Abstract
Diketopyrrolo[3,4-c]pyrroles (DPP) are high-performance organic optoelectronic materials. They have applications in solar cells, fluorescent probes, bioimaging, photodynamic/photothermal therapy, and in many other areas. This article reports a convenient two-step synthesis of various DPP dyes from Pigment Red 254, an inexpensive commercial pigment. The synthesis includes a Suzuki–Miyaura cross-coupling reaction of a bis(4-chlorophenyl)DPP derivative with aryl and hetaryl boronic acids under mild reaction conditions. The new dyes show large Stokes shifts and high fluorescence quantum yields, important features for their potential use in technical and biological applications.
1. Introduction
3,6-Diaryl-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-diones (also known as diketopyrrolopyrroles, DPP) are a class of brilliant and strongly fluorescent pigments and dyes. These high-performance compounds gained wide attention in recent years due their outstanding properties, namely large extinction coefficients, high fluorescent quantum yields, and environment and heat stability, which make them excellent candidates for a range of technical and biological applications [1]. In fact, DPP derivatives have applications in materials technology, from paint pigments to dye-sensitized solar cells (DSSC) [2,3,4,5,6], organic solar cells [7,8,9,10,11,12,13], organic electronics [14,15,16,17], fluorescent probes [18,19,20,21,22,23], materials for lithium-ion batteries [24], for ionic charge storage [25], or for the removal of micropollutants from water [26], etc. Besides that, the studies concerning their potential use in biological applications, namely as antibacterial agents [27,28,29], in photodynamic/photothermal therapy [30,31,32,33,34,35,36,37,38], or bioimaging/theranostics [39,40,41,42,43], have increased in recent years.
The DPP bicyclic system is usually generated from the reaction of aryl nitriles with diethyl (or diisopropyl) succinate in the presence of a strong base, and frequently the expected symmetrical DPP derivatives are obtained in low to moderate yields [44]. Other approaches, developed for the synthesis of non-symmetrical DPP derivatives, require precursors not easily available or long synthetic routes [1,45,46,47]. An attractive approach to new DPPs involves the modification of adequately functionalized DPP derivatives. The Suzuki–Miyaura, Stille, Sonogashira, and Heck coupling reactions using bromophenyl, bromothienyl, and bromofuryl DPP derivatives have been extensively used for the preparation of small DPP molecules and polymeric DPP-based materials [16,17,26,48,49,50,51,52,53]. Although these are very versatile and efficient reactions, again, the required brominated derivatives are expensive or not easily accessible. In this paper we report a simple procedure for the synthesis of DPP derivatives bearing functional groups adequate for further transformations starting from an inexpensive commercial DPP pigment.
2. Results and Discussion
2.1. Design and Synthesis
Pigment Red 254 (also known as Ferrari red) is an inexpensive commercial DPP pigment. However, it has scarcely been used as precursor to other DPP derivatives and the reported transformations are mainly N-alkylations [54,55,56,57]. Although it contains two 4-chlorophenyl groups that can be used to get access to other DPP derivatives by direct substitution of the chlorine atoms, this approach has been rarely used [28,58,59,60]. Here we report that this pigment can be successfully converted into other DPP derivatives, with adequate functional groups for further transformations, by Suzuki–Miyaura cross-coupling reaction with aryl and hetaryl boronic acids. This method is an excellent alternative to the previously reported one that requires the synthesis of the corresponding diboronate followed by reaction with iodoarenes, with both steps requiring Pd catalysis [28,60]. This new route involves only one Pd catalyzed step, requires mild conditions, and provides the new compounds in higher yields.
The synthetic procedures to obtain the DPP derivatives are summarized in Scheme 1. The first step involved the N,N’-dialkylation of Pigment Red 254 with 1-iodopentane as previously reported [28]. This step is essential to convert the pigment (insoluble) into a dye (soluble), thus facilitating the following reactions and the purification process of the resulting products. Dye 1, which is soluble in most common organic solvents, was then used in Suzuki–Miyaura cross-coupling reactions with various boronic acid derivatives affording dyes 2a–g in moderate to excellent yields (42–96%). This method is compatible with the presence of a diversity of functional groups, namely formyl, acetyl, amino, hydroxy, vinyl, and others not shown here, that may be used for further transformations as already shown by us [28]. Comparing the yield for compound 2a by the previous method (42% overall yield from 1) [28] with the one reported here (80% yield), it is evident that this new approach affords the expected DPP derivatives in much higher yields.

Scheme 1.
Synthesis of DPP dyes 2a–g.
2.2. Structural and Photophysical Characterization
The structures of dyes 2a–g were unambiguously established from their 1H and 13C NMR and mass spectra. Their absorption and emission spectra were also obtained (see Supplementary Materials). As expected, the 1H NMR spectra of all compounds show the resonance of the N-CH2 protons as a triplet at ca. 3.8 ppm and the signals of the remaining protons of pentyl groups between 0.75–1.75 ppm. The signals of the p-substituted phenyl rings linked to the DPP core appear as AB systems centered at ca. 7.9 ppm, and the signals of the protons of the peripheric aryl/hetaryl groups appear at slightly higher fields (between 6.75 and 7.75 ppm). In addition, for compounds 2a, 2b, and 2f, the 1H NMR spectra show the diagnostic signals corresponding to the formyl and acetyl groups at 10.08, 2.66, and 9.71 ppm, respectively. The 13C NMR spectra of compounds 2a–g are also consistent with the proposed structures, showing all the expected peaks. The mass spectra of compounds 2a–g show, in all cases, the protonated molecular ion [M + H]+ as the base peak.
The UV–Vis and fluorescence spectra of compounds 2a–g in DMF are shown in Figure 1 and their photophysical properties are summarized in Table 1. Compared with DPP 1 (λmax = 476 nm), the λmax for all dyes are bathochromically shifted, with the largest shift observed for the furan derivative 2f. Stokes shifts for dyes 2 were typically in the range of 68 and 73 nm, as observed for other 3,6-bis(biphenyl)DPP derivatives [49]. However, due to the presence of strong electron withdrawing and donating groups, compounds 2a and 2c show Stokes shifts of 83 and 54 nm, respectively. The fluorescence quantum yield (ØF) for each dye is also shown in Table 1. Considering those values, dyes 2 can be divided into two groups: those with electron withdrawing groups or heterocyclic rings (2a, 2b, 2f, and 2g) have ØF in the range 0.8–0.9, while compounds 2c, 2d, and 2e, bearing electron donating groups, have ØF near 0.4.
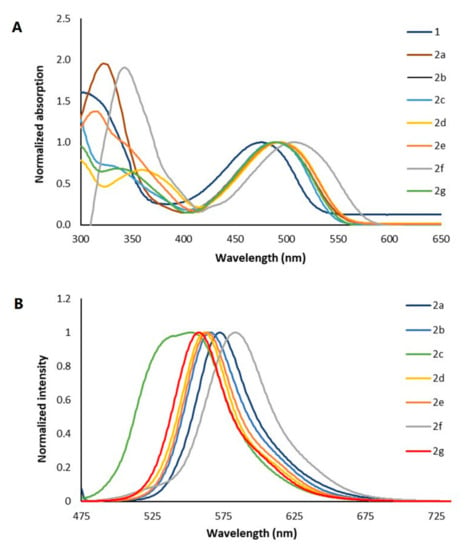
Figure 1.
Normalized (A) absorption and (B) fluorescence spectra of 2a–g in DMF.

Table 1.
Spectroscopic data for compounds 2a–g a.
As highlighted in the introduction, the use of DPP derivatives in a large diversity of applications is being actively studied. In this context, considering the fluorescence properties displayed by dyes 2, they, or their adequately functionalized derivatives, are potentially useful compounds as molecular probes for bioimaging/theranostics.
3. Materials and Methods
3.1. Chemicals and Instrumentation
The reagents used in this work were purchased from Merck Life Science (Algés, Portugal) and were used as received. The solvents were used as received or distilled and dried by standard procedures. Analytical thin-layer chromatography (TLC) was carried out on precoated sheets with silica gel (Merck 60, 0.2 mm thick). Preparative thin-layer chromatography was carried out on 20 cm × 20 cm glass plates precoated with a layer of silica gel 60 (0.5 mm thick) and activated in an oven at 100 ℃ for 12 h. 1H and 13C NMR spectra were recorded on a Bruker Avance 300 or Bruker Avance 500. CDCl3 was used as a solvent and tetramethylsilane (TMS) as an internal reference. The chemical shifts are expressed in δ (ppm) and the coupling constants (J) in hertz (Hz). UV–Vis spectra were recorded on a Shimadzu UV-2501PC spectrophotometer using DMF as the solvent. The emission spectra were recorded with a Jasco FP-8300 spectrofluorometer using DMF as the solvent. Mass spectra were recorded using a Micromass Q-TOF-2TM mass spectrometer and CHCl3 as the solvent. Fluorescence quantum yields (ØF) were calculated using fluorescein as a reference (ØF = 0.55 in DMF) [61]. Melting points were determined with a Büchi B-540 apparatus.
3.2. Synthesis
General Procedure for the Suzuki–Miyaura Cross-Coupling Reactions
To a suspension of K3PO4 (4 equiv.) in degassed THF (20.0 mL), DPP 1 (80 mg, 0.161 mmol), the corresponding boronic acid (0.644 mmol, 4 equiv.) and catalytic amounts of Pd2(Pdba)3 (5 mol%) and SPhos (10 mol%) were added. The resulting mixture was refluxed overnight under a nitrogen atmosphere. It was then cooled down to room temperature and the solvent was removed under reduced pressure. The products were purified by preparative TLC using mixtures of dichloromethane/hexane or ethyl acetate/hexane as eluents. For the synthesis of 2d, Pd(OAc)2 was used as a catalyst and butan-1-ol as a solvent.
4′,4‴-(3,6-Dioxo-2,5-dipentyl-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrole-1,4-diyl)bis([1,1′-biphenyl]-4-carbaldehyde) (2a): 82 mg, 80% yield; mp: 177.4–179.2 ℃; 1H NMR (300 Hz, CDCl3) δ (ppm), 10.08 (s, 2H), 8.01–7.94 (m, 8H), 7.83–7.79 (m, 8H), 3.81 (t, J = 7.6 Hz, 4H), 1.70–1.62 (m, 4H), 1.28–1.22 (m, 8H), 0.83 (t, J = 6.7 Hz, 6H); 13C NMR (CDCl3, 75 MHz) δ (ppm) 191.8, 162.8, 147.9, 145.8, 142.3, 135.8, 130.5, 129.4, 128.2, 127.9, 127.8, 110.2, 42.1, 29.3, 28.9, 22.2, 13.9; MS (ESI+) m/z: 637.4 ([M + H]+, 100%).
3,6-Bis(4′-acetyl-[1,1′-biphenyl]-4-yl)-2,5-dipentyl-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (2b): 82 mg, 77% yield; mp: 279.8–280.4 ℃; 1H NMR (CDCl3, 300 MHz), δ (ppm): 8.08 (d, J = 8 Hz, 4H), 7.97 (d, J = 9 Hz, 4H), 7.83–7.75 (m, 8H) 3.82 (t, J = 7.5 Hz, 4H), 2.66 (s, 6H), 1.69–1.64 (m, 4H), 1.30–1.25 (m, 8H), 0.85 (t, J = 7 Hz, 6H); 13C NMR (CDCl3, 75 MHz) δ (ppm) 197.7, 162.8, 147.9, 144.4, 142.5, 136.5, 129.1, 129.0, 127.9, 127.5, 110.2, 42.1, 29.3, 28.9, 26.8, 22.2, 13.9; MS (ESI+) m/z: 665.6 ([M + H]+, 100%).
3,6-Bis(3′-amino-[1,1′-biphenyl]-4-yl)-2,5-dipentyl-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (2c): 41 mg, 42% yield; mp: 233.5–235.1 ℃; 1H NMR (CDCl3, 300 MHz), δ (ppm): 7.90 (d, J = 8.6 Hz, 4H), 7.72 (d, J = 8.6 Hz, 4H), 7.26 (t, J = 8 Hz, 2H), 7.04 (ddd, J = 8, 2 and 1 Hz, 2H), 6.95 (t, J = 2 Hz, 2H), 6.72 (ddd, J = 8, 2, and 1 Hz, 2H), 3.80 (t, J = 7.5 Hz, 4H), 1.70–1.63 (m, 4H), 1.29–1.24 (m, 8H), 0.83 (t, J = 7.0 Hz, 6H); 13C NMR (CDCl3 + (CD3)2CO, 75 MHz), δ (ppm): 162.6, 149.4, 141.1, 130.2, 129.7, 127.5, 116.2, 115.1, 113.4, 41.8, 29.3, 29.1, 22.3, 13.8; MS (ESI+) m/z: 611.4 ([M + H]+, 100%).
3,6-Bis(4′-hydroxy-[1,1′-biphenyl]-4-yl)-2,5-dipentyl-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (2d): (61 mg, 62% yield); mp: 316.7–317.2 ℃ (crystalized from CH2Cl2/MeOH); 1H NMR (DMSO, 300 MHz), δ (ppm): 9.81 (s, 2H), 7.92 (d, J = 8.5 Hz, 4H), 7.83 (d, J = 8.5 Hz, 4H), 7.65 (d, J = 8.6 Hz, 4H), 6.9 (d, J = 8.6 Hz, 2H), 3.77 (t, J = 7.5 Hz, 4H), 1.51–1.43 (m, 4H), 1.20–1.13 (m, 8H), 0.78 (t, J = 7 Hz, 6H); 13C NMR (DMSO, 75 MHz), δ (ppm): 162.1, 158.5, 147.9, 143.1, 129.9, 129.7, 128.5, 126.5, 126.1, 116.4, 109.1, 41.4, 28.8, 28.7, 21.9, 14.2; MS (ESI+) m/z: 643.5 ([M + H]+, 100%).
2,5-Dipentyl-3,6-bis(4′-vinyl-[1,1′-biphenyl]-4-yl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (2e): 98 mg, 96% yield; mp: 233.4–235.1 ℃; 1H NMR (CDCl3, 300 MHz), δ (ppm): 7.93 (d, J = 9 Hz, 4H), 7.78 (d, J = 9 Hz, 4H), 7.64 (d, J = 9 Hz, 4H), 7.53 (d, J = 9 Hz, 4H), 6.83–6.73 (m, 2H), 5.83 (d, J = 12 Hz, 2H), 5.32 (d, J = 12 Hz, 2H), 3.82 (t, J = 7.5 Hz, 4H), 1.72–1.63 (m, 4H), 1.31–1.25 (m, 8H), 0.85 (t, J = 7 Hz, 6H); 13C NMR (CDCl3, 75 MHz), δ (ppm): 162.9, 148.1, 143.2, 139.3, 137.4, 136.3, 129.3, 127.3, 127.1, 126.9, 114.5, 109.9, 42.1, 29.3, 28.9, 22.2, 13.9; MS (ESI+) m/z: 633.6 ([M + H]+, 100%).
5,5′-((3,6-Dioxo-2,5-dipentyl-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrole-1,4-diyl)bis(4,1-phenylene))bis(furan-2-carbaldehyde) (2f): 63 mg, 63% yield; mp: 233.4–235.1 ℃. 1H NMR (CDCl3, 300 MHz), δ (ppm): 9.71 (s, 2H), 8.0 (d, J = 9 Hz, 4H), 7.95 (d, J = 9 Hz, 4H), 7.36 (d, J = 3 Hz, 2H), 6.98 (d, J = 6 Hz, 2H), 3.79 (t, J = 7.5 Hz, 4H), 1.66–1.61 (m, 4H), 1.28–1.24 (m, 8H), 0.85 (t, J = 6.9 Hz, 6H); 13C NMR (CDCl3, 75 MHz), δ (ppm): 177.5, 162.6, 158.0, 152.5, 147.6, 141.2, 131.2, 129.3, 128.9, 125.6, 109.3, 42.2, 29.7, 28.9, 22.2, 13.9; MS (ESI+) m/z: 617.5 ([M + H]+, 100%).
2,5-Dipentyl-3,6-bis(4-(thiophen-2-yl)phenyl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (2g): 70 mg, 73% yield; mp: 170.3–172.1 ℃. 1H NMR (CDCl3, 300 MHz), δ (ppm): 7.90 (d, J = 9 Hz, 4H), 7.77 (d, J = 9 Hz, 4H), 7.58–7.60 (m, 2H), 7.47–7.42 (m, 4H), 3.81 (t, J = 7.5 Hz, 4H), 1.69–1.61 (m, 4H), 1.30–1.24 (m, 8H), 0.85 (t, J = 7.5 Hz, 6H); 13C NMR (CDCl3, 75 MHz), δ (ppm): 162.9, 147.9, 141.3, 138.4, 137.2, 129.9, 126.8, 126.1, 121.8, 109.9, 42.1, 29.3, 28.9, 22.2, 13.9; MS (ESI+) m/z: 593.4 ([M + H]+, 100%).
4. Conclusions
Pigment Red 254 was used as starting material to prepare, in two steps only, adequately functionalized DPP derivatives. The resulting compounds bear a range of functional groups that may be used for further transformations, namely for the introduction of functional units with specific physical/electronic properties or biological functions. The seven compounds reported here show large Stokes shifts and high fluorescence quantum yields, important features for their potential application in various fields.
Supplementary Materials
The following are available online: 1H and 13C NMR spectra, and absorption and emission spectra.
Author Contributions
Conceptualization, A.C.T.; synthesis and structural characterization, V.A.S.A. Both authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação para a Ciência e a Tecnologia (FCT), through project PTDC/QEQ-QOR/6160/2014 and the LAQV-REQUIMTE (UIDB/50006/2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in supplementary material.
Acknowledgments
Thanks are due to the University of Aveiro and Fundação para a Ciência e a Tecnologia (FCT) for the financial support to project PTDC/QEQ-QOR/6160/2014 and the LAQV-REQUIMTE (UIDB/50006/2020) through national funds and, where applicable, co-financed by FEDER, within the PT2020 Partnership Agreement, and to the Portuguese NMR Network. Vítor A. S. Almodovar thanks FCT for his doctoral grant (SFRH/BD/135598/2018).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of compounds 2a–g are available from the authors.
References
- Grzybowski, M.; Gryko, D.T. Diketopyrrolopyrroles: Synthesis, Reactivity, and Optical Properties. Adv. Opt. Mater. 2015, 3, 280–320. [Google Scholar] [CrossRef]
- Yum, J.-H.; Holcombe, T.W.; Kim, Y.; Rakstys, K.; Moehl, T.; Teuscher, J.; Delcamp, J.H.; Nazeeruddin, M.K.; Grätzel, M. Blue-Coloured Highly Efficient Dye-Sensitized Solar Cells by Implementing the Diketopyrrolopyrrole Chromophore. Sci. Rep. 2013, 3, 2446. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.H.; Jiang, K.J.; Zhang, F.; Wu, W.; Li, S.G.; Yang, L.M.; Song, Y.L. Engineering Diketopyrrolopyrrole Sensitizers for Highly Efficient Dye-sensitized Solar Cells: Enhanced Light Harvesting and Intramolecular Charge Transfer. RSC Adv. 2014, 4, 16906–16912. [Google Scholar] [CrossRef]
- Ganesan, P.; Yella, A.; Holcombe, T.W.; Gao, P.; Rajalingam, R.; Al-Muhtaseb, S.A.; Grätzel, M.; Nazeeruddin, M.K. Unravel the Impact of Anchoring Groups on the Photovoltaic Performances of Diketopyrrolopyrrole Sensitizers for Dye-Sensitized Solar Cells. ACS Sustain. Chem. Eng. 2015, 3, 2389–2396. [Google Scholar] [CrossRef]
- Lim, D.; Choi, K.; Hayati, D.; Park, D.-H.; Ghifari, A.; Lee, K.M.; Ko, Y.; Jun, Y.; Suk, H.-J.; Hong, J. Blue-Colored Dyes Featuring a Diketopyrrolopyrrole Spacer for Translucent Dye-Sensitized Solar Cells. Dye. Pigment. 2020, 173, 107840. [Google Scholar] [CrossRef]
- Heath-Apostolopoulos, I.; Vargas-Ortiz, D.; Wilbraham, L.; Jelfs, K.E.; Zwijnenburg, M.A. Using High-Throughput Virtual Screening to Explore the Optoelectronic Property Space of Organic Dyes; Finding Diketopyrrolopyrrole Dyes for Dye-Sensitized Water Splitting and Solar Cells. Sustain. Energy Fuels 2021, 5, 704–719. [Google Scholar] [CrossRef]
- Gao, K.; Li, L.; Lai, T.; Xiao, L.; Huang, Y.; Huang, F.; Peng, J.; Cao, Y.; Liu, F.; Russell, T.P.; et al. Deep Absorbing Porphyrin Small Molecule for High-Performance Organic Solar Cells with Very Low Energy Losses. J. Am. Chem. Soc. 2015, 137, 7282–7285. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.J.; Brendel, M.; Ruckdeschel, P.; Pflaum, J.; Thelakkat, M. Diketopyrrolopyrroles with a Distinct Energy Level Cascade for Efficient Charge Carrier Generation in Organic Solar Cells. Adv. Energy Mater. 2015, 5, 1500914. [Google Scholar] [CrossRef]
- Tang, A.; Zhan, C.; Yao, J.; Zhou, E. Design of Diketopyrrolopyrrole (DPP)-Based Small Molecules for Organic-Solar-Cell Applications. Adv. Mater. 2017, 29, 1600013. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, J.; Jiao, J.; Huang, L.; Tang, J. Recent Advances of Polymer Acceptors for High-Performance Organic Solar Cells. J. Mater. Chem. C 2020, 8, 28–43. [Google Scholar] [CrossRef]
- Gao, K.; Kan, Y.; Chen, X.; Liu, F.; Kan, B.; Nian, L.; Wan, X.; Chen, Y.; Peng, X.; Russell, T.P.; et al. Low-Bandgap Porphyrins for Highly Efficient Organic Solar Cells: Materials, Morphology, and Applications. Adv. Mater. 2020, 32, 1906129. [Google Scholar] [CrossRef]
- Privado, M.; Malhotra, P.; de la Cruz, P.; Singhal, R.; Cerdá, J.; Aragó, J.; Ortí, E.; Sharma, G.D.; Langa, F. Ternary Organic Solar Cell with a Near-Infrared Absorbing Selenophene–Diketopyrrolopyrrole-Based Nonfullerene Acceptor and an Efficiency above 10%. Sol. RRL 2020, 4, 1900471. [Google Scholar] [CrossRef]
- Piradi, V.; Xu, X.; Peng, Q.; Zhu, X. Diketopyrrolopyrrole Linked Porphyrin Dimers for Visible-near-infrared photoresponsive nonfullerene organic solar cells. Mater. Adv. 2020, 1, 2520–2525. [Google Scholar] [CrossRef]
- Liu, Q.; Bottle, S.E.; Sonar, P. Developments of Diketopyrrolopyrrole-Dye-Based Organic Semiconductors for a Wide Range of Applications in Electronics. Adv. Mater. 2020, 32, 1903882. [Google Scholar] [CrossRef]
- Liu, Q.; Kanahashi, K.; Matsuki, K.; Manzhos, S.; Feron, K.; Bottle, S.E.; Tanaka, K.; Nanseki, T.; Takenobu, T.; Tanaka, H.; et al. Triethylene Glycol Substituted Diketopyrrolopyrrole- and Isoindigo-Dye Based Donor–Acceptor Copolymers for Organic Light-Emitting Electrochemical Cells and Transistors. Adv. Electron. Mater. 2020, 6, 1901414. [Google Scholar] [CrossRef]
- Moser, M.; Savva, A.; Thorley, K.; Paulsen, B.D.; Hidalgo, T.C.; Ohayon, D.; Chen, H.; Giovannitti, A.; Marks, A.; Gasparini, N.; et al. Polaron Delocalization in Donor–Acceptor Polymers and its Impact on Organic Electrochemical Transistor Performance. Angew. Chemie 2021, 133, 7777–7785. [Google Scholar] [CrossRef]
- Fusco, S.; Barra, M.; Bonomo, M.; Cassinese, A.; Centore, R.; Chiarella, F.; Senneca, F.; Carella, A. Novel DPP Derivatives Functionalized with Auxiliary Electron-Acceptor Groups and Characterized by Narrow Bandgap and Ambipolar Charge Transport Properties. Dye. Pigment. 2021, 186, 109026. [Google Scholar] [CrossRef]
- Kaur, M.; Choi, D.H. Diketopyrrolopyrrole: Brilliant Red Pigment Dye-Based Fluorescent Probes and Their Applications. Chem. Soc. Rev. 2015, 44, 58–77. [Google Scholar] [CrossRef]
- Hang, Y.; Wang, J.; Jiang, T.; Lu, N.; Hua, J. Diketopyrrolopyrrole-Based Ratiometric/Turn-on Fluorescent Chemosensors for Citrate Detection in the Near-Infrared Region by an Aggregation-Induced Emission Mechanism. Anal. Chem. 2016, 88, 1696–1703. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Yang, Z.; Yan, Y.; Xie, X.; Qu, N.; Wang, Y.; Wang, C.; Hua, J. New Diketopyrrolopyrrole-Based Ratiometric Fluorescent Probe for Intracellular Esterase Detection and Discrimination of Live and Dead Cells in Different Fluorescence Channels. ACS Appl. Mater. Interfaces 2018, 10, 31088–31095. [Google Scholar] [CrossRef]
- Veríssimo, M.I.S.; Almodôvar, V.A.S.; Tomé, A.C.; Gomes, M.T.S.R. Fluorescent Optrode for Proteins Based on a Diketopyrrolopyrrole Derivative: Practical Application to Total Protein Determination in Urine. Opt. Laser Technol. 2020, 130, 106364. [Google Scholar] [CrossRef]
- Praveen, V.K.; Vedhanarayanan, B.; Mal, A.; Mishra, R.K.; Ajayaghosh, A. Self-Assembled Extended π-Systems for Sensing and Security Applications. Acc. Chem. Res. 2020, 53, 496–507. [Google Scholar] [CrossRef]
- Auwalu, M.A.; Cheng, S. Diketopyrrolopyrrole Fluorescent Probes, Photophysical and Biological Applications. Chemosensors 2021, 9, 44. [Google Scholar] [CrossRef]
- Xu, Z.; Hou, S.; Zhu, Z.; Zhou, P.; Xue, L.; Lin, H.; Zhou, J.; Zhuo, S. Functional Thiophene-Diketopyrrolopyrrole-Based Polymer Derivatives as Organic Anode Materials for Lithium-Ion Batteries. Nanoscale 2021, 13, 2673–2684. [Google Scholar] [CrossRef]
- Samuel, J.J.; Karrothu, V.K.; Canjeevaram Balasubramanyam, R.K.; Mohapatra, A.A.; Gangadharappa, C.; Kankanallu, V.R.; Patil, S.; Aetukuri, N.P.B. Ionic Charge Storage in Diketopyrrolopyrrole-Based Redox-Active Conjugated Polymers. J. Phys. Chem. C 2021, 125, 4449–4457. [Google Scholar] [CrossRef]
- Wang, X.; Xie, L.; Lin, K.; Ma, W.; Zhao, T.; Ji, X.; Alyami, M.; Khashab, N.M.; Wang, H.; Sessler, J.L. Calix[4]pyrrole-Crosslinked Porous Polymeric Networks for the Removal of Micropollutants from Water. Angew. Chemie Int. Ed. 2021, 60, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, J.; Yu, M.; Jia, W.; Duan, S.; Cao, D.; Ding, X.; Yu, B.; Zhang, X.; Xu, F.-J. Molecular Sizes and Antibacterial Performance Relationships of Flexible Ionic Liquid Derivatives. J. Am. Chem. Soc. 2020, 142, 20257–20269. [Google Scholar] [CrossRef]
- Agazzi, M.L.; Almodovar, V.A.S.; Gsponer, N.S.; Bertolotti, S.; Tomé, A.C.; Durantini, E.N. Diketopyrrolopyrrole-Fullerene C60 Architectures as Highly Efficient Heavy Atom-Free Photosensitizers: Synthesis, Photophysical Properties and Photodynamic Activity. Org. Biomol. Chem. 2020, 18, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, Y.; Zhao, H.; Qi, R.; Chen, Z.; Yuan, H.; Liang, H.; Wang, L. Dual-Mode Antibacterial Conjugated Polymer Nanoparticles for Photothermal and Photodynamic Therapy. Macromol. Biosci. 2020, 20, 1900301. [Google Scholar] [CrossRef]
- Schmitt, J.; Heitz, V.; Sour, A.; Bolze, F.; Kessler, P.; Flamigni, L.; Ventura, B.; Bonnet, C.S.; Tõth, É. A Theranostic Agent Combining a Two-Photon-Absorbing Photosensitizer for Photodynamic Therapy and a Gadolinium(III) Complex for MRI Detection. Chem. A Eur. J. 2016, 22, 2775–2786. [Google Scholar] [CrossRef]
- Cai, Y.; Liang, P.; Tang, Q.; Yang, X.; Si, W.; Huang, W.; Zhang, Q.; Dong, X. Diketopyrrolopyrrole-Triphenylamine Organic Nanoparticles as Multifunctional Reagents for Photoacoustic Imaging-Guided Photodynamic/Photothermal Synergistic Tumor Therapy. ACS Nano 2017, 11, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, X.; Tang, Y.; Zou, J.; Wang, P.; Zhang, Y.; Si, W.; Huang, W.; Dong, X. A Light-Induced Nitric Oxide ControlLable Release Nano-Platform Based on Diketopyrrolopyrrole Derivatives for pH-Responsive Photodynamic/Photothermal Synergistic Cancer Therapy. Chem. Sci. 2018, 9, 8103–8109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Xu, W.; Wang, J.; Liu, L.; Chu, Y.; Wang, Y.; Hu, Y.; Yi, T.; Hua, J. Diketopyrrolopyrrole-Based Multifunctional Ratiometric Fluorescent Probe and γ-Glutamyltranspeptidase-Triggered Activatable Photosensitizer for Tumor Therapy. J. Mater. Chem. C 2020, 8, 8183–8190. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, L.; Tang, H.; Cao, D.; Chen, W. Diketopyrrolopyrrole: An Emerging Phototherapy Agent in Fighting Cancer. Dye. Pigment. 2020, 181, 108599. [Google Scholar] [CrossRef]
- Wei, Z.; Zhu, B.; Cai, Y.; Yan, D.; Lou, Y.; Guo, Z.; Deng, P. Near-Infrared-Absorbing Diketopyrrolopyrrole-Based Semiconducting Polymer Nanoparticles for Photothermal Therapy. Part. Part. Syst. Charact. 2020, 37, 1900433. [Google Scholar] [CrossRef]
- Jenni, S.; Picci, G.; Fornasier, M.; Mamusa, M.; Schmidt, J.; Talmon, Y.; Sour, A.; Heitz, V.; Murgia, S.; Caltagirone, C. Multifunctional Cubic Liquid Crystalline Nanoparticles for Chemo- and Photodynamic Synergistic Cancer Therapy. Photochem. Photobiol. Sci. 2020, 19, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, F.; Chen, Q.; Zhou, E.; Zhang, P.; Cui, Z.; Liu, Z.; Huang, Y. Synergistic Non-Bonding Interactions based on Diketopyrrolo-pyrrole for Elevated Photoacoustic Imaging-guided Photothermal Therapy. Biomater. Sci. 2021, 9, 908–916. [Google Scholar] [CrossRef]
- Jin, X.; Xing, X.; Deng, Q.; Qing, W.; Liu, Z.; Huang, Y. Molecular Engineering of Diketopyrrolopyrrole-Conjugated Polymer Nanoparticles by Chalcogenide Variation for Photoacoustic Imaging Guided Photothermal Therapy. J. Mater. Chem. B 2021, 9, 3153–3160. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.; Tang, H.; Cao, D. Diketopyrrolopyrrole-based Fluorescent Probes for Detection and Bioimaging: Current Progresses and Perspectives. Dye. Pigment. 2019, 162, 934–950. [Google Scholar] [CrossRef]
- Chiminazzo, A.; Borsato, G.; Favero, A.; Fabbro, C.; McKenna, C.E.; Dalle Carbonare, L.G.; Valenti, M.T.; Fabris, F.; Scarso, A. Diketopyrrolopyrrole Bis-Phosphonate Conjugate: A New Fluorescent Probe for In Vitro Bone Imaging. Chem. A Eur. J. 2019, 25, 3617–3626. [Google Scholar] [CrossRef]
- Sun, W.; Tang, F.; Cui, J.-X.; Lu, Z.-L. Fluorescent Nanoparticles for Targeted Tumor Imaging and DNA Tracking Gene Delivery In Vitro / In Vivo. ACS Omega 2020, 5, 31700–31705. [Google Scholar] [CrossRef] [PubMed]
- Abelha, T.F.; Morris, G.; Lima, S.M.; Andrade, L.H.C.; McLean, A.J.; Alexander, C.; Calvo-Castro, J.; McHugh, C.J. Development of a Neutral Diketopyrrolopyrrole Phosphine Oxide for the Selective Bioimaging of Mitochondria at the Nanomolar Level. Chem. A Eur. J. 2020, 26, 3173–3180. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Xu, X.; Liu, J.; Lu, X.; Huang, W.; Fan, Q. Diketopyrrolopyrrole Derivatives-based NIR-II Fluorophores for Theranostics. Dye. Pigment. 2021, 193, 109480. [Google Scholar] [CrossRef]
- Potrawa, T.; Langhals, H. Fluoreszenzfarbstoffe mit großen Stokes-Shifts – lösliche Dihydropyrrolopyrroldione. Chem. Ber. 1987, 120, 1075–1078. [Google Scholar] [CrossRef] [Green Version]
- Pieczykolan, M.; Sadowski, B.; Gryko, D.T. An Efficient Method for the Programmed Synthesis of Multifunctional Diketopyrrolopyrroles. Angew. Chemie. 2020, 59, 7528–7535. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.D.; Banasiewicz, M.; Jacquemin, D.; Gryko, D.T. Switch-On Diketopyrrolopyrrole-Based Chemosensors for Cations Possessing Lewis Acid Character. Chem. Asian J. 2021, 16, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Bronstein, H. Synthesis of Fully Asymmetric Diketopyrrolopyrrole Derivatives. RSC Adv. 2021, 11, 5276–5283. [Google Scholar] [CrossRef]
- Qu, Y.; Hua, J.; Tian, H. Colorimetric and Ratiometric Red Fluorescent Chemosensor for Fluoride Ion Based on Diketopyrrolopyrrole. Org. Lett. 2010, 12, 3320–3323. [Google Scholar] [CrossRef] [PubMed]
- Beninatto, R.; Borsato, G.; De Lucchi, O.; Fabris, F.; Lucchini, V.; Zendri, E. New 3,6-Bis(biphenyl)diketopyrrolopyrrole Dyes and Pigments via Suzuki–Miyaura Coupling. Dye. Pigment. 2013, 96, 679–685. [Google Scholar] [CrossRef]
- Ftouni, H.; Bolze, F.; Nicoud, J.-F. Water-Soluble Diketopyrrolopyrrole Derivatives for Two-Photon Excited Fluorescence Microscopy. Dye. Pigment. 2013, 97, 77–83. [Google Scholar] [CrossRef]
- Papadopoulos, I.; Álvaro-Martins, M.J.; Molina, D.; McCosker, P.M.; Keller, P.A.; Clark, T.; Sastre-Santos, Á.; Guldi, D.M. Solvent-Dependent Singlet Fission in Diketopyrrolopyrrole Dimers: A Mediating Charge Transfer versus a Trapping Symmetry-Breaking Charge Separation. Adv. Energy Mater. 2020, 10, 2001496. [Google Scholar] [CrossRef]
- Popli, C.; Jang, Y.; Patil, Y.; Misra, R.; D’Souza, F. Formation of Highly Efficient, Long-Lived Charge Separated States in Star-Shaped Ferrocene-Diketopyrrolopyrrole-Triphenylamine Donor–Acceptor–Donor Conjugates. Chem. A Eur. J. 2020, 26, 15109–15115. [Google Scholar] [CrossRef]
- Neto, A.L.; Scalon, L.; Octavio de Araujo, L.; Lopes de Araújo, F.; Spada, E.R.; Pereira da Cunha, M.R.; Desordi, J.C.; Barreto, R.C.; Macedo, A.G.; Faria, R.M.; et al. New Insights into DPP3Th and C70 based Planar Solar Cells: A Study Combining DFT and Experimental Approach. Mater. Chem. Phys. 2021, 262, 124271. [Google Scholar] [CrossRef]
- Jung, H.-S.; Kim, Y.-J.; Ha, S.-W.; Lee, J.-K. White Light-Emitting Diodes Using Thermally and Photochemically Stable Fluorescent Silica Nanoparticles as Color-Converters. J. Mater. Chem. C 2013, 1, 5879. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Kim, J.-H.; Yoo, J.-S.; Bae, J.-H.; Yoon, C.; Choi, J.-H. Synthesis and Thermal Stability of Solvent Soluble Dyes Based on Dimerized Diketo-pyrrolo-pyrrole Pigment. Bull. Korean Chem. Soc. 2014, 35, 659–662. [Google Scholar] [CrossRef] [Green Version]
- Honda, A.; Tamaki, Y.; Miyamura, K. The Effects of Noncovalent Interactions on Surface Structures Formed by Diketopyrrolopyrrole Pigment and Its Alkyl-Derivatives on HOPG Substrate. Bull. Chem. Soc. Jpn. 2015, 88, 969–975. [Google Scholar] [CrossRef]
- Wang, K.; Feng, J.; Xu, J.; Li, J.; Mai, M.; Wang, X.; Wang, L. Engineering Aromatic Heterocycle Strategy: Improving Copper Electrodeposition Performance via Tuning the Bandgap of Diketopyrrolopyrrole-based Leveler. Tetrahedron 2020, 76, 130882. [Google Scholar] [CrossRef]
- Yamagata, T.; Kuwabara, J.; Kanbara, T. Synthesis of Highly Fluorescent Diketopyrrolopyrrole Derivative and Two-Step Response of Fluorescence to Acid. Tetrahedron Lett. 2010, 51, 1596–1599. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, J.; Yamagata, T.; Kanbara, T. Solid-state Structure and Optical Properties of Highly Fluorescent Diketopyrrolopyrrole Derivatives Synthesized by Cross-Coupling Reaction. Tetrahedron 2010, 66, 3736–3741. [Google Scholar] [CrossRef] [Green Version]
- Yamagata, T.; Kuwabara, J.; Kanbara, T. Synthesis and Characterization of Dioxopyrrolopyrrole Derivatives Having Electron-Withdrawing Groups. European J. Org. Chem. 2012, 2012, 5282–5290. [Google Scholar] [CrossRef]
- Hargreaves, J.S.; Webber, S.E. Photon Harvesting Polymers: Intracoil Energy Transfer in Anthryl- and Fluorescein-Tagged Polystyrene. Can. J. Chem. 1985, 63, 1320–1327. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).