Monitoring Site-Specific Fermentation Outcomes via Oxidation Reduction Potential and UV-Vis Spectroscopy to Characterize “Hidden” Parameters of Pinot Noir Wine Fermentations
Abstract
:1. Introduction
2. Results
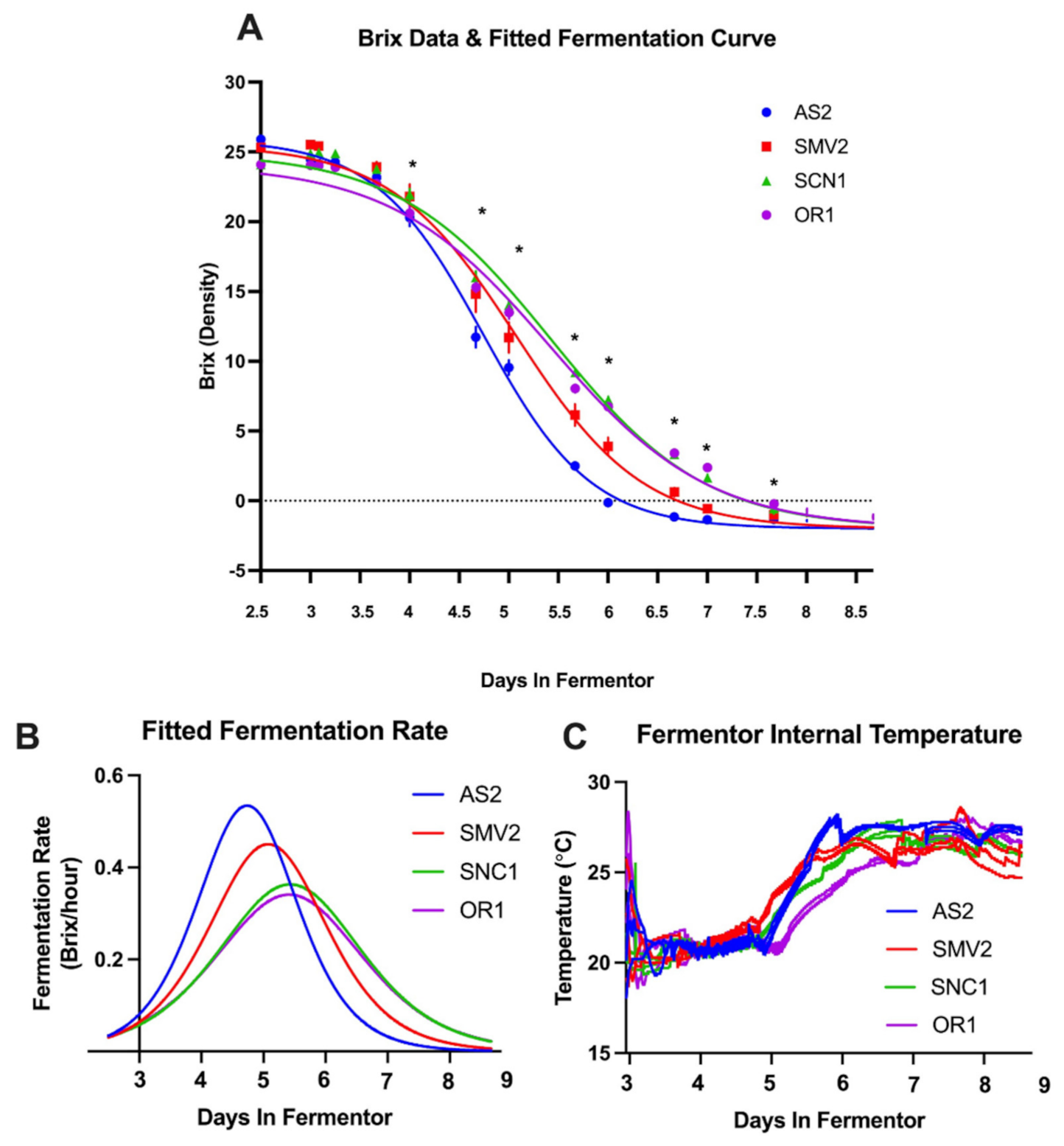
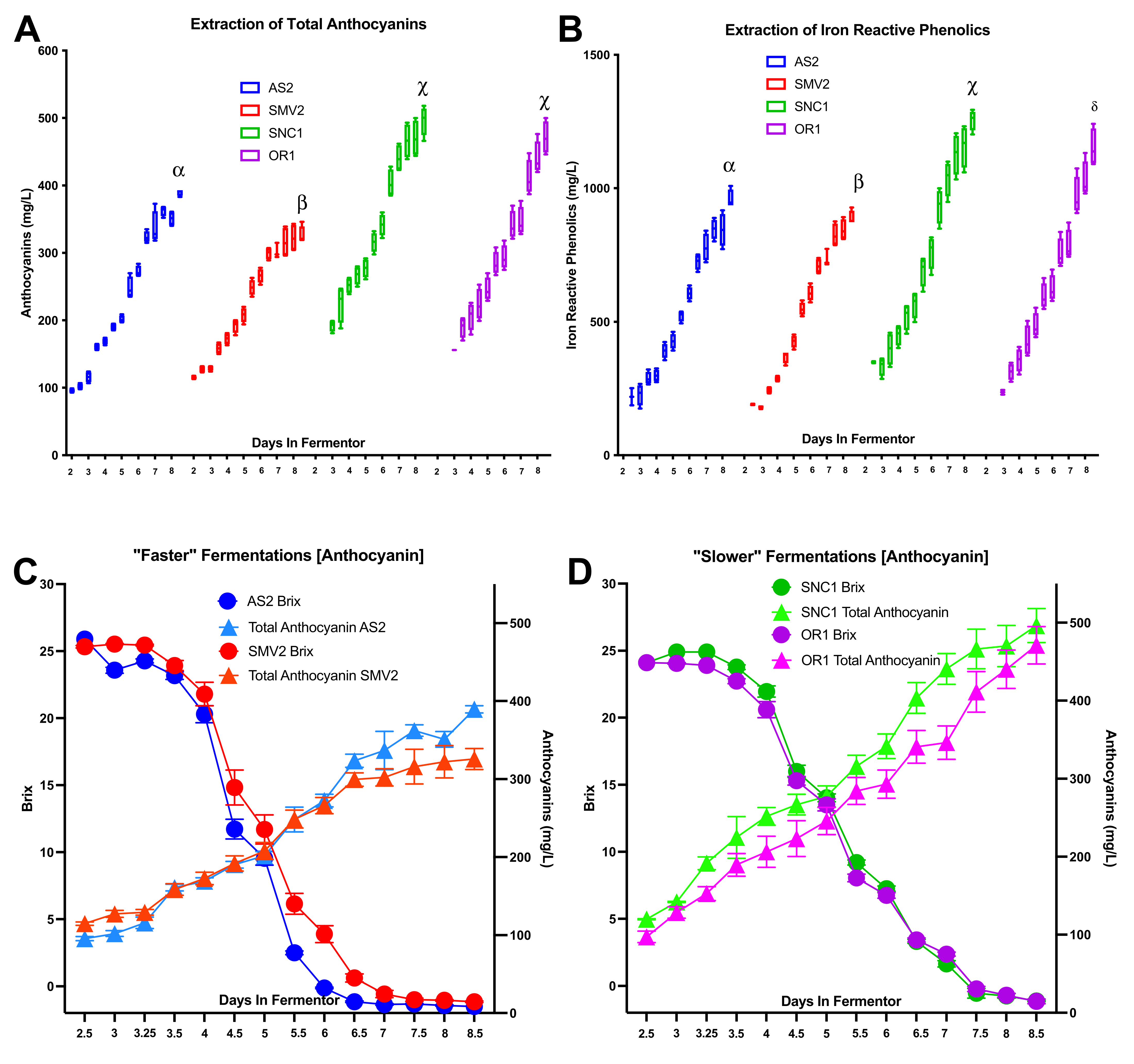
2.1. Grapes from Different Vineyard Sites Result in “Slower” or “Faster” Fermentations
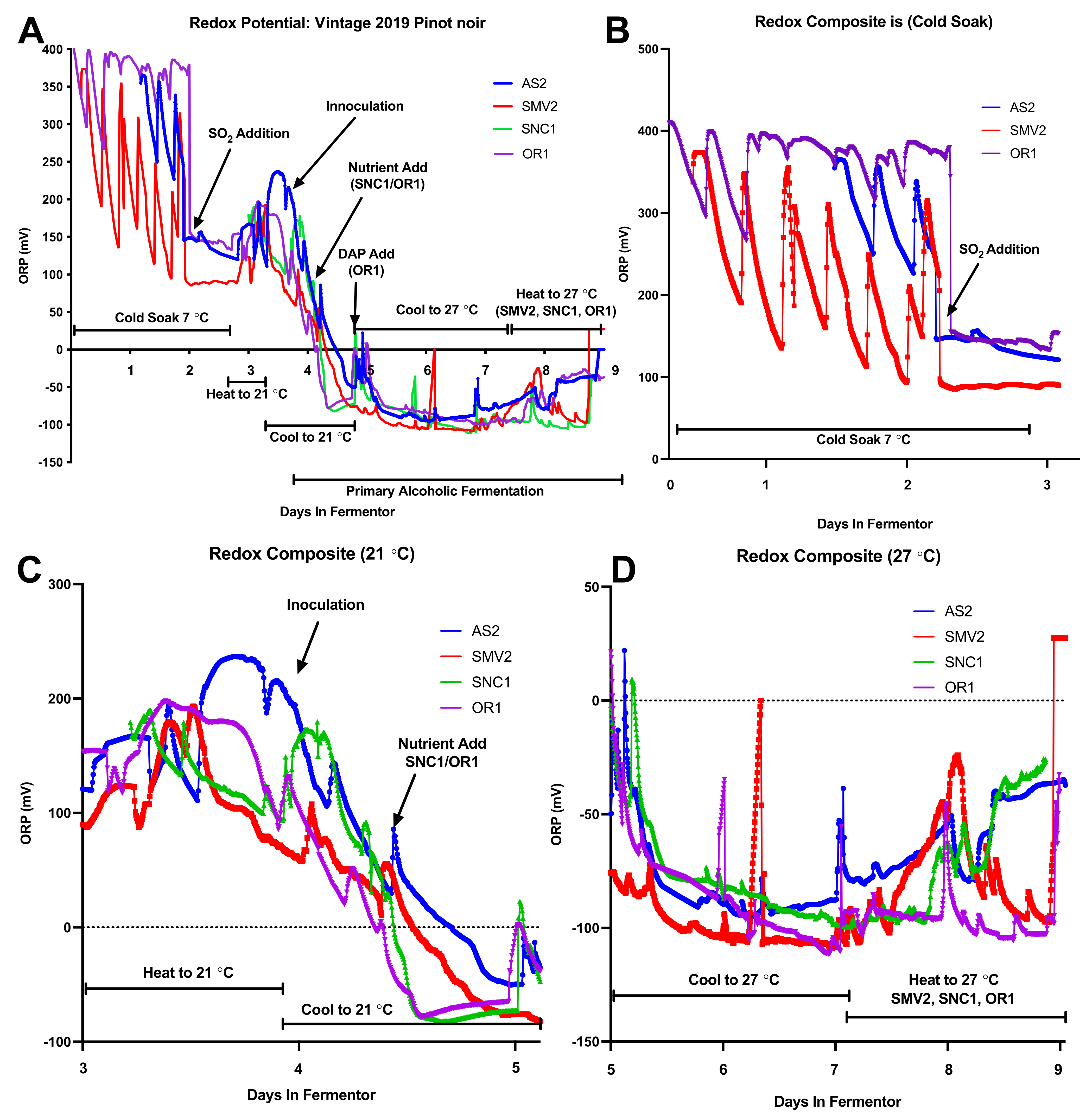
2.2. Composite Redox Potential during Fermentation
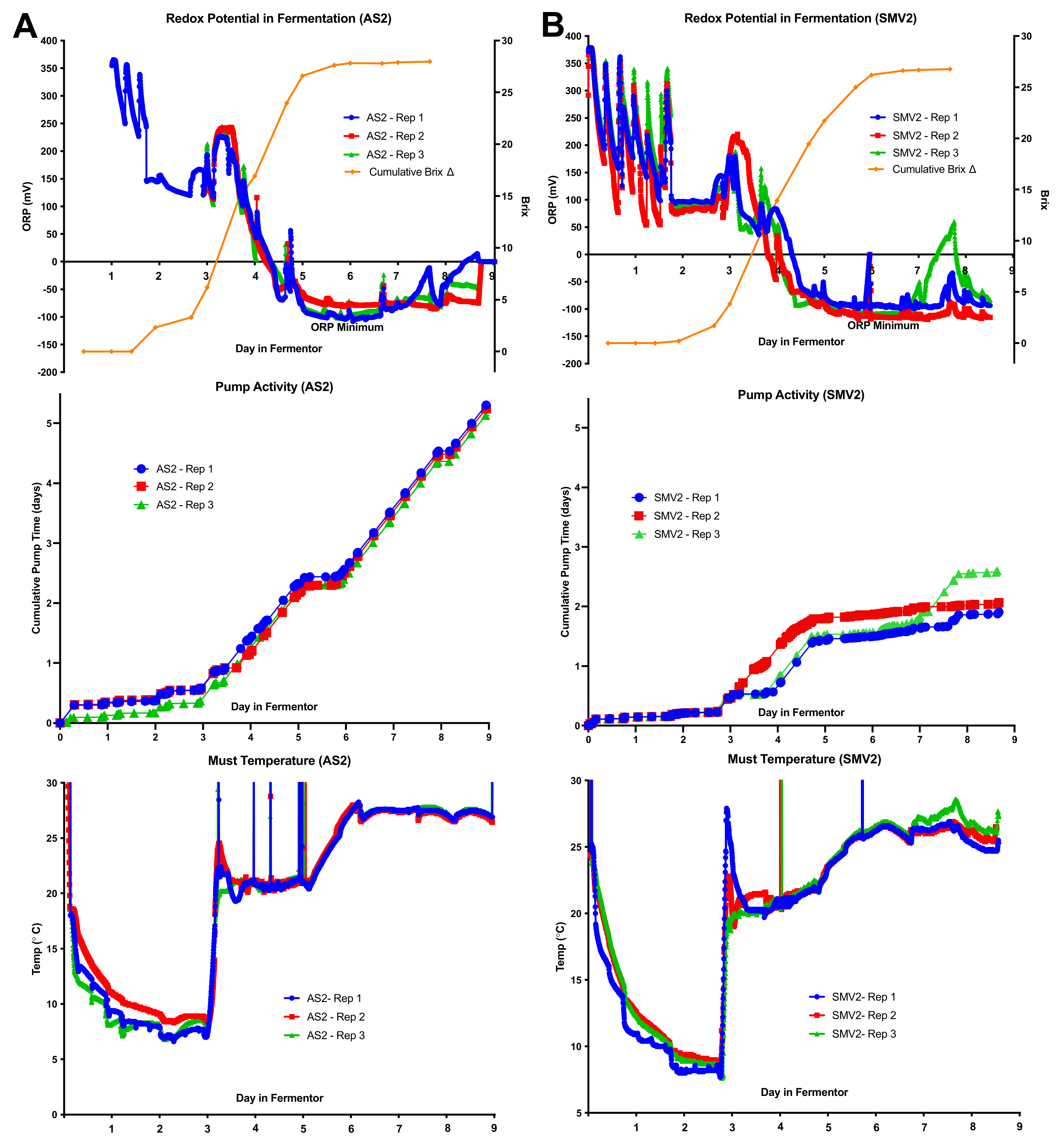
2.3. Individual Redox Potential Profiles and Pump Activity
2.4. Anthocyanin and Iron-Reactive Phenolics
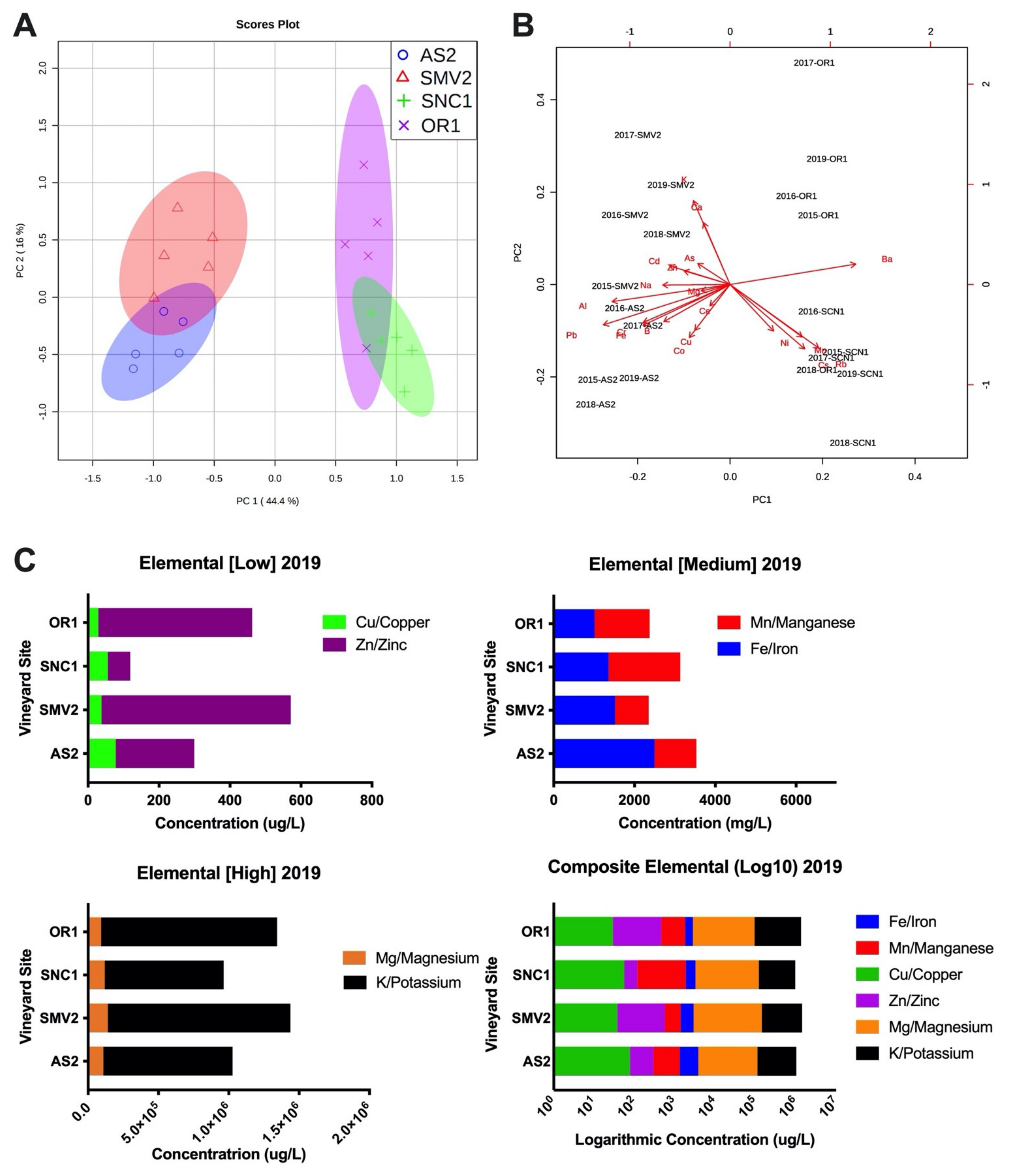
2.5. Elemental Composition and Metal Ion Concentration
3. Discussion
4. Materials and Methods
4.1. Vineyard Sites and Viticultural Practices
4.2. Fermentation and Inoculation Protocol
4.3. Initial Must and Final Wine Chemistry Analysis
4.4. Process Control and Probe Installation
4.5. Elemental Analysis of Must
4.6. Brix/Phenolic Sampling and Analysis by UV-Vis Spectroscopy
4.7. Figure Generation, Data Processing, Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Aspen Publishers: Gaithersburg, MD, USA, 1999; ISBN 978-0-8342-1270-1. [Google Scholar]
- Bisson, L.F.; Walker, G.A. 19—The microbial dynamics of wine fermentation. In Advances in Fermented Foods and Beverages; Holzapfel, W., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 435–476. ISBN 978-1-78242-015-6. [Google Scholar]
- Butzke, C.E. (Ed.) Yeast fermentation in wine. In Winemaking Problems Solved; Elsevier: Amsterdam, The Netherlands, 2010; pp. 52–76. ISBN 978-1-84569-475-3. [Google Scholar]
- Bisson, L.F.; Karpel, J.E. Genetics of yeast impacting wine quality. Annu. Rev. Food Sci. Technol. 2010, 1, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Lerno, L.; Reichwage, M.; Ponangi, R.; Hearne, L.; Block, D.E.; Oberholster, A. Effects of Cap and Overall Fermentation Temperature on Phenolic Extraction in Cabernet Sauvignon Fermentations. Am. J. Enol. Vitic. 2015, 66, 444–453. [Google Scholar] [CrossRef]
- Bisson, L.F. Stuck and sluggish fermentations. Am. J. Enol. Vitic. 1999, 50, 107–119. [Google Scholar]
- Kishida, N.; Kim, J.-H.; Chen, M.; Sasaki, H.; Sudo, R. Effectiveness of oxidation-reduction potential and pH as monitoring and control parameters for nitrogen removal in swine wastewater treatment by sequencing batch reactors. J. Biosci. Bioeng. 2003, 96, 285–290. [Google Scholar] [CrossRef]
- Liu, C.-G.; Lin, Y.-H.; Bai, F.-W. Development of redox potential-controlled schemes for very-high-gravity ethanol fermentation. J. Biotechnol. 2011, 153, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Goncharuk, V.V.; Bagrii, V.A.; Mel’nik, L.A.; Chebotareva, R.D.; Bashtan, S.Y. The use of redox potential in water treatment processes. J. Water Chem. Technol. 2010, 32, 1–9. [Google Scholar] [CrossRef]
- Martin, F.; Cachon, R.; Pernin, K.; De Coninck, J.; Gervais, P.; Guichard, E.; Cayot, N. Effect of oxidoreduction potential on aroma biosynthesis by lactic acid bacteria in nonfat yogurt. J. Dairy Sci. 2011, 94, 614–622. [Google Scholar] [CrossRef]
- Reichart, O.; Szakmár, K.; Jozwiak, Á.; Felföldi, J.; Baranyai, L. Redox potential measurement as a rapid method for microbiological testing and its validation for coliform determination. Int. J. Food Microbiol. 2007, 114, 143–148. [Google Scholar] [CrossRef]
- Coleman, R.E. Kinetics of Oxygen Consumption in Solutions of Iron and Tartaric Acid. Ph.D. Thesis, University of California Davis, Davis, CA, USA, 2019. [Google Scholar]
- Killeen, D.J. Advance Monitoring and Control of Wine Fermentation. Master’s Thesis, University of California Davis, Davis, CA, USA, 2015. [Google Scholar]
- Coleman, R.E.; Boulton, R.B.; Stuchebrukhov, A.A. Kinetics of autoxidation of tartaric acid in presence of iron. J. Chem. Phys. 2020, 153, 064503. [Google Scholar] [CrossRef]
- Kjaergaard, L. The redox potential: Its use and control in biotechnology. In Advances in Biochemical Engineering; Springer: Berlin/Heidelberg, Germany, 1977; Volume 7, pp. 131–150. [Google Scholar]
- Danilewicz, J.C. Review of Oxidative Processes in Wine and Value of Reduction Potentials in Enology. Am. J. Enol. Vitic. 2012, 63, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Danilewicz, J.C. Fe(II):Fe(III) Ratio and Redox Status of White Wines. Am. J. Enol. Vitic. 2016, 67, 146–152. [Google Scholar] [CrossRef]
- Rankine, B.C. Nature, origin and prevention of hydrogen sulphide aroma in wines. J. Sci. Food Agric. 1963, 14, 79–91. [Google Scholar] [CrossRef]
- Kukec, A.; Berovic, M.; Wondra, M. The Role of On-line Redox Potential Measurement in Sauvignon blanc Fermentation. Food Technol. Biotechnol. 2002, 40, 49–55. [Google Scholar]
- Killeen, D.J.; Boulton, R.; Knoesen, A. Advanced Monitoring and Control of Redox Potential in Wine Fermentation. Am. J. Enol. Vitic. 2018, 69, 394–399. [Google Scholar] [CrossRef]
- Liu, C.-G.; Qin, J.-C.; Lin, Y.-H. Fermentation and Redox Potential. In Fermentation Processes; Jozala, A.F., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-2927-1. [Google Scholar]
- Schütz, M.; Kunkee, R.E. Formation of Hydrogen Sulfide from Elemental Sulfur During Fermentation by Wine Yeast. Am. J. Enol. Vitic. 1977, 28, 137–144. [Google Scholar]
- Sohn, H.-Y.; Murray, D.B.; Kuriyama, H. Ultradian oscillation of Saccharomyces cerevisiae during aerobic continuous culture: Hydrogen sulphide mediates population synchrony. Yeast 2000, 16, 1185–1190. [Google Scholar] [CrossRef]
- Casassa, L.F.; Harbertson, J.F. Extraction, Evolution, and Sensory Impact of Phenolic Compounds During Red Wine Maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef]
- Cauduro Girardello, R.; Cooper, M.L.; Lerno, L.A.; Brenneman, C.; Eridon, S.; Sokolowsky, M.; Heymann, H.; Oberholster, A. Impact of Grapevine Red Blotch Disease on Cabernet Sauvignon and Merlot Wine Composition and Sensory Attributes. Molecules 2020, 25, 3299. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.-T.; Machado, N.; Moutinho-Pereira, J. Grapevine abiotic stress assessment and search for sustainable adaptation strategies in Mediterranean-like climates. A review. Agron. Sustain. Dev. 2018, 38, 66. [Google Scholar] [CrossRef] [Green Version]
- Keller, M. Chapter 6—Developmental Physiology. In The Science of Grapevines; Keller, M., Ed.; Academic Press: San Diego, CA, USA, 2010; pp. 169–225. ISBN 978-0-12-374881-2. [Google Scholar]
- Costa, C.; Graça, A.; Fontes, N.; Teixeira, M.; Gerós, H.; Santos, J.A. The Interplay between Atmospheric Conditions and Grape Berry Quality Parameters in Portugal. Appl. Sci. 2020, 10, 4943. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Kennedy, J.A.; Adams, D.O. Tannin in Skins and Seeds of Cabernet Sauvignon, Syrah, and Pinot noir Berries during Ripening. Am. J. Enol. Vitic. 2002, 53, 54. [Google Scholar]
- Skogerson, K.; Downey, M.; Mazza, M.; Boulton, R. Rapid Determination of Phenolic Components in Red Wines from UV-Visible Spectra and the Method of Partial Least Squares. Am. J. Enol. Vitic. 2007, 58, 318. [Google Scholar]
- LLC, W. Winexray. Available online: https://www.winexray.com/how-we-do-it (accessed on 3 February 2021).
- Hopfer, H.; Nelson, J.; Collins, T.S.; Heymann, H.; Ebeler, S.E. The combined impact of vineyard origin and processing winery on the elemental profile of red wines. Food Chem. 2015, 172, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Grainger, C.; Yeh, A.; Byer, S.; Hjelmeland, A.; Lima, M.M.M.; Runnebaum, R.C. Vineyard site impact on the elemental composition of Pinot noir wines. Food Chem. 2021, 334, 127386. [Google Scholar] [CrossRef] [PubMed]
- McHargue, J.S.; Calfee, R.K. Effect of Manganese, Copper and Zinc on the Growth of Yeast. Plant Physiol. 1931, 6, 559–566. [Google Scholar] [CrossRef]
- Sun, X.; Liu, L.; Ma, T.; Yu, J.; Huang, W.; Fang, Y.; Zhan, J.; Sun, X.; Liu, L.; Ma, T.; et al. Effect of high Cu2+ stress on fermentation performance and copper biosorption of Saccharomyces cerevisiae during wine fermentation. Food Sci. Technol. 2019, 39, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Garay, C.A.; de Llanos, R.; Romero, A.M.; Martínez-Pastor, M.T.; Puig, S. Responses of Saccharomyces cerevisiae Strains from Different Origins to Elevated Iron Concentrations. Appl. Environ. Microbiol. 2016, 82, 1906–1916. [Google Scholar] [CrossRef] [Green Version]
- Cantu, A.; Lafontaine, S.; Frias, I.; Sokolowsky, M.; Yeh, A.; Lestringant, P.; Hjelmeland, A.; Byer, S.; Heymann, H.; Runnebaum, R.C. Investigating the impact of regionality on the sensorial and chemical aging characteristics of Pinot noir grown throughout the U.S. West coast. Food Chem. 2021, 337, 127720. [Google Scholar] [CrossRef] [PubMed]
- White, R.E. The Value of Soil Knowledge in Understanding Wine Terroir. Front. Environ. Sci. 2020, 8, 12. [Google Scholar] [CrossRef]
- Belda, I.; Gobbi, A.; Ruiz, J.; de Celis, M.; Ortiz-Álvarez, R.; Acedo, A.; Santos, A. Microbiomics to Define Wine Terroir. In Comprehensive Foodomics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 438–451. ISBN 978-0-12-816396-2. [Google Scholar]
- Zhang, Y.; Lin, Y.-H. Metabolic flux analysis of Saccharomyces cerevisiae during redox potential–controlled very high-gravity ethanol fermentation. Biotechnol. Appl. Biochem. 2020, 67, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Chiorcea-Paquim, A.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.M.M.; Hernandez, D.; Yeh, A.; Reiter, T.; Runnebaum, R.C. Reproducibility of elemental profile across two vintages in Pinot noir wines from fourteen different vineyard sites. Food Res. Int. 2021, 141, 110045. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M. Metals in yeast fermentation processes. Adv. Appl. Microbiol. 2004, 54, 197–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, I.E.; Kowalski, B.R. prediction of wine quality and geographic origin from chemical measurements by parital least-squares regression modeling. Anal. Chim. Acta 1984, 162, 241–251. [Google Scholar] [CrossRef]
- Ledderhof, D.; Reynolds, A.G.; Brown, R.; Jollineau, M.; Kotsaki, E. Spatial Variability in Ontario Pinot noir Vineyards: Use of Geomatics and Implications for Precision Viticulture. Am. J. Enol. Vitic. 2017, 68, 151–168. [Google Scholar] [CrossRef]
- Tomasino, E.; Harrison, R.; Sedcole, R.; Frost, A. Regional Differentiation of New Zealand Pinot noir Wine by Wine Professionals Using Canonical Variate Analysis. Am. J. Enol. Vitic. 2013, 64, 357–363. [Google Scholar] [CrossRef]
- Bell, S.-J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Liu, C.-G.; Hao, X.-M.; Lin, Y.-H.; Bai, F.-W. Redox potential driven aeration during very-high-gravity ethanol fermentation by using flocculating yeast. Sci. Rep. 2016, 6, 25763. [Google Scholar] [CrossRef] [Green Version]
- Bisson, L.F.; Fan, Q.; Walker, G.A. Sugar and Glycerol Transport in Saccharomyces cerevisiae. Adv. Exp. Med. Biol. 2016, 892, 125–168. [Google Scholar] [CrossRef]
- Panprivech, S.; Lerno, L.A.; Brenneman, C.A.; Block, D.E.; Oberholster, A. Investigating the Effect of Cold Soak Duration on Phenolic Extraction during Cabernet Sauvignon Fermentation. Molecules 2015, 20, 7974–7989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, R.; Alwazeer, D. Study of relationship between oxido-reduction potential and polyphenol oxidase activity. Res. J. Al-Baath Univ. Basic Sci. Ser. 2009, 31, 86–90. [Google Scholar]
- Al-Amrani, M.; Al-Alawi, A.; Al-Marhobi, I. Assessment of Enzymatic Browning and Evaluation of Antibrowning Methods on Dates. Int. J. Food Sci. 2020, 2020, 8380461. [Google Scholar] [CrossRef]
- Quirós, M.; Martínez-Moreno, R.; Albiol, J.; Morales, P.; Vázquez-Lima, F.; Barreiro-Vázquez, A.; Ferrer, P.; Gonzalez, R. Metabolic Flux Analysis during the Exponential Growth Phase of Saccharomyces cerevisiae in Wine Fermentations. PLoS ONE 2013, 8, e71909. [Google Scholar] [CrossRef] [Green Version]
- Thorsen, M.; Jacobson, T.; Vooijs, R.; Navarrete, C.; Bliek, T.; Schat, H.; Tamás, M.J. Glutathione serves an extracellular defence function to decrease arsenite accumulation and toxicity in yeast. Mol. Microbiol. 2012, 84, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Zimdars, S.; Schrage, L.; Sommer, S.; Schieber, A.; Weber, F. Influence of Glutathione on Yeast Fermentation Efficiency under Copper Stress. J. Agric. Food Chem. 2019, 67, 10913–10920. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Manassa, P.; Dawson, M.; Fitzgerald, S.K. Oxidation reduction potential as a parameter to regulate micro-oxygen injection into anaerobic digester for reducing hydrogen sulphide concentration in biogas. Bioresour. Technol. 2014, 173, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fariña, L.; Medina, K.; Urruty, M.; Boido, E.; Dellacassa, E.; Carrau, F. Redox effect on volatile compound formation in wine during fermentation by Saccharomyces cerevisiae. Food Chem. 2012, 134, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Tariba, B. Metals in Wine—Impact on Wine Quality and Health Outcomes. Biol. Trace Elem. Res. 2011, 144, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Bekker, M.Z.; Day, M.P.; Smith, P.A. Changes in Metal Ion Concentrations in a Chardonnay Wine Related to Oxygen Exposure during Vinification. Molecules 2019, 24, 1523. [Google Scholar] [CrossRef] [Green Version]
- Maante-Kuljus, M.; Rätsep, R.; Moor, U.; Mainla, L.; Põldma, P.; Koort, A.; Karp, K. Effect of Vintage and Viticultural Practices on the Phenolic Content of Hybrid Winegrapes in Very Cool Climate. Agriculture 2020, 10, 169. [Google Scholar] [CrossRef]
- Birch, R.M.; Ciani, M.; Walker, G.M. Magnesium, Calcium and Fermentative Metabolism in Wine Yeasts. J. Wine Res. 2003, 14, 3–15. [Google Scholar] [CrossRef]
- Novozymes. Yeast Micronutrient and Growth Factor Requirements. Available online: https://www.novozymes.com/-/media/Project/Novozymes/Website/website/document-library/Advance-your-business/Bioenergy/Yeast-Micronutrient-Requirements-2017.pdf (accessed on 13 May 2021).
- Stehlik-Tomas, V.; Gulan Zetić, V.; Stanzer, D.; Grba, S.; Vahčić, N. Zinc, Copper and Manganese Enrichment in Yeast Saccharomyces cerevisae. Food Technol. Biotechnol. 2004, 42, 115–120. [Google Scholar]
- Danilewicz, J.C. Chemistry of Manganese and Interaction with Iron and Copper in Wine. Am. J. Enol. Vitic. 2016, 67, 377–384. [Google Scholar] [CrossRef]
- Cyert, M.S.; Philpott, C.C. Regulation of Cation Balance in Saccharomyces cerevisiae. Genetics 2013, 193, 677–713. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
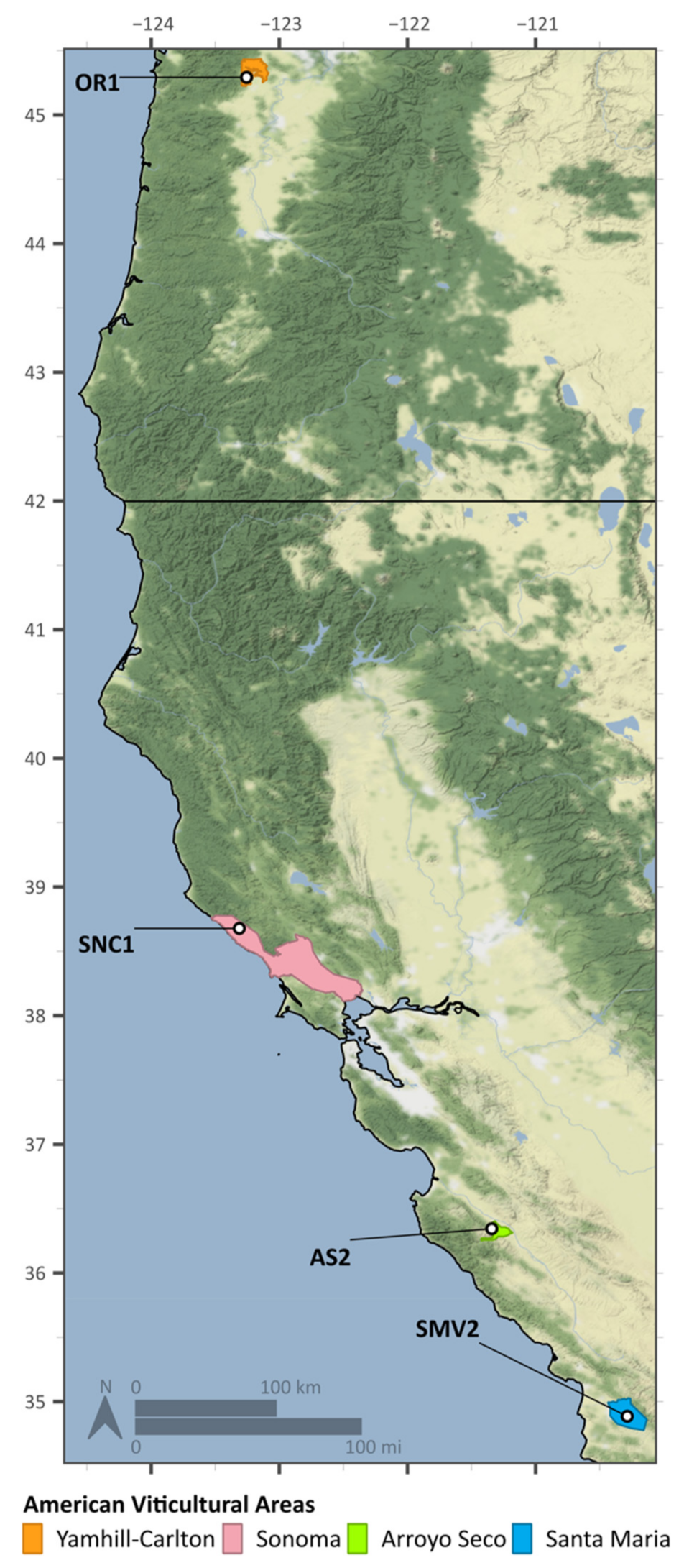

| Vineyard Site | American Vineyard Area | Elevation (ft) | Year of Planting | Soil Texture | Topography | Vine Spacing (ft) | Row Orientation |
|---|---|---|---|---|---|---|---|
| AS2 | Arroyo Seco | 591 | 2005 | Loam | Hillside | 4 × 3 | E/W |
| SMV2 | Santa Maria Valley | 503 | 2004 | Loam | Bench | 6 × 5 | NE/SW |
| SNC1 | Sonoma Coast | 667 | 2000 | Sandy Loam | Hillside | 9 × 5 | NE/SW |
| OR1 | Willamette Valley | 309 | 2005 | Clay Loam | Bench | 3.28 × 5.25 | N/S |
| Vineyard Site | Harvest Date | Initial Brix | Stem:Must Ratio | pH | Titratable Acidity (g/L) | Malic Acid (mg/L) | NH3 (mg/L) | NOPA (mg/L) | YAN (mg/L) | NutriStart (g) | DAP (g) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AS2 | 9/16/19 | 25.8 | 0.057 | 3.69 | 5.23 | 3772 | 127 | 255 | 317 | 0 | 0 |
| SMV2 | 9/24/19 | 24.6 | 0.068 | 3.69 | 4.89 | 3105 | 90.3 | 186 | 258 | 0 | 0 |
| SNC1 | 9/13/19 | 25.1 | 0.066 | 3.58 | 4.23 | 3067 | 98 | 133 | 211 | 36.1 | 1.3 |
| OR1 | 9/30/19 | 23.1 | 0.049 | 3.43 | 5.35 | 4307 | 26 | 74 | 95 | 32.6 | 39.2 |
| Vineyard Site | ALC % | pH | Titratable Acidity (g/L) | Acetic Acid (g/L) | Free SO2 (mg/L) | Rep 1 RS at Press (g/L) | Rep 2 RS at Press (g/L) | Rep 3 RS at Press (g/L) | RS in Bottle (g/L) |
|---|---|---|---|---|---|---|---|---|---|
| AS2 | 13.93 ± 0.01 | 3.75 ± 0.01 | 5.19 ± 0.05 | 0.42 ± 0.01 | 31.33 ± 0.03 | 0.96 | 0.84 | 1.05 | 0.60 ± 0.58 |
| SMV2 | 14.09 ± 0.01 | 3.74 ± 0.00 | 5.35 ± 0.03 | 0.44 ± 0.02 | 31.67 ± 0.02 | 17.35 | 3.96 | 1.68 | 0.57 ± 0.58 |
| SNC1 | 13.33 ± 0.01 | 3.53 ± 0.01 | 5.58 ± 0.02 | 0.25 ± 0.01 | 17.67 ± 0.03 | 4.06 | 3.25 | 4.74 | 0.55 ± 0.58 |
| OR1 | 12.71 ± 0.01 | 3.66 ± 0.01 | 5.34 ± 0.05 | 0.33 ± 0.01 | 26.67 ± 0.02 | 9.29 | 10.4 | 10.78 | 0.23 ± 1.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, G.A.; Nelson, J.; Halligan, T.; Lima, M.M.M.; Knoesen, A.; Runnebaum, R.C. Monitoring Site-Specific Fermentation Outcomes via Oxidation Reduction Potential and UV-Vis Spectroscopy to Characterize “Hidden” Parameters of Pinot Noir Wine Fermentations. Molecules 2021, 26, 4748. https://doi.org/10.3390/molecules26164748
Walker GA, Nelson J, Halligan T, Lima MMM, Knoesen A, Runnebaum RC. Monitoring Site-Specific Fermentation Outcomes via Oxidation Reduction Potential and UV-Vis Spectroscopy to Characterize “Hidden” Parameters of Pinot Noir Wine Fermentations. Molecules. 2021; 26(16):4748. https://doi.org/10.3390/molecules26164748
Chicago/Turabian StyleWalker, Gordon A., James Nelson, Thomas Halligan, Maisa M. M. Lima, Andre Knoesen, and Ron C. Runnebaum. 2021. "Monitoring Site-Specific Fermentation Outcomes via Oxidation Reduction Potential and UV-Vis Spectroscopy to Characterize “Hidden” Parameters of Pinot Noir Wine Fermentations" Molecules 26, no. 16: 4748. https://doi.org/10.3390/molecules26164748
APA StyleWalker, G. A., Nelson, J., Halligan, T., Lima, M. M. M., Knoesen, A., & Runnebaum, R. C. (2021). Monitoring Site-Specific Fermentation Outcomes via Oxidation Reduction Potential and UV-Vis Spectroscopy to Characterize “Hidden” Parameters of Pinot Noir Wine Fermentations. Molecules, 26(16), 4748. https://doi.org/10.3390/molecules26164748






