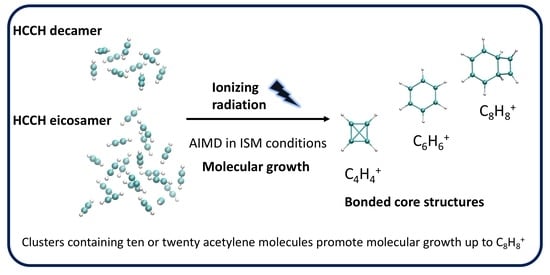
The Effect of Cluster Size on the Intra-Cluster Ionic Polymerization Process
Abstract
:1. Introduction
2. Results
2.1. AIMD Simulations
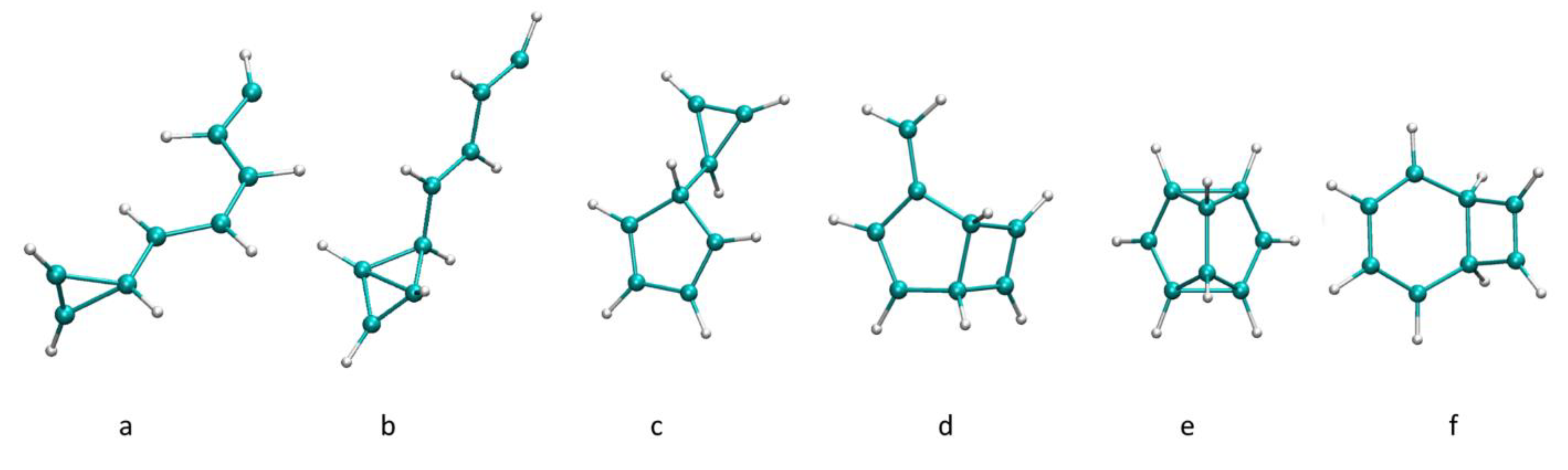
2.2. AIMD of the Acetylene Decamer Clusters
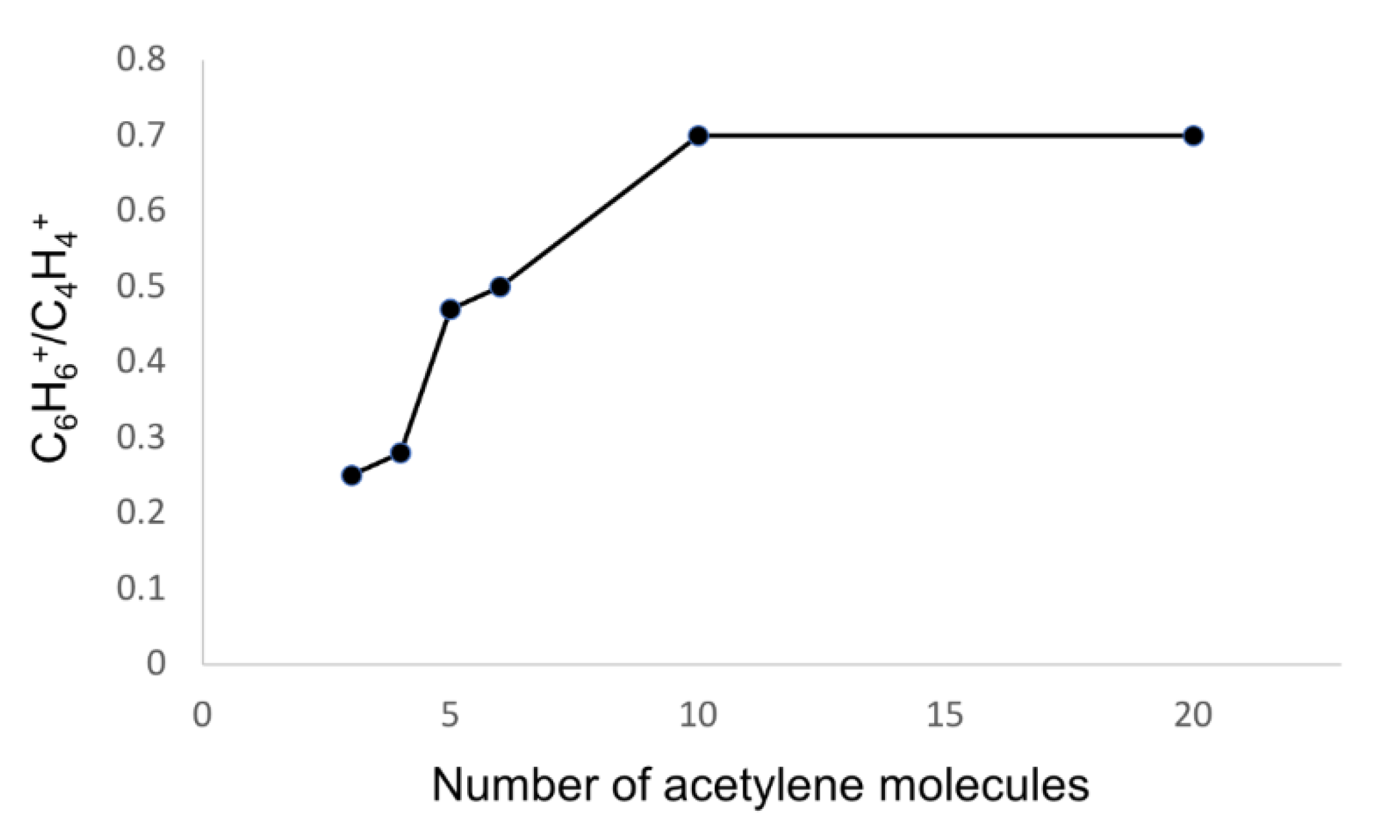
2.3. Comparison of the Growth Process in Different Cluster Sizes
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sampleav Ailability
References
- Ehrenfreund, P.; Sephton, M.A. Carbon Molecules in Space: From Astrochemistry to Astrobiology. Faraday Discuss. 2006, 133, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.M.; Lee, T.J.; Gudipati, M.S.; Allamandola, L.J.; Head-Gordon, M. Charged Polycyclic Aromatic Hydrocarbon Clusters and The Galactic Extended Red Emission. Proc. Natl. Acad. Sci. USA 2007, 104, 5274–5278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joblin, C.; Mulas, G. Interstellar Polycylic Aromatic Hydrocarbons: From Space to The Laboratory. Eas Publ. 2009, 35, 133–152. [Google Scholar] [CrossRef]
- Leger, A.; Puget, J.L. Identification of the ‘Unidentified’ IR Emission Features of Interstellar Dust? Astron. Astrophys. 1984, 137, L5–L8. [Google Scholar]
- Allamandola, L.J.; Tielens, A.G.G.M.; Barker, J.R. Interstellar Polycyclic Aromatic Hydrocarbons–The Infrared Emission Bands, The Excitation/Emission Mechanism, and The Astrophysical Implications. Astrophys. J. Suppl. Ser. 1989, 71, 733–775. [Google Scholar] [CrossRef]
- Tielens, A.G.G.M. Interstellar Polycyclic Aromatic Hydrocarbon Molecules. Annu. Rev. Astron. Astrophys. 2008, 46, 289–337. [Google Scholar] [CrossRef] [Green Version]
- Sellgrem, K. The Near-Infrared Continuum Emission of Visual Reflection Nebulae. Astrophys. J. Part 1 1984, 277, 623–633. [Google Scholar] [CrossRef]
- Puget, J.L.; Léger, A. A New Component of The Interstellar Matter: Small Grains and Large Aromatic Molecules. Annu. Rev. Astron. Astrophys. 1989, 27, 161–198. [Google Scholar] [CrossRef]
- Frenklach, M.; Wang, H. Twenty-Third Symposium (International) on CombustionDetailed Modeling of Soot Particle Nucleation and Growth. Symp. (Int.) Combust. 1991, 23, 1559–1566. [Google Scholar] [CrossRef]
- Bittner, J.D.; Howard, J.B. Eighteenth Symposium (International) on Combustion Composition Profiles and Reaction Mechanisms in a Near-Sooting Premixed Benzene/Oxygen/Argon Flame. Symp. (Int.) Combust. 1981, 18, 1105–1116. [Google Scholar] [CrossRef]
- Wang, H.; Frenklach, M. Calculations of Rate Coefficients for the Chemically Activated Reactions of Acetylene with Vinylic and Aromatic Radicals. J. Phys. Chem. 1994, 98, 11465–11489. [Google Scholar] [CrossRef]
- Marinov, N.M.; Pitz, W.J.; Westbrook, C.K.; Vincitore, A.M.; Castaldi, M.J.; Senkan, S.M.; Melius, C.F. Aromatic and Polycyclic Aromatic Hydrocarbon Formation in a Laminar Premixed n-Butane Flame. Combust. Flame 1998, 114, 192–213. [Google Scholar] [CrossRef]
- Parker, D.S.N.; Kaiser, R.I.; Troy, T.P.; Ahmed, M. Hydrogen Abstraction/Acetylene Addition Revealed. Angew. Chem. Int. Ed. 2014, 53, 7740–7744. [Google Scholar] [CrossRef]
- Hopf, H. From Acetylenes to Aromatics: Novel Routes–Novel Products. In Modern Arene Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2004; pp. 169–195. [Google Scholar]
- Peverati, R.; Platt, S.P.; Attah, I.K.; Aziz, S.G.; El-Shall, M.S.; Head-Gordon, M. Nucleophilic Aromatic Addition in Ionizing Environments: Observation and Analysis of New C–N Valence Bonds in Complexes between Naphthalene Radical Cation and Pyridine. J. Am. Chem. Soc. 2017, 139, 11923–11932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghesquière, P.; Talbi, D. On the Formation of Naphthalene Cation in Space from Small Hydrocarbon Molecules: A Theoretical Study. Chem. Phys. Lett. 2013, 564, 11–15. [Google Scholar] [CrossRef]
- Paul, M.W.; Karen, W. Benzene Formation in The Inner Regions of Protostellar Disks. Astrophys. J. Lett. 2007, 655, L49. [Google Scholar]
- Woods, P.M. The Formation of Benzene in Dense Environments. EAS Publ. Ser. 2011, 46, 235–240. [Google Scholar] [CrossRef]
- CBrown, R.F. Some Developments in The High Temperature Gas Phase Chemistry of Alkynes, Arynes and Aryl Radicals. Eur. J. Org. Chem. 1999, 1999, 3211–3222. [Google Scholar] [CrossRef]
- Hopf, H.; Berger, H.; Zimmermann, G.; Nüchter, U.; Jones, P.G.; Dix, I. Formation of Isobenzenes by Thermal Isomerization of 1,3-Hexadiene-5-yne Derivatives. Angew. Chem. Int. Ed. Engl. 1997, 36, 1187–1190. [Google Scholar] [CrossRef]
- Jones, B.M.; Zhang, F.; Kaiser, R.I.; Jamal, A.; Mebel, A.M.; Cordiner, M.A.; Charnley, S.B. Formation of Benzene in The Interstellar Medium. Proc. Natl. Acad. Sci. 2011, 108, 452–457. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.M.; McEwan, M.J.; Scott, G.B.; Adams, N.G.; Babcock, L.M.; Terzieva, R.; Herbst, E. New H and H2 Reactions with Small Hydrocarbon Ions and Their Roles in Benzene Synthesis in Dense Interstellar Clouds. Astrophys. J. 1999, 513, 287. [Google Scholar]
- Paul, M.W.; Woods, P.M.; Millar, T.J.; Zijlstra, A.A.; Herbst, E. The Synthesis of Benzene in The Proto-Planetary Nebula CRL 618. Astrophys. J. Lett. 2002, 574, L167. [Google Scholar]
- Kaiser, R.I.; Parker, D.S.; Mebel, A.M. Reaction Dynamics in Astrochemistry: Low-Temperature Pathways toPolycyclic Aromatic Hydrocarbons in The Interstellar Medium. Annu. Rev. Phys. Chem. 2015, 66, 43–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Li, Z.; Zhao, Y.; Wan, S.; Liu, H.; Huang, X.; Sun, C. Mechanism for The Formation of Benzene in The Titan’s Atmosphere: A Theoretical Study on The Mechanism of Reaction. Comput. Theor. Chem. 2012, 991, 66–73. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, W.; Ahmed, M.; Zagidullin, M.V.; Azyazov, V.N.; Morozov, A.N.; Kaiser, R.I. Gas-Phase Synthesis of Benzene via The Propargyl Radical Self-Reaction. Sci. Adv. 2021, 7, eabf0360. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, W.B.; Ono, Y.; Linn, S.H.; Ng, C.Y. A Study of The Unimolecular Decomposition of the (C2H4) +3 Complex. J. Chem. Phys. 1985, 83, 2813–2817. [Google Scholar] [CrossRef]
- Ono, Y.; Ng, C.Y. A Study of The Unimolecular Decomposition of the (C2H2)3+ Complex. J. Am. Chem. Soc. 1982, 104, 4752–4758. [Google Scholar] [CrossRef]
- Shinohara, H.; Sato, H.; Washida, N. Photoionization Mass Spectroscopic Studies of Ethylene and Acetylene Clusters: Intracluster Excess Energy Dissipation. J. Phys. Chem. 1990, 94, 6718–6723. [Google Scholar] [CrossRef]
- Booze, J.A.; Baer, T. The Photoionization and Dissociation Dynamics of Energy-Selected Acetylene Dimers,Trimers, and Tetramers. J. Chem. Phys. 1993, 98, 186–200. [Google Scholar] [CrossRef]
- Coolbaugh, M.T.; Whitney, S.G.; Vaidyanathan, G.; Garvey, J.F. Intracluster Polymerization Reactions within Acetylene and Methylacetylene Clusters Ions. J. Phys. Chem. 1992, 96, 9139–9144. [Google Scholar] [CrossRef]
- Momoh, P.O.; Attah, I.K.; El-Shall, M.S.; Kanters, R.P.; Pinski, J.M.; Abrash, S.A. Formation of Covalently Bonded Polycyclic Hydrocarbon Ions by Intracluster Polymerization of Ionized Ethynylbenzene Clusters. J. Phys. Chem. A 2014, 118, 8251–8263. [Google Scholar] [CrossRef]
- Momoh, P.O.; Hamid, A.M.; Soliman, A.R.; Abrash, S.A.; El-Shall, M.S. Structure of the C8H8•+ Radical Cation Formed by Electron Impact Ionization of Acetylene Clusters. Evidence for a (Benzene•+·Acetylene) Complex. J. Phys. Chem. Lett. 2011, 2, 2412–2419. [Google Scholar] [CrossRef]
- Momoh, P.O.; Hamid, A.M.; Abrash, S.A.; Samy El-Shall, M. Structure and Hydration of the C4H4•+ Ion Formed by Electron Impact Ionization of Acetylene Clusters. J. Chem. Phys. 2011, 134, 204315. [Google Scholar] [CrossRef] [Green Version]
- Momoh, P.O.; El-Shall, M.S. Stepwise Hydration of Ionized Acetylene Trimer. Further Evidence for TheFormation of Benzene Radical Cation. Chem. Phys. Lett. 2007, 436, 25–29. [Google Scholar] [CrossRef]
- Momoh, P.O.; Abrash, S.A.; Mabrouki, R.; El-Shall, M.S. Polymerization of Ionized Acetylene Clusters Into Covalent Bonded Ions: Evidence for The Formation of Benzene Radical Cation. J. Am. Chem. Soc. 2006, 128, 12408–12409. [Google Scholar] [CrossRef] [Green Version]
- Relph, R.A.; Bopp, J.C.; Roscioli, J.R.; Johnson, M.A. Structural Characterization of (C2H2)1–6+ Cluster Ions by Vibrational Predissociation Spectroscopy. J. Chem. Phys. 2009, 131, 114305. [Google Scholar] [CrossRef]
- Stein, T.; Bandyopadhyay, B.; Troy, T.P.; Fang, Y.; Kostko, O.; Ahmed, M.; Head-Gordon, M. Ab Initio Dynamics and Photoionization Mass Spectrometry Reveal Ion-Molecule Pathways from Ionized Acetylene Clusters to Benzene Cation. Proc. Natl. Acad. Sci. USA 2017, 114, E4125–E4133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.; Kostko, O. From Atoms to Aerosols: Probing Clusters and Nanoparticles with Synchrotron based Mass Spectrometry and X-ray Spectroscopy. Phys. Chem. Chem. Phys. 2020, 22, 2713–2737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandyopadhyay, B.; Stein, T.; Fang, Y.; Kostko, O.; White, A.; Head-Gordon, M.; Ahmed, M. Probing Ionic Complexes of Ethylene and Acetylene with Vacuum-ultraviolet Radiation. J. Phys. Chem. A 2016, 120, 5053–5064. [Google Scholar] [CrossRef] [Green Version]
- Jose, J.; Zamir, A.; Stein, T. Molecular Dynamics Reveals Formation Path of Benzonitrile and Other Molecules in Conditions Relevant to The Interstellar Medium. Proc. Natl. Acad. Sci. USA 2021, 118, e2101371118. [Google Scholar] [CrossRef]
- Stein, T.; Jose, J. Molecular Formation upon Ionization of van der Waals Clusters and Implication to Astrochemistry. Isr. J. Chem. 2020, 60, 1–9. [Google Scholar] [CrossRef]
- Stein, T.; Bera, P.P.; Lee, T.J.; Head-Gordon, M. Molecular Growth Upon Ionization of van der Waals Clusters Containing HCCH and HCN is a Pathway to Prebiotic Molecules. Phys. Chem. Chem. Phys. 2020, 22, 20337–20348. [Google Scholar] [CrossRef]
- McGuire, B.A.; Burkhardt, A.M.; Kalenskii, S.; Shingledecker, C.N.; Remijan, A.J.; Herbst, E.; McCarthy, M.C. Detection of The Aromatic Molecule Benzonitrile in The Interstellar Medium. Science 2018, 359, 202. [Google Scholar] [CrossRef] [Green Version]
- Bera, P.P.; Head-Gordon, M.; Lee, T.J. Association Mechanisms of Unsaturated C2 Hydrocarbons with their Cations: Acetylene and Ethylene. Phys. Chem. Chem. Phys. 2013, 15, 2012–2023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Doney, K.D.; Linnartz, H. Laboratory Gas-phase Detection of the Cyclopropenyl Cation (c-C 3 H 3 +). Astrophys. J. 2014, 791, L28. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Gan, Z.; Epifanovsky, E.; Gilbert, A.T.; Wormit, M.; Kussmann, J.; Rassolov, V.A. Advances in Molecular Quantum Chemistry Contained in the Q-Chem 4 Program Package. Mol. Phys. 2015, 113, 184–215. [Google Scholar] [CrossRef] [Green Version]
- Mardirossian, N.; Head-Gordon, M. [Small Omega]B97X-V: A 10-Parameter, Range-Separated Hybrid,Generalized Gradient Approximation Density Functional with Nonlocal Correlation, Designed by a Survival-of-The-Fittest Strategy. Phys. Chem. Chem. Phys. 2014, 16, 9904–9924. [Google Scholar] [CrossRef] [Green Version]
- Dunning, T.H., Jr. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Systematic Optimization of Long-Range Corrected Hybrid Density Functionals. J. Chem. Phys. 2008, 128, 084106. [Google Scholar] [CrossRef] [PubMed]
- Rassolov, V.A.; Pople, J.A.; Ratner, M.A.; Windus, T.L. 6-31G* Basis Set for Atoms K through Zn. J. Chem. Phys. 1998, 109, 1223–1229. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossich Molina, E.; Stein, T. The Effect of Cluster Size on the Intra-Cluster Ionic Polymerization Process. Molecules 2021, 26, 4782. https://doi.org/10.3390/molecules26164782
Rossich Molina E, Stein T. The Effect of Cluster Size on the Intra-Cluster Ionic Polymerization Process. Molecules. 2021; 26(16):4782. https://doi.org/10.3390/molecules26164782
Chicago/Turabian StyleRossich Molina, Estefania, and Tamar Stein. 2021. "The Effect of Cluster Size on the Intra-Cluster Ionic Polymerization Process" Molecules 26, no. 16: 4782. https://doi.org/10.3390/molecules26164782
APA StyleRossich Molina, E., & Stein, T. (2021). The Effect of Cluster Size on the Intra-Cluster Ionic Polymerization Process. Molecules, 26(16), 4782. https://doi.org/10.3390/molecules26164782