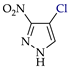
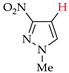
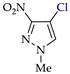
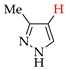
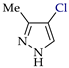
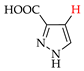
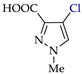
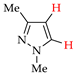
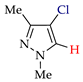
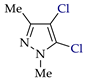
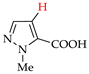
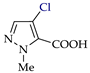
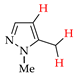
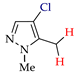
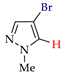
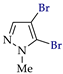
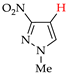
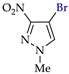
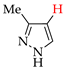
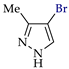
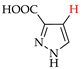
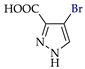
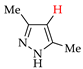
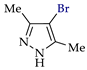
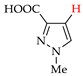
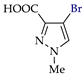
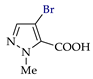
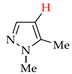
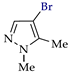
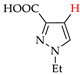
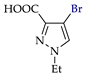
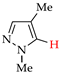
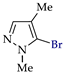
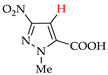
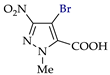
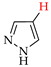
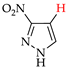
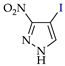
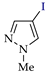
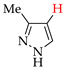
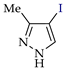
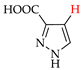
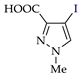
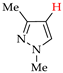
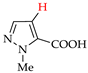
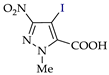
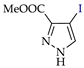
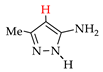
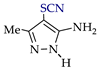
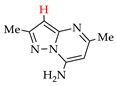
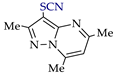
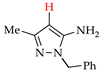
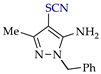
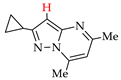
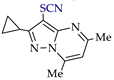
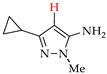
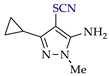
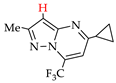
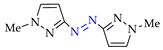
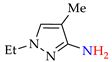
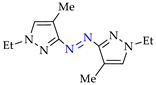
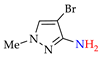
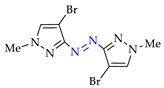
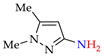
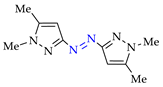
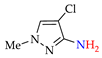
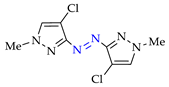
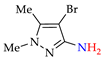
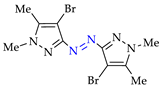
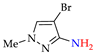
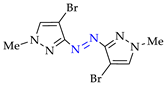
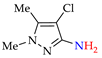
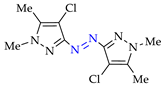
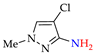
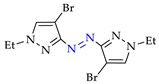
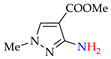
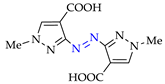
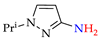
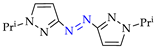
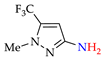
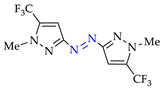
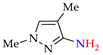
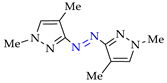
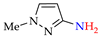
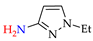
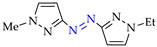
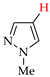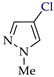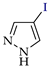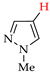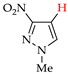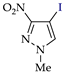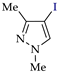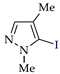Abstract
The review summarizes for the first time the poorly studied electrooxidative functionalization of pyrazole derivatives leading to the C–Cl, C–Br, C–I, C–S and N–N coupling products with applied properties. The introduction discusses some aspects of aromatic hydrogen substitution. Further, we mainly consider our works on effective synthesis of the corresponding halogeno, thiocyanato and azo compounds using cheap, affordable and environmentally promising electric currents.
1. Introduction
The functionalization of arenes is the key to their diversity, opening the way to practically useful substances. At the beginning of the 21st century, C‒H functionalization of arenes became a popular tool for the implementation of such processes [1], and the center for selective C‒H functionalization (mainly based on metal complex catalysis) was organized in the USA [2,3]. At the same time, a more attractive metal-free C‒H functionalization of arenes has existed for many years. Its development is discussed below, since the review is related to it.
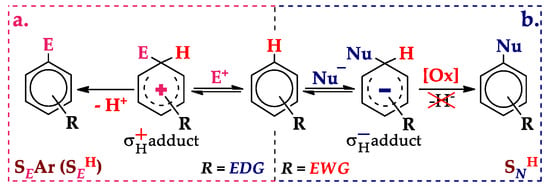
The first to consider is the electrophilic aromatic hydrogen substitution (SEH) [4]. It proceeds via the σH+ adduct formation and proton elimination leading to the target product (Scheme 1a).
Scheme 1.
Electrophilic (a) and nucleophilic (b) aromatic hydrogen substitution.
The nucleophilic aromatic hydrogen substitution (SNH, Scheme 1b) is problematic due to the difficulty of the hydride ion direct elimination from the σH− adduct. Nevertheless, in the mid-1970s, Chupakhin and Postovsky proposed an indirect way of obtaining the target product, the chemical oxidation of the σH− adduct [5]. To date, such processes have been extensively developed by Chupakhin and Charushin [6,7,8,9].
This review is devoted to electrooxidative functionalization of pyrazoles. Why is it so attractive? The answer is given by Seebach [10], who showed that the interaction of two near-polar co-reactants is provided by the polarity inversion of one of them. This is the essence of electrochemistry, where the electrode transformation of the substrate is accompanied by its polarity inversion. For example, 1,4-dimethoxybenzene (DMB) and pyrazole (Scheme 2) are two non-interacting nucleophiles, but polarity inversion of DMB by electrooxidation leads to the electrophilic radical cation that reacts with pyrazole. In the electrochemical literature, this C‒H functionalization is called anodic substitution, which has been actively studied since the mid-1950s [11,12,13,14,15,16,17,18,19,20].
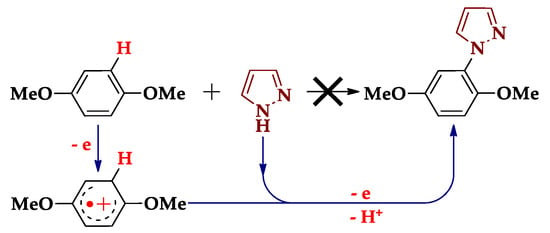
Scheme 2.
Electrooxidative N-arylation of pyrazole by 1,4-dimethoxybenzene.
Note that chemical (electrophilic and nucleophilic) substitution and electrochemical (anodic) substitution in arenes have been being developed in parallel and independently of each other for a long time. In addition, the role of anodic substitution among the corresponding chemical processes was unconsidered.
Only recently we have introduced the concept of anodic substitution as an electrochemical aromatic substitution of hydrogen [21,22]. Such reactions were designated as SNH An (An is anode), since the key stage is anodic oxidation of the substrate. For electron-rich arenes, the processes proceed along two main routes (Scheme 3), depending on the ease of oxidation of nucleophile vs. arene [21]. Route I (arene oxidizes easier than Nu−) proceeds via the interaction between Nu− and the arene radical cation. Route II (Nu− oxidizes easier than arene) proceeds via the formation of Nu•, which either interacts with the arenes homolytically (route IIa) or forms a dimer that interacts with the arene as an electrophile (route IIb).
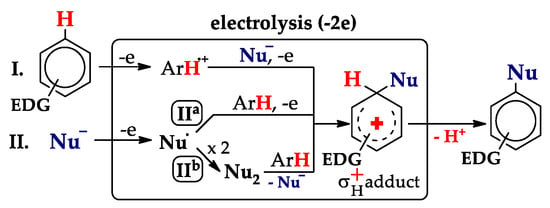
Scheme 3.
Main routes of SNH An processes: I (EpoxAr < EpoxNu−) or II (EpoxNu− < EpoxAr).
In general, the multitude of SNH (An) processes, where hydrogen is displaced with a nucleophile, are electrooxidative C‒H functionalizations (C–H An) and can be described by Scheme 4. Such strategy opens up the direct method of C–H functionalization of arenes with the C–C and C–Het coupling realization. The latter is especially shown in the examples of electrooxidative C–H halogenation and thiocyanation of pyrazole derivatives (Section 2 and Section 3). A special place is occupied by the N–N coupling of amino pyrazoles via the N–H functionalization (Section 4), since its patterns are somehow similar to those described above, but not sufficiently studied.

Scheme 4.
General scheme of the electrooxidative C‒H functionalization (C–H An).
Such processes are attractive for green chemistry [23,24] because they use cheap, affordable and environmentally promising electric current instead of chemical oxidants (often toxic, unrecyclable and used in excess) and complex catalysts (sometimes expensive and toxic). In addition, the varying anode potential eliminates the difficulties of an empirical search for suitable chemical oxidants, while Pt, a frequently used electrode material [25], can be replaced by more attractive ones, e.g., glassy carbon, or ruthenium–titanium oxide (for more information on electrode materials see [25,26,27]).
2. Electrooxidative C–H Halogenation of Pyrazole and Its Substituted Derivatives
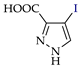
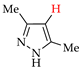
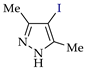
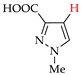
Halogenated pyrazoles are widely used in organic synthesis; in particular, iodo and bromo pyrazoles are the key reagents in transition metal-catalyzed cross-coupling [28]. Moreover, they are important precursors of drugs, such as antihepatitis, anti-Alzheimer, antiparkinsonian and anti-schizophrenic drugs (chloro-pyrazoles) [29,30,31], antiglaucoma drugs (bromo-pyrazoles) [32] and antiatherosclerotic, antimalarial, anti-inflammatory and immunocorrective drugs (iodo-pyrazoles) [33,34,35,36,37,38]. At the same time, chloro- and iodo-pyrazoles are used for preparation of antidiabetic drugs [39,40], bromo- and iodo-pyrazoles—of anticancer [41,42,43,44] and antimicrobial [45,46] agents, and chloro- and bromo-pyrazoles—of agrochemicals [47,48,49,50,51].
The active use of halogeno-pyrazoles has stimulated interest in their efficient and ecologically attractive synthesis, including electrosynthesis (see Introduction). At the same time, the electrochemical halogenation of pyrazoles has practically not been studied before us, but it was preceded by chemical halogenation, the aspects of which are briefly given below.
2.1. Chemical Halogenation of Pyrazoles
The processes (Scheme 5) are usually carried out by the interaction of pyrazoles and halogens (or halogenating reagents), and they occur first at position 4 and only then at other positions [28].
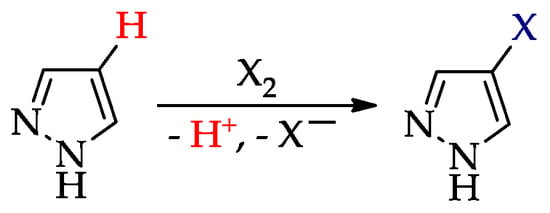
Scheme 5.
Chemical halogenation of pyrazoles (X = Cl, Br, I).
2.1.1. Chlorination
The most common is the interaction of pyrazoles and Cl2. It was used to convert the pyrazole and its alkyl derivatives into the 4-chloro pyrazoles (yields 40–85%, at 0–40 °C, in CH2Cl2 or CCl4). Under severe conditions (at 80–100 °C, in AcOH) dichloropyrazoles and the chloro products of the alkyl groups were also formed [52,53]. At the same time, the corresponding 4-chloro derivatives were obtained by the reaction of 3,5-dimethyl-1H-pyrazole (and its N-substituted derivatives) with N-chlorosuccinimide (yield 95–98%, at 20–25 °C, in CCl4 or H2O) [54,55].
2.1.2. Bromination
The reaction of alkyl-substituted pyrazoles and their carboxylic acids with Br2 (at 20–25 °C) proceeded in yields of 75–96% in non-aqueous media (CH2Cl2, CHCl3 or CCl4) [45,56,57,58], or 50–75% in water [59,60]. The NaOH additives (which binds with HBr formed) allows bromination of low-reactive pyrazole-3-carboxylic acid in the yield of 90% [61]. A number of 4-bromopyrazoles were also obtained by the reaction of 3,5-dimethyl-1H-pyrazole (and its N-substituted derivatives) with N-bromosuccinimide (yields 90–99%, at 20–25 °C, in CCl4 or H2O) [55].
2.1.3. Iodination
Good results in the iodination of pyrazoles with donor substituents were obtained using the I2–NaI–K2CO3 system (yields 75–90% at 20–25 °C in aq. EtOH) [62,63,64]. A solution of N-iodosuccinimide in acidic media (50% aq. H2SO4, CF3SO3H, CF3COOH, AcOH) was also efficient for the iodination [44,62,65]. Finally, the iodination of practically any pyrazoles proceeded efficiently and without toxic waste using the I2–HIO3 system in AcOH–CCl4 [66,67].
In general, the above methods are quite effective, but not ecologically attractive enough due to the frequent use of halogens in their pure form or waste of other halogenating agents (e.g., succinimide). Such problems can be solved using electrochemical methods—C–H An halogenation.
2.2. C-H An Halogenation of Pyrazoles
The halogenation (Scheme 6) usually proceeds [26] via the electrogeneration of a halogen followed by its interaction with pyrazole (cf. Scheme 3, route IIb).
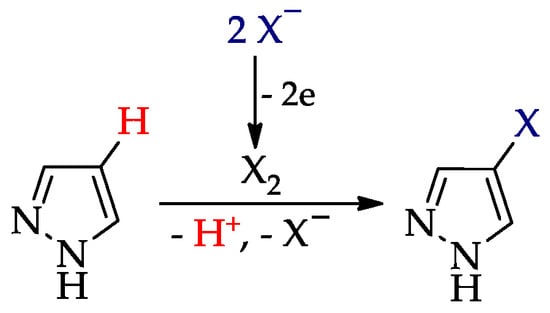
Scheme 6.
C–H An halogenation of pyrazoles (X = Cl, Br, I).
Such processes are mainly carried out under mild conditions in an anodic compartment of a divided cell on a Pt-anode under galvanostatic electrolysis with alkali metal halides in H2O or in H2O–CHCl3. A series of N–H and N–Alk pyrazoles, including those with donor (acceptor) substituents, were objects of study (Table 1, Table 2 and Table 3).

Table 1.
C‒H An chlorination of pyrazoles (Az‒H) 1.

Table 2.
C‒H An bromination of pyrazoles (Az‒H) 1.

Table 3.
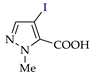
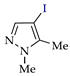
C‒H (An) iodination of pyrazoles (Az‒H).
2.2.1. Chlorination
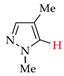
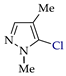
C–H An chlorination of pyrazole 1a (Table 1, entry 1) led to 4-chloropyrazole 1b (yield 46%) and to by-product 1b′ (yield 8%) in H2O–CHCl3 at the theoretical amount of electricity passed (Q/Qt = 1). Apparently, the product 1b undergoes chlorination to 1,4-dichloropyrazole 1b′ (Scheme 7), followed by C–N dehydrogenative cross-coupling to by-product 1b-1b′.
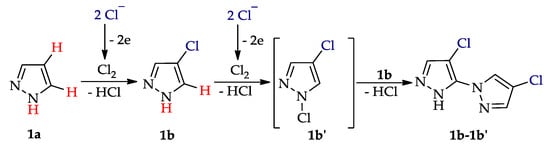
Scheme 7.
C‒H An chlorination of pyrazole 1a.
The need for CHCl3 (as an extractant of target product) should be noted, since its absence decreased the yield of product 1b to 34% and increased the yield of by-product 1b-1b′ to 15%. Pyrazoles 2a–7a (entries 2–7), gave the monochlorinated products 2b–7b with different yields (8–71%) depending on the position of Me groups. Di- and trichloroproducts were obtained in entries 5 and 6.
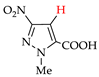
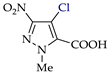
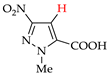
Pyrazoles with acceptor groups (NO2 or COOH) were chlorinated without CHCl3 additives: the yields of the target products 8b–14b were 41–93% (entries 8–14). Only pyrazole 14a, containing both NO2 and COOH groups, was the least reactive. Therefore, the electrochemical method for the synthesis of 4-chloropyrazolcarboxylic acids [68] is noticeably superior to the corresponding chemical one [69].
2.2.2. Bromination
Compared with chlorination, the C‒H An bromination (Table 2) proceeded more effectively for pyrazole and its methyl derivatives (yields of products 1c–6c 55–94%). In some cases, dibromo by-products (entries 2 and 5), low yield (entry 9), or the absence of any reactions (entries 7 and 14) were observed.
2.2.3. Iodination
C‒H An iodination by weakly electrophilic I2 (Table 3) was generally less effective than bromination [73]. Traces of the target products or no reaction were observed in half of the cases (entries 2, 7–9, 11–13). In other cases, the yields were 35–86% (for pyrazole and its methyl derivatives in entries 1, 3–6) and 30–40% (for nitro- and carboxypyrazoles in entries 10 and 14).
A much more effective iodinating agent was HOI, which can be obtained by the reaction of KIO3 with KI (or I2) and H2SO4 [75,76,77,78,79]. The original process [74] includes the electrogeneration of KIO3 (on the NiO(OH) anode [80]), followed by the interaction of HOI generated in situ with the pyrazole (Scheme 8). As a result, the yields of target products increased to 74–93% (entries 1, 2, 4, 8, 10–12, 13).
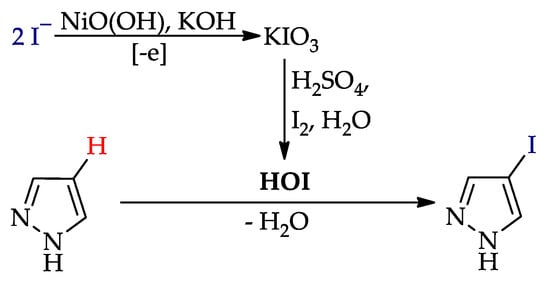
Scheme 8.
C–H iodination of pyrazoles via HOI.
2.3. The Mechanistic Aspects of C–H (An) Halogenation of Pyrazoles
Since I−, Br−, and Cl− are commonly oxidized at lower anodic potentials than the studied pyrazoles, the process proceeds via the electrooxidation of Hal− to Hal2 followed by interaction of the latter with arenes (see Scheme 3, route IIb and Scheme 6). The possible mechanism [26,71,79] (Scheme 9) includes the initial attack of the halogen on the N2 of Az–H with the formation of σH+ adduct 1. The latter, depending on the R, gives either N–X intermediate (R = H) or σH+ adduct 2 (R = Alk). Therefore, the target Az–X is formed either due to N–C rearrangement of N–X derivative or due to the deprotonation of σH+ adduct 2.
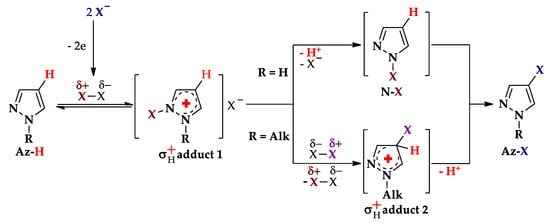
Scheme 9.
C–H An halogenation of pyrazoles (possible mechanisms, X = Cl, Br, I).
Iodination by HOI proceeds similarly, but for highly basic N-unsubstituted pyrazole and its alkyl derivatives it most likely occurs (Scheme 10) via C–I adduct (the result of protonation of N2 and HOI attack on C4) [74,75,77,78,79].
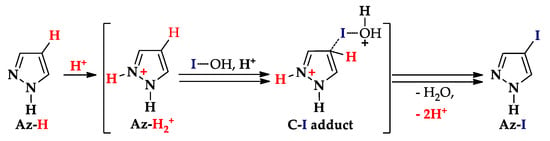
Scheme 10.
Iodination of highly basic pyrazoles by HOI.
Additional control experiments showed different properties of N–Cl and N–Br intermediates (Scheme 11). Therefore, the N–C rearrangement of the N–Cl derivative is significantly lower than that for the N–Br (cf. stages N–X→Az–Cl and N–X→Az–Br). At the same time, for the N–Cl bond, homolytic cleavage is observed, while for N–Br it is heterolytic (cf. stages N–X→Ar–CH2-Cl and N–X→Ar–Br).
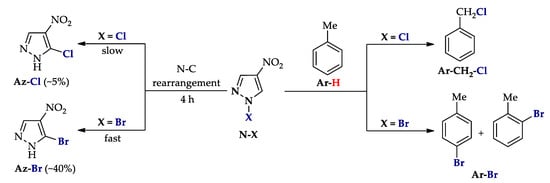
Scheme 11.
Different behavior of N–Cl and N–Br intermediates (unpublished control experiments).
The above data not only reveal the essence of pyrazoles C‒H An halogenation, but also explain the difference in its efficiency (e.g., the anomalously less efficient chlorination of N-unsubstituted pyrazoles compared to bromination (cf. entries 1, 3 and 4, Table 1 and Table 2), and the formation of by-products (e.g., entries 1 and 6, Table 1).
Therefore, this Section describes the basic patterns of C–H An halogenation, and the efficient (up to 94% yield) gram-scale synthesis of a series of chloro-, bromo- and iodo-pyrazoles in aqueous or aqueous-organic media. The following Section reflects the main points on the related C‒H An thiocyanation of pyrazole derivatives.
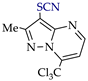
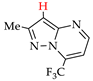
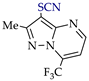
3. Electrooxidative C–H Thiocyanation of 5-Aminopyrazoles and Pyrazolo [1,5-a]pyrimidines
Thiocyanation of the C–H bond of arenes is an effective tool for C–S coupling [81,82,83,84]. The resulting aryl thiocyanates are valuable precursors of sulfur and nitrogen-containing compounds (thiols [85], (di)sulfides [86,87], dithiocarbamates [88], thiazoles [89], tetrazoles [90]), and are highly bioactive compounds (antifungal [91], antitumor [92], antiparasitic [93]). Recently synthesized thiocyanates of pyrazole derivatives also have sufficient antifungal [94] and antitumor [95] activity.
One of the key intermediates of C‒H thiocyanation of arenes is the well-known [96,97] pseudohalogene thiocyanogen (SCN)2. It is usually obtained in situ by chemical or electrochemical oxidation of the thiocyanate ion (Scheme 12).
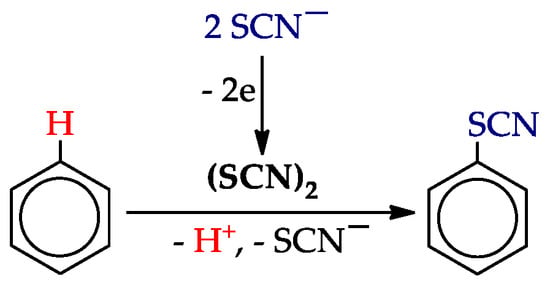
Scheme 12.
C–H thiocyanation of arenes via the thiocyanogen.
The chemical approach has been actively developed over the past 10–15 years, but it is often associated with the use of an excess of unrecyclable oxidants, which can sometimes be toxic, scalding or poorly available (e.g., Br2 [98], I2 [99], DEAD [100], HIO3 [101], H5IO6 [102], I2O5 [103], H2O2 [102,104,105,106,107], K2S2O8 [108,109], CAN [110], Mn(OAc)3 [111], p-TSA [112], NCS [113], NBS [100], NIS [114], NTS [115], DDQ [116,117]). The electrochemical approach (see [22], Scheme 3, route IIb, and Scheme 13) is devoid of such disadvantages, but it is poorly studied in general [118,119,120]. For pyrazole derivatives, C–H An thiocyanation is studied for the first time in a series of works [22,121,122,123,124,125,126], which are reflected in this Section.
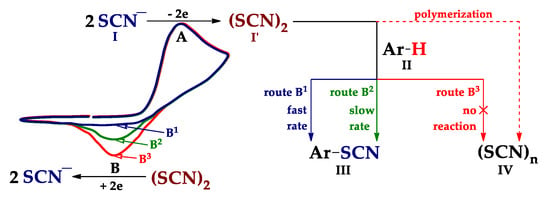
Scheme 13.
C–H An thiocyanation of arenes (II) via the thiocyanogen (I′) and voltammetric test of the process efficiency.
3.1. C–H An Thiocyanation: General Patterns and Approaches
According to the above and developed [22,118,119,120,121,122,123,124,125,126] concepts, C–H An thiocyanation occurs during the anodic oxidation of the thiocyanate ion in the presence of arene, as a rule, via the thiocyanogen (Scheme 13, step I→I′). The latter either interacts with arene (step I′ + II→III), or gives polythiocyanogen [127] (step I′→IV).
These processes were investigated by cyclic voltammetry (CV) [22,123,125,126]. Scheme 13 shows a typical CV curve of SCN−. Peak A corresponds to the oxidation of thiocyanate ion I to thiocyanogen I′, which is detected on the reverse scan by its reduction peak B (B3). If after the addition of arene II, peak B disappears (cf. peaks B1 and B3) or decreases (cf. peaks B2 and B3), then Ar–H II interacts with (SCN)2, respectively, via the route B1 or B2 to form the target Ar–SCN III. If the peak B does not change, then Ar–H II does not react with (SCN)2 (see peak B3 and route B3). In this case the main reaction product is polythiocyanogen IV.
Further, we proposed [122,123] the original system of approaches to the C–H An thiocyanation of arenes (Scheme 14) depending on the reactivity of arenes with respect to (SCN)2: via the generation (SCN)2 at the oxidation potential (Epox) of thiocyanate ion (approach A, cf. Scheme 3, route IIb, and Scheme 13), via electrogeneration (SCN)2 in the presence of ZnCl2 activating additives (approach B) or via the generation of a highly reactive radical cation at Epox of arene (approach C, cf. Scheme 3, route I).
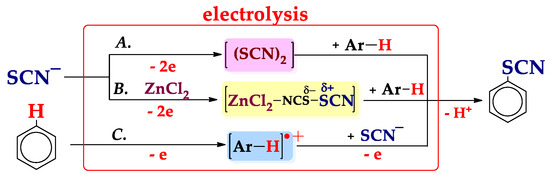
Scheme 14.
Approaches and possible mechanisms of C–H An thiocyanation.
Approach A is used for arenes that react with (SCN)2 (Scheme 13, routes B1 and B2), whereas approaches B and C are used for arenes that do not interact with non-activated (SCN)2.
These patterns and approaches are considered below on the examples of C–H An thiocyanation of the practically useful [128,129,130] derivatives of 5-aminopyrazole and pyrazolo[1,5-a]pyrimidine and the original electrosynthesized 1-(hetero)arylpyrazoles [124,131].
3.2. C–H An Thiocyanation of Pyrazole Derivatives
The studies included a preliminary CV test in addition to electrosynthesis. The initial pyrazoles 1e–15e and their thiocyanation products 1f–15f are presented in Table 4 and Table 5.

Table 4.
C–H An thiocyanation of pyrazole derivatives (Az‒H) via (SCN)2 (approach A) 1.

Table 5.
C–H An thiocyanation of hardly oxidizable pyrazolo[1,5-a]pyrimidines (Az‒H) (approaches B and C) 1.
3.2.1. CV studies and the Choice of Optimal Approach
Figure 1 shows CV curves of NH4SCN and its mixtures with 3-methyl-1H-pyrazol-5-amine (1e), 2-methyl-5-thiophen-2-yl-7-(trifluoromethyl) pyrazolo[1,5-a]pyrimidine (10e), as well as curves of individual compounds and their thiocyanato products (1e,10e,1f,10f) [125,126]. The CV of NH4SCN (curve 1, Figure 1A,B) has the anodic peak A1 (Epox = 0.70 V) of the thiocyanate ion and a cathodic peak B1 (Epred = 0.34 V) of the thiocyanogen. The peak B1 disappeared after the addition of pyrazole 1e and did not change after the addition of pyrazole 10e (cf. corresponding curves 1 and 4). This clearly shows that pyrazole 1e reacts rapidly with (SCN)2 (see Scheme 13, route B1) and approach A (Scheme 14) is suitable for its thiocyanation. From the other side, the pyrazole 10e does not react with (SCN)2 and approaches B and C may be suitable for its thiocyanation. Note also that on full scans, peaks A3 of thiocyanates 1f,10f were observed, in addition to the peaks A2 of pyrazoles 1e,10e (see curve 5, Figure 1A,B).
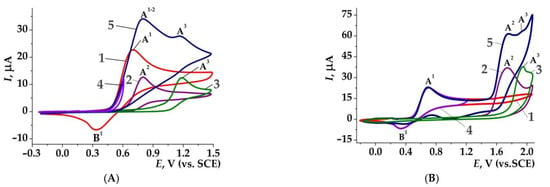
Figure 1.
CV curves on Pt working electrode in 0.1M NaClO4 in MeCN, ν = 0.10 V·s−1. (A) NH4SCN (0.002M)—1; 3-methyl-1H-pyrazol-5-amine 1e (0.002M)—2; 3-methyl-4-thiocyanato-1H-pyrazol-5-amine 1f (0.002M)—3; mixture NH4SCN/azole 1e (1:1) with the reverse scan from 0.60 V—4; the same on the reverse scan from 1.45V—5; (B) NH4SCN (0.002M)—1; 2-methyl-5-thiophen-2-yl-7-(trifluoromethyl)pyrazolo[1,5-a]pyrimidine 10e—2; 2-methyl-3-thiocyanato-5-thiophen-2-yl-7-(trifluoromethyl)pyrazolo[1,5-a]pyrimidine 10f—3; mixture NH4SCN/ azole 10e (1:1) on the reverse scan from 1.20 V—4; the same on the reverse scan from 2.10 V—5.
3.2.2. Electrosynthesis
Electrolyses were carried out in 0.1M solution of NaClO4 in MeCN (MeCN–H2O) in undivided or divided cells (UC or DC) in controlled-potential or galvanostatic mode (CPE or GE), passing a theoretical or excess amounts of electricity (Q/Qt = 1–3). Pt or glassy carbon (GC) electrodes were used.
The amino compounds 1e–6e gave thiocyanates 1f–6f with yields 64–89% (under CPE at EpoxSCN−) and 57–71% (under GE) at Q/Qt = 1 (Table 4, entries 1–6) when implementing approach A (see Scheme 14).
From the less reactive pyrazolo[1,5-a]pyrimidines 7e–9e, products 7f–9f were obtained with yields 66–85% at Q/Qt = 2 (entries 7–9). The electrode material affected things differently: the yield of thiocyanate 2a (entry 1) was 83% (GC) and 72% (Pt), while the yield of thiocyanate 2g (entry 7) was 64% (GC) and 89% (Pt). Most of the processes (entries 3–9) were successfully carried out in an undivided cell. The possibility of scaling the process was also shown (entries 1, 2 and 7).
It was noted [121,122,123,125] that approach A is not suitable for the thiocyanation of hardly oxidizable (Epox > 1.70 V) pyrazoles 10e–15e with acceptor substituents (Table 5) and leads to trace amounts of target thiocyanates 10f–15f and polythiocyanogen (see Scheme 13, route B3). In this case, the process proceeds quite efficiently with an increase in the reactivity of the thiocyanogen (Scheme 14, approach B) or the initial pyrazole (approach C). As a result, CPE in the presence of ZnCl2 activating additives (approach B) allowed us to obtain products 10f–15f with yields 69–81% [121,123], while metal-free CPE at EpoxAzH with Q/Qt = 3 (approach C) also led to the products 10f–15f with smaller yields of 47–65% [122,123].
Note that the possibility of approach C realization can also be tested by CV: a decrease in the peak B1 is observed on the full scan (cf. curves 5 and 1, Figure 1B), which corresponds to the interaction of the thiocyanate ion and the pyrazole cation radical via the ECE mechanism [122,123,132] (see Scheme 14, approach C, and Scheme 3, route I).
In addition to approaches A–C, an equally effective approach was developed [41] based on the HCl-catalyzed condensation of previously obtained 4-thiocyanatopyrazoles 1e, 2e (see entries 1 and 2, Table 4) with 1,3-dicarbonyl compounds or their derivatives (Scheme 15). As a result, 3-thiocyanatopyrazolo[1,5-a]pyrimidines both without substituents and with donor (acceptor) substituents in the pyrimidine ring were obtained with yields of 77–96% [126].
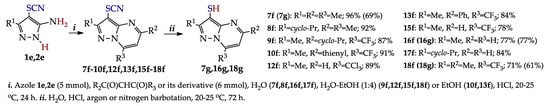
Scheme 15.
Synthesis of 3-thiocyanatopyrazolo[1,5-a]pyrimidines and pyrazolo[1,5-a]pyrimidine-3-thiols.
Developing this direction, the opportunity of transformation of the SCN group into the SH group [94,123] was shown, which opens the way to thiols as promising nucleophiles for C–H functionalization (e.g., see [133,134,135]). Hydrolysis with HCl was the most effective (yields of thiols 7g, 16g, 18g were 61–77%), while the use of chemical reductants or strong acids (LiAlH4, NaBH4, Zn in AcOH, HClO4, H2SO4) was ineffective.
Thus, a series of thiocyanates of substituted pyrazoles and pyrazolo[1,5-a]pyrimidines were obtained on the basis of electrolysis of the “thiocyanate ion/pyrazole” mixture.
In addition, during the development of research on the electrosynthesis of aryl thiocyanates [22] and N-arylpyrazoles [131,136,137,138] we showed [124] the possibility of synthesizing new molecules with pyrazole and thiocyanate fragments (Scheme 16) as promising hybrid polyfunctional [139] structures.
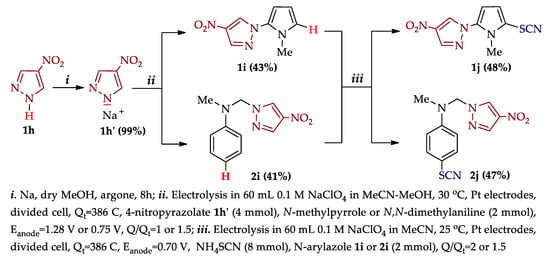
Scheme 16.
N–H An arylation of 4-nitropyrazole followed by C–H An thiocyanation of resulting N-arylazoles.
The N–H arylation of pyrazole 1h was carried out by activating its N–H bond (i) followed by the introduction of electrolysis with N-methylpyrrole or N,N-dimethylaniline (ii). In the latter case, the reaction proceeded selectively at the Me group without affecting the aromatic ring. Subsequent C–H An thiocyanation (iii) of the isolated N-arylazoles 1i, 2i led to the target products 1j, 2j.
3.3. Antifungal and Antibacterial Activity of Thiocyanated Pyrazole Derivatives
Tests for antifungal (C. albicans, A. niger) and antibacterial (S. aureus, E. coli) activity [91,94,123,124] showed that thiocyanate-pyrazoles are more active against fungi than bacteria. The greatest activity is observed against A. niger at thiocyanate 7f [94] and thiocyanatoazolylaniline 2j [124], whose minimum inhibitory concentration (MIC) is 0.24–0.48 µg/mL (it is superior to the antifungal drugs amphotericin B and fluconazole and is comparable to itraconazole).
The contribution of thiocyanate and pyrazole fragments to antifungal and antibacterial activity was clearly shown in the individual examples (Figure 2). Thus, the activity of compound 1j increased more than 2000-fold for A. niger and more than 16-fold for C. albicans after the introduction of the SCN group. The presence of 4-nitropyrazole in 4-thiocyanatoaniline 2j’ provided a selective increase in antifungal activity by a factor of 16–64, while N-arylazole 2i was inactive in all cases.
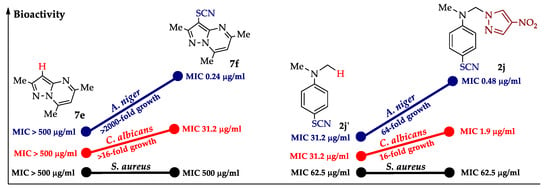
Figure 2.
Effect of thiocyanate and pyrazole fragments on antifungal and antibacterial activity.
Therefore, this Section is devoted to the efficient C–H An thiocyanation of various pyrazole derivatives (in some cases, their N–H An arylation), leading to pharmacologically active target mono- and polyfunctional products. The next Section is devoted to the N–H functionalization of amino pyrazoles followed by their N–N coupling and obtaining azopyrazoles.
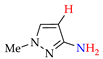
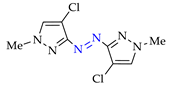
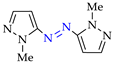
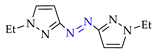
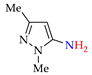
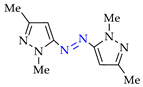
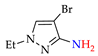
4. (Electro)oxidative N–N Coupling of Aminopyrazoles
Azoarenes are widely used in practice: from dyes and pharmaceuticals [140,141,142] to reagents in syntheses [143,144] and energy-rich materials [145,146]. One of the most popular methods for the synthesis of azoarenes is the oxidation of corresponding amines (Scheme 17), predominantly by chemical oxidants (BaMnO4 [147], Pb(OAc)4 [148], HgO [149], K2FeO4 [150], TCICA [151], t-BuOI [152]) or oxidation systems (CuBr-pyridine-O2 [153], I2-t-BuOOH [154], t-BuOCl-NaI [155]). The synthesis of polyfunctional azopyrazoles by silver catalyzed cascade conversion of diazo compounds [156] is also of interest.
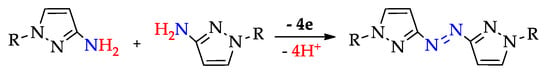
Scheme 17.
Synthesis of azopyrazoles by (electro)oxidative N–N coupling of aminopyrazoles.
At the same time, a more promising electrochemical approach is poorly studied. In particular, electrosyntheses of azobenzene on the Pt anode [157,158] or N,N’-bis(morpholino)diazene on the NiO(OH) anode [159] are described. Note that NiO(OH) is one of the popular electrogenerated redox mediators [80,159]. The use of such redox-mediators is a trend in modern electroorganic chemistry [18], since it allows the processes to be carried out under milder conditions, increasing their efficiency and selectivity. This Section describes the original approaches to the synthesis of azopyrazoles using electrogenerated redox mediators NiO(OH) [160,161,162] and Br2 [163], or electrogenerated hypohalites as oxidants [164,165].
4.1. (Electro)oxidative N-N Coupling of Aminopyrazoles: Approaches and General Patterns
One-stage Approach A (Scheme 18) is carried out in alkaline medium via the anodic dissolution of the Ni and the formation of adsorbed Ni(OH)2, followed by its anodic oxidation to adsorbed NiO(OH). It oxidizes aminopyrazoles (Az–NH2) to azopyrazoles (Az–N = N–Az) and forms Ni(OH)2, after which the cycle repeats [80,161]. In Approach B, the metal-free oxidant is Br2 [164], which is effectively electro(re)generated on the ruthenium–titanium oxide anode (RTOA).
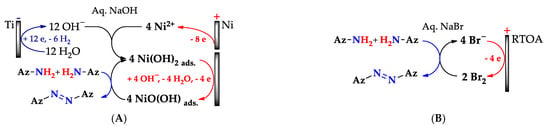
Scheme 18.
One-stage redox-mediated N–N coupling of aminopyrazoles using electrogenerated NiO(OH) (Approach A) and Br2 (Approach B).
In addition, special voltammetric tests showed that an increase in the Ni(OH)2 peak (Figure 3, A, peak A1, Epox = 0.46 V) or a decrease in the Br2 peak (Figure 3B, peak B2, Epred = 0.69 V) after the adding of aminopyrazole is proportional to the process efficiency [162,164].
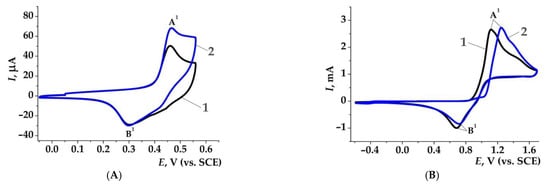
Figure 3.
CV curves, ν = 0.10 V·s−1. (A) On Ni working electrode in 0.2M aq. NaOH: electrogenerated Ni(OH)2—1; after addition of 1-methyl-1H-pyrazol-3-amine 1k (0.002M)—2; (B) on Pt working electrode in 1M aq. NaNO3: NaBr (0.3M)—1; after addition of 1-methyl-1H-pyrazol-3-amine 1k (0.002M)—2.
Two-stage approaches (Scheme 19) include preliminary electrogeneration of hypogalites followed by addition of aminopyrazoles [164,165]. Note, that hypohalites exist in equilibrium forms: predominantly HOCl (HOBr) in a neutral medium, and predominantly NaOCl (NaOBr) after adding NaOH (approaches C, D, C’, D’, respectively).
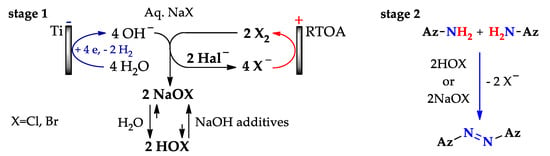
Scheme 19.
Two-stage N–N coupling of aminopyrazoles using electrogenerated HOCl (HOBr) or NaOCl (NaOBr) (Approaches C, D, C’, D’, respectively).
According to the data [80,152,154,155,159,163,164,165], the possible mechanisms (Scheme 20) involve the oxidation of 1-methyl-1H-pyrazol-3-amine 1k (step 1k→[Az–NH2]·+) or its N–H halogenation (step 1k→[NH–X]) followed by N–H amination to hydrazopyrazole [NH–NH] and its oxidation to the target azopyrazole 1k–1k (see also Scheme 3 and Scheme 9).
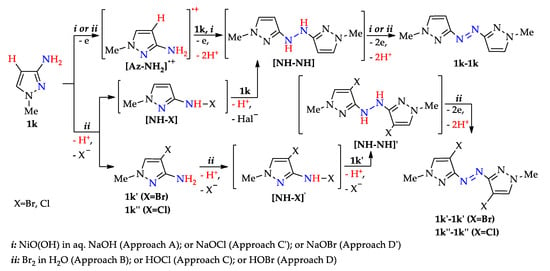
Scheme 20.
N–N coupling of aminopyrazoles (possible mechanisms).
When Br2 and HOX are used, C–H halogenation of aminopyrazole 1k also occurs (step 1k→1k′(1k″), see Scheme 9), followed by the formation of halogenated azopyrazole (steps 1k′→[NH–X]’→[NH–NH]’→1k′–1k′(1k″–1k″)). These patterns are consistent with the experimental results below.
4.2. Synthesis of Azopyrazoles
Approach A is most versatile and allows us to obtain target azopyrazoles with yields of 52–88% (Table 6, entries 1–6, 8–11). On the contrary, approaches B, C and D are more suitable for 4-substituted pyrazoles (entries 2, 6 and 7) or pyrazole with acceptor (CF3) group (entry 12), where the yields of the corresponding azo products were 62–93%.

Table 6.
N–N homo-coupling of aminopyrazoles (Az–NH2) using approaches A, B, C (C’), D (D’) 1.
Nevertheless, approaches C’ and D’ (entries 1 and 4) allow to obtain rather selectively azopyrazoles 1k–1k and 2k–2k (yields 72–75%), and approaches C and D (entry 8) open the way to azohalogenopyrazoles 6k″–6k″ and 6k″–6k″ (yields 79–80%).
Moreover, the approach A is useful for previously unexplored chemical and electrochemical N–N cross-coupling of aminopyrazoles [162] (Table 7), and yields of the target azo compounds 1k–2k and 4k–5k were 48–50%. Such results create prospects for obtaining useful multifunctional azo compounds [152,156].

Table 7.
N–N cross-coupling of aminopyrazoles (Az–NH2) using approach A 1.
5. Conclusions
This review is the first step in summarizing the data on promising, but poorly studied electrooxidative functionalization of C‒H and N‒H bonds in pyrazole derivatives. It paves the way for the efficient synthesis of C‒Cl, C‒Br, C‒I, C‒S and N‒N coupling products using cheap, affordable and environmentally promising electric currents.
Additional advantages are the predominantly galvanostatic electrolysis mode and the reusability of commercially available electrodes, salts and solvents, as well as the gram-scalability of the processes. In half of the cases, a simple isolation of pure target products without chromatography is also possible. Moreover, the key regularities of the corresponding processes are considered, including the dependence of the efficiency of functionalization of pyrazoles on their structure and oxidation potential. An increasingly important role is played by cyclic voltammetry, which makes it possible both to study mechanisms and to predict the efficiency of synthesis.
All this makes the electrooxidative functionalization of pyrazole-type compounds very viable for further application and development.
Author Contributions
Conceptualization, V.A.P.; writing—original draft preparation, V.A.K. and V.A.P.; writing—review and editing, B.V.L., V.L.S., A.S.K. and S.V.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation. Grant 19-73-20259.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 2b, 4b, 9b–13b, 1c–4c, 6c, 8c, 10c–13c, 1d–4d, 8d, 10d–14d, 1f–15f, 1j, 2j, 1k-1k–8k-8k are available from the authors.
References
- Godula, K.; Sames, D. C-H Bond Functionalization in Complex Organic Synthesis. Science 2006, 312, 67–72. [Google Scholar] [CrossRef]
- Davies, H.M.L.; Morton, D. C-H Functionalization: Collaborative Methods to Redefine Chemical Logic. Angew. Chem. Int. Ed. 2014, 53, 10256–10258. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.M.L.; Morton, D. Recent Advances in C–H Functionalization. J. Org. Chem. 2016, 81, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.B. Chapter 11. Aromatic Substitution, Electrophilic. In March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 607–686. [Google Scholar]
- Chupakhin, O.N.; Postovskii, I.Y. Nucleophilic Substitution of Hydrogen in Aromatic Systems. Russ. Chem. Rev. 1976, 45, 454–468. [Google Scholar] [CrossRef]
- Chupakhin, O.N.; Charushin, V.N.; van der Plas, H.C. Nucleophilic Aromatic Substitution of Hydrogen; Academic Press: New York, NY, USA, 1994. [Google Scholar]
- Charushin, V.N.; Chupakhin, O.N. Nucleophilic aromatic substitution of hydrogen and related reactions. Mendeleev Commun. 2007, 17, 249–254. [Google Scholar] [CrossRef]
- Chupakhin, O.N.; Charushin, V.N. Recent advances in the field of nucleophilic aromatic substitution of hydrogen. Tetrahedron Lett. 2016, 57, 2665–2672. [Google Scholar] [CrossRef]
- Charushin, V.N.; Chupakhin, O.N. Nucleophilic C—H functionalization of arenes: A contribution to green chemistry. Russ. Chem. Bull. 2019, 68, 453–471. [Google Scholar] [CrossRef]
- Seebach, D. Methods of Reactivity Umpolung. Angew. Chem. Int. Ed. 1979, 18, 239–258. [Google Scholar] [CrossRef]
- Weinberg, N.L.; Weinberg, H.R. Electrochemical oxidation of organic compounds. Chem. Rev. 1968, 68, 449–523. [Google Scholar] [CrossRef]
- Organic Electrochemistry, 1st ed.; Baizer, M.M., Ed.; M. Dekker: New York, NY, USA, 1973. [Google Scholar]
- Organic Electrochemistry, 2nd ed.; Baizer, M.M., Ed.; M. Dekker: New York, NY, USA, 1983. [Google Scholar]
- Yoshida, K. Electrooxidation in Organic Chemistry. The Role of Cation Radicals as Synthetic Intermediates; Wiley: New York, NY, USA, 1984. [Google Scholar]
- Organic Electrochemistry, 3rd ed.; Lund, H., Baizer, M.M., Eds.; M. Dekker: New York, NY, USA, 1991. [Google Scholar]
- Hammerich, O.; Utley, J.H.P.; Eberson, L. Organic Electrochemistry, 4th ed.; Lund, H., Hammerich, O., Eds.; M. Dekker: New York, NY, USA, 2001. [Google Scholar]
- Shchepochkin, A.V.; Chupakhin, O.N.; Charushin, V.N.; Petrosyan, V.A. Direct nucleophilic functionalization of C(sp2)–H-bonds in arenes and hetarenes by electrochemical methods. Russ. Chem. Rev. 2013, 82, 747–771. [Google Scholar] [CrossRef]
- Francke, R.; Little, R.D. Redox catalysis in organic electrosynthesis: Basic principles and recent developments. Chem. Soc. Rev. 2014, 43, 2492–2521. [Google Scholar] [CrossRef]
- Karkas, M.D. Electrochemical strategies for C-H functionalization and C-N bond formation. Chem. Soc. Rev. 2018, 47, 5786–5865. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.-L.; Fang, P.; Mei, T.-S. Recent Advances in Organic Electrochemical C–H Functionalization. Chin. J. Chem. 2018, 36, 338–352. [Google Scholar] [CrossRef]
- Petrosyan, V.A. Reactions of anodic and chemical aromatic substitution. Mendeleev Commun. 2011, 21, 115–121. [Google Scholar] [CrossRef]
- Kokorekin, V.A.; Sigacheva, V.L.; Petrosyan, V.A. New data on heteroarene thiocyanation by anodic oxidation of NH4SCN. The processes of electroinduced nucleophilic aromatic substitution of hydrogen. Tetrahedron Lett. 2014, 55, 4306–4309. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.; Hanefeld, U. Green Chemistry and Catalysis; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Frontana-Uribe, B.A.; Little, R.D.; Ibanez, J.G.; Palma, A.; Vasquez-Medrano, R. Organic electrosynthesis: A promising green methodology in organic chemistry. Green Chem. 2010, 12, 2099–2119. [Google Scholar] [CrossRef]
- Heard, D.M.; Lennox, A.J.J. Electrode Materials in Modern Organic Electrochemistry. Angew. Chem. Int. Ed. 2020, 59, 18866–18884. [Google Scholar] [CrossRef]
- Lyalin, B.V.; Petrosyan, V.A. Electrochemical halogenation of organic compounds. Russ. J. Electrochem. 2013, 49, 497–529. [Google Scholar] [CrossRef]
- Beer, H.B. Dimensionally Stable Anodes. In Electrochemistry in Industry: New Directions; Landau, U., Yeager, E., Kortan, D., Eds.; Springer: Boston, MA, USA, 1982; pp. 19–28. [Google Scholar] [CrossRef]
- Janin, Y.L. Preparation and Chemistry of 3/5-Halogenopyrazoles. Chem. Rev. 2012, 112, 3924–3958. [Google Scholar] [CrossRef] [PubMed]
- Slomczynska, U.; Olivo, P.; Beattie, J.; Starkey, G.; Noueiry, A.; Roth, R. Compounds, Compositions and Methods for Control of Hepatitis C Viral Infections. International Patent 2010096115, 26 August 2010. [Google Scholar]
- Tanigchi, T.; Kawada, A.; Kondo, M.; Quinn, J.F.; Kanitomo, J.; Yoshikawa, M.; Fashimi, M. Pyridazinone Compounds. U.S. Patent 20100197651, 5 August 2010. [Google Scholar]
- Alberati, D.; Alvares, R.S.; Bleicher, K.; Flohr, A.; Markus, R.; Groeke, K.Z.; Koerner, M.; Kuhn, B.; Peters, J.-U.; Rudolph, M. Novel Imidazopyridines. U.S. Patent 20110071128, 24 March 2011. [Google Scholar]
- Linn, D.M.; Fong, E.H. Nicotinic Acetylcholine Agonists in the Treatment of Glaucoma and Retinal Neuropathy. International Patent 2004039366, 13 May 2004. [Google Scholar]
- Tomokazu, N. Therapeutic Agent for Hyperlipemia, Arteriosclerosis, and/or Metabolic Syndrome. Japan Patent 2006182668, 13 July 2006. [Google Scholar]
- D’orchumont, H.; Fraisse, L.; Zimmerman, A. Use of Indazolecarboxamide Derivatives for the Preparation of a Medicament that is Intended for the Treatment and Prevention of Paludism. International Patent 2005099703, 27 October 2005. [Google Scholar]
- Pennell, A.M.K.; Aggen, J.B.; Wright, J.J.; Sen, S.; McMaster, B.E.; Brian, E.; Dairaghi, J.D. 1-Aryl-4-substituted Piperazines Derivatives for Use as CCR1 Antagonists for the Treatment of Inflammation and Immune Disorders. International Patent 03105853, 24 December 2003. [Google Scholar]
- Singh, J.; Gurney, M.E.; Burgin, A.; Sandanayaka, V.; Kiselyov, A.; Motta, A.; Schultz, G.; Hategan, G. Biaryl as PDE4 inhibitors for treating inflammation. International Patent 2009067600, 28 May 2009. [Google Scholar]
- Talley, J.J.; Sprott, K.; Pearson, J.P.; Game, P.; Milene, G.T.; Schairer, W.; Yang, J.J.; Kim, C.; Barden, T.; Lundigran, R. Indole compounds. International Patent 2008019357, 14 February 2008. [Google Scholar]
- Pelcman, B.; Sanin, A.; Nilsson, P.; Groth, T.; Kromann, H. Pyrazoles Useful in the Treatment of Inflammation. International Patent 2007051981, 10 May 2007. [Google Scholar]
- Banner, D.; Helpert, H.; Mauser, H.; Mayweg, V.A.; Rogers-Evans, M. Amino oxazine Derivatives. International Patent 2011070029, 16 June 2011. [Google Scholar]
- Bamba, M.; Eiki, J.; Mitsuya, M.; Nishimura, T.; Sakai, F.; Sasaki, Y.; Watanabe, H. Novel 2-Pyridinecarboxamide Derivatives. International Patent 2004081001, 23 September 2004. [Google Scholar]
- Fix-Stenzel, S.; Judd, A.; Michaelides, M. Pyrrolopyridine and Pyrrolopyrimidine Inhibitors of Kinases. International Patent 2011143459, 17 November 2011. [Google Scholar]
- You, H.; Youn, H.-S.; Im, I.; Bae, M.-H.; Lee, S.-K.; Ko, H.; Eom, S.H.; Kim, Y.-C. Design, synthesis and X-ray crystallographic study of NAmPRTase inhibitors as anti-cancer agents. Eur. J. Med. Chem. 2011, 46, 1153–1164. [Google Scholar] [CrossRef]
- Ahearn, S.P.; Altman, M.; Chichetti, S.; Czako, B.; Daniels, M.H.; Falcone, D.; Guerin, D.; Lipford, K.; Martinez, M.; Osimboni, E.; et al. Tyrosine kinase Inhibitors. International Patent 2011084402, 14 July 2011. [Google Scholar]
- Basso, A.D. Anti-mitotic Agent and Aurora Kinase Inhibitor Combination as Anti-cancer Treatment. International Patent 2009017701, 5 February 2009. [Google Scholar]
- Hattori, K.; Matsuda, K.; Murata, M.; Nakajima, T.; Tsutsumi, H. Substituted 3-Pyrrolidinylthio-carbapenems as Antimicrobial Agents. International Patent 9321186, 28 October 1993. [Google Scholar]
- Barrett, D.; Matsuda, H.; Matsuda, K.; Matsuya, T.; Mizuno, H.; Murano, K.; Ogino, T.; Toda, A. New Compound. International Patent 02072621, 19 September 2002. [Google Scholar]
- Kudo, N.; Furuta, S.; Taniguchi, M.; Endo, T.; Sato, K. Synthesis and Herbicidal Activity of 1,5-Diarylpyrazole Derivatives. Chem. Pharm. Bull. 1999, 47, 857–868. [Google Scholar] [CrossRef]
- Fustero, S.; Román, R.; Sanz-Cervera, J.F.; Simón-Fuentes, A.; Cuñat, A.C.; Villanova, S.; Murguía, M. Improved Regioselectivity in Pyrazole Formation through the Use of Fluorinated Alcohols as Solvents: Synthesis and Biological Activity of Fluorinated Tebufenpyrad Analogs. J. Org. Chem. 2008, 73, 3523–3529. [Google Scholar] [CrossRef]
- Higashino, Y.; Ikeda, Y.; Koike, S.; Kyomura, N.; Okui, S.; Tomita, H. Pyrazoles and Agricultural Chemicals Containing Them as Active Ingredients. International Patent 9737990, 16 October 1997. [Google Scholar]
- Auler, T.; Bojack, G.; Bretschneider, T.; Drewes, M.W.; Feucht, D.; Fischer, R.; Hills, M.; Kehne, H.; Konze, J.; Kuck, K.-H.; et al. N-Heterocyclyl Phenyl-substituted Cyclic Ketoenols. International Patent 2004111042, 23 October 2004. [Google Scholar]
- Fischer, R.; Gomibuchi, T.; Nakakura, N.; Otsu, Y.; Shibuya, K.; Wada, K.; Yoneta, Y. N1-((Pyrazol-1-ymethyl)-2-methylphenyl)-phatalamide Derivatives and Related Compounds Insecticides. International Patent 2005095351, 13 October 2005. [Google Scholar]
- Hüttel, R.; Schäfer, O.; Welzel, G. Die Chlorierung der Pyrazole. Justus Liebigs Ann. Chem. 1956, 598, 186–197. [Google Scholar] [CrossRef]
- Ohsawa, A.; Kaihoh, T.; Itoh, T.; Okada, M.; Kawabata, C.; Yamaguchi, K.; Igeta, H. Reactions of N-Aminopyrazoles with Halogenating Reagents and Synthesis of 1, 2, 3-Triazines. Chem. Pharm. Bull. 1988, 36, 3838–3848. [Google Scholar] [CrossRef][Green Version]
- Stefani, H.A.; Pereira, C.M.P.; Almeida, R.B.; Braga, R.C.; Guzen, K.P.; Cella, R. A mild and efficient method for halogenation of 3,5-dimethyl pyrazoles by ultrasound irradiation using N-halosuccinimides. Tetrahedron Lett. 2005, 46, 6833–6837. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Wang, Z.X. Halogenation of Pyrazoles Using N-Halosuccinimides in CCl4 and in Water. Synthetic Commun. 2007, 37, 137–147. [Google Scholar] [CrossRef]
- Mullens, P.R. An improved synthesis of 1-methyl-1H-pyrazole-4-boronic acid pinacol ester and its corresponding lithium hydroxy ate complex: Application in Suzuki couplings. Tetrahedron Lett. 2009, 50, 6783–6786. [Google Scholar] [CrossRef]
- Khan, K.M.; Maharvi, G.M.; Choudhary, M.I.; Rahman, A.-U.; Perveen, S. A modified, economical and efficient synthesis of variably substituted pyrazolo[4,3-d]pyrimidin-7-ones. J. Het. Chem. 2005, 42, 1085–1093. [Google Scholar] [CrossRef]
- Kerr, E.R.; Mather, W.B. Electrochemical Chlorination of Hydrocarbon in Hydrochloric Acid-Acetic Acid Solytion. U.S. Patent 3692646, 19 September 1972. [Google Scholar]
- Ehlert, M.K.; Storr, A.; Thompson, R.C. Metal pyrazolate polymers. Part 3. Synthesis and study of Cu(I) and Cu(II) complexes of 4-Xdmpz (where X = H, Cl, Br, I, and CH3 for Cu(I) and X = H, Cl, Br, and CH3 for Cu(II); dmpz = 3,5-dimethylpyrazolate). Can. J. Chem. 1992, 70, 1121–1128. [Google Scholar] [CrossRef]
- Petko, K.; Sokolenko, T.; Yagupolskii, L. Chemical properties of derivatives of N-difluoromethyl-and N-2-H-tetrafluoroethylpyrazoles. Chem. Heterocycl. Compd. 2006, 42, 1177–1184. [Google Scholar] [CrossRef]
- Akopyan, G.A. Bromination of pyrazole-3(5)-carboxylic acid. Russ. J. Gen. Chem. 2007, 77, 1652–1653. [Google Scholar] [CrossRef]
- Guillou, S.; Bonhomme, F.J.; Ermolenko, M.S.; Janin, Y.L. Simple preparations of 4 and 5-iodinated pyrazoles as useful building blocks. Tetrahedron 2011, 67, 8451–8457. [Google Scholar] [CrossRef]
- Salanouve, E.; Guillou, S.; Bizouarne, M.; Bonhomme, F.J.; Janin, Y.L. 3-Methoxypyrazoles from 1,1-dimethoxyethene, few original results. Tetrahedron 2012, 68, 3165–3171. [Google Scholar] [CrossRef]
- Guillou, S.; Janin, Y.L. 5-Iodo-3-Ethoxypyrazoles: An Entry Point to New Chemical Entities. Chem. Eur. J. 2010, 16, 4669–4677. [Google Scholar] [CrossRef]
- Sinyakov, A.; Vasilevskii, S.; Shvartsberg, M. A new rearrangement of chloroethynylpyrazoles. Russ. Chem. Bull. 1977, 26, 2142–2147. [Google Scholar] [CrossRef]
- Vasilevskii, S.F.; Shvartsberg, M.S. Oxidative iodination of substituted N-methylpyrazoles. Russ. Chem. Bull. 1980, 29, 778–784. [Google Scholar] [CrossRef]
- Tret’yakov, E.V.; Vasitevsky, S.F. Nitrodeiodination of 4-iodo-l-methylpyrazoles. Russ. Chem. Bull. 1996, 45, 2581–2584. [Google Scholar] [CrossRef]
- Lyalin, B.V.; Petrosyan, V.A.; Ugrak, B.I. Electrosynthesis of 4-chloropyrazolecarboxylic acids. Russ. Chem. Bull. 2009, 58, 291–296. [Google Scholar] [CrossRef]
- Perevalov, V.P.; Manaev, Y.A.; Bezborodov, B.V.; Stepanov, B.I. Chlorination of 1,5-dimethylpyrazole. Chem. Heterocycl. Compd. 1990, 26, 301–303. [Google Scholar] [CrossRef]
- Lyalin, B.V.; Petrosyan, V.A.; Ugrak, B.I. Electrosynthesis of 4-chloro derivatives of pyrazole and alkylpyrazoles. Russ. J. Electrochem. 2008, 44, 1320–1326. [Google Scholar] [CrossRef]
- Lyalin, B.V.; Petrosyan, V.A. The Reactivity Trends in Electrochemical Chlorination and Bromination of N-Substituted and N-Unsubstituted Pyrazoles. Chem. Heterocycl. Compd. 2014, 49, 1599–1610. [Google Scholar] [CrossRef]
- Lyalin, B.V.; Petrosyan, V.A.; Ugrak, B.I. Electrosynthesis of 4-bromosubstituted pyrazole and its derivatives. Russ. J. Electrochem. 2010, 46, 123–129. [Google Scholar] [CrossRef]
- Lyalin, B.V.; Petrosyan, V.A.; Ugrak, B.I. Electrosynthesis of 4-iodopyrazole and its derivatives. Russ. Chem. Bull. 2010, 59, 1549–1555. [Google Scholar] [CrossRef]
- Lyalin, B.V.; Petrosyan, V.A. New approach to electrochemical iodination of arenes exemplified by the synthesis of 4-iodopyrazoles of different structures. Russ. Chem. Bull. 2014, 63, 360–367. [Google Scholar] [CrossRef]
- Lyalin, B.V.; Petrosyan, V.A. Efficient iodination of structurally varying pyrazoles in heterophase medium. Russ. Chem. Bull. 2013, 62, 1044–1051. [Google Scholar] [CrossRef]
- Noszticzius, Z.; Noszticzius, E.; Schelly, Z.A. Use of ion-selective electrodes for monitoring oscillating reactions. 1. Potential response of the silver halide membrane electrodes to hypohalous acids. J. Am. Chem. Soc. 1982, 104, 6194–6199. [Google Scholar] [CrossRef]
- Kanishchev, M.I.; Korneeva, N.V.; Shevelev, S.A.; Fainzil’berg, A.A. Nitropyrazoles (review). Chem. Heterocycl. Compd. 1988, 24, 353–370. [Google Scholar] [CrossRef]
- Belen’kii, L.I.; Chuvylkin, N.D. Relationships and features of electrophilic substitution reactions in the azole series. Chem. Heterocycl. Compd. 1996, 32, 1319–1343. [Google Scholar] [CrossRef]
- Pevzner, M.S. Aromatic N-Haloazoles. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 75, pp. 1–77. [Google Scholar]
- Lyalin, B.V.; Petrosyan, V.A. Oxidation of organic compounds on NiOOH electrode. Russ. J. Electrochem. 2010, 46, 1199–1214. [Google Scholar] [CrossRef]
- Nikoofar, K. A Brief on Thiocyanation of N-Activated Arenes and N-Bearing Heteroaromatic Compounds. Chem. Sci. Trans. 2013, 3, 691–700. [Google Scholar] [CrossRef][Green Version]
- Castanheiro, T.; Suffert, J.; Donnard, M.; Gulea, M. Recent advances in the chemistry of organic thiocyanates. Chem. Soc. Rev. 2016, 45, 494–505. [Google Scholar] [CrossRef]
- Majedi, S.; Sreerama, L.; Vessally, E.; Behmagham, F. Metal-Free Regioselective Thiocyanation of (Hetero) Aromatic C-H Bonds using Ammonium Thiocyanate: An Overview. J. Chem. Lett. 2020, 1, 25–31. [Google Scholar] [CrossRef]
- Rezayati, S.; Ramazani, A. A review on electrophilic thiocyanation of aromatic and heteroaromatic compounds. Tetrahedron 2020, 76, 131382. [Google Scholar] [CrossRef]
- Maurya, C.K.; Mazumder, A.; Gupta, P.K. Phosphorus pentasulfide mediated conversion of organic thiocyanates to thiols. Beilstein J. Org. Chem. 2017, 13, 1184–1188. [Google Scholar] [CrossRef]
- Noland, W.E.; Lanzatella, N.P.; Dickson, R.R.; Messner, M.E.; Nguyen, H.H. Access to Indoles via Diels–Alder Reactions of 5-Methylthio-2-vinylpyrroles with Maleimides. J. Heterocycl. Chem. 2013, 50, 795–808. [Google Scholar] [CrossRef]
- Maurya, C.K.; Mazumder, A.; Kumar, A.; Gupta, P.K. Synthesis of Disulfanes from Organic Thiocyanates Mediated by Sodium in Silica Gel. Synlett 2016, 27, 409–411. [Google Scholar] [CrossRef]
- Biswas, K.; Ghosh, S.; Ghosh, P.; Basu, B. Cyclic ammonium salts of dithiocarbamic acid: Stable alternative reagents for the synthesis of S-alkyl carbodithioates from organyl thiocyanates in water. J. Sulfur Chem. 2016, 37, 361–376. [Google Scholar] [CrossRef]
- Li, W.-T.; Wu, W.-H.; Tang, C.-H.; Tai, R.; Chen, S.-T. One-Pot Tandem Copper-Catalyzed Library Synthesis of 1-Thiazolyl-1,2,3-triazoles as Anticancer Agents. ACS Comb. Sci. 2011, 13, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Baghershiroudi, M.; Safa, K.D.; Adibkia, K.; Lotfipour, F. Synthesis and antibacterial evaluation of new sulfanyltetrazole derivatives bearing piperidine dithiocarbamate moiety. Synth. Commun. 2018, 48, 323–328. [Google Scholar] [CrossRef]
- Kokorekin, V.A.; Terent’ev, A.O.; Ramenskaya, G.V.; Grammatikova, N.É.; Rodionova, G.M.; Ilovaiskii, A.I. Synthesis and Antifungal Activity of Arylthiocyanates. Pharm. Chem. J. 2013, 47, 422–425. [Google Scholar] [CrossRef]
- Fortes, M.P.; da Silva, P.B.N.; da Silva, T.G.; Kaufman, T.S.; Militão, G.C.G.; Silveira, C.C. Synthesis and preliminary evaluation of 3-thiocyanato-1H-indoles as potential anticancer agents. Eur. J. Med. Chem. 2016, 118, 21–26. [Google Scholar] [CrossRef]
- Chao, M.N.; Matiuzzi, C.E.; Storey, M.; Li, C.; Szajnman, S.H.; Docampo, R.; Moreno, S.N.J.; Rodriguez, J.B. Aryloxyethyl Thiocyanates Are Potent Growth Inhibitors of Trypanosoma cruzi and Toxoplasma gondii. ChemMedChem 2015, 10, 1094–1108. [Google Scholar] [CrossRef]
- Kokorekin, V.A.; Khodonov, V.M.; Neverov, S.V.; Grammatikova, N.É.; Petrosyan, V.A. “Metal-free” synthesis and antifungal activity of 3-thiocyanatopyrazolo[1,5-a]pyrimidines. Russ. Chem. Bull. 2021, 70, 600–604. [Google Scholar] [CrossRef]
- Yi, X.-J.; El-Idreesy, T.T.; Eldebss, T.M.A.; Farag, A.M.; Abdulla, M.M.; Hassan, S.A.; Mabkhot, Y.N. Synthesis, biological evaluation, and molecular docking studies of new pyrazol-3-one derivatives with aromatase inhibition activities. Chem. Biol. Drug Des. 2016, 88, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.L. Substitution and Addition Reactions of Thiocyanogen. In Organic Reactions; John Wiley & Sons, Inc.: New York, NY, USA, 1946; Volume 3, pp. 240–266. [Google Scholar]
- Guy, R.G. Syntheses and preparative applications of thiocyanates. In Cyanates and Their Thio Derivatives; Patai, S., Ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 1977; Volume 2, pp. 819–886. [Google Scholar]
- Jason, A.; Phadke, A.S.; Deshpande, M.; Agarwak, A.; Chen, D.; Gadhachanda, V.R.; Hashimoto, A.; Pais, G.; Wang, Q.; Wang, X. Aryl, Heteroaryl, and Heterocyclic Compounds for Treatment of Medical Disorders. WO2017035353A1, 2 March 2017. [Google Scholar]
- Rodríguez, R.; Camargo, P.; Sierra, C.A.; Soto, C.Y.; Cobo, J.; Nogueras, M. Iodine mediated an efficient and greener thiocyanation of aminopyrimidines by a modification of the Kaufmann’s reaction. Tetrahedron Lett. 2011, 52, 2652–2654. [Google Scholar] [CrossRef]
- Reddy, B.V.S.; Reddy, S.M.S.; Madan, C. NBS or DEAD as effective reagents in α-thiocyanation of enolizable ketones with ammonium thiocyanate. Tetr. Lett. 2011, 52, 1432–1435. [Google Scholar] [CrossRef]
- Mahajan, U.S.; Akamanchi, K.G. Facile Method for Thiocyanation of Activated Arenes Using Iodic Acid in Combination with Ammonium Thiocyanate. Synthetic Commun. 2009, 39, 2674–2682. [Google Scholar] [CrossRef]
- Khazaei, A.; Zolfigol, M.A.; Mokhlesi, M.; Panah, F.D.; Sajjadifar, S. Simple and Highly Efficient Catalytic Thiocyanation of Aromatic Compounds in Aqueous Media. Helv. Chim. Acta 2012, 95, 106–114. [Google Scholar] [CrossRef]
- Wu, J.; Wu, G.; Wu, L. Thiocyanation of Aromatic and Heteroaromatic Compounds using Ammonium Thiocyanate and I2O5. Synthetic Commun. 2008, 38, 2367–2373. [Google Scholar] [CrossRef]
- Ali, D.; Panday, A.K.; Choudhury, L.H. Hydrogen Peroxide-Mediated Rapid Room Temperature Metal-Free C(sp2)-H Thiocyanation of Amino Pyrazoles, Amino Uracils, and Enamines. J. Org. Chem. 2020, 85, 13610–13620. [Google Scholar] [CrossRef]
- Sajjadifar, S.; Louie, O. Regioselective Thiocyanation of Aromatic and Heteroaromatic Compounds by Using Boron Sulfonic Acid as a New, Efficient, and Cheap Catalyst in Water. J. Chem. 2013, 2013, 6. [Google Scholar] [CrossRef]
- Khalili, D. Highly efficient and regioselective thiocyanation of aromatic amines, anisols and activated phenols with H2O2/NH4SCN catalyzed by nanomagnetic Fe3O4. Chin. Chem. Lett. 2015, 26, 547–552. [Google Scholar] [CrossRef]
- Khazaei, A.; Zolfigol, M.A.; Mokhlesi, M.; Pirveysian, M. Citric acid as a trifunctional organocatalyst for thiocyanation of aromatic and heteroaromatic compounds in aqueous media. Can. J. Chem. 2012, 90, 427–432. [Google Scholar] [CrossRef]
- Songsichan, T.; Katrun, P.; Khaikate, O.; Soorukram, D.; Pohmakotr, M.; Reutrakul, V.; Kuhakarn, C. Thiocyanation of Pyrazoles Using KSCN/K2S2O8 Combination. SynOpen 2018, 2, 6–16. [Google Scholar] [CrossRef]
- Mete, T.B.; Khopade, T.M.; Bhat, R.G. Transition-metal-free regioselective thiocyanation of phenols, anilines and heterocycles. Tetrahedron Lett. 2017, 58, 415–418. [Google Scholar] [CrossRef]
- Karimi Zarchi, M.A.; Banihashemi, R. Thiocyanation of aromatic and heteroaromatic compounds using polymer-supported thiocyanate ion as the versatile reagent and ceric ammonium nitrate as the versatile single-electron oxidant. J. Sulfur Chem. 2016, 37, 282–295. [Google Scholar] [CrossRef]
- Pan, X.-Q.; Lei, M.-Y.; Zou, J.-P.; Zhang, W. Mn(OAc)3-promoted regioselective free radical thiocyanation of indoles and anilines. Tetrahedron Lett. 2009, 50, 347–349. [Google Scholar] [CrossRef]
- Das, B.; Kumar, A.S. Efficient Thiocyanation of Indoles Using Para-Toluene Sulfonic Acid. Synth. Commun. 2010, 40, 337–341. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, Q.; Wei, S.; Qu, J.; Wang, B. Highly Efficient and Practical Thiocyanation of Imidazopyridines Using an N-Chlorosuccinimide/NaSCN Combination. Eur. J. Org. Chem. 2016, 2016, 3373–3379. [Google Scholar] [CrossRef]
- Muniraj, N.; Dhineshkumar, J.; Prabhu, K.R. N-Iodosuccinimide Catalyzed Oxidative Selenocyanation and Thiocyanation of Electron Rich Arenes. ChemistrySelect 2016, 1, 1033–1038. [Google Scholar] [CrossRef]
- Mokhtari, B.; Azadi, R.; Rahmani-Nezhad, S. In situ-generated N-thiocyanatosuccinimide (NTS) as a highly efficient reagent for the one-pot thiocyanation or isothiocyanation of alcohols. Tetrahedron Lett. 2009, 50, 6588–6589. [Google Scholar] [CrossRef]
- Memarian, H.R.; Mohammadpoor-Baltork, I.; Nikoofar, K. DDQ-promoted thiocyanation of aromatic and heteroaromatic compounds. Can. J. Chem. 2007, 85, 930–937. [Google Scholar] [CrossRef]
- Memarian, H.R.; Mohammadpoor-Baltork, I.; Nikoofar, K. Ultrasound-assisted thiocyanation of aromatic and heteroaromatic compounds using ammonium thiocyanate and DDQ. Ultrason. Sonochem. 2008, 15, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Gitkis, A.; Becker, J.Y. Anodic thiocyanation of mono- and disubstituted aromatic compounds. Electrochim. Acta 2010, 55, 5854–5859. [Google Scholar] [CrossRef]
- Fotouhi, L.; Nikoofar, K. Electrochemical thiocyanation of nitrogen-containing aromatic and heteroaromatic compounds. Tetrahedron Lett. 2013, 54, 2903–2905. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Jiang, H.; Sun, L. A low-cost electrochemical thio- and selenocyanation strategy for electron-rich arenes under catalyst- and oxidant-free conditions. RSC Adv. 2018, 8, 22042–22045. [Google Scholar] [CrossRef]
- Kokorekin, V.A.; Yaubasarova, R.R.; Neverov, S.V.; Petrosyan, V.A. Reactivity of electrogenerated thiocyanogen in the thiocyanation of pyrazolo[1,5-a]pyrimidines. Mendeleev Commun. 2016, 26, 413–414. [Google Scholar] [CrossRef]
- Kokorekin, V.A.; Mel’nikova, E.I.; Yaubasarova, R.R.; Gorpinchenko, N.V.; Petrosyan, V.A. “Metal-free” electrooxidative C—H thiocyanation of arenes. Russ. Chem. Bull. 2019, 68, 2140–2141. [Google Scholar] [CrossRef]
- Kokorekin, V.A.; Yaubasarova, R.R.; Neverov, S.V.; Petrosyan, V.A. Electrooxidative C–H Functionalization of Heteroarenes. Thiocyanation of Pyrazolo[1,5-a]pyrimidines. Eur. J. Org. Chem. 2019, 2019, 4233–4238. [Google Scholar] [CrossRef]
- Yaubasarova, R.R.; Kokorekin, V.A.; Ramenskaya, G.V.; Petrosyan, V.A. Double electrooxidative C–H functionalization of (het)arenes with thiocyanate and 4-nitropyrazolate ions. Mendeleev Commun. 2019, 29, 334–336. [Google Scholar] [CrossRef]
- Kokorekin, V.A.; Melnikova, E.I.; Yaubasarova, R.R.; Petrosyan, V.A. Electrooxidative C–H thiocyanation of hetarenes: Voltammetric assessment of thiocyanogen reactivity. Mendeleev Commun. 2020, 30, 70–72. [Google Scholar] [CrossRef]
- Kokorekin, V.A.; Neverov, S.V.; Kuzina, V.N.; Petrosyan, V.A. A New Method for the Synthesis of 3-Thiocyanatopyrazolo[1,5-a]pyrimidines. Molecules 2020, 25, 4169. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F. New Developments in the Study of the Structure of Parathiocyanogen: (SCN)x, An Inorganic Polymer. J. Inorg. Organomet. Polym. 1997, 7, 35–50. [Google Scholar] [CrossRef]
- Shaabani, A.; Nazeri, M.T.; Afshari, R. 5-Amino-pyrazoles: Potent reagents in organic and medicinal synthesis. Mol. Divers. 2019, 23, 751–807. [Google Scholar] [CrossRef] [PubMed]
- Cherukupalli, S.; Karpoormath, R.; Chandrasekaran, B.; Hampannavar, G.A.; Thapliyal, N.; Palakollu, V.N. An insight on synthetic and medicinal aspects of pyrazolo[1,5-a]pyrimidine scaffold. Eur. J. Med. Chem. 2017, 126, 298–352. [Google Scholar] [CrossRef] [PubMed]
- Arias-Gómez, A.; Godoy, A.; Portilla, J. Functional Pyrazolo[1,5-a]pyrimidines: Current Approaches in Synthetic Transformations and Uses as an Antitumor Scaffold. Molecules 2021, 26, 2708. [Google Scholar] [CrossRef] [PubMed]
- Sigacheva, V.L.; Kokorekin, V.A.; Strelenko, Y.A.; Neverov, S.V.; Petrosyan, V.A. Electrochemical Azolation of N-substituted Pyrroles: A New Case in SNH(An) Reactions. Mendeleev Commun. 2012, 22, 270–272. [Google Scholar] [CrossRef]
- Henton, D.R.; McCreery, R.A.; Swenton, J.S. Anodic oxidation of 1,4-dimethoxy aromatic compounds. A facile route to functionalized quinone bisketals. J. Org. Chem. 1980, 45, 369–378. [Google Scholar] [CrossRef]
- Adibi, H.; Rashidi, A.; Khodaei, M.M.; Alizadeh, A.; Majnooni, M.B.; Pakravan, N.; Abiri, R.; Nematollahi, D. Catecholthioether Derivatives: Preliminary Study of in-Vitro Antimicrobial and Antioxidant Activities. Chem. Pharm. Bull. 2011, 59, 1149–1152. [Google Scholar] [CrossRef]
- Kokorekin, V.A.; Solomatin, Y.A.; Gening, M.L.; Petrosyan, V.A. Acid-catalyzed SNH(An) thiolation of p-dihydroxybenzene. Mendeleev Commun. 2017, 27, 586–588. [Google Scholar] [CrossRef]
- Chen, C.; Niu, P.; Shen, Z.; Li, M. Electrochemical Sulfenylation of Indoles with Disulfides Mediated by Potassium Iodide. J. Electrochem. Soc. 2018, 165, G67–G74. [Google Scholar] [CrossRef]
- Petrosyan, V.A.; Burasov, A.V. Anodic azolation of 1,2- and 1,3-dimethoxybenzenes. Russ. Chem. Bull. 2010, 59, 522–527. [Google Scholar] [CrossRef]
- Feng, P.; Ma, G.; Chen, X.; Wu, X.; Lin, L.; Liu, P.; Chen, T. Electrooxidative and Regioselective C−H Azolation of Phenol and Aniline Derivatives. Angew. Chem. Int. Ed. 2019, 58, 8400–8404. [Google Scholar] [CrossRef]
- Wang, J.-H.; Lei, T.; Nan, X.-L.; Wu, H.-L.; Li, X.-B.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Regioselective Ortho Amination of an Aromatic C–H Bond by Trifluoroacetic Acid via Electrochemistry. Org. Lett. 2019, 21, 5581–5585. [Google Scholar] [CrossRef] [PubMed]
- Ananikov, V.P.; Eremin, D.B.; Yakukhnov, S.A.; Dilman, A.D.; Levin, V.V.; Egorov, M.P.; Karlov, S.S.; Kustov, L.M.; Tarasov, A.L.; Greish, A.A.; et al. Organic and hybrid systems: From science to practice. Mendeleev Commun. 2017, 27, 425–438. [Google Scholar] [CrossRef]
- Hunger, K. Industrial Dyes: Chemistry, Properties, Applications; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Sandborn, W.J. Rational selection of oral 5-aminosalicylate formulations and prodrugs for the treatment of ulcerative colitis. Am. J. Gastroenterol. 2002, 97, 2939–2941. [Google Scholar] [CrossRef]
- Ovchinnikov, I.V.; Kulikov, A.S.; Makhova, N.N.; Tosco, P.; Di Stilo, A.; Fruttero, R.; Gasco, A. Synthesis and vasodilating properties of N-alkylamide derivatives of 4-amino-3-furoxancarboxylic acid and related azo derivatives. Il Farmaco 2003, 58, 677–681. [Google Scholar] [CrossRef]
- Iranpoor, N.; Firouzabadi, H.; Khalili, D.; Motevalli, S. Easily Prepared Azopyridines as Potent and Recyclable Reagents for Facile Esterification Reactions. An Efficient Modified Mitsunobu Reaction. J. Org. Chem. 2008, 73, 4882–4887. [Google Scholar] [CrossRef]
- Iranpoor, N.; Firouzabadi, H.; Shahin, R.; Khalili, D. 2,2′-Azobenzothiazole as a New Recyclable Oxidant for Heterogeneous Thiocyanation of Aromatic Compounds with Ammonium Thiocyanate. Synth. Commun. 2011, 42, 2040–2047. [Google Scholar] [CrossRef]
- Ovchinnikov, I.V.; Makhova, N.N.; Khmel’nitsky, L.I.; Kuz’min, V.S.; Akimova, L.N.; Pepekin, V.I. Dinitrodiazenofuroxan as a new energetic explosive. Dokl. Chem. 1998, 359, 67–70. [Google Scholar]
- Veauthier, J.M.; Chavez, D.E.; Tappan, B.C.; Parrish, D.A. Synthesis and Characterization of Furazan Energetics ADAAF and DOATF. J. Energ. Mater. 2010, 28, 229–249. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Mostafavipoor, Z. Barium Manganate. A Versatile Oxidant in Organic Synthesis. Bull. Chem. Soc. Jpn. 1983, 56, 914–917. [Google Scholar] [CrossRef]
- Birchall, J.M.; Haszeldine, R.N.; Kemp, J.E.G. Polyfluoroarenes. Part X. Polyfluoroaromatic azo-compounds. J. Chem. Soc. C 1970, 449–455. [Google Scholar] [CrossRef]
- Farhadi, S.; Zaringhadama, P.; Sahamiehb, R.Z. Photo-assisted Oxidation of Anilines and Other Primary Aromatic Amines to Azo Compounds Using Mercury (II) Oxide as a Photo-Oxidant. Acta Chim. Slov. 2007, 54, 647–653. [Google Scholar]
- Huang, H.; Sommerfeld, D.; Dunn, B.C.; Lloyd, C.R.; Eyring, E.M. Ferrate(VI) oxidation of aniline. J. Chem. Soc. Dalton Trans. 2001, 1301–1305. [Google Scholar] [CrossRef]
- Chavez, D.E.; Parrish, D.A.; Leonard, P. The Synthesis and Characterization of a New Furazan Heterocyclic System. Synlett 2012, 23, 2126–2128. [Google Scholar] [CrossRef]
- Okumura, S.; Lin, C.-H.; Takeda, Y.; Minakata, S. Oxidative Dimerization of (Hetero)aromatic Amines Utilizing t-BuOI Leading to (Hetero)aromatic Azo Compounds: Scope and Mechanistic Studies. J. Org. Chem. 2013, 78, 12090–12105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiao, N. Copper-Catalyzed Aerobic Oxidative Dehydrogenative Coupling of Anilines Leading to Aromatic Azo Compounds using Dioxygen as an Oxidant. Angew. Chem. 2010, 122, 6310–6313. [Google Scholar] [CrossRef]
- Jiang, B.; Ning, Y.; Fan, W.; Tu, S.-J.; Li, G. Oxidative Dehydrogenative Couplings of Pyrazol-5-amines Selectively Forming Azopyrroles. J. Org. Chem. 2014, 79, 4018–4024. [Google Scholar] [CrossRef]
- Takeda, Y.; Okumura, S.; Minakata, S. Oxidative Dimerization of Aromatic Amines using tBuOI: Entry to Unsymmetric Aromatic Azo Compounds. Angew. Chem. Int. Ed. 2012, 51, 7804–7808. [Google Scholar] [CrossRef]
- Pandit, R.P.; Lee, Y.R. Construction of Multifunctionalized Azopyrazoles by Silver- Catalyzed Cascade Reaction of Diazo Compounds. Adv. Synth. Catal. 2015, 357, 2657–2664. [Google Scholar] [CrossRef]
- Wawzonek, S.; McIntyre, T. Electrolytic oxidation of aromatic amines. J. Electrochem. Soc. 1967, 114, 1025. [Google Scholar] [CrossRef]
- Wawzonek, S.; McIntyre, T. Electrolytic preparation of azobenzenes. J. Electrochem. Soc. 1972, 119, 1350. [Google Scholar] [CrossRef]
- Schäfer, H.-J. Oxidation of organic compounds at the nickel hydroxide electrode. In Electrochemistry I. Top. Curr. Chem; Steckhan, E., Ed.; Springer: Berlin, Germany; New York, NY, USA, 1987; Volume 142, pp. 101–129. [Google Scholar]
- Lyalin, B.V.; Sigacheva, V.L.; Kokorekin, V.A.; Petrosyan, V.A. A new synthesis of azopyrazoles by oxidation of C-aminopyrazoles on a NiO(OH) electrode. Mendeleev Commun. 2015, 25, 479–481. [Google Scholar] [CrossRef]
- Lyalin, B.V.; Sigacheva, V.L.; Kokorekin, V.A.; Petrosyan, V.A. Electrosynthesis of azopyrazoles via the oxidation of N-alkylaminopyrazoles on a NiO(OH) anode in aqueous alkali—A green method for N-N homocoupling. Tetrahedron Lett. 2018, 59, 2741–2744. [Google Scholar] [CrossRef]
- Lyalin, B.V.; Sigacheva, V.L.; Petrosyan, V.A. Electrocatalytic N—N cross-coupling of N-alkyl-3-aminopyrazoles at a nickel anode. Russ. Chem. Bull. 2020, 69, 2020–2021. [Google Scholar] [CrossRef]
- Lyalin, B.V.; Sigacheva, V.L.; Kokorekin, V.A.; Dutova, T.Y.; Rodionova, G.M.; Petrosyan, V.A. Oxidative transformation of N-substituted 3-aminopyrazoles to azopyrazoles using electrogenerated bromine as a mediator. Russ. Chem. Bull. 2018, 67, 510–516. [Google Scholar] [CrossRef]
- Lyalin, B.V.; Sigacheva, V.L.; Kokorekin, V.A.; Petrosyan, V.A. Oxidative conversion of N-substituted 3-aminopyrazoles to azopyrazoles using electrogenerated NaOCl as the mediator. Arkivoc 2017, 2017, 55–62. [Google Scholar] [CrossRef]
- Lyalin, B.V.; Sigacheva, V.L.; Ugrak, B.I.; Petrosyan, V.A. Oxidative N—N coupling of N-alkyl-3-aminopyrazoles to azopyrazoles in aqueous solutions of NaOCl and NaOBr. Russ. Chem. Bull. 2021, 70, 164–170. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).