temperature Phase Behavior of Poly(ethylene oxide) in Ionic Liquids. Macromolecules 2012, 45, 3627–3633. [Google Scholar] [CrossRef]
- Costa, L.T.; Ribeiro, M.C.C. Molecular dynamics simulation of polymer electrolytes based on poly(ethylene oxide) and ionic liquids. I. Structural properties. J. Chem. Phys. 2006, 124, 164902. [Google Scholar] [CrossRef]
- Costa, L.T.; Ribeiro, M.C.C. Molecular dynamics simulation of polymer electrolytes based on poly(ethylene oxide) and ionic liquids. II. Dynamical properties. J. Chem. Phys. 2007, 127, 164901. [Google Scholar] [CrossRef]
- Lee, H.-N.; Lodge, T.P. Poly(n-butyl methacrylate) in Ionic Liquids with Tunable Lower Critical Solution Temperatures (LCST). J. Phys. Chem. B 2011, 115, 1971–1977. [Google Scholar] [CrossRef]
- Hoarfrost, M.L.; He, Y.; Lodge, T.P. Lower Critical Solution Temperature Phase Behavior of Poly(n-butyl methacrylate) in Ionic Liquid Mixtures. Macromolecules 2013, 46, 9464–9472. [Google Scholar] [CrossRef]
- Ueki, T.; Watanabe, M. Lower Critical Solution Temperature Behavior of Linear Polymers in Ionic Liquids and the Corresponding Volume Phase Transition of Polymer Gels. Langmuir 2007, 23, 988–990. [Google Scholar] [CrossRef] [PubMed]
- Ueki, T.; Karino, T.; Kobayashi, Y.; Shibayama, M.; Watanabe, M. Difference in Lower Critical Solution Temperature Behavior between Random Copolymers and a Homopolymer Having Solvatophilic and Solvatophobic Structures in an Ionic Liquid †. J. Phys. Chem. B 2007, 111, 4750–4754. [Google Scholar] [CrossRef]
- Ueki, T.; Arai, A.A.; Kodama, K.; Kaino, S.; Takada, N.; Morita, T.; Nishikawa, K.; Watanabe, M. Thermodynamic Study of Phase Transitions of Poly(benzyl methacrylate) in Ionic Liquid Solvents. Pure Appl. Chem. 2009, 81, 1829–1841. [Google Scholar] [CrossRef]
- Fujii, K.; Ueki, T.; Niitsuma, K.; Matsunaga, T.; Watanabe, M.; Shibayama, M. Structural aspects of the LCST phase behavior of poly(benzyl methacrylate) in room-temperature ionic liquid. Polymer 2011, 52, 1589–1595. [Google Scholar] [CrossRef]
- Fujii, K.; Ueki, T.; Hashimoto, K.; Kobayashi, Y.; Kitazawa, Y.; Hirosawa, K.; Matsugami, M.; Ohara, K.; Watanabe, M.; Shibayama, M. Microscopic Structure of Solvated Poly(benzyl methacrylate) in an Imidazolium-Based Ionic Liquid: High-Energy X-ray Total Scattering and All-Atom MD Simulation Study. Macromolecules 2017, 50, 4780–4786. [Google Scholar] [CrossRef]
- Kharel, A.; Hall, C.; Ernoch, P.C.; Stepanek, P.; Lodge, T.P. Dilute Solution Properties of Poly(benzyl methacrylate) in Ionic Liquids. Macromolecules 2020, 53, 885–894. [Google Scholar] [CrossRef]
- Kaiser Custodio, K.S.; Claudio, G.C.; Nellas, R.B. Structural Dynamics of Neighboring Water Molecules of N-Isopropylacrylamide Pentamer. ACS Omega 2020, 5, 1408–1413. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kitazawa, Y.; Hashimoto, K.; Ueki, T.; Kokubo, H.; Watanabe, M. Thermosensitive Phase Separation Behavior of Poly(benzyl methacrylate)/Solvate Ionic Liquid Solutions. Langmuir 2017, 33, 14105–14114. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Kobayashi, Y.; Kokubo, H.; Ueki, T.; Ohara, K.; Fujii, K.; Watanabe, M. Solvation Structure of Poly(benzyl methacrylate) in a Solvate Ionic Liquid: Preferential Solvation of Li-Glyme Complex Cation. J. Phys. Chem. B 2019, 123, 4098–4107. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Gonçalves, A.M.M.; Sintra, T.; Pereira, J.L.; Gonçalves, F.; Coutinho, J.A.P. Designing ionic liquids: The chemical structure role in the toxicity. Ecotoxicology 2013, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Halperin, A.; Krçger, M.; Winnik, F.M. Polymer Phase Diagrams Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef] [PubMed]
- Rackaitis, M.; Strawhecker, K.; Manias, E. Water-soluble polymers with tunable temperature sensitivity: Solution behavior. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2339–2342. [Google Scholar] [CrossRef]
- White, R.P.; Lipson, J.E.G. Origins of Unusual Phase Behavior in Polymer/Ionic Liquid Solutions. Macromolecules 2013, 46, 5714–5723. [Google Scholar] [CrossRef]
- Gennes, P.G. Special Features of Water Soluble Polymers. Pure Appl. Chem. 2009, 64, 1585–1588. [Google Scholar] [CrossRef][Green Version]
- Schäfer-Soenen, H.; Moerkerke, R.; Berghmans, H.; Koningsveld, R.; Dušek, K.; Šolc, K. Zero and off-zero critical concentrations in systems containing polydisperse polymers† with very high molar masses. 2. The system water-poly(vinyl methyl ether). Macromolecules 1997, 30, 410–416. [Google Scholar] [CrossRef]
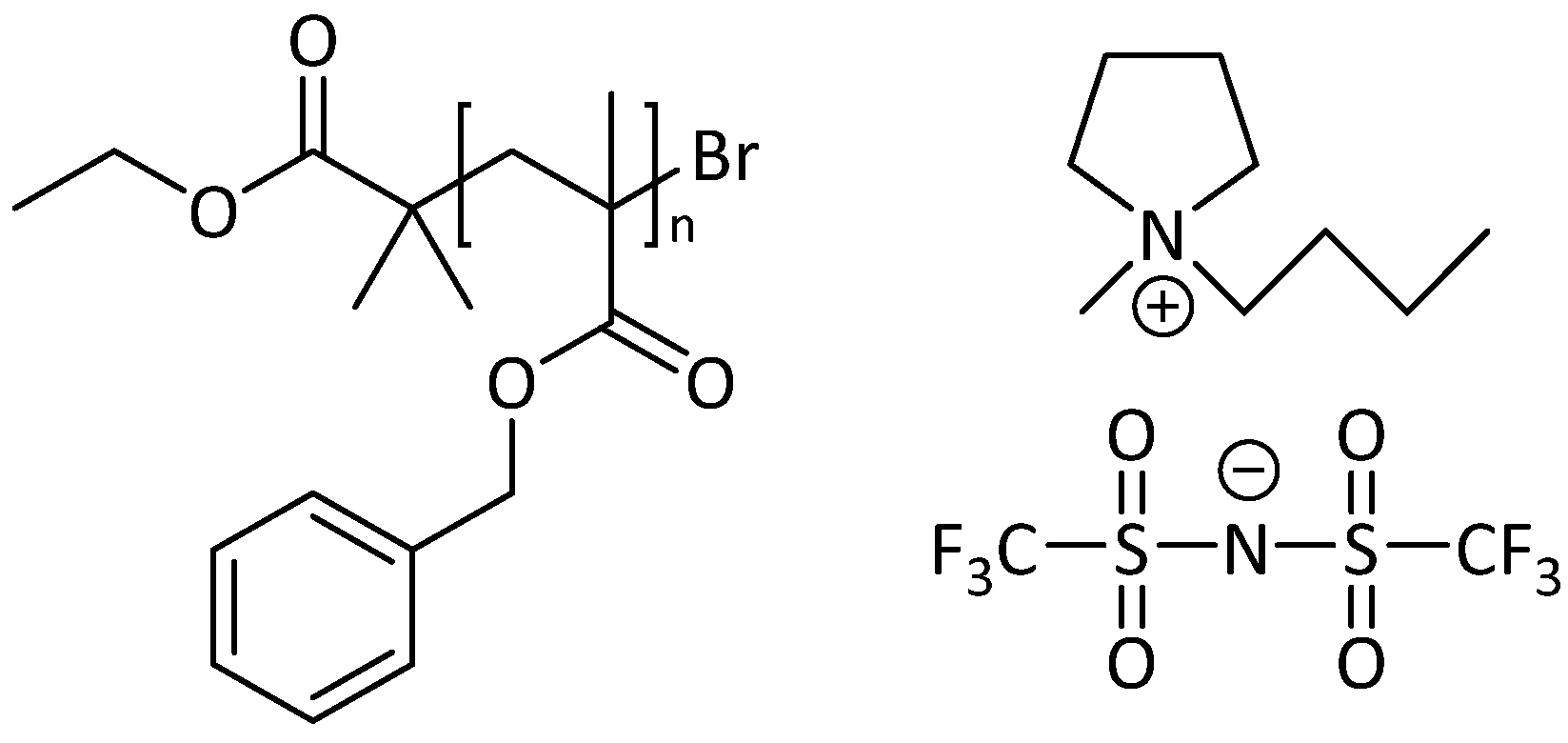


| Sample | Mw (kDa) 1 | Ð 1 | N 2 | Φc,th 3 |
|---|---|---|---|---|
| PBzMA-32 | 32 | 1.20 | 90 | 11 |
| PBzMA-63 | 63 | 1.15 | 177 | 8 |
| PBzMA-76 | 76 | 1.18 | 213 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrick, B.R.; Seitzinger, C.L.; Lodge, T.P. Unusual Lower Critical Solution Temperature Phase Behavior of Poly(benzyl methacrylate) in a Pyrrolidinium-Based Ionic Liquid. Molecules 2021, 26, 4850. https://doi.org/10.3390/molecules26164850
Carrick BR, Seitzinger CL, Lodge TP. Unusual Lower Critical Solution Temperature Phase Behavior of Poly(benzyl methacrylate) in a Pyrrolidinium-Based Ionic Liquid. Molecules. 2021; 26(16):4850. https://doi.org/10.3390/molecules26164850
Chicago/Turabian StyleCarrick, Brian R., Claire L. Seitzinger, and Timothy P. Lodge. 2021. "Unusual Lower Critical Solution Temperature Phase Behavior of Poly(benzyl methacrylate) in a Pyrrolidinium-Based Ionic Liquid" Molecules 26, no. 16: 4850. https://doi.org/10.3390/molecules26164850
APA StyleCarrick, B. R., Seitzinger, C. L., & Lodge, T. P. (2021). Unusual Lower Critical Solution Temperature Phase Behavior of Poly(benzyl methacrylate) in a Pyrrolidinium-Based Ionic Liquid. Molecules, 26(16), 4850. https://doi.org/10.3390/molecules26164850






