IEDDA: An Attractive Bioorthogonal Reaction for Biomedical Applications
Abstract
:1. The Emergence of the Pretargeting Approach
1.1. Limitations of Direct Targeting
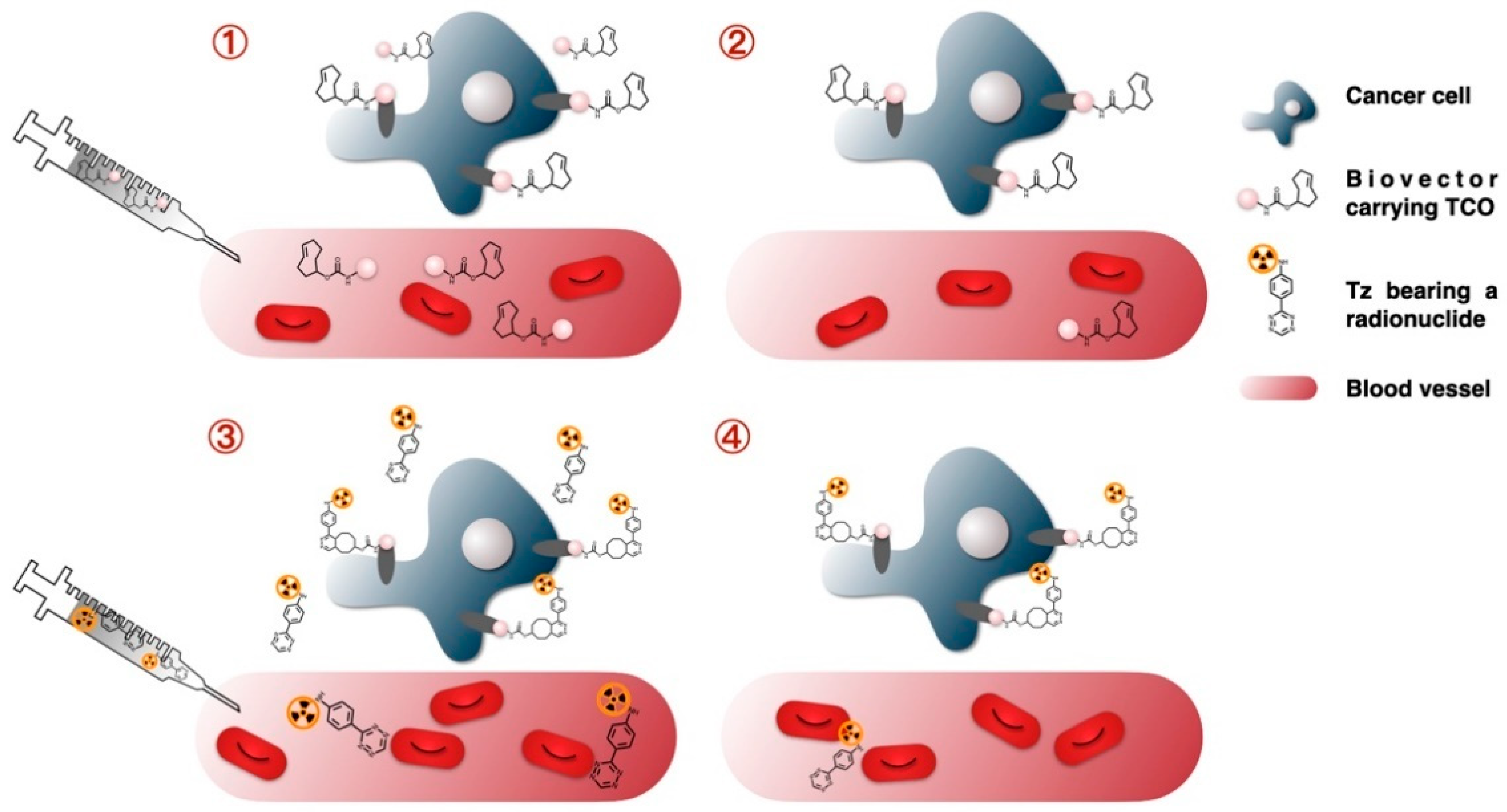
1.2. Basic Principle of the Pretargeting Approach
2. Bioorthogonal Reactions
2.1. The Staudinger Ligation
2.2. Copper-Catalyzed [3 + 2] Azide–Alkyne Cycloaddition (CuAAC)
2.3. Strain-Promoted [3 + 2] Azide–Alkyne Cycloaddition (SPAAC)
2.4. Inverse Electron-Demand Diels-Alder (IEDDA)
3. Dienes
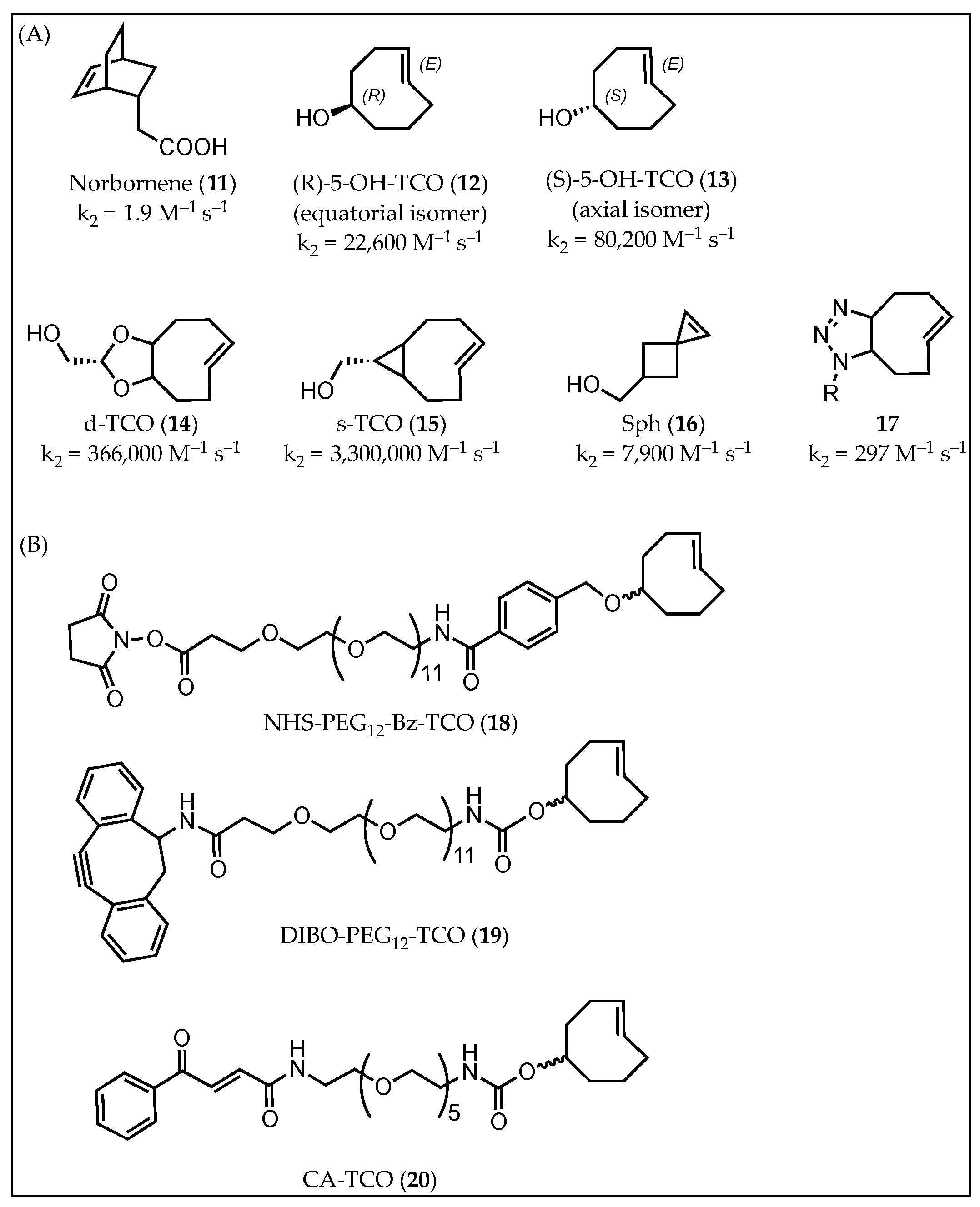
4. Dienophiles
5. Applications
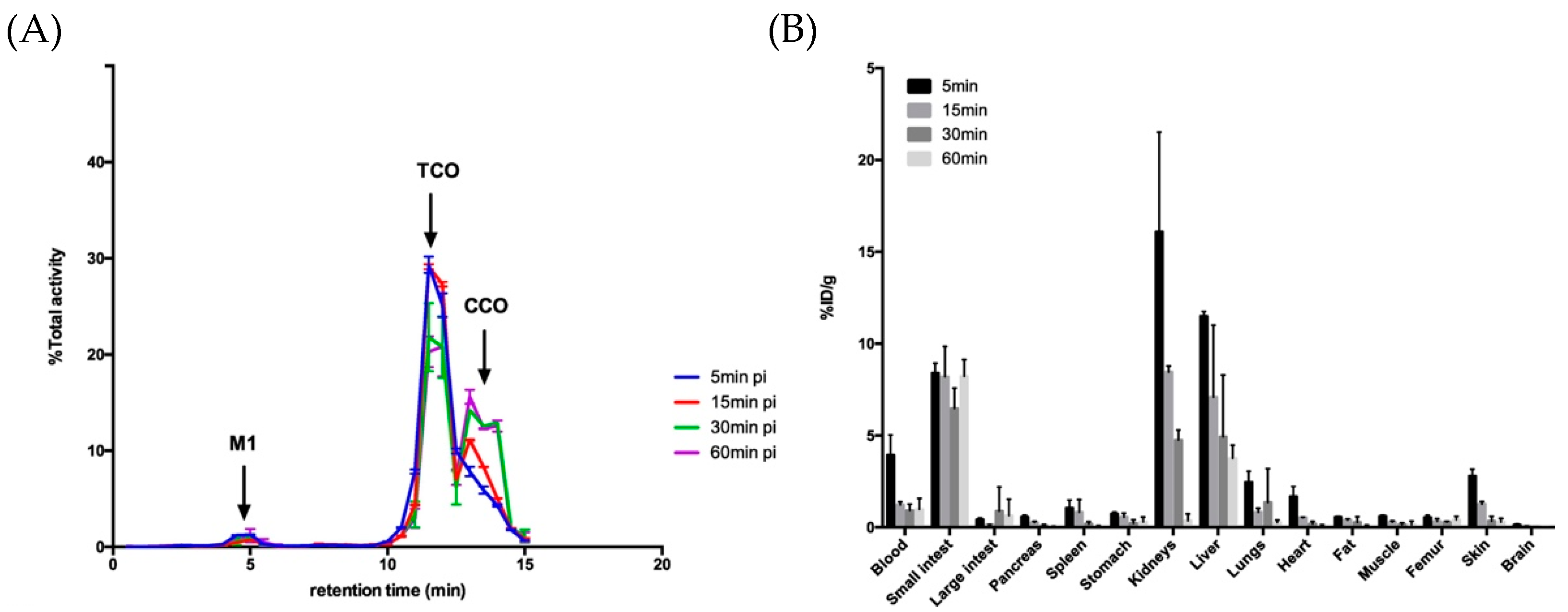
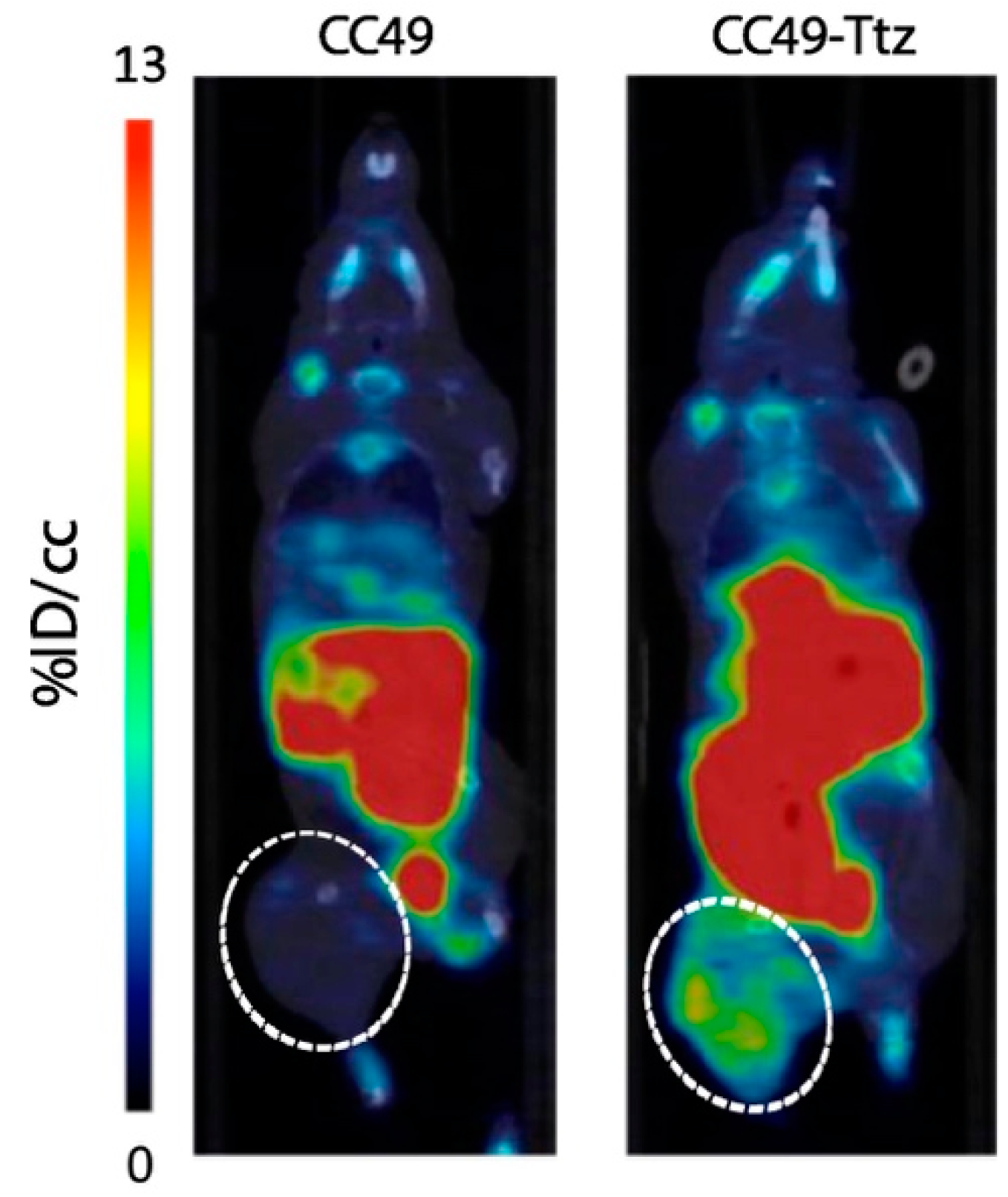
5.1. Radiolabeling of Monoclonal Antibodies (mAbs) with Short-Lived Radionuclides and Pretargeting
5.2. Radiolabeling of Nanoparticles
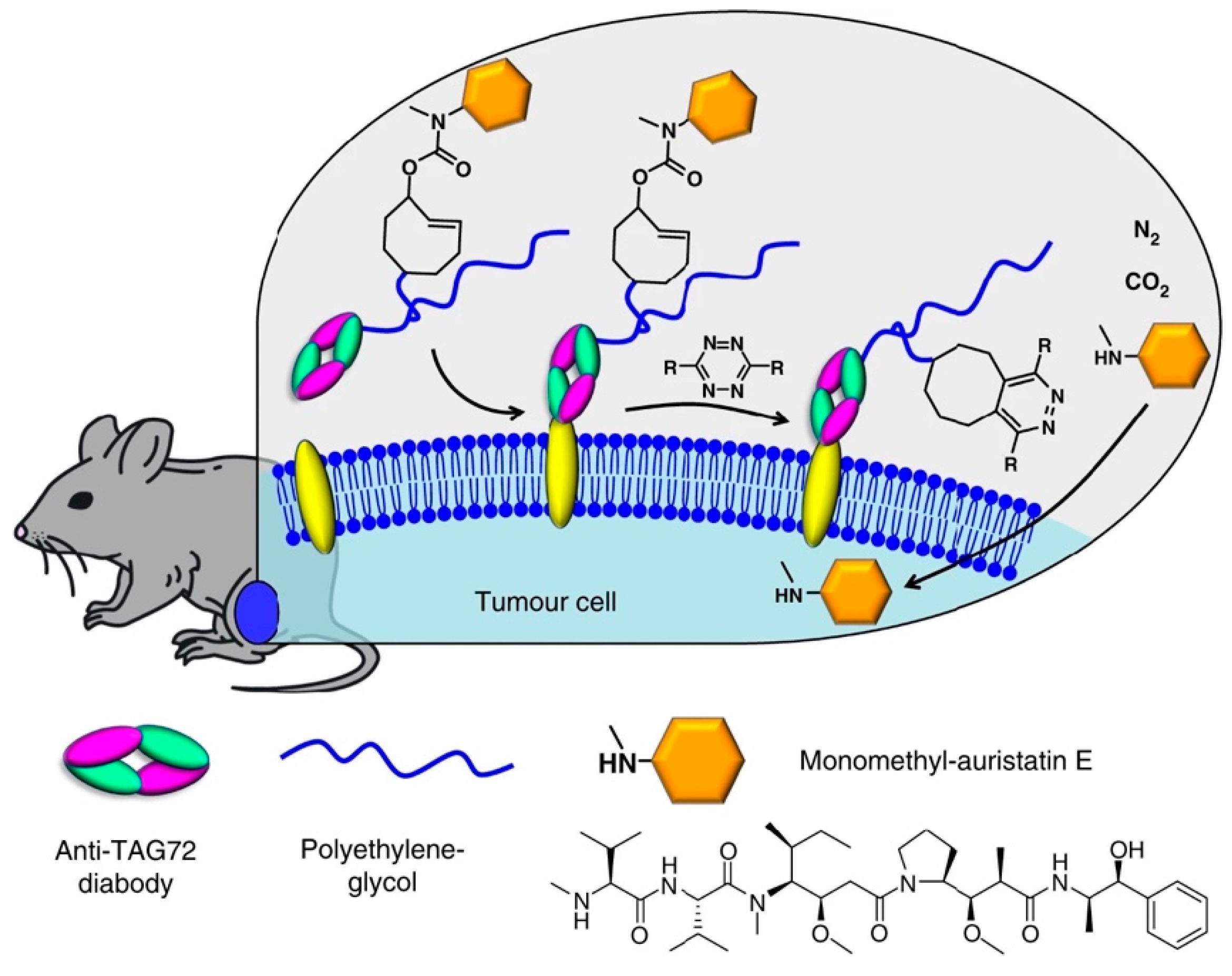
5.3. Drug to Release
5.4. Activatable Fluorescence Probes
5.5. Photodynamic Therapy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sharkey, R.M.; Goldenberg, D.M. Advances in Radioimmunotherapy in the Age of Molecular Engineering and Pretargeting. Cancer Investig. 2006, 24, 82–97. [Google Scholar] [CrossRef]
- Carroll, V.; Demoin, D.; Hoffman, T.; Jurisson, S. Inorganic chemistry in nuclear imaging and radiotherapy: Current and future directions. Radiochim. Acta 2012, 100, 653–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keire, D.A.; Jang, Y.H.; Li, L.; Dasgupta, S.; Goddard, W.A.; Shively, J.E. Chelators for radioimmunotherapy: I. NMR and ab initio calculation studies on 1,4,7,10-tetra(carboxyethyl)-1,4,7,10-tetraazacyclododecane (DO4Pr) and 1,4,7-tris(carboxymethyl)-10-(carboxyethyl)-1,4,7,10-tetraazacyclododecane (DO3A1Pr). Inorg. Chem. 2001, 40, 4310–4318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boerman, O.C.; Van Schaijk, F.G.; Oyen, W.J.G.; Corstens, F.H.M. Pretargeted radioimmunotherapy of cancer: Progress step by step. J. Nucl. Med. 2003, 44, 400–411. [Google Scholar]
- Zeglis, B.M.; Sevak, K.K.; Reiner, T.; Mohindra, P.; Carlin, S.; Zanzonico, P.; Weissleder, R.; Lewis, J.S. A Pretargeted PET Imaging Strategy Based on Bioorthogonal Diels–Alder Click Chemistry. J. Nucl. Med. 2013, 54, 1389–1396. [Google Scholar] [CrossRef] [Green Version]
- Keinänen, O.; Fung, K.; Pourat, J.; Jallinoja, V.; Vivier, D.; Pillarsetty, N.K.; Airaksinen, A.J.; Lewis, J.S.; Zeglis, B.M.; Sarparanta, M. Pretargeting of internalizing trastuzumab and cetuximab with a 18F-tetrazine tracer in xenograft models. EJNMMI Res. 2017, 7, 95. [Google Scholar] [CrossRef] [Green Version]
- Altai, M.; Membreno, R.; Cook, B.; Tolmachev, V.; Zeglis, B.M. Pretargeted Imaging and Therapy. J. Nucl. Med. 2017, 58, 1553–1559. [Google Scholar] [CrossRef]
- Batra, S.K.; Jain, M.; Wittel, U.; Chauhan, S.C.; Colcher, D. Pharmacokinetics and biodistribution of genetically engineered antibodies. Curr. Opin. Biotechnol. 2002, 13, 603–608. [Google Scholar] [CrossRef]
- Sharkey, R.M.; Blumenthal, R.D.; Hansen, H.J.; Goldenberg, D.M. Biological considerations for radioimmunotherapy. Cancer Res. 1990, 50, 964–970. [Google Scholar]
- Patra, M.; Zarschler, K.; Pietzsch, H.-J.; Stephan, H.; Gasser, G. New insights into the pretargeting approach to image and treat tumours. Chem. Soc. Rev. 2016, 45, 6415–6431. [Google Scholar] [CrossRef] [Green Version]
- Reardan, D.T.; Meares, C.F.; Goodwin, D.; McTigue, M.; David, G.S.; Stone, M.R.; Leung, J.P.; Bartholomew, R.M.; Frincke, J.M. Antibodies against metal chelates. Nature 1985, 316, 265–268. [Google Scholar] [CrossRef]
- Goodwin, D.A.; Mears, C.F.; McTigue, M.; David, G.S. Goodwin1986.Pdf. Nucl. Med. Commun. 1986, 7, 569–580. [Google Scholar] [CrossRef]
- Goodwin, D.; Meares, C.; Diamanti, C.; McCall, M.; Lai, C.; Torti, F.; McTigue, M.; Martin, B. Use of specific antibody for rapid clearance of circulating blood background from radiolabeled tumor imaging proteins. Eur. J. Nucl. Med. Mol. Imaging 1984, 9, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Stéen, E.J.L.; Edem, P.; Norregaard, K.; Jørgensen, J.T.; Shalgunov, V.; Kjaer, A.; Herth, M.M. Pretargeting in nuclear imaging and radionuclide therapy: Improving efficacy of theranostics and nanomedicines. Biomaterials 2018, 179, 209–245. [Google Scholar] [CrossRef]
- Bailly, C.; Bodet-Milin, C.; Rousseau, C.; Faivre-Chauvet, A.; Kraeber-Bodéré, F.; Barbet, J. Pretargeting for imaging and therapy in oncological nuclear medicine. EJNMMI Radiopharm. Chem. 2017, 2, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G. A Revisit to the Pretargeting Concept—A Target Conversion. Front. Pharmacol. 2018, 9, 1476. [Google Scholar] [CrossRef]
- Tienken, L.; Drude, N.; Schau, I.; Winz, O.H.; Temme, A.; Weinhold, E.; Mottaghy, F.M.; Morgenroth, A. Evaluation of a Pretargeting Strategy for Molecular Imaging of the Prostate Stem Cell Antigen with a Single Chain Antibody. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, B.E.; Adumeau, P.; Membreno, R.; Carnazza, K.E.; Brand, C.; Reiner, T.; Agnew, B.J.; Lewis, J.; Zeglis, B.M. Pretargeted PET Imaging Using a Site-Specifically Labeled Immunoconjugate. Bioconjug. Chem. 2016, 27, 1789–1795. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Brand, C.; Abdel-Atti, D.; Carnazza, K.E.; Cook, B.E.; Carlin, S.; Reiner, T.; Lewis, J.S. Optimization of a Pretargeted Strategy for the PET Imaging of Colorectal Carcinoma via the Modulation of Radioligand Pharmacokinetics. Mol. Pharm. 2015, 12, 3575–3587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adumeau, P.; Carnazza, K.E.; Brand, C.; Carlin, S.D.; Reiner, T.; Agnew, B.J.; Lewis, J.S.; Zeglis, B.M. A Pretargeted Approach for the Multimodal PET/NIRF Imaging of Colorectal Cancer. Theranostics 2016, 6, 2267–2277. [Google Scholar] [CrossRef]
- Houghton, J.L.; Membreno, R.; Abdel-Atti, D.; Cunanan, K.M.; Carlin, S.; Scholz, W.W.; Zanzonico, P.B.; Lewis, J.S.; Zeglis, B.M. Establishment of the invivo efficacy of pretargeted radioimmunotherapy utilizing inverse electron demand diels-alder click chemistry. Mol. Cancer Ther. 2017, 16, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Rossin, R.; Verkerk, P.R.; Van Den Bosch, S.M.; Vulders, R.C.M.; Verel, I.; Lub, J.; Robillard, M.S. In vivo chemistry for pretargeted tumor imaging in live mice. Angew. Chem. Int. Ed. 2010, 49, 3375–3378. [Google Scholar] [CrossRef]
- Liu, G.; Dou, S.; Chen, X.; Chen, L.; Liu, X.; Rusckowski, M.; Hnatowich, D.J. Adding a Clearing Agent to Pretargeting Does Not Lower the Tumor Accumulation of the Effector as Predicted. Cancer Biother. Radiopharm. 2010, 25, 757–762. [Google Scholar] [CrossRef]
- Karmani, L.; Levêque, P.; Bouzin, C.; Bol, A.; Dieu, M.; Walrand, S.; Borght, T.V.; Feron, O.; Grégoire, V.; Bonifazi, D.; et al. Biodistribution of 125I-labeled anti-endoglin antibody using SPECT/CT imaging: Impact of in vivo deiodination on tumor accumulation in mice. Nucl. Med. Biol. 2016, 43, 415–423. [Google Scholar] [CrossRef]
- Rossin, R.; Lappchen, T.; Bosch, S.M.V.D.; Laforest, R.; Robillard, M.S. Diels-Alder Reaction for Tumor Pretargeting: In Vivo Chemistry Can Boost Tumor Radiation Dose Compared with Directly Labeled Antibody. J. Nucl. Med. 2013, 54, 1989–1995. [Google Scholar] [CrossRef] [Green Version]
- Mirallié, E.; Saï-Maurel, C.; Faivre-Chauvet, A.; Regenet, N.; Chang, C.-H.; Goldenberg, D.M.; Chatal, J.-F.; Barbet, J.; Thedrez, P. Improved pretargeted delivery of radiolabelled hapten to human tumour xenograft in mice by avidin chase of circulating bispecific antibody. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Myrhammar, A.; Vorobyeva, A.; Westerlund, K.; Yoneoka, S.; Orlova, A.; Tsukahara, T.; Tolmachev, V.; Karlström, A.E.; Altai, M. Evaluation of an antibody-PNA conjugate as a clearing agent for antibody-based PNA-mediated radionuclide pretargeting. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cheal, S.M.; Patel, M.; Yang, G.; Veach, D.; Xu, H.; Guo, H.-F.; Zanzonico, P.B.; Axworthy, D.B.; Cheung, N.-K.V.; Ouerfelli, O.; et al. An N-Acetylgalactosamino Dendron-Clearing Agent for High-Therapeutic-Index DOTA-Hapten Pretargeted Radioimmunotherapy. Bioconjug. Chem. 2020, 31, 501–506. [Google Scholar] [CrossRef]
- Zheng, M.; Zheng, L.; Zhang, P.; Li, J.; Zhang, Y. Development of Bioorthogonal Reactions and Their Applications in Bioconjugation. Molecules 2015, 20, 3190–3205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaraj, N.K. The Future of Bioorthogonal Chemistry. ACS Cent. Sci. 2018, 4, 952–959. [Google Scholar] [CrossRef] [Green Version]
- Liu, B. Bio-orthogonal Click Chemistry for In Vivo Bioimaging. Trends Chem. 2019, 1, 763–778. [Google Scholar] [CrossRef]
- Knight, J.; Cornelissen, B. Bioorthogonal chemistry: Implications for pretargeted nuclear (PET/SPECT) imaging and therapy. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 96–113. [Google Scholar] [PubMed]
- Porte, K.; Riberaud, M.; Châtre, R.; Audisio, D.; Papot, S.; Taran, F. Bioorthogonal Reactions in Animals. ChemBioChem 2021, 22, 100–113. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal Reactions. Acc. Chem. Res. 2011, 44, 666–676. [Google Scholar] [CrossRef]
- McKay, C.; Finn, M. Click Chemistry in Complex Mixtures: Bioorthogonal Bioconjugation. Chem. Biol. 2014, 21, 1075–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem. Int. Ed. 2009, 48, 6974–6998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jewett, J.C.; Bertozzi, C.R. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 2010, 39, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Bertozzi, C.R. Bioorthogonal Click Chemistry: Covalent Labeling in Living Systems. QSAR Comb. Sci. 2007, 26, 1211–1219. [Google Scholar] [CrossRef]
- Best, M.D. Click Chemistry and Bioorthogonal Reactions: Unprecedented Selectivity in the Labeling of Biological Molecules. Biochemistry 2009, 48, 6571–6584. [Google Scholar] [CrossRef]
- Ariza, X.; Urpí, F.; Vilarrasa, J. A practical procedure for the preparation of carbamates from azides. Tetrahedron Lett. 1999, 40, 7515–7517. [Google Scholar] [CrossRef]
- Staudinger, H.; Hauser, E. Über neue organische Phosphorverbindungen IV Phosphinimine. Helv. Chim. Acta 1921, 4, 861–886. [Google Scholar] [CrossRef] [Green Version]
- Saxon, E.; Bertozzi, C.R. Cell surface engineering by a modified Staudinger reaction. Science 2000, 287, 2007–2010. [Google Scholar] [CrossRef] [Green Version]
- Van Berkel, S.S.; Van Eldijk, M.B.; Van Hest, J.C.M. Staudinger ligation as a method for bioconjugation. Angew. Chem. Int. Ed. 2011, 50, 8806–8827. [Google Scholar] [CrossRef]
- Saxon, E.; Armstrong, J.I.; Bertozzi, C.R. A “traceless” Staudinger ligation for the chemoselective synthesis of amide bonds. Org. Lett. 2000, 2, 2141–2143. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.; Asfura, K.G.; Stabler, C. Microencapsulation of islets within alginate/poly(ethylene glycol) gels cross-linked via Staudinger ligation. Acta Biomater. 2011, 7, 614–624. [Google Scholar] [CrossRef] [Green Version]
- Vugts, D.J.; Vervoort, A.; Walsum, M.S.-V.; Visser, G.W.M.; Robillard, M.S.; Versteegen, R.; Vulders, R.; Herscheid, J.D.M.; Van Dongen, G.A.M.S. Synthesis of Phosphine and Antibody–Azide Probes forin VivoStaudinger Ligation in a Pretargeted Imaging and Therapy Approach. Bioconjug. Chem. 2011, 22, 2072–2081. [Google Scholar] [CrossRef]
- Oliveira, B.; Guo, Z.; Bernardes, G.J.L. Inverse electron demand Diels–Alder reactions in chemical biology. Chem. Soc. Rev. 2017, 46, 4895–4950. [Google Scholar] [CrossRef] [Green Version]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Koo, H. Biomedical applications of copper-free click chemistry: In vitro, in vivo, and ex vivo. Chem. Sci. 2019, 10, 7835–7851. [Google Scholar] [CrossRef] [Green Version]
- Uttamapinant, C.; Sanchez, M.I.; Liu, D.S.; Yao, J.Z.; White, K.A.; Grecian, S.; Clark, S.; Gee, K.R.; Ting, A.Y.; Clarke, S. Site-specific protein labeling using PRIME and chelation-assisted click chemistry. Nat. Protoc. 2013, 8, 1620–1634. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wu, Y.-W. Selective chemical labeling of proteins. Org. Biomol. Chem. 2016, 14, 5417–5439. [Google Scholar] [CrossRef] [Green Version]
- Presolski, S.I.; Hong, V.; Cho, S.-H.; Finn, M. Tailored Ligand Acceleration of the Cu-Catalyzed Azide−Alkyne Cycloaddition Reaction: Practical and Mechanistic Implications. J. Am. Chem. Soc. 2010, 132, 14570–14576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soriano Del Amo, D.; Wang, W.; Jiang, H.; Besanceney, C.; Yan, A.C.; Levy, M.; Liu, Y.; Marlow, F.L.; Wu, P. Biocompatible copper(I) catalysts for in vivo imaging of glycans. J. Am. Chem. Soc. 2010, 132, 16893–16899. [Google Scholar] [CrossRef] [Green Version]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A Strain-Promoted [3 + 2] Azide−Alkyne Cycloaddition for Covalent Modification of Biomolecules in Living Systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef]
- Debets, M.; Van Berkel, S.S.; Dommerholt, J.; Dirks, A.J.; Rutjes, F.P.J.T.; Van Delft, F.L. Bioconjugation with Strained Alkenes and Alkynes. Acc. Chem. Res. 2011, 44, 805–815. [Google Scholar] [CrossRef]
- Ning, X.; Guo, J.; Wolfert, M.; Boons, G.-J. Visualizing Metabolically Labeled Glycoconjugates of Living Cells by Copper-Free and Fast Huisgen Cycloadditions. Angew. Chem. Int. Ed. 2008, 47, 2253–2255. [Google Scholar] [CrossRef] [PubMed]
- Rondon, A.; Degoul, F. Antibody Pretargeting Based on Bioorthogonal Click Chemistry for Cancer Imaging and Targeted Radionuclide Therapy. Bioconjug. Chem. 2019, 31, 159–173. [Google Scholar] [CrossRef]
- White, B.W. Effect of temporal ordering on visual recognition. Percept. Mot. Ski. 1962, 15, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Bosch, S.V.D.; Rossin, R.; Verkerk, P.R.; Hoeve, W.T.; Janssen, H.; Lub, J.; Robillard, M. Evaluation of strained alkynes for Cu-free click reaction in live mice. Nucl. Med. Biol. 2013, 40, 415–423. [Google Scholar] [CrossRef]
- Lee, D.-E.; Na, J.H.; Lee, S.; Kang, C.M.; Kim, H.N.; Han, S.J.; Kim, H.; Choe, Y.S.; Jung, K.-H.; Lee, K.C.; et al. Facile Method to Radiolabel Glycol Chitosan Nanoparticles with 64Cu via Copper-Free Click Chemistry for MicroPET Imaging. Mol. Pharm. 2012, 10, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Au, K.M.; Tripathy, A.; Lin, C.P.-I.; Wagner, K.; Hong, S.; Wang, A.; Park, S.I. Bespoke Pretargeted Nanoradioimmunotherapy for the Treatment of Non-Hodgkin Lymphoma. ACS Nano 2018, 12, 1544–1563. [Google Scholar] [CrossRef]
- Boutureira, O.; Bernardes, G.J. Advances in Chemical Protein Modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar] [CrossRef]
- Soellner, M.B.; Nilsson, B.L.; Raines, R.T. Reaction Mechanism and Kinetics of the Traceless Staudinger Ligation. J. Am. Chem. Soc. 2006, 128, 8820–8828. [Google Scholar] [CrossRef] [PubMed]
- Blackman, M.L.; Royzen, M.; Fox, J.M. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels−Alder Reactivity. J. Am. Chem. Soc. 2008, 130, 13518–13519. [Google Scholar] [CrossRef] [Green Version]
- Pagel, M. Inverse electron demand Diels-Alder (IEDDA) reactions in peptide chemistry. J. Pept. Sci. 2019, 25, e3141. [Google Scholar] [CrossRef] [Green Version]
- Jamroz, D.; Fischer-Durand, N.; Palusiak, M.; Wojtulewski, S.; Jarzyński, S.; Stępniewska, M.; Salmain, M.; Rudolf, B. Inverse electron-demand Diels-Alder (iEDDA) bioorthogonal conjugation of half-sandwich transition metallocarbonyl entities to a model protein. Appl. Organomet. Chem. 2020, 34, e5507. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Weissleder, R. Biomedical Applications of Tetrazine Cycloadditions. Acc. Chem. Res. 2011, 44, 816–827. [Google Scholar] [CrossRef] [Green Version]
- Sauer, J. Structure-reactivity problem in cycloaddition reactions to form heterocyclic compounds. Chem. Heterocycl. Compd. 1995, 31, 1140–1154. [Google Scholar] [CrossRef]
- Mushtaq, S.; Yun, S.-J.; Jeon, J. Recent Advances in Bioorthogonal Click Chemistry for Efficient Synthesis of Radiotracers and Radiopharmaceuticals. Molecules 2019, 24, 3567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggi, A.; Ruivo, E.; Fissers, J.; Vangestel, C.; Chatterjee, S.; Joossens, J.; Sobott, F.; Staelens, S.; Stroobants, S.; Van Der Veken, P.; et al. Development of a novel antibody–tetrazine conjugate for bioorthogonal pretargeting. Org. Biomol. Chem. 2016, 14, 7544–7551. [Google Scholar] [CrossRef]
- Wijnen, J.W.; Zavarise, S.; Engberts, J.B.F.N.; Charton, M. Substituent Effects on an Inverse Electron Demand Hetero Diels−Alder Reaction in Aqueous Solution and Organic Solvents: Cycloaddition of Substituted Styrenes to Di(2-pyridyl)-1,2,4,5-tetrazine. J. Org. Chem. 1996, 61, 2001–2005. [Google Scholar] [CrossRef]
- Stéen, J.E.L.; Jørgensen, J.T.; Christoph, D.; Battisti, U.M.; Nørregard, K.; Edem, P.E.; Bratteby, K.; Shalgunov, V.; Wilkovitsch, M.; Svatunek, D.; et al. Lipophilicity and click reactivity determine the performance of bioorthogonal tetrazine tools in pretargeted in vivo chemistry. ACS Pharmacol. Transl. Sci. 2021, 4, 824–833. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Weissleder, R.; Hilderbrand, S.A. Tetrazine-Based Cycloadditions: Application to Pretargeted Live Cell Imaging. Bioconjug. Chem. 2008, 19, 2297–2299. [Google Scholar] [CrossRef] [Green Version]
- Rossin, R.; Bosch, S.M.V.D.; Hoeve, W.T.; Carvelli, M.; Versteegen, R.; Lub, J.; Robillard, M.S. Highly Reactive trans-Cyclooctene Tags with Improved Stability for Diels–Alder Chemistry in Living Systems. Bioconjug. Chem. 2013, 24, 1210–1217. [Google Scholar] [CrossRef]
- Wang, M.; Svatunek, D.; Rohlfing, K.; Liu, Y.; Wang, H.; Giglio, B.; Yuan, H.; Wu, Z.; Li, Z.; Fox, J. Conformationally Strained trans-Cyclooctene (sTCO) Enables the Rapid Construction of 18F-PET Probes via Tetrazine Ligation. Theranostics 2016, 6, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Ramil, C.P.; Dong, M.; An, P.; Lewandowski, T.M.; Yu, Z.; Miller, L.J.; Lin, Q. Spirohexene-Tetrazine Ligation Enables Bioorthogonal Labeling of Class B G Protein-Coupled Receptors in Live Cells. J. Am. Chem. Soc. 2017, 139, 13376–13386. [Google Scholar] [CrossRef]
- Stöckmann, H.; Neves, A.A.; Day, H.A.; Stairs, S.; Brindle, K.M.; Leeper, F.J. (E,E)-1,5-Cyclooctadiene: A small and fast click-chemistry multitalent. Chem. Commun. 2011, 47, 7203–7205. [Google Scholar] [CrossRef] [PubMed]
- Longo, B.; Zanato, C.; Piras, M.; Dall’Angelo, S.; Windhorst, A.D.; Vugts, D.J.; Baldassarre, M.; Zanda, M. Design, Synthesis, Conjugation, and Reactivity of Novel trans,trans-1,5-Cyclooctadiene-Derived Bioorthogonal Linkers. Bioconjug. Chem. 2020, 31, 2201–2210. [Google Scholar] [CrossRef]
- Ferreira, V.F.C.; Oliveira, B.L.; D’Onofrio, A.; Farinha, C.M.; Gano, L.; Paulo, A.; Bernardes, G.J.L.; Mendes, F. In Vivo Pretargeting Based on Cysteine-Selective Antibody Modification with IEDDA Bioorthogonal Handles for Click Chemistry. Bioconjug. Chem. 2020, 32, 121–132. [Google Scholar] [CrossRef]
- Van Dongen, G.A.M.S.; Poot, A.J.; Vugts, D.J. PET imaging with radiolabeled antibodies and tyrosine kinase inhibitors: Immuno-PET and TKI-PET. Tumor Biol. 2012, 33, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Zaroff, S.; Tan, G. Hybridoma technology: The preferred method for monoclonal antibody generation for in vivo applications. Biotechology 2019, 67, 90–92. [Google Scholar] [CrossRef] [Green Version]
- Treglia, G.; Salsano, M. PET imaging using radiolabelled antibodies: Future direction in tumor diagnosis and correlate applications. Res. Rep. Nucl. Med. 2013, 3, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Bailly, C.; Cléry, P.-F.; Faivre-Chauvet, A.; Bourgeois, M.; Guérard, F.; Haddad, F.; Barbet, J.; Chérel, M.; Kraeber-Bodere, F.; Carlier, T.; et al. Immuno-PET for clinical theranostic approaches. Int. J. Mol. Sci. 2016, 18, 57. [Google Scholar] [CrossRef] [Green Version]
- Ruivo, E.; Adhikari, K.; Elvas, F.; Fissers, J.; Vangestel, C.; Staelens, S.; Stroobants, S.; Van Der Veken, P.; Wyffels, L.; Augustyns, K. Improved stability of a novel fluorine-18 labeled TCO analogue for pretargeted PET imaging. Nucl. Med. Biol. 2019, 76–77, 36–42. [Google Scholar] [CrossRef]
- Houghton, J.L.; Zeglis, B.M.; Abdel-Atti, D.; Sawada, R.; Scholz, W.W.; Lewis, J.S. Pretargeted Immuno-PET of Pancreatic Cancer: Overcoming Circulating Antigen and Internalized Antibody to Reduce Radiation Doses. J. Nucl. Med. 2016, 57, 453–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, J.-P.; Houghton, J.L.; Kozlowski, P.; Abdel-Atti, D.; Reiner, T.; Pillarsetty, N.V.K.; Scholz, W.W.; Zeglis, B.M.; Lewis, J.S. 18F-Based Pretargeted PET Imaging Based on Bioorthogonal Diels–Alder Click Chemistry. Bioconjug. Chem. 2016, 27, 298–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keinänen, O.; Li, X.-G.; Chenna, N.K.; Lumen, D.; Ott, J.; Molthoff, C.F.M.; Sarparanta, M.; Helariutta, K.; Vuorinen, T.; Windhorst, A.D.; et al. A New Highly Reactive and Low Lipophilicity Fluorine-18 Labeled Tetrazine Derivative for Pretargeted PET Imaging. ACS Med. Chem. Lett. 2015, 7, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Poty, S.; Membreno, R.; Glaser, J.M.; Ragupathi, A.; Scholz, W.W.; Zeglis, B.M.; Lewis, J.S. The inverse electron-demand Diels-Alder reaction as a new methodology for the synthesis of 225Ac-labelled radioimmunoconjugates. ChemComm. 2018, 54, 2599–2602. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.-P.; Tully, K.; Jackson, J.; Dilling, T.R.; Reiner, T.; Lewis, J.S. Bioorthogonal Masking of Circulating Antibody–TCO Groups Using Tetrazine-Functionalized Dextran Polymers. Bioconjug. Chem. 2018, 29, 538–545. [Google Scholar] [CrossRef]
- Membreno, R.; Keinänen, O.; Cook, B.E.; Tully, K.M.; Fung, K.C.; Lewis, J.S.; Zeglis, B.M. Toward the Optimization of Click-Mediated Pretargeted Radioimmunotherapy. Mol. Pharm. 2019, 16, 2259–2263. [Google Scholar] [CrossRef]
- Membreno, R.; Cook, B.E.; Fung, K.; Lewis, J.S.; Zeglis, B.M. Click-Mediated Pretargeted Radioimmunotherapy of Colorectal Carcinoma. Mol. Pharm. 2018, 15, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Keinänen, O.; Brennan, J.M.; Membreno, R.; Fung, K.; Gangangari, K.; Dayts, E.J.; Williams, C.J.; Zeglis, B.M. Dual Radionuclide Theranostic Pretargeting. Mol. Pharm. 2019, 16, 4416–4421. [Google Scholar] [CrossRef]
- Cook, B.E.; Membreno, R.; Zeglis, B.M. Dendrimer Scaffold for the Amplification of In Vivo Pretargeting Ligations. Bioconjug. Chem. 2018, 29, 2734–2740. [Google Scholar] [CrossRef]
- Rahim, M.K.; Kota, R.; Haun, J.B. Enhancing Reactivity for Bioorthogonal Pretargeting by Unmasking Antibody-Conjugated trans-Cyclooctenes. Bioconjug. Chem. 2015, 26, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Cai, H.; Hassink, M.; Blackman, M.L.; Brown, R.C.D.; Conti, P.S.; Fox, J.M. Tetrazine–trans-cyclooctene ligation for the rapid construction of 18F labeled probes. Chem. Commun. 2010, 46, 8043–8045. [Google Scholar] [CrossRef] [Green Version]
- Wyffels, L.; Thomae, D.; Waldron, A.-M.; Fissers, J.; Dedeurwaerdere, S.; Van Der Veken, P.; Joossens, J.; Stroobants, S.; Augustyns, K.; Staelens, S. In vivo evaluation of 18F-labeled TCO for pre-targeted PET imaging in the brain. Nucl. Med. Biol. 2014, 41, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Billaud, E.M.F.; Belderbos, S.; Cleeren, F.; Maes, W.; Van De Wouwer, M.; Koole, M.; Verbruggen, A.; Himmelreich, U.; Geukens, N.; Bormans, G. Pretargeted PET Imaging Using a Bioorthogonal 18F-Labeled trans-Cyclooctene in an Ovarian Carcinoma Model. Bioconjug. Chem. 2017, 28, 2915–2920. [Google Scholar] [CrossRef]
- Goos, J.; Cho, A.; Carter, L.M.; Dilling, T.R.; Davydova, M.; Mandleywala, K.; Puttick, S.; Gupta, A.; Price, W.S.; Quinn, J.F.; et al. Delivery of polymeric nanostars for molecular imaging and endoradiotherapy through the enhanced permeability and retention (EPR) effect. Theranostics 2020, 10, 567–584. [Google Scholar] [CrossRef]
- Van Onzen, A.H.A.M.; Rossin, R.; Schenning, A.P.H.J.; Nicolay, K.; Milroy, L.G.; Robillard, M.S.; Robillard, M.S.; Brunsveld, L. Tetrazine—Trans-Cyclooctene Chemistry Applied to Fabricate Self-Assembled Fluorescent and Radioactive Nanoparticles for in Vivo Dual Mode Imaging. Bioconjug. Chem. 2019, 30, 547–551. [Google Scholar] [CrossRef]
- Petkau-Milroy, K.K.; Kaeser, A.A.; Fischer, I.I.; Brunsveld, L.L.; Schenning, A.P.H.J. Pre- and Postfunctionalized Self-Assembled π-Conjugated Fluorescent Organic Nanoparticles for Dual Targeting. J. Am. Chem. Soc. 2011, 133, 17063–17071. [Google Scholar] [CrossRef] [PubMed]
- Keinänen, O.; Mäkilä, E.M.; Lindgren, R.; Virtanen, H.; Liljenbäck, H.; Oikonen, V.; Sarparanta, M.; Molthoff, C.; Windhorst, A.D.; Roivainen, A.; et al. Pretargeted PET Imaging of trans-Cyclooctene-Modified Porous Silicon Nanoparticles. ACS Omega 2017, 2, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Rossin, R.; Versteegen, R.M.; Wu, J.; Khasanov, A.; Wessels, H.J.; Steenbergen, E.J.; Hoeve, W.T.; Janssen, H.M.; Van Onzen, A.H.A.M.; Hudson, P.J.; et al. Chemically triggered drug release from an antibody-drug conjugate leads to potent antitumour activity in mice. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Versteegen, R.M.; Hoeve, W.T.; Rossin, R.; de Geus, M.; Janssen, H.M.; Robillard, M.S. Click-to-Release from trans-Cyclooctenes: Mechanistic Insights and Expansion of Scope from Established Carbamate to Remarkable Ether Cleavage. Angew. Chem. Int. Ed. 2018, 57, 10494–10499. [Google Scholar] [CrossRef]
- Rossin, R.; Van Duijnhoven, S.M.J.; Ten Hoeve, W.; Janssen, H.M.; Kleijn, L.H.J.; Hoeben, F.J.M.; Versteegen, R.M.; Robillard, M.S. Triggered Drug Release from an Antibody-Drug Conjugate Using Fast “click-to-Release”. Chemistry in Mice. Bioconjug. Chem. 2016, 27, 1697–1706. [Google Scholar] [CrossRef]
- Li, H.; Conde, J.; Guerreiro, A.; Bernardes, G.J.L. Tetrazine Carbon Nanotubes for Pretargeted In Vivo “Click-to-Release” Bioorthogonal Tumour Imaging. Angew. Chem. Int. Ed. 2020, 59, 16023–16032. [Google Scholar] [CrossRef] [PubMed]
- Kozma, E.; Demeter, O.; Kele, P. Bio-orthogonal Fluorescent Labelling of Biopolymers through Inverse-Electron-Demand Diels-Alder Reactions. ChemBioChem 2017, 18, 486–501. [Google Scholar] [CrossRef] [Green Version]
- Devaraj, N.K.; Hilderbrand, S.; Upadhyay, R.; Mazitschek, R.; Weissleder, R. Bioorthogonal Turn-On Probes for Imaging Small Molecules inside Living Cells. Angew. Chem. Int. Ed. 2010, 49, 2869–2872. [Google Scholar] [CrossRef]
- Meimetis, L.G.; Carlson, J.; Giedt, R.J.; Kohler, R.; Weissleder, R. Ultrafluorogenic Coumarin-Tetrazine Probes for Real-Time Biological Imaging. Angew. Chem. Int. Ed. 2014, 53, 7531–7534. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.; Meimetis, L.G.; Hilderbrand, S.A.; Weissleder, R. BODIPY-Tetrazine Derivatives as Superbright Bioorthogonal Turn-on Probes. Angew. Chem. Int. Ed. 2013, 52, 6917–6920. [Google Scholar] [CrossRef] [Green Version]
- Knorr, G.; Kozma, E.; Herner, A.; Lemke, E.; Kele, P. New Red-Emitting Tetrazine-Phenoxazine Fluorogenic Labels for Live-Cell Intracellular Bioorthogonal Labeling Schemes. Chem. A Eur. J. 2016, 22, 8972–8979. [Google Scholar] [CrossRef]
- Németh, E.; Knorr, G.; Németh, K.; Kele, P. A Bioorthogonally Applicable, Fluorogenic, Large Stokes-Shift Probe for Intracellular Super-Resolution Imaging of Proteins. Biomolecules 2020, 10, 397. [Google Scholar] [CrossRef] [Green Version]
- Kormos, A.; Kern, D.; Egyed, A.; Söveges, B.; Németh, K.; Kele, P. Microscope laser assisted photooxidative activation of bioorthogonal ClickOx probes. Chem. Commun. 2020, 56, 5425–5428. [Google Scholar] [CrossRef] [PubMed]
- Kozma, E.; Girona, G.E.; Paci, G.; Lemke, E.A.; Kele, P. Bioorthogonal double-fluorogenic siliconrhodamine probes for intracellular super-resolution microscopy. Chem. Commun. 2017, 53, 6696–6699. [Google Scholar] [CrossRef] [Green Version]
- Bojtár, M.; Németh, K.; Domahidy, F.; Knorr, G.; Verkman, A.; Kállay, M.; Kele, P. Conditionally Activatable Visible-Light Photocages. J. Am. Chem. Soc. 2020, 142, 15164–15171. [Google Scholar] [CrossRef]
- Renard, E.; Camps, E.C.; Canovas, C.; Kip, A.; Gotthardt, M.; Rijpkema, M.; Denat, F.; Goncalves, V.; van Lith, S. Site-Specific Dual-Labeling of a VHH with a Chelator and a Photosensitizer for Nuclear Imaging and Targeted Photodynamic Therapy of EGFR-Positive Tumors. Cancers 2021, 13, 428. [Google Scholar] [CrossRef]
- Linden, G.; Zhang, L.; Pieck, F.; Linne, U.; Kosenkov, D.; Tonner, R.; Vázquez, O. Conditional Singlet Oxygen Generation through a Bioorthogonal DNA-targeted Tetrazine Reaction. Angew. Chem. Int. Ed. 2019, 58, 12868–12873. [Google Scholar] [CrossRef] [PubMed]
- Linden, G.; Vázquez, O. Bioorthogonal Turn-On BODIPY-Peptide Photosensitizers for Tailored Photodynamic Therapy. Chem. A Eur. J. 2020, 26, 10014–10023. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Tu, Y.; Wang, K.; Xu, C.; Yuan, Y.; Wang, J. A General Strategy for Macrotheranostic Prodrug Activation: Synergy between the Acidic Tumor Microenvironment and Bioorthogonal Chemistry. Angew. Chem. Int. Ed. 2020, 59, 7168–7172. [Google Scholar] [CrossRef]


| Bioorthogonal Reaction | Reaction Scheme | k (M−1 s−1) | Advantages | Drawbacks |
|---|---|---|---|---|
| Staudinger ligation | A: Staudinger reaction | ∼10−3 [64] |
|
|
B: Staudinger ligation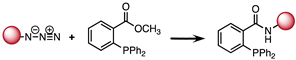 | 10−3 |
|
| |
C: “Traceless” Staudinger ligation | 7.7 × 10−3 [65] |
|
| |
| CuAAC | 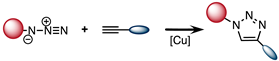 | 10 |
|
|
| SPAAC | 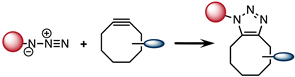 | 0.1 |
|
|
| IEDDA | 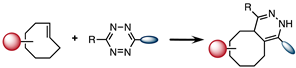 | 1–106 |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handula, M.; Chen, K.-T.; Seimbille, Y. IEDDA: An Attractive Bioorthogonal Reaction for Biomedical Applications. Molecules 2021, 26, 4640. https://doi.org/10.3390/molecules26154640
Handula M, Chen K-T, Seimbille Y. IEDDA: An Attractive Bioorthogonal Reaction for Biomedical Applications. Molecules. 2021; 26(15):4640. https://doi.org/10.3390/molecules26154640
Chicago/Turabian StyleHandula, Maryana, Kuo-Ting Chen, and Yann Seimbille. 2021. "IEDDA: An Attractive Bioorthogonal Reaction for Biomedical Applications" Molecules 26, no. 15: 4640. https://doi.org/10.3390/molecules26154640
APA StyleHandula, M., Chen, K.-T., & Seimbille, Y. (2021). IEDDA: An Attractive Bioorthogonal Reaction for Biomedical Applications. Molecules, 26(15), 4640. https://doi.org/10.3390/molecules26154640








