Abstract
The structure, stability, and bonding character of some exemplary LAr and L-ArBeO (L = He, Ne, Ar, N2, CO, F2, Cl2, ClF, HF, HCl, NH3) were investigated by MP2 and coupled-cluster calculations, and by symmetry-adapted perturbation theory. The nature of the stabilizing interactions was also assayed by the method recently proposed by the authors to classify the chemical bonds in noble-gas compounds. The comparative analysis of the LAr and L-ArBeO unraveled geometric and bonding effects peculiarly related to the σ-hole at the Ar atom of ArBeO, including the major stabilizing/destabilizing role of the electrostatic interactionensuing from the negative/positive molecular electrostatic potential of L at the contact zone with ArBeO. The role of the inductive and dispersive components was also assayed, making it possible to discern the factors governing the transition from the (mainly) dispersive domain of the LAr, to the σ-hole domain of the L-ArBeO. Our conclusions could be valid for various types of non-covalent interactions, especially those involving σ-holes of respectable strength such as those occurring in ArBeO.
1. Introduction
Non-covalent interactions (NCIs) play a major role in natural and synthetic processes [1,2,3]. Over the years, the traditional interest for the hydrogen bond (HB) [4,5] has been progressively extended to other NCIs, and greatest attention is, in particular, currently paid to σ-hole [6,7,8,9] interactions. The tetrel, pnictogen, chalcogen, and halogen bonds [10,11] (the latter actually rivaling in interest [12,13] that of the HB) are just elder sisters of a large family that embraces most groups of the periodic table [14,15].
σ-holes are regions of charge depletion centered on the outer extension of covalent bonds. They are typically associated with maximum points (VS,max) of the molecular electrostatic potential (MEP) [16] (customarily taken at or at around the 0.0010 ea0−3 isodensity surface [17]), and the value of the MEP at VS,max is generally (but not always) positive. Thus, σ-holes typically behave as electrophilic centers, able to promote by contact with a ligand a complex blend of electrostatic, polarization (including induction and charge transfer), and dispersion contributions. The detailed assay of these terms, and of their dependence on the type and the strength of the interaction, are, indeed, of major interest in the study of NCIs. In a recent study [18], we sought to acquireinsights into σ-hole effects by exploring the changes occurring when pure (or nearly pure) dispersive contacts wereconverted into σ-hole interactions. The investigated systems were inspired, in particular, by already-established evidence concerning the complexes of the noble-gas atoms (Ng) with beryllium Lewis acids, particularly BeO [19]. It is, thus, well known that the NgBeO feature remarkably high binding energies, mainly arisingfrom the appreciable polarization of Ng by BeO, and the ensuing appreciable inductive stabilization. We thus surmisedthat such a major electronic effect could induce a σ-hole on Ng, andexplored the MEP of the NgBeO. The calculations actually confirmed a σ-hole already appreciable in ArBeO, and only slightly more pronounced in KrBeO and XeBeO. We thus focused on the simplest L-ArBeO (L = He, Ne, Ar, HF), and compared their bonding situation with that of their “dispersive” cousins, LAr [18]. To this end, we employed the symmetry-adapted perturbation theory (SAPT) [20,21], and the method that we recently proposed [22,23,24] to analyze the bonding situation of Ng compounds. Compared with the LAr, the L-ArBeO were unraveled as structurally more compact, and this mirrored increased values of the SAPT interaction energies (with respect to the L + Ar and L + ArBeO dissociation limits). The two most affected binding components were, in particular, the electrostatic and the inductive, theirincreases seemingly being related to the electrostatic potential of L, and to its polarizability [18]. Further evidence in this regard came from the failed location of a bound FH-ArBeO, that we ascribed [18], likewise to other “counterintuitive” NCIs [25,26],to the unfavorable electrostatic contact between the positive H atom of HF and the positive Ar atom of ArBeO. To reinforce the interpretation, we decided to explore other exemplary LAr and L-ArBeO, including L = N2, CO, F2, Cl2, ClF, HCl, and NH3. The MEP of these ligands ranges, in fact, between definitely-negative to definitely-positive values, and this should magnify any effect arising from the contact with the σ-hole of ArBeO. The obtained results, discussed in the present article, actually confirmed this expectation, and furnished insights that could be useful in discussing other types ofσ-holecomplexes.
The paper is organized as follows. Section 2 and Section 3 provide, respectively, a brief account of the methods employed to perform the bonding analysis, and the most relevant computational details. Section 4 first presents the investigated LAr and L-ArBeO, their predicted data, and an estimate of their accuracy. Then follows the SAPT analysis of the LAr and L-ArBeO, the former being discussed first as reference systems to best highlight the σ-hole effects occurring in the complexes of L-ArBeO. The LAr and L-ArBeO are then compared in terms of our proposed descriptors of bonding character. The most relevant conclusions are providedin Section 5.
2. Methods of Bonding Analysis
The bonding analysis was first accomplished by SAPT [20,21]. In this approach, the total Hamiltonian of the dimer is partitioned as H = F + V + W, where F = FA + FB is the sum of the Fock operators for monomers A and B, V is the intermolecular interaction operator, and W = WA + WB is the sum of the Møller-Plesset fluctuation operators. The latter are defined as WX = HX – FX, where HX is the total Hamiltonian of monomer X. The is expanded as a perturbative series, and, in particular, we included the following terms:
the first (1/2) and the second (0/1/2/3) number superscript in parenthesesindicating the first-/second-order, and the zeroth-/first-/second-/third-order intramonomer electron correlation correction to V and W, respectively. The notations in subscript indicate the classical (Coulombic) electrostatic energy (elst), the exchange term that results from the antisymmetrization of the wave-function (exch), the induction energy (ind) (including the charge transfer), and the dispersion energy (disp). The “r” indicates that a given component has been computed by including the coupled Hartree-Fock (HF) response for the perturbed system. The term collects the contributions to the supermolecular HF energy beyond the second-order of intermolecular operator, the is the part of not included in , and is the part of with intra-monomer excitations at the CCSD level of theory.
The terms of Equation (1) were grouped so to express as the sum of the electrostatic (), inductive (), dispersive (), and exchange () components:
Our method of bonding analysis [22,23,24] relies on the examination of the plotted shape of the local electron energy density H(r) [22,27,28], and on the values that this function takes over the volume Ωs enclosed by the s(r) = 0.4 reduced-density gradient (RDG) isosurface [29,30] associated with the bond-critical point (BCP) located for a given Ng-X (X = binding partner) from the topological analysis of the electron density ρ(r) [31]. Ancillary indices include the size of Ωs, the total electronic charge enclosed by Ωs, N(Ωs), the average electron density over Ωs, ρs(ave) = N(Ωs)/Ωs, and the average, maximum, and minimum values of H(r) over Ωs, Hs(ave, max, min). As discussed previously [22], the H(r) partitions the atomic space in two well-recognizable regions, namely an inner one of negative values, indicated as H–(r), and an outer one of positive values, indicated as H+(r). The boundary of these regions falls at a distance R– that is typical of each atom; at this distance, H(r = R−) = 0. Interestingly, when two atoms form a chemical bond, their H−(r) and H+(r) regions combine in modes that signal the nature of the interaction. Particularly for the Ng-X bonds, it is possible to distinguish three types of interactions, indicated as A, B, or C.
In interactions of type A, the atoms overlap all the contour lines of their H+(r) regions, and part of the contour lines of their inner H−(r) regions, the bond appearing as a continuous region of negative values of H(r), plunged in a zone of positive values. The interaction is topologically-signed by a (3, +1) critical point of the H(r) (denoted as the HCP) falling on the bond axis. Exemplary in this regard is the N-N bond of the N2 moiety of the N2-Ar and the linear N2-ArBeO shown in Figure 1. The Ar-Be bond of N2-ArBeO is, instead, a showcase of interactions of type B. In these contacts, the H−(r) region of Ng is, again, overlapped with the H−(r) region of the binding partner, but (i) no HCP exists on the bond axis, and (ii) the Ng-X inter-nuclear region includes a (more or less wide) region of positive H(r). Finally, the N-Ar bonds of N2-Ar and N2-ArBeO are illustrative examples of interactions of type C. In contacts like these, the Ng and the binding partner overlap only part of their H+(r) regions, their H−(r) regions remaining, instead, perfectly closed, and separated by a (more or less wide) region of positive H(r). The bond thus appears as two clearly distinguishable H−(r) regions, separated by a region of positive values of H(r).
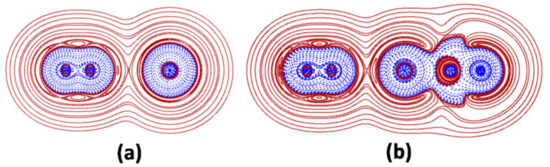
Figure 1.
2D-plots of H(r) in the main plane of (a) N2-Ar and (b) linear N2-ArBeO (solid/brown and dashed/blue lines correspond, respectively, to positive and negative values).
Any Ng-X is assigned as covalent (Cov) if (i) it is of type A, and (ii) the electron density at the BCP, ρ(BCP) is at least 0.08 ea0−3. This is the case of the N2 moiety of the N2-Ar and N2-ArBeO shown in Figure 1a,b. Any Ng-X not fulfilling these criteria wasfurther assayed by integrating the H(r) over Ωs. If the function was, invariably, positive over the entire volume, the interaction wasassigned as non-covalent (nCov). If the function waspartially or fully negative, the Ng-X wasassigned as partially-covalent (pCov), and distinguished as H+/−, H−/+, and H−, the superscript indicating that, over Ωs, the H(r) was ranging from negative to positive, but, on the average, was positive (H+/−) or negative (H−/+) or, that it is invariably negative (H−). Interactions of type C involving a single Ng atom, such as the N2-Ar shown in Figure 1a, and all the other presently investigated LAr (vide infra) were also assayed in terms of the degree of polarization of Ng, DoP(Ng). This index measures, in essence, the deformation of the H−(r) region of Ng arising from the interaction with X [23,32]. The DoPis, in particular, defined by the equation:
where is the radius of the H−(r) region of Ng along the axis formed by Ng and the Ng-X BCP, and is the radius of the H−(r) region of the free atom. The positive/negative sign of the DoP signal Ng atoms polarized toward/opposite to X, and the magnitude of the DoP is related to the extent of the polarization. Illustrative examples of the effective use of this index are providedin Refs. [23,32].
3. Computational Details
The employed levels of theory were the Møller-Plesset truncated at the second order (MP2) [33], the coupled cluster with inclusion of single and double substitutions, CCSD, and an estimate of connected triples, CCSD(T) [34]. The calculations were performed with the Dunning’s correlation consistent aug-cc-pVTZ [35] (denoted here as aVTZ) by explicitly correlating the outer valence electrons (frozen-core approximation). The MP2 and CCSD(T) calculations were performed, respectively, with the Gaussian 09 (G09) (Revision D1) [36], and the CFOUR (version 2.1) [37].
The SAPT [20,21] calculations were performed with the SAPT2016 [38], using the G09 for the integrals calculation. The employed basis set, denoted here as aVTZ/mbf, combined the aVTZ with a set of extra 3s3p2d1f mid-bond functions [39,40] (three s and three p functions with exponents 0.9, 0.3, 0.1, two d functions with exponents 0.6 and 0.2, and one f function with exponent 0.3) placed at the mid-point of any Ar-X distance (X = atom closest to Ar in the linear complexes, or center-of-mass of X2 in the T-shaped complexes).
The functions investigated in the bonding analysis werethe ρ(r) [31], the H(r) [22,27,28], and the RDG and its related NCI indices [29,30]. The ρ(r) is defined by the equation [31]:
where is the occupation number of the natural orbital , in turn expanded as a linear combination of the basis functions.
The H(r) is the sum of the kinetic energy density G(r) and the potential energy density V(r):
The presentlyemployed definition [31,41] of the G(r) is given by the equation:
where the sum runs over all the occupied natural orbitals of occupation numbers . The potential energy density V(r) is evaluated [31] from the local form of the virial theorem:
The RDG is defined by the equation [29,30]:
Low-value s(r) isosurfaces (typically 0.3–0.6) appear among atoms undergoing any type of interaction, the NCIs emerging, in particular, by considering the spatial regions of low ρ(r). The low-s(r)/low-ρ(r) isosurfaces are, in turn, mapped in terms of the sign (λ2) × ρ(r), λ2 being the second eigenvalue (λ1<λ2<λ3) of the Hessian matrix of ρ(r). In essence, the sign of λ2 is used to distinguish between attractive (λ2< 0) and repulsive (λ2> 0) interactions, and the value of ρ(r) is exploited to rank the corresponding strength. In the present study, we also calculated the integral of a given property P (particularly the ρ(r) and the H(r))over the volume Ωs enclosed by the s(r) = 0.4 isosurface at around the BCP located on any Ar-X bond path, P(Ωs). This integration was accomplished by producing an orthogonal grid of points that enclosed the isosurface and applying the formula:
where P(ri) is the value of P at the grid point ri, and dx, dy, and dz are the grid step sizes in the x, y, and z directions, respectively (dx = dy = dz = 0.025 a0). The summation is carried out on all grid points ri having RDG < s.
The ρ(r), the H(r), and the s(r) were analyzed with the Multiwfn (version 3.8.dev) [42] using the MP2/aVTZ wave functions stored in the wfx files generated with the G09 or the CCSD(T)/aVTZ wave functions stored in the molden files generated with CFOUR, and subsequently formatted with the Molden2AIM utility [43]. The two-(2D) plots of the H(r) were also produced with the Multiwfn, and included the standard contour lines belonging to the patterns ±k × 10n (k = 1, 2, 4, 8; n = −5 ÷ 6), together with the contour lines corresponding to the critical points specifically located from the topological analysis of the H(r).
4. Results and Discussion
4.1. The Investigated LAr and L-ArBeO: Predicted Data and Their Accuracy
The investigated LAr includedthe diatomic HeAr, NeAr, and ArAr, the linear and T-shaped (N2)Ar, (F2)Ar, and (Cl2)Ar, the linear isomeric (CO)Ar, (FCl)Ar, (HF)Ar, and (HCl)Ar, and the H3N-Ar structure of C3v symmetry. These species were explored at both the MP2/aVTZ and CCSD(T)/aVTZ level of theory, andinvariably characterized as true minima on the potential energy surface (PES). The only exceptions were the OC-Ar and the H3N-Ar, both featuring a doublydegenerate imaginary absorption (number of imaginary frequencies NIMAG = 2). As shown in Table S1 of the Supplementary Materials, the MP2/aVTZ and CCSD(T)/aVTZ harmonic frequencies of the LAr were, invariably, quite similar, and the MP2/aVTZAr-X distances featured a mean unsigned deviation (MUD) from the CCSD(T)/aVTZ values of only 0.0385 Å.We also ascertained (see Table S1 of Supplementary Materials) the high similarity between the MP2/aVTZ and CCSD(T)/aVTZ predicted data of the simplest He-ArBeO, Ne-ArBeO, Ar-ArBeO, and HF-ArBeO. Based on these findings, all the other L-ArBeO (L = N2, CO, F2, Cl2, ClF, HCl, NH3) were investigated at the predictably accurate MP2/aVTZ. The connectivities and bond distances of all the presently investigated LAr and L-ArBeO are thus shown in Figure 2 (their Cartesian coordinates are also availableas Data S1 of the Supplementary Materials).Their CCSD/aVTZT1 diagnostics (the norm of the vector t1 of the single-excitation amplitudes from the CCSD calculation divided by the square root of the number of correlated electrons N, ) resulted invariably within the accepted threshold of 0.02 [44], thus confirming the validity of a mono-determinantal description of their wave functions.
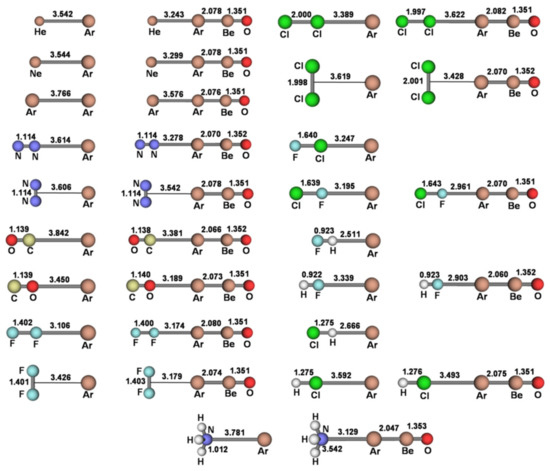
Figure 2.
MP2/aVTZconnectivities and bond distances (Å) of the LAr and L-ArBeO (L = He, Ne, Ar, N2, CO, F2, Cl2, ClF, HF, HCl, NH3).
The MP2/aVTZ structures of the LAr and L-ArBeO were then employed to perform the SAPT calculations (dissociation limits: L + Ar and L + ArBeO), and the bonding analysis. The obtained results are shown in Table 1 and Table 2. For the LAr, the computed were, in general, well consistent (MUD = 0.02 kcal mol−1) with other accurate theoretical estimates already available from the literature [45,46,47,48,49,50,51,52,53,54,55]. In addition, as shown in Table S2 of the Supplementary Materials, the results of the bonding analysis of the LAr performed at the MP2/aVTZ and CCSD(T)/aVTZ levels of theory furnished strictly similar results. These findings, overall, support the good accuracy of the data reported in Table 1 and Table 2. Their detailed discussion is providedin the forthcoming paragraphs.

Table 1.
SAPT analysis (kcal mol−1) of the LAr(dissociation limits: L + Ar) and L-ArBeO(dissociation limits: L + ArBeO) (see Figure 2) performed with the aVTZ/mbf basis set. Eelst(%), Eind(%), and Edisp(%) are the percentage contributions, respectively, of |Eelst|, |Eind|, and |Edisp| to (|Eelst|+ |Eind| + |Edisp|).

Table 2.
MP2/aVTZ properties of the nCov(C) bonds (see text) of the LAr and L-ArBeO (see Figure 2). N(Ωs), ρs(ave), and Hs(ave/max/min) are, respectively, the total electronic charge (me), the average electron density (e a0−3), and the average, maximum, and minimum of H(r) (hartree a0−3) over the volume Ωs (a03) enclosed by the s(r) = 0.4 isosurfaceat around the BCP.
4.2. SAPT Analysis of the LAr: The Role of the MEP of L
We first discussthe SAPT data of the LAr(dissociation limit: L + Ar). The complexes of Ng with neutral speciesare, in general, perceived as typical van der Waalsmolecules [56], held together by the favorable balance between dispersion and exchange repulsion. The leading interaction components of any LNg may include, however, electrostatic, inductive, and even charge-transfer contributions [57]. Illustrative in this regard are also the presently-investigated LAr, whose decomposed (see Table 1) clearlyunraveledan invariably major , but also non-negligible contributions of and . Especially relevant in the present contextis the dependence of these terms on the MEP of L, particularly on the values that this function takes for the various ligands at the VS,max or VS,min occurring on the outer elongation of the bond axis or perpendicular to it. We could locate these critical points on any probed isodensity surface (namely 0.0005, 0.0010, 0.0015, and 0.0020 ea0−3), and, as shown in Table S3 of the Supplementary Materials, the values of the MEP at these points wereonly less sensitive to the employed geometry(experimental, MP2/aVTZ or CCSD(T)/aVTZ), and tothe methodused to calculate the electron density (MP2/aVTZ or CCSD(T)/aVTZ). The forthcoming discussion is based, in particular, on the values quoted in Table 3, computed at the MP2/aVTZ0.0010 ea0−3 isodensity surfaces using the MP2/aVTZ-optimized geometries.

Table 3.
MP2/aVTZ values (kcal mol−1, 0.0010 ea0−3 isodensity surface) at the minimum (VS,Min) or maximum (VS,Max) points of the MEP calculated at the MP2/aVTZ optimized geometries (Å and °), and experimental polarizabilities α (Å3) of L.
We first note from Figure 2 the strict relationship between the connectivity of any LAr and the position of the VS,max or VS,min of the involved ligand. In essence, the anisotropy of the electron density of L directed the Ar atom toward the stationary point(s) on the PES. Depending on L, however, the potential locally-experienced by Ar was quite different, and ranged (see Table 3) from the definitelynegative one at the VS,min(N) of NH3 (−37.25 kcal mol−1) to the definitely positive one at the VS,max(H) of HF (68.78 kcal mol−1), passing through the only slightly-positive values of He (1.26 kcal mol−1), Ne (1.02 kcal mol−1), Ar (1.85 kcal mol−1), and at the VS,max(perp) of F2 (0.76 kcal mol−1) and Cl2 (1.26 kcal mol−1). Interestingly, as expected from the results of our recent studies on other numerous Ng complexes [23,32], these different local fields produced a well-recognizable effect, namely the polarization of Ar in modes and extents that strictly mirror the values of the MEP. This phenomenon is effectively caught by the DoP(Ar) (vide supra) of the various LAr (see Table 1) that is positively correlated with the MEP at the VS,max or VS,min of L (see Table 3). This is graphically shown in Figure 3.
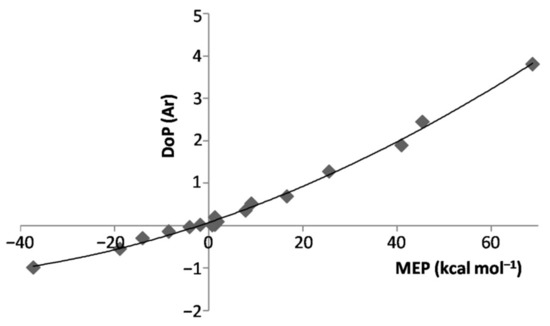
Figure 3.
DoP(Ar) of the LAr vs. MEP at the VS,max/VS,min of L.
In essence, when exposed to the MEP of the ligand, the Ar atom polarized toward/opposite to it, the effect increasing by increasing the magnitude of the experienced positive/negative MEP. However, the polarization of Ar was, expectedly, the major physical phenomenon behindthe inductive component of the interaction. As a matter of fact, as shown in Figure S1 of the Supplementary Materials, for both positive and negative values of the MEP of L, the of the various LAr generally increased by increasing the magnitude of the MEP. This positive correlation becomes even more clear by inspecting Figure 4, showing the dependence on the MEP of Lof the percentage contribution of to the total attractive part of the SAPT interaction, . On the other hand, neither the absolute nor the percentage contribution of the LAr correlated with the MEP of L. This was, indeed, not unexpected, as this termsolely arises from non-specific electrostatic interactions between the frozen densities of L and of the apolar Ar. One also notes from Table 1 thatthe of the various LAr spanned in the small range between ca. 12 and ca. 22%. These nearly constant contributions produced an inverse dependence between and (Figure S2 of Supplementary Materials), a positive correlation between and the MEP of L (Figure S3 of Supplementary Materials), and the positive correlation shown in Figure 5 between the ratio of the LAr, and the MEP of L. The absolute values of are, in any case, totally uncorrelated with the MEP. The dispersion is, in fact, also a form of polarization [67], but different in originfrom the inductive effects exerted by the MEP.
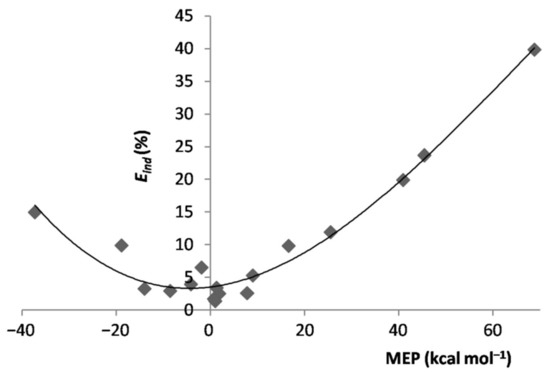
Figure 4.
of the LAr vs. MEP at the VS,max/VS,min of L.
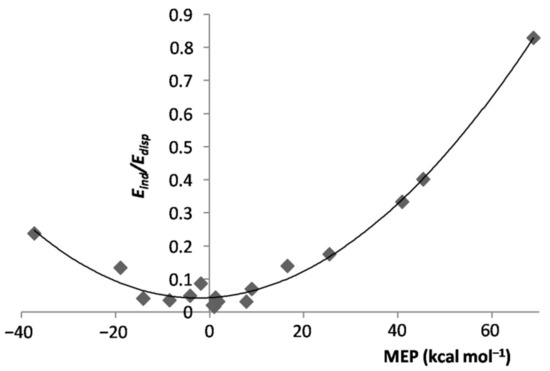
Figure 5.
ratio of the LArvs. MEP at the VS,max/VS,min of L.
The ligation of BeO to the Ar atom of any LAr produced major structural and stability effects that wereclearly referable to the σ-hole occurring at Ar. This is best discussed in the subsequent paragraph.
4.3. From the LAr to the L-ArBeO: The Role of the σ-Hole of ArBeO
The only slightly positive electrostatic potential of Ar (1.85 kcal mol−1 at the MP2/aVTZ0.0010 ea0−3 isodensity surface) was dramatically affected by the ligation with BeO. As shown in Figure 6a, plotting the MEP of ArBeO in the scale between −20 and 48 kcal mol−1, the Ar atom appeared as definitely positive, with a seemingly uniform MEP at around 48 kcal mol−1. However, as shown in Figure 6b, using a narrower scale between 42.0 and 50.0 kcal mol−1, the MEP of Ar emerged as clearly anisotropic, and featured a VS,max of 50.9 kcal mol−1 on the outer prolongation of the Ar-Be axis. This maximum signs a σ-hole arising from the polarization of Ar by BeO toward the inner region of the Ar-Be bond.
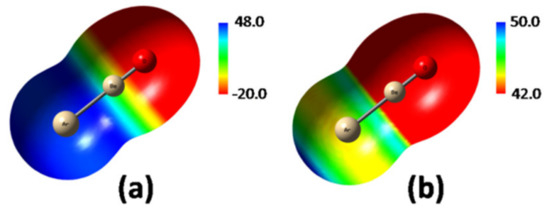
Figure 6.
MP2/aVTZ MEP (0.0010 ea0−3 isodensity surface) of ArBeO plotted using a wider (a) and narrower (b) scale (values in kcal mol−1).
The major ensuing effects are best appreciated by comparing the SAPT data of the L-ArBeO(dissociation limit: L + ArBeO) with those of the corresponding LAr. We first discuss the changes occurring in the electrostatic component of the interaction. In this regard, based on the data quoted in Table 1, it is possible to recognize three major situations. Thus, when Aris in contact with ligands whose MEP at the VS,max or VS,min was negative or only slightly-positive (up to less than 2 kcal mol−1), the of the L-ArBeO was invariably negative, and higher in magnitude than that of the corresponding LAr. The was also definitely more negative than that of the LAr, and the Ar-X distance wasshorterby ca. 0.2−0.4 Å, the contraction arriving up to ca. 0.65 Å for L = NH3. These systems include the Ng-ArBeO (Ng = He, Ne, Ar), the linear N2-ArBeO, OC-ArBeO, CO-ArBeO, ClF-ArBeO, and HF-ArBeO, the T-shaped F2-ArBeO and Cl2-ArBeO, and the C3v isomer H3N-ArBeO.All these species are also characterized as true minima on the MP2/aVTZ PES, the only exception being the T-shaped Cl2-ArBeO, featuring a single imaginary frequency (NIMAG = 1) (see Table S1 of the Supplementary Materials). According to the electrostatic model of NCIs [67,68], these contacts must be viewed as “intuitive” interactions [25,26] driven from the favorable attraction between MEPs of opposite sign. When Ar was in contact with VS,max or VS,min featuring positive values of the MEP up to ca. 25 kcal mol−1, the of the L-ArBeO was lower in magnitude than that of the corresponding LAr, and even positive. The was also less negative than that of the LAr, or even positive. These systems include the T-shaped N2-ArBeO, and the linear F2-ArBeO and Cl2-ArBeO, invariably characterized as higher-order saddle points on the corresponding PES (see Table S1 of the Supplementary Materials). All these features are typical of “counterintuitive” complexes [25,26], whose bound characters arise from inductive and dispersive components still sufficient to compensate for the repulsive electrostatic contributionfrom MEPs of the same sign.Finally, the contact of Ar with ligands featuring definitely positive values of the MEP at their VS,max was totally repulsive, and the corresponding L-ArBeO were unbound on the PES. This happened, in particular, for the FH-ArBeO, FCl-ArBeO, and ClH-ArBeO. The major role of the MEP of L in determining these different situations clearly emerges by examining Figure 7, showing the positive correlation between this quantity andthe of the L-ArBeO. The percentage contributions , covering the wide range between ca. 9 and ca. 72%, werealso regularly dependent on the MEP of L (see Figure S4 of the Supplementary Materials).
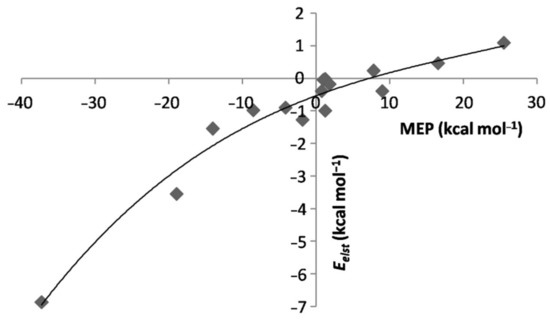
Figure 7.
of the L-ArBeO vs. MEP at the VS,max/VS,min of L.
The major role of the electrostatic component in determining the stability of the L-ArBeO clearly emerges by examining some exemplary situations. Thus, the higly negative VS,Min(N) of NH3of −37.25kcal mol−1produced a Eelst of the H3N-ArBeO as negative as ca. −6.9 kcal mol−1 (see Table 1). The of the H3N-Ar was, instead, only ca. −0.2 kcal mol−1, and the difference of −6.7 kcal mol−1 decisively contributed to the huge increase of the when going from H3N-Ar (−0.33 kcal mol−1) to H3N-ArBeO (−5.4 kcal mol−1). It is also of interest to compare the N2-ArBeO with the isomeric OC-ArBeO and CO-ArBeO. The VS,Min(N) of N2, −8.53 kcal mol−1was more negative than the VS,Min(O) of CO, −4.12, and this produced a of N2-ArBeO (−0.98 kcal mol−1) that was more negative than that of the CO-ArBeO (−0.90 kcal mol−1). This is opposite to the trend across N2-Ar and CO-Ar, whose amounts to ca. −0.11 and −0.12 kcal mol−1, respectively. Conversely, the VS,Min(C) of CO of -14.04 kcal mol−1produced a of OC-ArBeO (ca. −1.55 kcal mol−1) that was more negative than that of N2-ArBeO, the difference of −0.57 kcal mol−1 decisively determining the of OC-ArBeO as more negative than that of N2-ArBeO (−1.49 vs. −1.16 kcal mol−1). As a matter of fact, in the absence of this electrostatic stabilization, the of N2-Ar and OC-Ar were, essentially, the same (−0.25 and −0.24 kcal mol−1, respectively).
As for the inductive component of the interaction, its magnitude invariably increased(becamemore negative) when going from any LAr to the corresponding L-ArBeO. At variance with the LAr, however, the values of were not correlated with the MEP of the L. This finding was, indeed, not unexpected, as this energy term expectedly mirrors the mutual polarization of L and ArBeO. This suggestion is partially supported by the graph plotted in Figure S5 of the Supplementary Materials, showing the dependence of the of the L-ArBeO on the experimental polarizability α of L [60,61,62,63,64,65,66,67,68] (see Table 3). For the majority of the complexes, the two quantities were, indeed, positively correlated. There were, however, at least three major deviations (HF-ArBeO, H3N-ArBeO, and the linear Cl2-ArBeO), whose occurrencesclearly suggestthat various factors conceivably contributed to this energy component. In any case, for any L-ArBeO, the percentage contribution , wasonly minor, and spannedover the rather limited range of ca. 9–21% (see Table 1). As shown in Figures S6 and S7 of the Supplementary Materials, this producedan inverse relationship between and , and a positive correlation between the of the various L-ArBeO, and the MEP of L. This also producedthe positive correlation shown in Figure 8 between the ratio of the L-ArBeO, and the MEP of L. In any case, not unexpectedly, the absolute values of were totally uncorrelated with respect to the MEP.
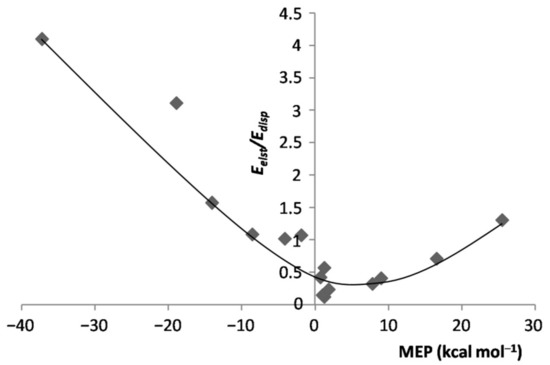
Figure 8.
ratio of the L-ArBeOvs. MEP at the VS,max/VS,min of L.
4.4. Bonding Analysi of the LAr and the L-ArBeO
In keeping with the results of the SAPT analysis, all the presently-investigated LAr and L-ArBeO were assigned as nCov(C). Likewise, the exemplary N2-Ar and linear N2-ArBeO shown in Figure 1, the H−(r) regions of any L and Ar or ArBeO, were, in fact, invariably well separated and plunged in a zone of positive values of H(r); the latter wasalso invariably positive over Ωs. However, as shown in Table 3, when going from any LAr to the corresponding L-ArBeO, the bond indices, particularly the N(Ωs) and the ρs(ave), underwentappreciable changes that were, in particular, clearly related to the changes occurring in their SAPT terms (vide supra). Thus, for the Ng-ArBeO (Ng = He, Ne, Ar), the linear N2-ArBeO, OC-ArBeO, CO-ArBeO, ClF-ArBeO, and HF-ArBeO, the T-shaped F2-ArBeO and Cl2-ArBeO, and the C3v isomer H3N-ArBeO, the values of both N(Ωs) and ρs(ave) were higher than those predicted for the corresponding LAr. This suggests tighter interactions, well consistent with the SAPT prediction of L-ArBeO complexes more stable than the LAr. On the other hand, for the T-shaped N2-ArBeO, and the linear F2-ArBeO and Cl2-ArBeO, the values of the two bond indices werelower than those predicted for the corresponding LAr, in good agreement with the lower stability of the L-ArBeO predicted by the SAPT analysis. This confirms the sensitivity of our proposed method in catching even subtle differences in the bonding character of the Ng complexes.
5. Conclusions
The comparison of the L-ArBeO with their “dispersive” cousins LAr allowed us to highlight structural and stability effects peculiarly related to the σ-hole of ArBeO. Consistent with the electrostatic model of NCIs [16,59], the most-affected SAPT term wasthe . The values predicted for the L-ArBeO were clearly related to the values of the MEP at the VS,max or VS,min of L, and ranged from negative (attractive) to positive (repulsive) values depending on the negative/positive value of the MEP. The percentage contribution of may actually arrive up to ca 80% of the attractive interaction, and decisively determined the overall increased or decreased stability of any L-ArBeO with respect to the LAr. The positive σ-hole of ArBeO also increased the inductive component of the interactionby an extent roughly related to the polarizability of L. The latter effects were, however, less pronounced than those occurring for the electrostatic component, the accounting for only ca. 9−21% of the overall stabilization. The nearly constant values of actually resulted in a regular inverse dependence between the electrostatic and the dispersive component, even though the absolute contribution of wasnot directly correlated with the MEP of L. All these major effects become even clearer if one considers the bonding situation of the LAr. In the absence of the σ-hole on Ar, the contribution of the electrostatic component wasonly minor, and nearly constant at around ca. 12−22% of the attractive stabilization. The interaction was, indeed, invariably dominated by the dispersion, even though the nearly null value of the electrostatic potential of Ar allowed us to recognize the direct influence of the MEP of L on the inductive component of the interaction. Our reached conclusions could be valid for other types of NCIs, especially those involving σ-holes of respectable strength such as those occurring in ArBeO. In this regard, there is, certainly, room for further future investigation.
Supplementary Materials
The following are available online. Table S1: MP2/aVTZ and CCSDT(T)/aVTZAr-X distances and harmonic vibrational frequencies of the LAr and L-ArBeO. Data S1: Cartesian coordinates of the LAr and L-ArBeO. Table S2: MP2/aVTZ and CCSD(T)/aVTZ properties of the nCov(C) bonds of the LAr and L-ArBeO. Table S3: Values of the MEP of L at the points of minimum (VS,Min) or maximum (VS,Max) calculated at the experimental, and at the CCSD(T)/aVTZ and MP2/aVTZ-optimized geometries. Figure S1: of the LAr vs. MEP at the VS,max/VS,min of L. Figure S2: vs. of the LAr. Figure S3: of the LAr vs. MEP at the VS,max/VS,min of L. Figure S4: of the L-ArBeO vs. MEP at the VS,max/VS,min of L. Figure S5: of the L-ArBeO vs. polarizability α of L. Figure S6: vs. of the L-ArBeO. Figure S7: of the L-ArBeO vs. MEP at the VS,max/VS,min of L.
Author Contributions
Methodology, data curation, software, S.B., F.G., N.S.; writing—original draft preparation, F.G.; writing—review and editing, S.B., N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Education, University and Research (MIUR) and the DIBAF Department of the University of Tuscia through the program “Departments of Excellence 2018–2022” (“Dipartimenti di Eccellenza2018–2022”), grant “Landscape 4.0—food, wellbeing and environment”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Hobza, P.; Müller-Dethlefs, K. Noncovalent Interactions. Theory and Experiment; RSC Theoretical and Computational Chemistry Series; Royal Society of Chemistry: Cambridge, UK, 2010. [Google Scholar]
- Noncovalent Interactions in the Synthesis and Design of New Compounds; Maharramov, A.M.; Mahmudov, K.T.; Kopylovich, M.N.; Pombeiro, A.J.L. (Eds.) John Wiley and Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- He, H.; Tan, W.; Guo, J.; Yi, M.; Shy, A.N.; Xu, B. Enzymatic Noncovalent Synthesis. Chem. Rev. 2020, 120, 9994–10078. [Google Scholar] [CrossRef]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the Hydrogen Bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Scheiner, S. Forty Years of Progress in the Study of the Hydrogen Bond. Struct. Chem. 2019, 30, 1119–1128. [Google Scholar] [CrossRef]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen Bonding: The σ-Hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef]
- Clark, T. σ-Holes. WIREs Comput. Mol. Sci. 2013, 3, 13–20. [Google Scholar]
- Politzer, P.; Murray, J.S.; Clark, T. HalogenBonding and other σ-HoleInteractions: A Perspective. Phys. Chem. ChemPhys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef]
- Kolar, M.H.; Hobza, P. Computer Modeling of Halogen Bonds and other σ-Hole Interactions. Chem. Rev. 2016, 116, 5155–5187. [Google Scholar] [CrossRef] [Green Version]
- Brammer, L. Halogen Bonding, Chalcogen Bonding, Pnictogen Bonding, Tetrel Bonding: Origins, Current Status and Discussion. Faraday Discuss. 2017, 203, 485–507. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Li, Q.; Scheiner, S. Comparative Strengths of Tetrel, Pnicogen, Chalcogen, and Halogen Bonds and ContributingFactors. Molecules 2018, 23, 1681. [Google Scholar] [CrossRef] [Green Version]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the Halogen Bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [Green Version]
- Parajuli, R. Does the Recent IUPAC Definition of Hydrogen Bonding Lead to New Intermolecular Interactions? Curr. Sci. 2016, 110, 495–498. [Google Scholar]
- Alkorta, I.; Elguero, J.; Frontera, A. Not only Hydrogen Bonds: Other Noncovalent Interactions. Crystals 2020, 10, 180. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.S.; Politzer, P. Molecular Electrostatic Potentials and Noncovalent Interactions. WIREs Comput. Mol. Sci. 2017, 7, e1326. [Google Scholar] [CrossRef]
- Bader, R.F.; Carroll, M.T.; Cheeseman, J.R.; Chang, C. Properties of Atoms in Molecules: Atomic Volumes. J. Am. Chem. Soc. 1987, 109, 7968–7979. [Google Scholar] [CrossRef]
- Borocci, S.; Grandinetti, F.; Sanna, N. From LAr to L-ArBeO (L = He, Ne, Ar, HF): Switching on σ-Hole Effects in Non-Covalent Interactions. Chem. Phys. Lett. 2021, 768, 138402. [Google Scholar] [CrossRef]
- Grandinetti, F. Noble Gas. Chemistry: Structure, Bonding, and Gas.-Phase Chemistry; Wiley-VCH: Weinheim, Germany, 2018; Chapter 3; pp. 100–107. [Google Scholar]
- Jeziorski, B.; Moszyński, R.; Ratkiewicz, A.; Rybak, V.; Szalewicz, K.; Williams, H.L. Methods and Techniques in Computational Chemistry: METECC-94; Clementi, E., Ed.; STEF: Cagliari, Italy, 1993; Volume B. [Google Scholar]
- Jeziorski, B.; Moszyński, R.; Szalewicz, K. Perturbation Theory Approach to Intermolecular Potential Energy Surfaces of van der Waals Complexes. Chem. Rev. 1994, 94, 1887–1930. [Google Scholar] [CrossRef]
- Borocci, S.; Giordani, M.; Grandinetti, F. Bonding Motifs of Noble-Gas Compounds as Described by the Local Electron Energy Density. J. Phys. Chem. A 2015, 119, 6528–6541. [Google Scholar] [CrossRef] [PubMed]
- Borocci, S.; Grandinetti, F.; Sanna, N.; Antoniotti, P.; Nunzi, F. Non-Covalent Complexes of the Noble-Gas Atoms: Analyzing the Transition from Physical to Chemical Interactions. J. Comput. Chem. 2019, 40, 2318–2328. [Google Scholar] [CrossRef]
- Borocci, S.; Grandinetti, F.; Sanna, N.; Nunzi, F. Classifying the Chemical Bonds Involving the Noble-Gas Atoms. New J. Chem. 2020, 44, 14536–14550. [Google Scholar] [CrossRef]
- Murray, J.S.; Shields, Z.P.I.; Seybold, P.G.; Politzer, P. Intuitive and Counterintuitive Noncovalent Interactions of Aromatic πRegions with the Hydrogen and the Nitrogen of HCN. J. Comput. Sci. 2015, 10, 209–216. [Google Scholar] [CrossRef]
- Wang, C.; Danovich, D.; Shaik, S.; Wu, W.; Mo, Y. Attraction between Electrophilic Caps: A Counterintuitive Case of Noncovalent Interactions. J. Comput. Chem. 2019, 40, 1015–1022. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E. Chemical Bonds without Bonding Electron Density-Does the Difference Electron-Density Analysis Suffice for a Description of the Chemical Bond? Angew. Chem. Int. Ed. Engl. 1984, 23, 627–628. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E. A Description of the Chemical Bond in Terms of Local Properties of Electron Density and Energy. Croat. Chem. Acta 1984, 57, 1259–1281. [Google Scholar]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narth, C.; Maroun, Z.; Boto, R.A.; Chaudret, R.; Bonnet, M.L.; Piquemal, J.-P.; Contreras-García, J. A Complete NCI Perspective: From New Bonds to Reactivity. In Applications of Topological Methods in Molecular Chemistry; Springer: Cham, Switzerland, 2016; pp. 491–527. [Google Scholar]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Borocci, S.; Grandinetti, F.; Sanna, N.; Antoniotti, P.; Nunzi, F. Complexes of Helium with Neutral Molecules: Progress toward a Quantitative Scale of Bonding Character. J. Comput. Chem. 2020, 41, 1000–1011. [Google Scholar] [CrossRef]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618. [Google Scholar] [CrossRef] [Green Version]
- Raghavachari, K.; Trucks, G.W.; Pople, J.A.; Head-Gordon, M. A Fifth-Order Perturbation Comparison of Electron Correlation Theories. Chem. Phys. Lett. 1989, 157, 479–483. [Google Scholar] [CrossRef]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-To-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Welcome to the Website of CFOUR. Available online: http://www.cfour.de (accessed on 20 July 2021).
- Bukowski, R.; Cencek, W.; Jankowski, P.; Jeziorska, M.; Jeziorski, B.; Kucharski, S.A.; Lotrich, V.F.; Metz, M.P.; Misquitta, A.J.; Moszyński, R.; et al. SAPT2016: An Ab Initio Program for Symmetry-Adapted Perturbation Theory Calculations of Intermolecular Interactions Energies. Sequential and Parallel Versions; University of Delaware: Newark, DE, USA; University of Warsaw: Warsaw, Poland, 2016. [Google Scholar]
- Tao, F.-M.; Pan, Y.-K. Ab Initio Potential Energy Curves and Binding Energies of Ar2 and Mg2. Mol. Phys. 1994, 81, 507–518. [Google Scholar] [CrossRef]
- Tao, F.-M.; Klemperer, W. Accurate ab Initio Potential Energy Surfaces of Ar–HF, Ar–H2O, and Ar–NH3. J. Chem. Phys. 1994, 101, 1129–1145. [Google Scholar] [CrossRef]
- Saleh, G.; Gatti, C.; Lo Presti, L. Energetics of Non-Covalent Interactions from Electron and Energy Density Distributions. Comput. Theor. Chem. 2015, 1053, 53–59. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Zou, W. Molden2AIM. Available online: https://github.com/zorkzou/Molden2AIM (accessed on 01 February 2021).
- Lee, T.J.; Taylor, P.R. A Diagnostic for Determining the Quality of Single-Reference Electron Correlation Methods. Int. J. Quantum Chem. 1989, 36, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Keil, M.; Danielson, L.J.; Dunlop, P.J. On Obtaining Interatomic Potentials from Multiproperty Fits to Experimental Data. J. Chem. Phys. 1991, 94, 296–309. [Google Scholar] [CrossRef]
- Barrow, D.A.; Aziz, R.A. The Neon-Argon Potential Revisited. J. Chem. Phys. 1988, 89, 6189–6194. [Google Scholar] [CrossRef]
- Aziz, R.A. A Highly Accurate Interatomic Potential for Argon. J. Chem. Phys. 1993, 99, 4518–4525. [Google Scholar] [CrossRef]
- Dham, A.K.; Meath, W.J.; Jechow, J.W.; McCourt, F.R.W. New Exchange-Coulomb N2-Ar Potential-Energy Surface and its Comparison with other Recent N2-Ar Potential-Energy Surfaces. J. Chem. Phys. 2006, 124, 034308. [Google Scholar] [CrossRef]
- Sumiyoshi, Y.; Endo, Y. Three-Dimensional Potential Energy Surface of Ar-CO. J. Chem. Phys. 2015, 142, 024314. [Google Scholar] [CrossRef]
- Chan, K.W.; Power, T.D.; Jai-nhuknan, J.; Cybulski, S.M. An ab Initio Study of He-F2, Ne-F2, and Ar-F2 van der Waals Complexes. J. Chem. Phys. 1999, 110, 860–869. [Google Scholar] [CrossRef]
- Rohrbacher, A.; Janda, K.C.; Beneventi, L.; Casavecchia, P.; Volpi, G.G. Differential Scattering Cross Sections for HeCl2, NeCl2, and ArCl2: Multiproperty Fits of Potential Energy Surfaces. J. Phys. Chem. A 1997, 101, 6528–6537. [Google Scholar] [CrossRef]
- Prosmiti, R.; Villareal, P.; Delgado-Barrio, G. Structure and Bonding of ArClF: Intermolecular Potential Surface. Isr. J. Chem. 2003, 43, 279–286. [Google Scholar] [CrossRef]
- Hutson, J.M. Vibrational Dependence of the Anisotropic Intermolecular Potential of Ar-HF. J. Chem. Phys. 1992, 96, 6752–6767. [Google Scholar] [CrossRef]
- Jouypazadeh, H.; Solimannejad, M.; Farrokhpour, H. New Potential Energy Surface and Rovibrational Spectra of Ar···HCl. Comput. Theor. Chem. 2016, 1083, 64–71. [Google Scholar] [CrossRef]
- Loreau, J.; Liévin, J.; Scribano, Y.; van der Avoird, A. Potential Energy Surface and Bound States of the NH3-Ar and ND3-Ar Complexes. J. Chem. Phys. 2014, 141, 224303. [Google Scholar] [CrossRef] [Green Version]
- Chałasiński, G.; Szczęśniak, M.M. Origins of Structure and Energetics of van der Waals Clusters from ab Initio Calculations. Chem. Rev. 1994, 94, 1723–1765. [Google Scholar] [CrossRef]
- Nunzi, F.; Pannacci, G.; Tarantelli, F.; Belpassi, L.; Cappelletti, D.; Falcinelli, S.; Pirani, F. Leading Interaction Components in the Structure and Reactivity of Noble Gases Compounds. Molecules 2020, 25, 2367. [Google Scholar] [CrossRef] [PubMed]
- Maroulis, G. Accurate Electric Multipole Moment, Static Polarizability and Hyperpolarizability Derivatives for N2. J. Chem. Phys. 2003, 118, 2673–2687. [Google Scholar] [CrossRef]
- Maroulis, G. Electric Polarizability and Hyperpolarizability of Carbon Monoxide. J. Phys. Chem. 1996, 100, 13466–13473. [Google Scholar] [CrossRef]
- Maroulis, G. On the Bond-Length Dependence of the Static Electric Polarizability and Hyperpolarizability of F2. Chem. Phys. Lett. 2007, 442, 265–269. [Google Scholar] [CrossRef]
- Maroulis, G. Accurate Dipole Polarizability for Cl2 (X1Σg+). Mol. Phys. 1992, 77, 1085–1094. [Google Scholar] [CrossRef]
- Miller, K.J.; Savchik, J.A. A New Empirical Method to Calculate Average Molecular Polarizabilities. J. Am. Chem. Soc. 1979, 101, 7206–7213. [Google Scholar] [CrossRef]
- Sadlej, A.J. Electric Properties of Diatomic Interhalogens. A Study of the Electron Correlation and Relativistic Contributions. J. Chem. Phys. 1992, 96, 2048–2053. [Google Scholar] [CrossRef]
- Maroulis, G. Electric Multipole Moment, Dipole and Quadrupole (Hyper)Polarizability Derivatives for HF (X1Σ+). J. Mol. Struct. (THEOCHEM) 2003, 633, 177–197. [Google Scholar] [CrossRef]
- Maroulis, G. A Systematic Study of Basis Set, Electron Correlation, and Geometry Effects on the Electric Multipole Moments, Polarizability, and Hyperpolarizability of HCl. J. Chem. Phys. 1998, 108, 5432–5448. [Google Scholar] [CrossRef]
- Zeiss, G.D.; Meath, W.J. Dispersion Energy Constants C6 (A,B), Dipole Oscillator Strength Sums and Refractivities for Li, N, O, H2, N2, O2, NH3, H2O, NO and N2O. Mol. Phys. 1977, 33, 1155–1176. [Google Scholar] [CrossRef]
- Clark, T. Polarization, Donor-Acceptor Interactions, and Covalent Contributions in Weak Interactions: A Clarification. J. Mol. Model. 2017, 23, 297. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Politzer, P. Interaction and Polarization Energy Relationships in σ-Hole and π-Hole Bonding. Crystals 2020, 10, 76. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).