Effects of Ulva sp. Extracts on the Growth, Biofilm Production, and Virulence of Skin Bacteria Microbiota: Staphylococcus aureus, Staphylococcus epidermidis, and Cutibacterium acnes Strains
Abstract
:1. Introduction
2. Results
2.1. Poly- and Oligosaccharide Ulva sp. Fractions: Biochemical Composition and Molecular Weight Distribution
2.2. Effects of Poly- and Oligosaccharide Ulva sp. Fractions on the Bacterial Growth Kinetic
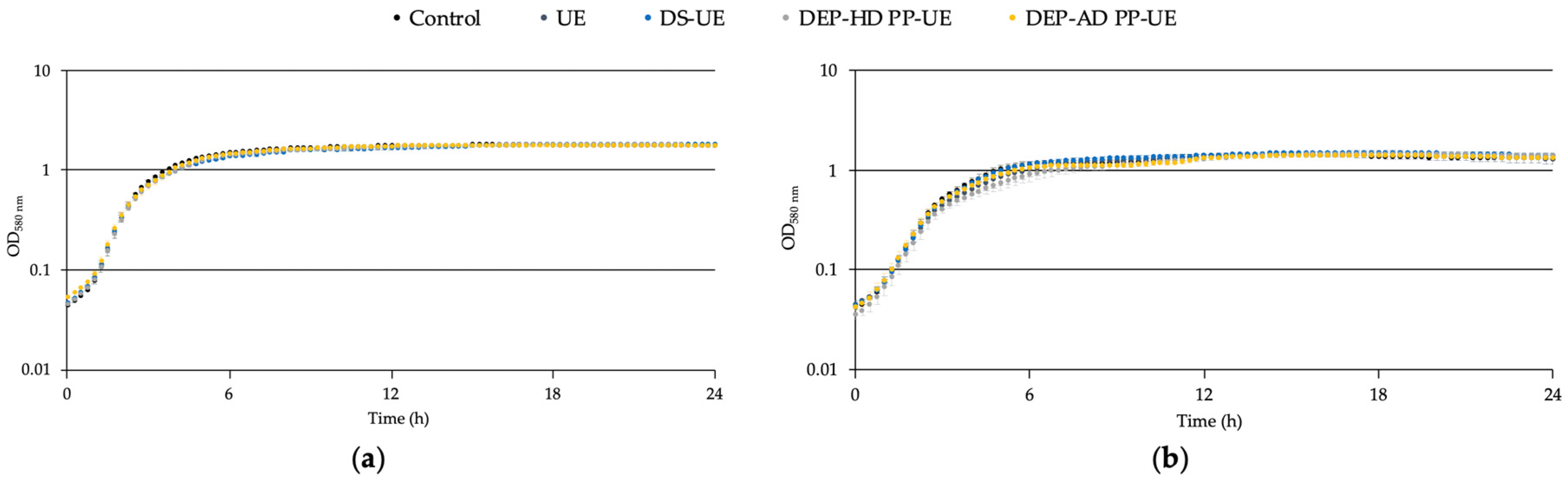
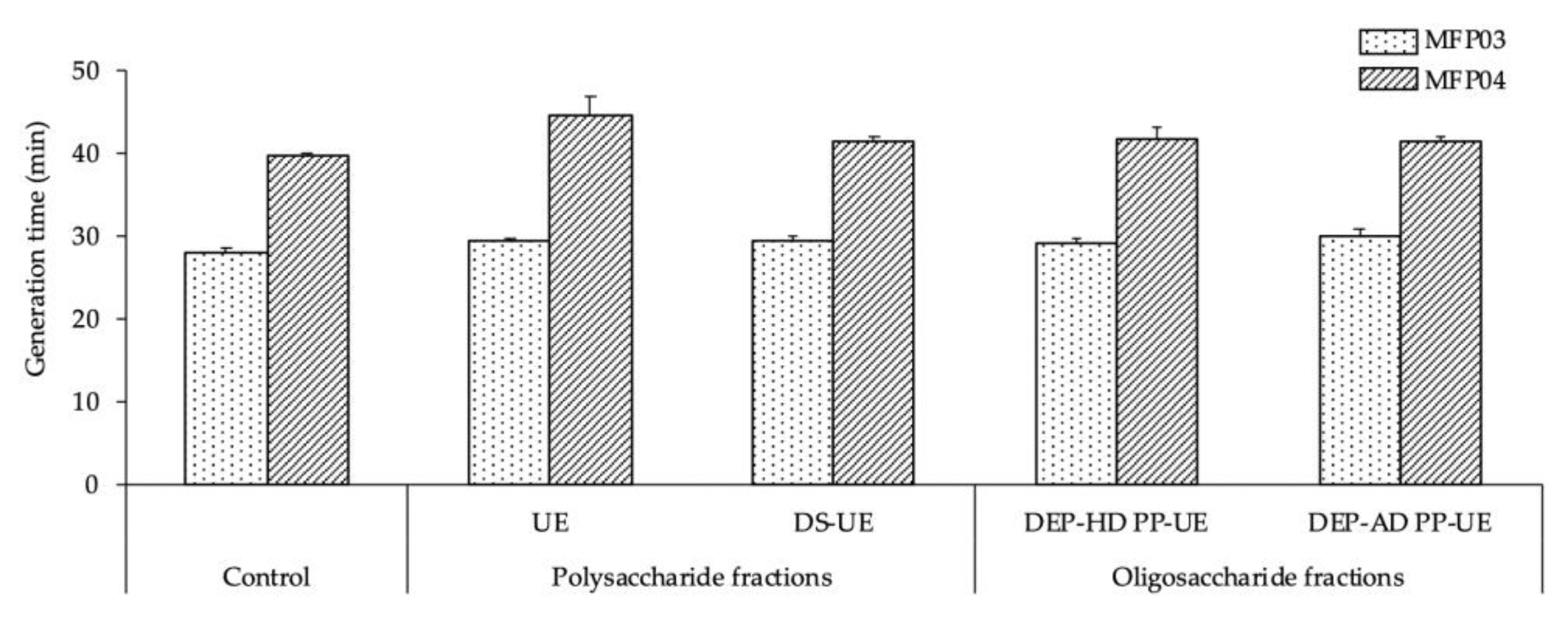
2.2.1. Effects on the Growth of S. aureus MFP03 and S. epidermidis MFP04
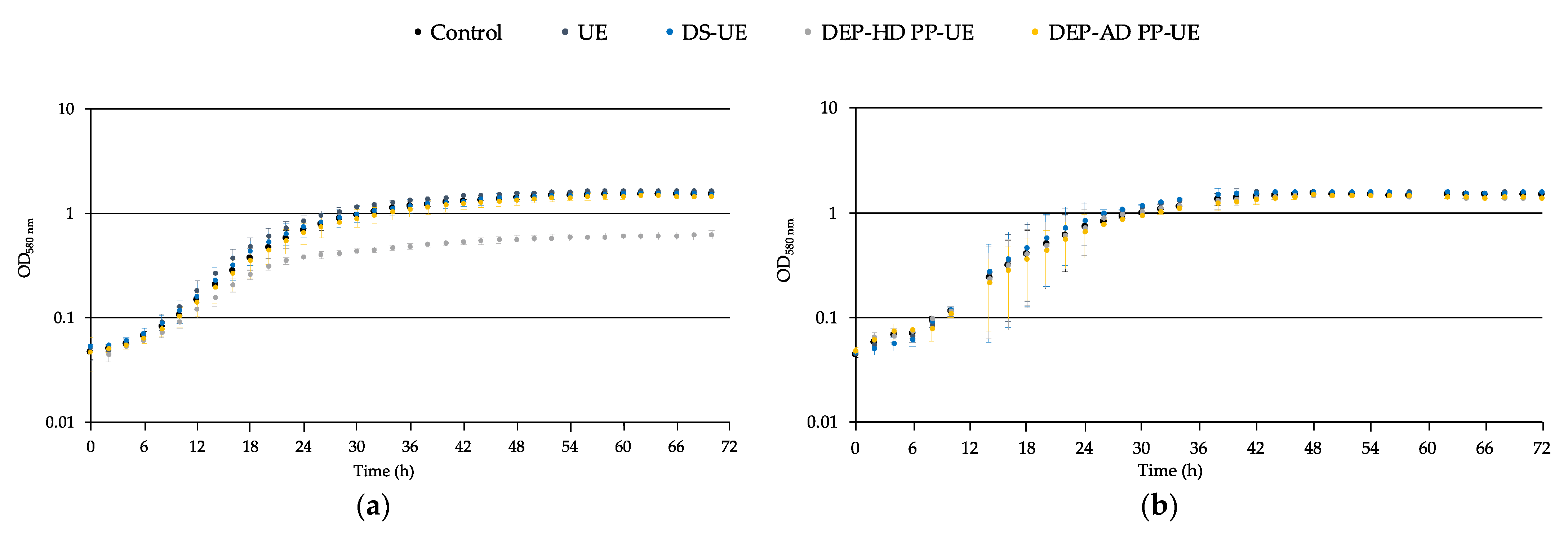
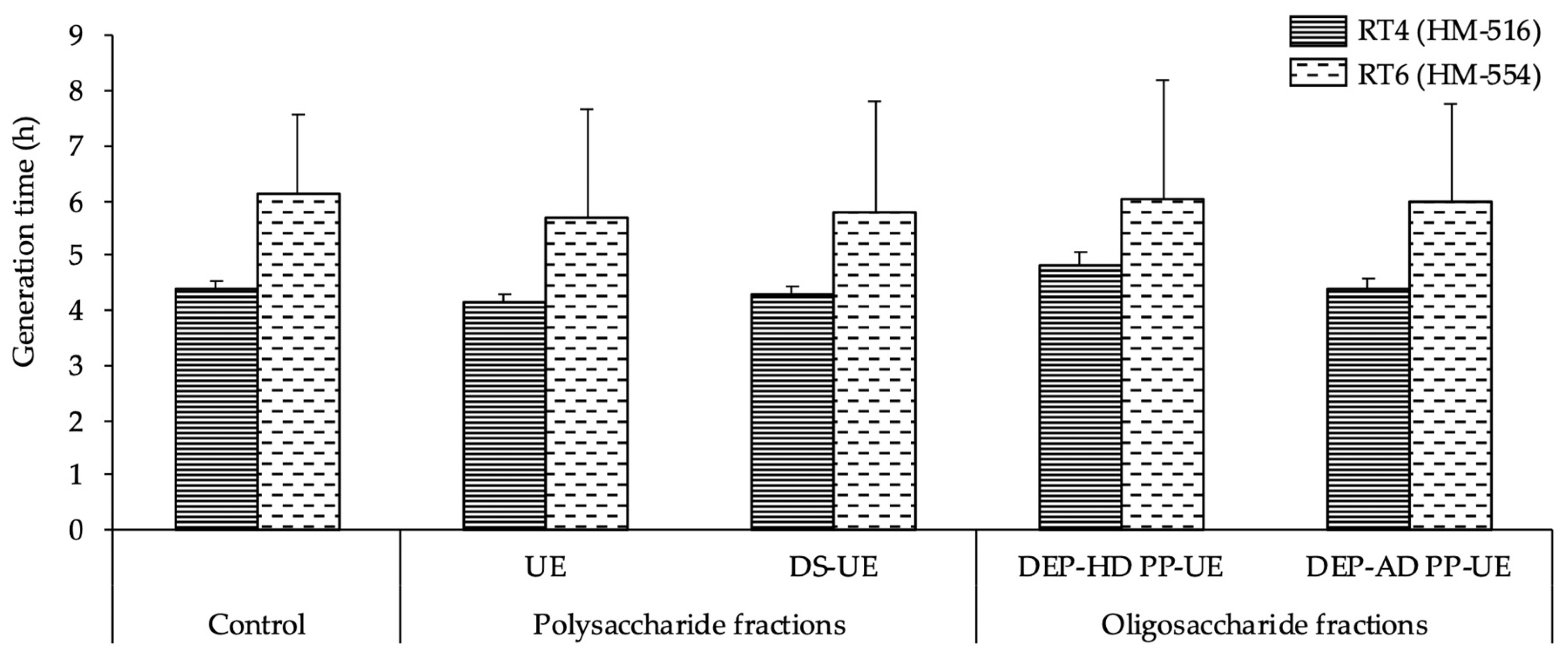
2.2.2. Effects on the Growth of Acneic and Non-Acneic C. acnes Strains
2.3. Effects of Poly- and Oligosaccharide Ulva sp. Fractions on Biofilm Production
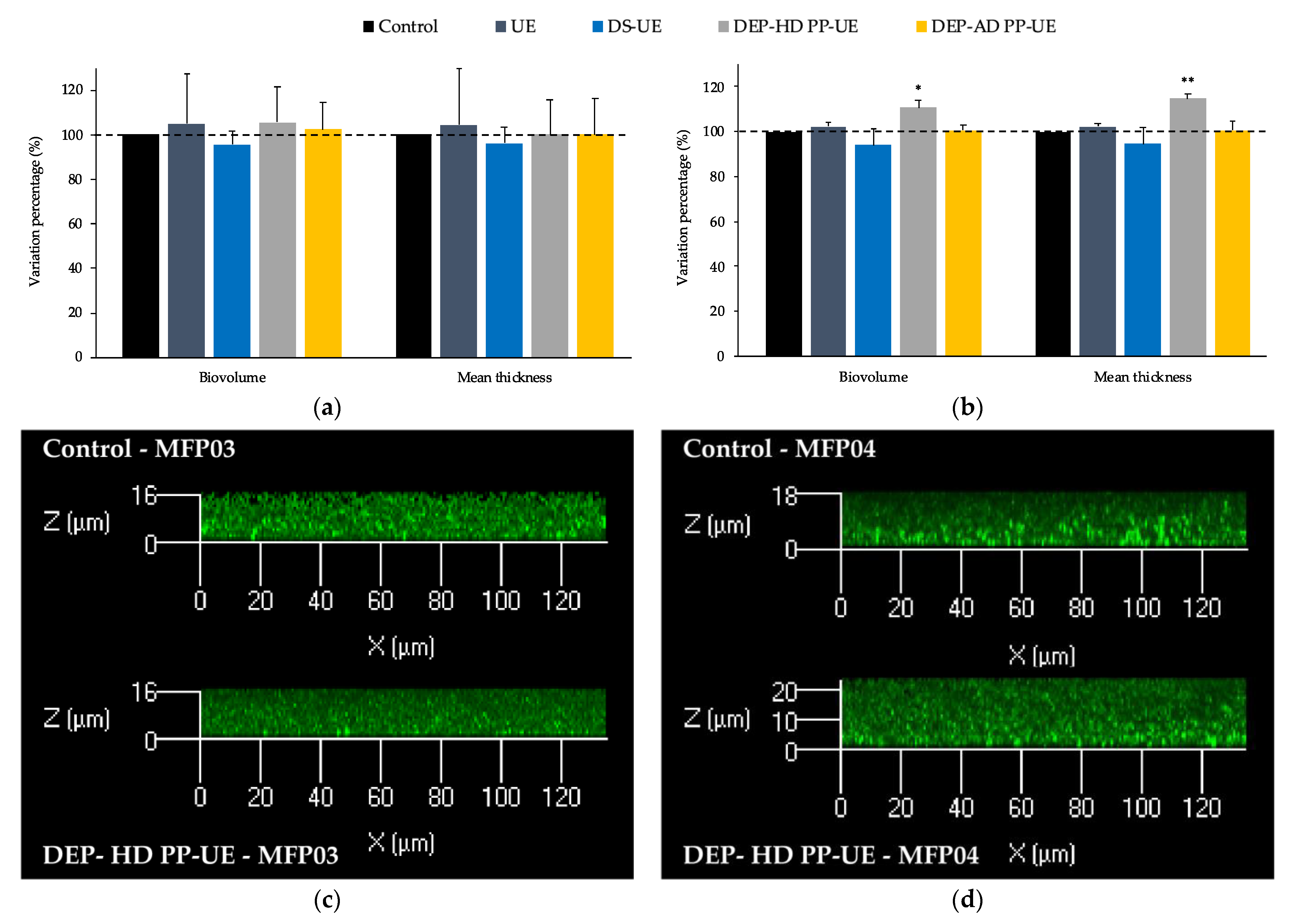
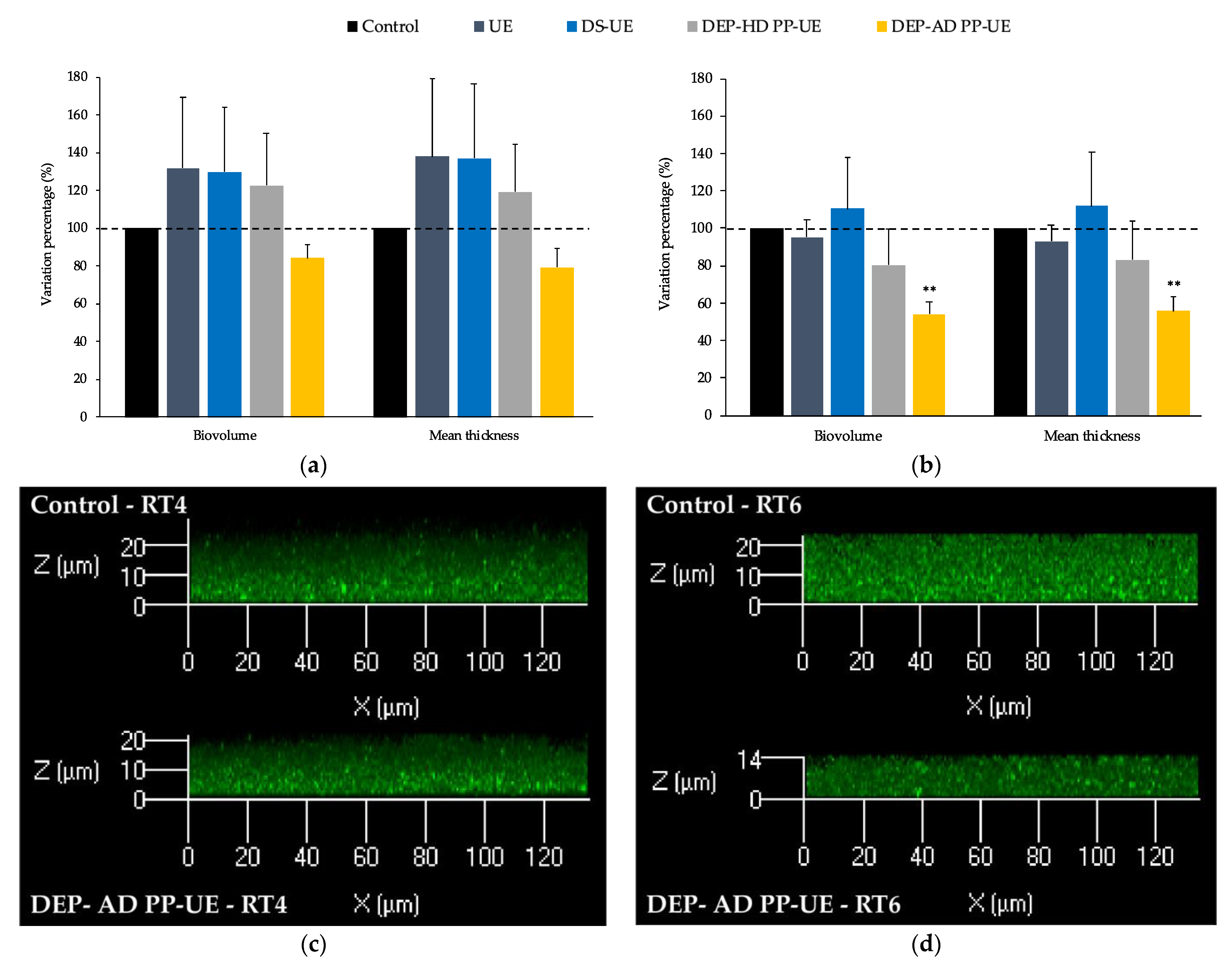
2.3.1. Effects on the Biofilm Production of S. aureus MFP03 and S. epidermidis MFP04
2.3.2. Effects on the Biofilm Production by Acneic and Non-Acneic C. acnes Strains
2.4. Effects of Poly- and Oligosaccharide Ulva sp. Fractions on the Cytotoxic Activity of S. aureus MFP03 and S. epidermidis MFP04 towards HaCaT Keratinocytes
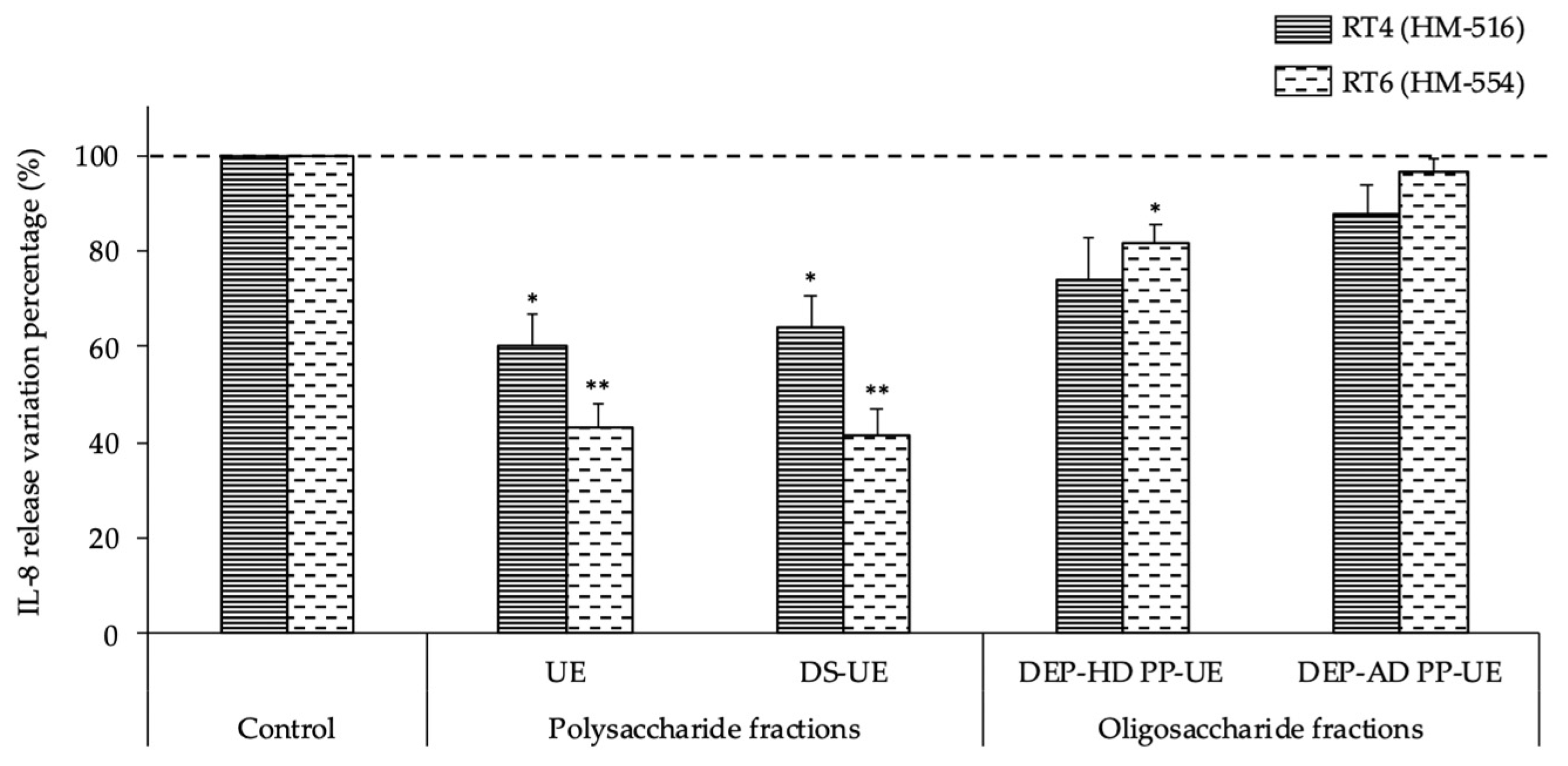
2.5. Effects of Poly- and Oligosaccharide Ulva sp. Fractions on the Inflammatory Potential of Acneic and Non-Acneic C. acnes Strains towards HaCaT Keratinocytes
3. Discussion
3.1. Effects of Poly- and Oligosaccharide Ulva sp.-Derived EAE Fractions on Commensal Staphylococcus Species and Acneic or Non-Acneic Cutibacterium acnes
3.2. Potential Implications of the Sulfate Composition and Molecular Weight of Ulvan Fractions and Strain Specificity in Skin Bacteria Modulation
4. Materials and Methods
4.1. Seaweed Material
4.2. Poly- and Oligosaccharide Ulva sp. Fractions Production and Characterization
4.3. Bacterial Strains and Culture Conditions
4.3.1. Staphylococcus aureus and Staphylococcus epidermidis Strains
4.3.2. Cutibacterium acnes Strains
4.4. Bacterial Growth Kinetic
4.4.1. Staphylococcus aureus and Staphylococcus epidermidis Strains
4.4.2. Cutibacterium acnes Strains
4.5. Biofilm Formation Activity: Confocal Laser Scanning Microscopy
4.6. HaCaT Keratinocytes Cell Culture and Infection
4.7. LDH Cytotoxicity Studies
4.8. Interleukin-8 Secretion Study
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Charlier, R.; Morand, P.; Finkl, C.; Thys, A. Green Tides on the Brittany Coasts. Environ. Res. Eng. Manag. 2007, 3, 52–59. [Google Scholar]
- Briand, X. Seaweed harvesting in Europe. In Seaweed Resources in Europe: Uses and Potential; Guiry, M.D., Blunden, G., Eds.; John Wiley & Sons: Chichester, UK, 1991; pp. 259–308. ISBN 0-471-92947-6. [Google Scholar]
- CEVA. Bilan 2019 Des Proliférations D’algues Vertes Sur Les Principales Baies Bretonnes. 2019, pp. 1–7. Available online: https://www.ceva-algues.com/document/etude-et-suivi-des-marees-d-algues-vertes-en-bretagne/ (accessed on 21 September 2020).
- Gao, G.; Clare, A.S.; Rose, C.; Caldwell, G.S. Eutrophication and warming-driven green tides (Ulva rigida) are predicted to increase under future climate change scenarios. Mar. Pollut. Bull. 2017, 114, 439–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Alstyne, K.L.; Nelson, T.A.; Ridgway, R.L. Environmental chemistry and chemical ecology of “green tide” Seaweed blooms. Integr. Comp. Biol. 2015, 55, 518–532. [Google Scholar] [CrossRef]
- Ye, N.H.; Zhang, X.W.; Mao, Y.Z.; Liang, C.W.; Xu, D.; Zou, J.; Zhuang, Z.M.; Wang, Q.Y. “Green tides” are overwhelming the coastline of our blue planet: Taking the world’s largest example. Ecol. Res. 2011, 26, 477–485. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and functional properties of Ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardouin, K.; Bedoux, G.; Burlot, A.-S.; Nyvall-Collén, P.; Bourgougnon, N. Enzymatic Recovery of Metabolites from Seaweeds: Potential Applications. Adv. Bot. Res. 2014, 71, 279–320. [Google Scholar]
- Pliego-Cortés, H.; Wijesekara, I.; Lang, M.; Bourgougnon, N.; Bedoux, G. Current knowledge and challenges in extraction, characterization and bioactivity of seaweed protein and seaweed-derived proteins. Adv. Bot. Res. 2020, 95, 289–326. [Google Scholar]
- Terme, N.; Hardouin, K.; Cortès, H.P.; Peñuela, A.; Freile-Pelegrín, Y.; Robledo, D.; Bedoux, G.; Bourgougnon, N. Emerging seaweed extraction techniques: Enzyme-assisted extraction a key step of seaweed biorefinery? In Sustainable Seaweed Technologies; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 225–256. ISBN 9780128179437. [Google Scholar]
- Fournière, M.; Latire, T.; Lang, M.; Terme, N.; Bourgougnon, N.; Bedoux, G. Production of Active Poly- and Oligosaccharidic Fractions from Ulva sp. by Combining Enzyme-Assisted Extraction (EAE) and Depolymerization. Metabolites 2019, 9, 182. [Google Scholar] [CrossRef] [Green Version]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine 2017, 45, 347–351. [Google Scholar] [CrossRef]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Pilot production of ulvans from Ulva sp. and their effects on hyaluronan and collagen production in cultured dermal fibroblasts. Carbohydr. Polym. 2017, 157, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Fournière, M.; Bedoux, G.; Lebonvallet, N.; Leschiera, R.; Le Goff-Pain, C.; Bourgougnon, N.; Latire, T. Poly- and Oligosaccharide Ulva sp. Fractions from Enzyme-Assisted Extraction Modulate the Metabolism of Extracellular Matrix in Human Skin Fibroblasts: Potential in Anti-Aging Dermo-Cosmetic Applications. Mar. Drugs 2021, 19, 156. [Google Scholar] [CrossRef]
- Ko, H.J.; Kim, G.B.; Lee, D.H.; Lee, G.S.; Pyo, H.B. The effect of hydrolyzed Jeju Ulva pertusa on the proliferation and type I collagen synthesis in replicative senescent fibroblasts. J. Soc. Cosmet. Sci. Korea 2013, 39, 177–186. [Google Scholar]
- Cai, C.; Guo, Z.; Yang, Y.; Geng, Z.; Tang, L.; Zhao, M.; Qiu, Y.; Chen, Y.; He, P. Inhibition of hydrogen peroxide induced injuring on human skin fibroblast by Ulva prolifera polysaccharide. Int. J. Biol. Macromol. 2016, 91, 241–247. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; Kong, H.H.; et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013, 498, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Perez-Perez, G.I.; Chen, Y.; Blaser, M.J. Quantitation of major human cutaneous bacterial and fungal populations. J. Clin. Microbiol. 2010, 48, 3575–3581. [Google Scholar] [CrossRef] [Green Version]
- Foulongne, V.; Sauvage, V.; Hebert, C.; Dereure, O.; Cheval, J.; Gouilh, M.A.; Pariente, K.; Segondy, M.; Burguière, A.; Manuguerra, J.-C.; et al. Human Skin Microbiota: High Diversity of DNA Viruses Identified on the Human Skin by High Throughput Sequencing. PLoS ONE 2012, 7, e38499. [Google Scholar] [CrossRef] [Green Version]
- Probst, A.J.; Auerbach, A.K.; Moissl-Eichinger, C. Archaea on Human Skin. PLoS ONE 2013, 8, e65388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacey, N.; Kavanagh, K.; Tseng, S.C.G. Under the lash: Demodex mites in human diseases. Biochem 2009, 31, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; Green, E.D.; et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; He, G.; Qian, J.; Zhan, Y.; Xiao, R. Potential role of the skin microbiota in Inflammatory skin diseases. J. Cosmet. Dermatol. 2021, 20, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, M.; Goldberg, D.; Aiello, A.; Larson, E.; Foxman, B. Skin microbiota: Microbial community structure and its potential association with health and disease. Infect. Genet. Evol. 2011, 11, 839–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef] [Green Version]
- Scholz, C.F.P.; Kilian, M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium. Int. J. Syst. Evol. Microbiol. 2016, 66, 4422–4432. [Google Scholar] [CrossRef] [PubMed]
- Fournière, M.; Latire, T.; Souak, D.; Feuilloley, M.G.J.; Bedoux, G. Staphylococcus epidermidis and Cutibacterium acnes: Two Major Sentinels of Skin Microbiota and the Influence of Cosmetics. Microorganisms 2020, 8, 1752. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-J.; Chang, T.-W.; Jiang, Y.; Kao, H.-J.; Chiou, B.-H.; Kao, M.-S.; Huang, C.-M. Commensal Staphylococcus aureus Provokes Immunity to Protect against Skin Infection of Methicillin-Resistant Staphylococcus aureus. Int. J. Mol. Sci. 2018, 19, 1290. [Google Scholar] [CrossRef] [Green Version]
- Christensen, G.J.M.; Brüggemann, H. Bacterial skin commensals and their role as host guardians. Benef. Microbes 2014, 5, 201–215. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Kong, H.H.; Segre, J.A. Skin Microbiome: Looking Back to Move Forward. J. Investig. Dermatol. 2012, 132, 933–939. [Google Scholar] [CrossRef] [Green Version]
- McDowell, A. Over a Decade of recA and tly Gene Sequence Typing of the Skin Bacterium Propionibacterium acnes: What Have We Learnt? Microorganisms 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagnelie, M.-A.; Khammari, A.; Dréno, B.; Corvec, S. Cutibacterium acnes molecular typing: Time to standardize the method. Clin. Microbiol. Infect. 2018, 24, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Fitz-Gibbon, S.; Tomida, S.; Chiu, B.-H.; Nguyen, L.; Du, C.; Liu, M.; Elashoff, D.; Erfe, M.C.; Loncaric, A.; Kim, J.; et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J. Investig. Dermatol. 2013, 133, 2152–2160. [Google Scholar] [CrossRef] [Green Version]
- Frenard, C.; Dagnelie, M.A.; Khammari, A.; Saint-Jean, M.; Boisrobert, A.; Corvec, S.; Dreno, B. Do Cutibacterium acnes and Staphylococcus aureus define two different types of folliculitis? Bacteriological study of scalp folliculitis. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e266–e268. [Google Scholar] [CrossRef]
- Tyner, H.; Patel, R. Propionibacterium acnes biofilm—A sanctuary for Staphylococcus aureus? Anaerobe 2016, 40, 63–67. [Google Scholar] [CrossRef]
- Racine, P.; Janvier, X.; Clabaut, M.; Catovic, C.; Souak, D.; Boukerb, A.M.; Groboillot, A.; Konto-Ghiorghi, Y.; Duclairoir-Poc, C.; Lesouhaitier, O.; et al. Dialog between skin and its microbiota: Emergence of “Cutaneous Bacterial Endocrinology. Exp. Dermatol. 2020, 29, 790–800. [Google Scholar] [CrossRef]
- Liu, Q.; Mazhar, M.; Miller, L.S. Immune and Inflammatory Reponses to Staphylococcus aureus Skin Infections. Curr. Dermatol. Rep. 2018, 7, 338–349. [Google Scholar] [CrossRef]
- Coenye, T.; Peeters, E.; Nelis, H.J. Biofilm formation by Propionibacterium acnes is associated with increased resistance to antimicrobial agents and increased production of putative virulence factors. Res. Microbiol. 2007, 158, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Achermann, Y.; Goldstein, E.J.C.; Coenye, T.; Shirtliffa, M.E. Propionibacterium acnes: From Commensal to Opportunistic Biofilm-Associated Implant Pathogen. Clin. Microbiol. Rev. 2014, 27, 419–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Domenico, E.G.; Cavallo, I.; Capitanio, B.; Ascenzioni, F.; Pimpinelli, F.; Morrone, A.; Ensoli, F. Staphylococcus aureus and the Cutaneous Microbiota Biofilms in the Pathogenesis of Atopic Dermatitis. Microorganisms 2019, 7, 301. [Google Scholar] [CrossRef] [Green Version]
- Wallen-Russell, C.; Wallen-Russell, S. Meta Analysis of Skin Microbiome: New Link between Skin Microbiota Diversity and Skin Health with Proposal to Use This as a Future Mechanism to Determine Whether Cosmetic Products Damage the Skin. Cosmetics 2017, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Sfriso, R.; Egert, M.; Gempeler, M.; Voegeli, R.; Campiche, R. Revealing the secret life of skin - with the microbiome you never walk alone. Int. J. Cosmet. Sci. 2019, 42, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Souak, D.; Barreau, M.; Courtois, A.; André, V.; Duclairoir-Poc, C.; Feuilloley, M.G.J.; Gault, M. Challenging Cosmetic Innovation: The Skin Microbiota and Probiotics Protect the Skin from UV-Induced Damage. Microorganisms 2021, 9, 936. [Google Scholar] [CrossRef]
- Holland, K.T.; Bojar, R.A. Cosmetics: What is their influence on the skin microflora? Am. J. Clin. Dermatol. 2002, 3, 445–449. [Google Scholar] [CrossRef]
- Sardari, R.R.R.; Nordberg Karlsson, E. Marine Poly- and Oligosaccharides as Prebiotics. J. Agric. Food Chem. 2018, 66, 11544–11549. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Emergent sources of prebiotics: Seaweeds and microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Morvan, P.-Y.; Vallee, R. Evaluation of the Effects of Stressful Life on Human Skin Microbiota. Appl. Microbiol. 2018, 4, 1000140. [Google Scholar]
- Filaire, E.; Vialleix, C.; Cadoret, J.-P.; Guénard, S.; Muller, C.; Dreux-Zigha, A.; Berthon, J.-Y. Characterization of Reactive and Sensitive Skin Microbiota: Effect of Halymenia durvillei (HD) Extract Treatment. Cosmetics 2019, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Hillion, M.; Mijouin, L.; Jaouen, T.; Barreau, M.; Meunier, P.; Lefeuvre, L.; Lati, E.; Chevalier, S.; Feuilloley, M.G.J. Comparative study of normal and sensitive skin aerobic bacterial populations. Microbiologyopen 2013, 2, 953–961. [Google Scholar] [CrossRef]
- Borrel, V.; Gannesen, A.V.; Barreau, M.; Gaviard, C.; Duclairoir-Poc, C.; Hardouin, J.; Konto-Ghiorghi, Y.; Lefeuvre, L.; Feuilloley, M.G.J. Adaptation of acneic and non acneic strains of Cutibacterium acnes to sebum-like environment. Microbiologyopen 2019, 8, e00841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardouin, K.; Bedoux, G.; Burlot, A.-S.; Donnay-Moreno, C.; Bergé, J.-P.; Nyvall-Collén, P.; Bourgougnon, N. Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Res. 2016, 16, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Assaw, S.; Rosli, N.L.; Azmi, N.A.M.; Mazlan, N.W.; Ismail, N. Antioxidant and antibacterial activities of polysaccharides and methanolic crude extracts of local edible red seaweed Gracilaria sp. Malays. Appl. Biol. 2018, 47, 135–144. [Google Scholar]
- Jesumani, V.; Du, H.; Pei, P.; Aslam, M.; Huang, N. Comparative study on skin protection activity of polyphenol-rich extract and polysaccharide-rich extract from Sargassum vachellianum. PLoS ONE 2020, 15, e0227308. [Google Scholar] [CrossRef] [Green Version]
- Van Tran, T.T.; Truong, H.B.; Tran, N.H.V.; Quach, T.M.T.; Nguyen, T.N.; Bui, M.L.; Yuguchi, Y.; Thanh, T.T.T. Structure, conformation in aqueous solution and antimicrobial activity of ulvan extracted from green seaweed Ulva reticulata. Nat. Prod. Res. 2018, 32, 2291–2296. [Google Scholar] [CrossRef]
- Gannesen, A.V.; Borrel, V.; Lefeuvre, L.; Netrusov, A.I.; Plakunov, V.K.; Feuilloley, M.G.J. Effect of two cosmetic compounds on the growth, biofilm formation activity, and surface properties of acneic strains of Cutibacterium acnes and Staphylococcus aureus. Microbiologyopen 2018, 8, e00659. [Google Scholar] [CrossRef]
- Hillion, M. Interactions Peau/Microbiote Cutané: Etude du Microbiote Cutané Cultivable et Influence de Produits Cosmétiques sur la Virulence Bactérienne. Apports de la Technique de Spectrométrie de Masse MALDI-TOF. Ph.D. Thesis, Université de Rouen, Évreux, France, January 2013. [Google Scholar]
- Lesouhaitier, O.; Clamens, T.; Rosay, T.; Desriac, F.; Louis, M.; Rodrigues, S.; Gannesen, A.V.; Plakunov, V.K.; Bouffartigues, E.; Tahrioui, A.; et al. Host Peptidic Hormones Affecting Bacterial Biofilm Formation and Virulence. J. Innate Immun. 2019, 11, 227–241. [Google Scholar] [CrossRef]
- N’Diaye, A.; Mijouin, L.; Hillion, M.; Diaz, S.; Konto-Ghiorghi, Y.; Percoco, G.; Chevalier, S.; Lefeuvre, L.; Harmer, N.J.; Lesouhaitier, O.; et al. Effect of Substance P in Staphylococcus aureus and Staphylococcus epidermidis virulence: Implication for skin homeostasis. Front. Microbiol. 2016, 7, 506. [Google Scholar] [CrossRef]
- N’Diaye, A.; Gannesen, A.V.; Borrel, V.; Maillot, O.; Enault, J.; Racine, P.J.; Plakunov, V.; Chevalier, S.; Lesouhaitier, O.; Feuilloley, M.G.J. Substance P and calcitonin gene-related peptide: Key regulators of cutaneous microbiota homeostasis. Front. Endocrinol. 2017, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Mijouin, L.; Hillion, M.; Ramdani, Y.; Jaouen, T.; Duclairoir-Poc, C.; Follet-Gueye, M.-L.; Lati, E.; Yvergnaux, F.; Driouich, A.; Lefeuvre, L.; et al. Effects of a Skin Neuropeptide (Substance P) on Cutaneous Microflora. PLoS ONE 2013, 8, e78773. [Google Scholar] [CrossRef]
- Brandwein, M.; Steinberg, D.; Meshner, S. Microbial biofilms and the human skin microbiome. Biofilms Microbiomes 2016, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Holmberg, A.; Lood, R.; Mörgelin, M.; Söderquist, B.; Holst, E.; Collin, M.; Christensson, B.; Rasmussen, M. Biofilm formation by Propionibacterium acnes is a characteristic of invasive isolates. Clin. Microbiol. Infect. 2009, 15, 787–795. [Google Scholar] [CrossRef] [Green Version]
- Mekni, M.A.; Bouchami, O.; Achour, W.; Ben Hassen, A. Strong biofilm production but not adhesion virulence factors can discriminate between invasive and commensal Staphylococcus epidermidis strains. APMIS 2012, 120, 605–611. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus epidermidis—The “accidental” pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Enault, J.; Saguet, T.; Yvergnaux, F.; Feuilloley, M.G.J. PS291®, a rhamnose-rich polysaccharide obtained by fermentation, is reducing Propionibacterium acnes adhesion and biofilm formation activity. In Proceedings of the International Federation of Societies Cosmetic Chemists, Paris, France, 27–30 October 2014; pp. 2801–2810. [Google Scholar]
- Holland, C.; Mak, T.N.; Zimny-Arndt, U.; Schmid, M.; Meyer, T.F.; Jungblut, P.R.; Brüggemann, H. Proteomic identification of secreted proteins of Propionibacterium acnes. BMC Microbiol. 2010, 10, 230. [Google Scholar] [CrossRef] [Green Version]
- Beylot, C.; Auffret, N.; Poli, F.; Claudel, J.P.; Leccia, M.T.; Del Giudice, P.; Dréno, B. Propionibacterium acnes: An update on its role in the pathogenesis of acne. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 271–278. [Google Scholar] [CrossRef]
- Lloyd, D.H.; Viac, J.; Werling, D.; Rème, C.A.; Gatto, H. Role of sugars in surface microbe-host interactions and immune reaction modulation. Vet. Dermatol. 2007, 18, 197–204. [Google Scholar] [CrossRef]
- Gadenne, V.; Lebrun, L.; Jouenne, T.; Thebault, P. Role of molecular properties of ulvans on their ability to elaborate antiadhesive surfaces. J. Biomed. Mater. Res. 2015, 103, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Bodin, J.; Adrien, A.; Bodet, P.-E.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Ulva intestinalis Protein Extracts Promote In Vitro Collagen and Hyaluronic Acid Production by Human Dermal Fibroblasts. Molecules 2020, 25, 2091. [Google Scholar] [CrossRef] [PubMed]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersboll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef] [Green Version]


| Fraction Name | Description | |
|---|---|---|
| Polysaccharide fractions | UE | Fraction rich in crude ulvans |
| DS-UE | Dialyzed fraction from crude ulvans | |
| Oligosaccharide fractions | DEP-HD PP-UE | Depolymerized fraction rich in ulvans obtained by radical H2O2 hydrolysis |
| DEP-AD PP-UE | Depolymerized fraction rich in ulvans obtained by acid resin hydrolysis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fournière, M.; Bedoux, G.; Souak, D.; Bourgougnon, N.; Feuilloley, M.G.J.; Latire, T. Effects of Ulva sp. Extracts on the Growth, Biofilm Production, and Virulence of Skin Bacteria Microbiota: Staphylococcus aureus, Staphylococcus epidermidis, and Cutibacterium acnes Strains. Molecules 2021, 26, 4763. https://doi.org/10.3390/molecules26164763
Fournière M, Bedoux G, Souak D, Bourgougnon N, Feuilloley MGJ, Latire T. Effects of Ulva sp. Extracts on the Growth, Biofilm Production, and Virulence of Skin Bacteria Microbiota: Staphylococcus aureus, Staphylococcus epidermidis, and Cutibacterium acnes Strains. Molecules. 2021; 26(16):4763. https://doi.org/10.3390/molecules26164763
Chicago/Turabian StyleFournière, Mathilde, Gilles Bedoux, Djouhar Souak, Nathalie Bourgougnon, Marc G. J. Feuilloley, and Thomas Latire. 2021. "Effects of Ulva sp. Extracts on the Growth, Biofilm Production, and Virulence of Skin Bacteria Microbiota: Staphylococcus aureus, Staphylococcus epidermidis, and Cutibacterium acnes Strains" Molecules 26, no. 16: 4763. https://doi.org/10.3390/molecules26164763
APA StyleFournière, M., Bedoux, G., Souak, D., Bourgougnon, N., Feuilloley, M. G. J., & Latire, T. (2021). Effects of Ulva sp. Extracts on the Growth, Biofilm Production, and Virulence of Skin Bacteria Microbiota: Staphylococcus aureus, Staphylococcus epidermidis, and Cutibacterium acnes Strains. Molecules, 26(16), 4763. https://doi.org/10.3390/molecules26164763







