Copper-Catalyzed One-Pot Synthesis of N-Sulfonyl Amidines from Sulfonyl Hydrazine, Terminal Alkynes and Sulfonyl Azides
Abstract
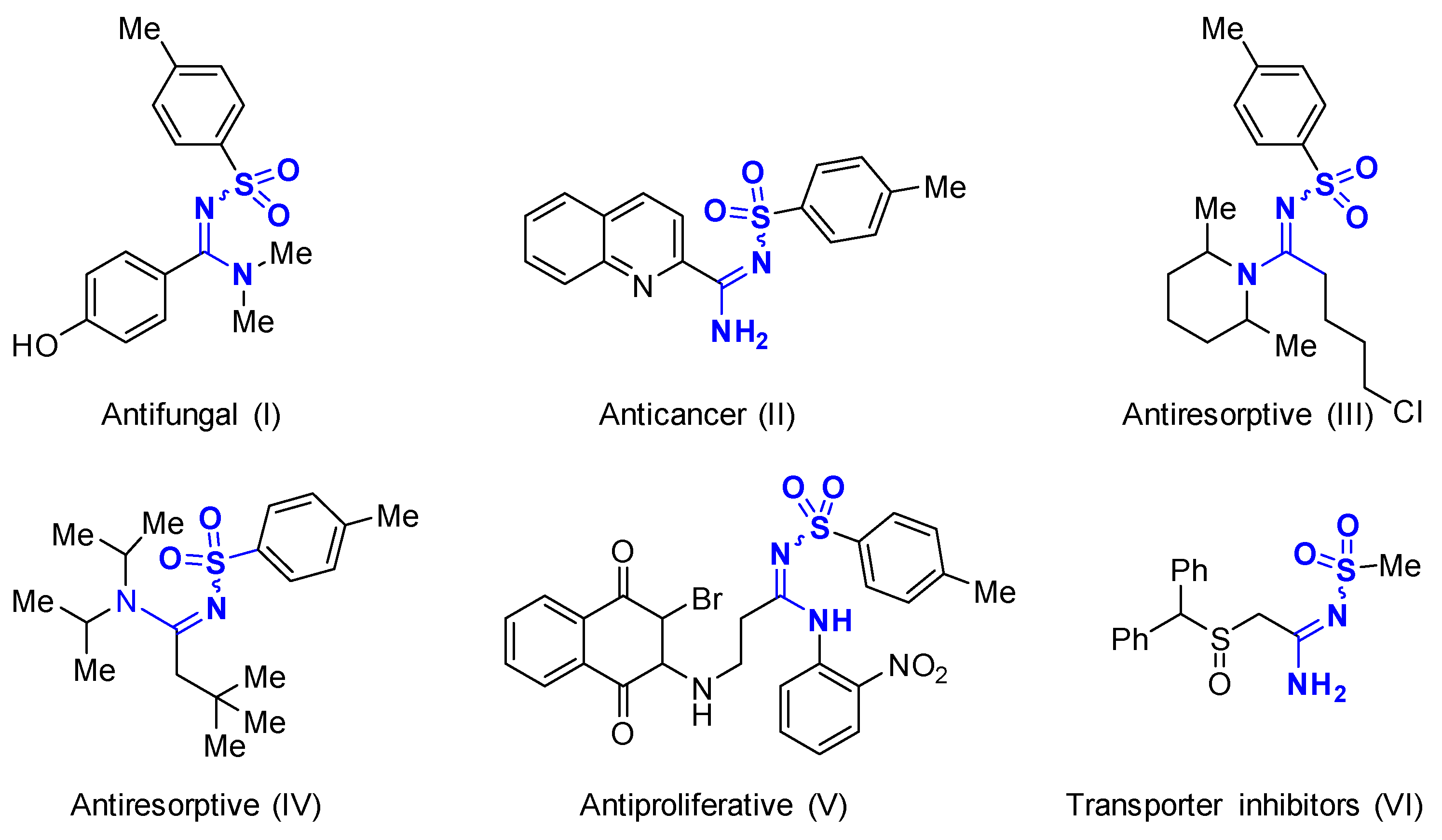
1. Introduction
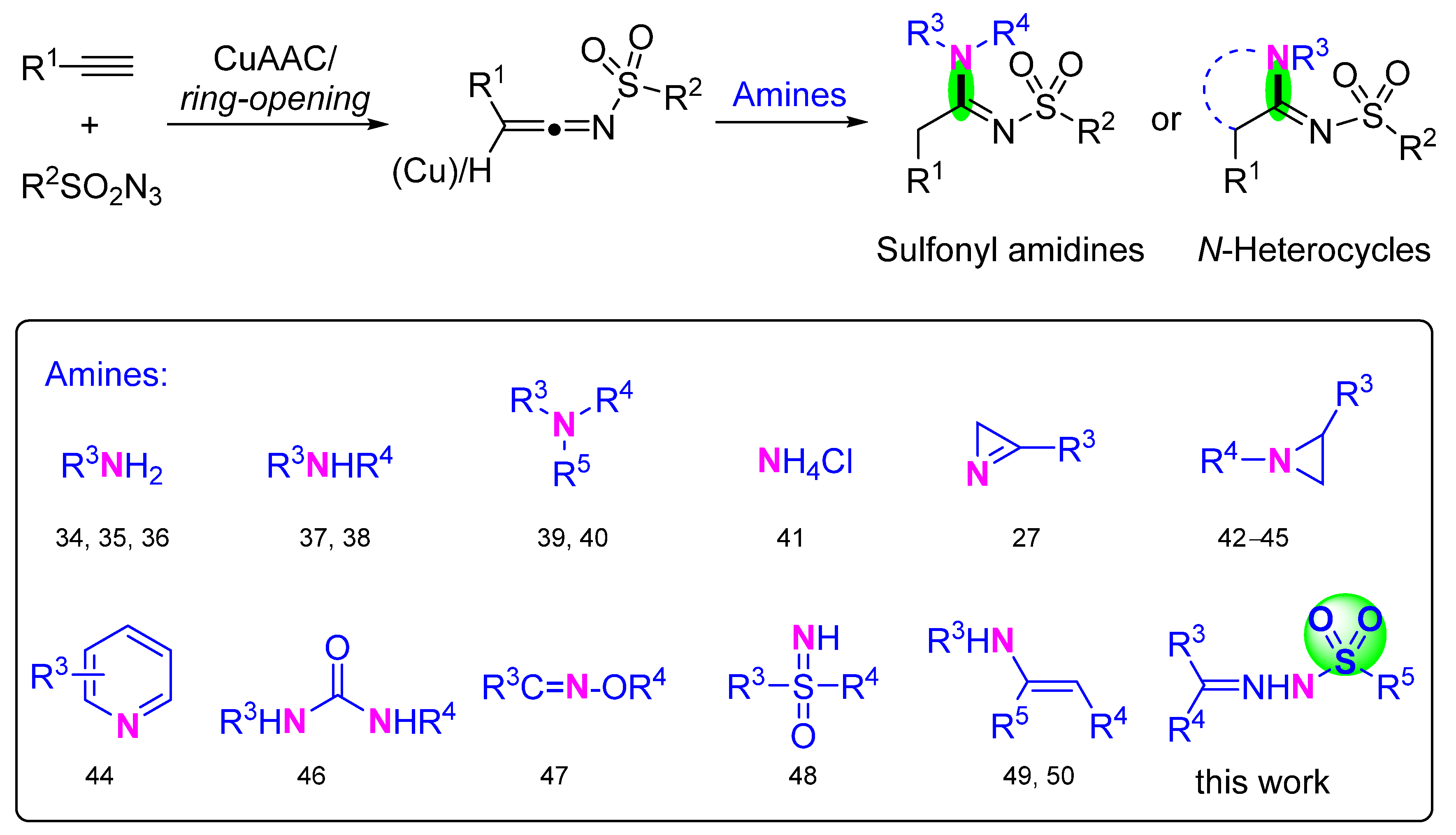
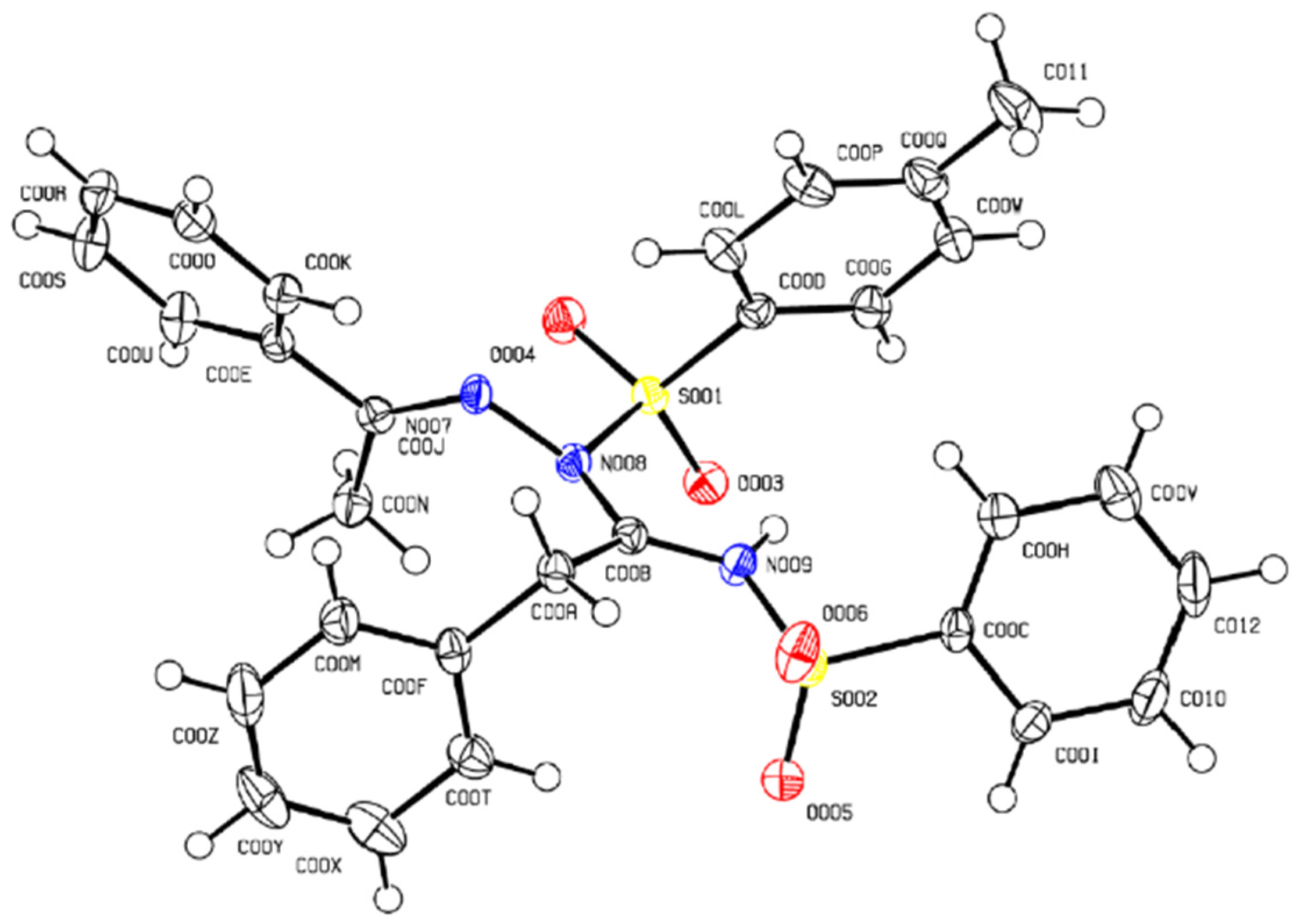
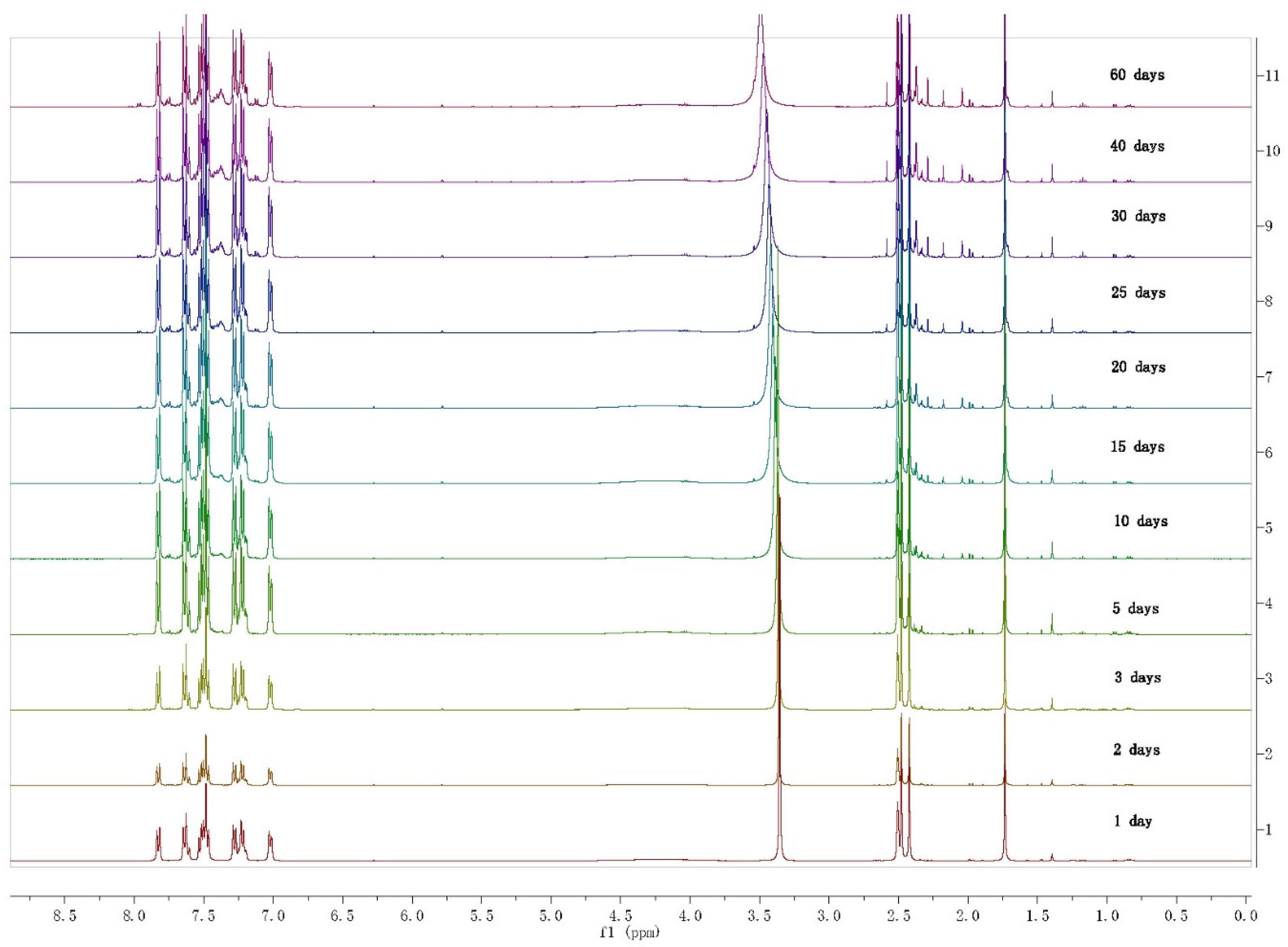
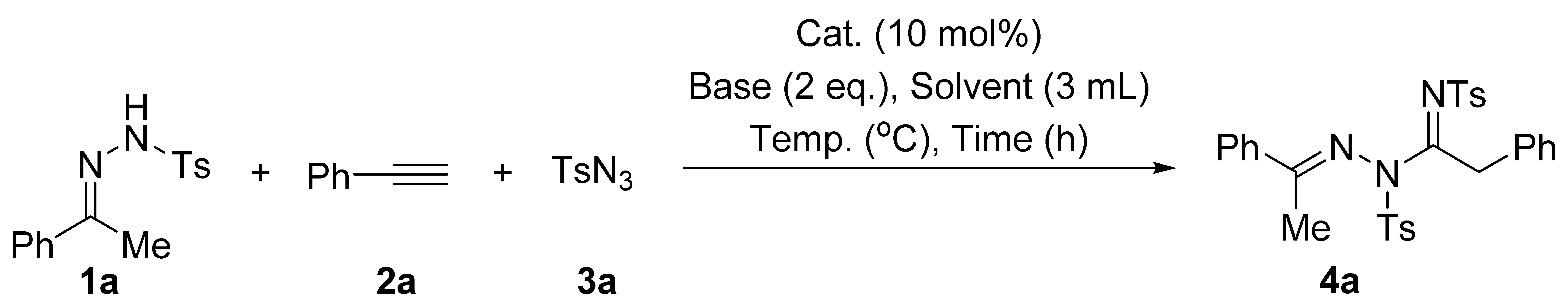
2. Results
3. Experimental
3.1. General Information
3.2. Compound Characterizations and Preparations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cheng, L.; Chen, S.; Wu, W.; Kuo, Z.C.; Wei, Z.; Meng, S.; Chen, C.; Zhang, C.; He, Y. Gastric cancer in young patients: A separate entity with aggressive features and poor prognosis. J. Cancer Res. Clin. Oncol. 2020, 146, 2937–2947. [Google Scholar] [CrossRef]
- Greenhill, J.V.; Lue, P. Amidines and guanidines in medicinal chemistry. Prog. Med. Chem. 1993, 30, 203–326. [Google Scholar] [PubMed]
- Adiche, C.; Hamadouche, M.; Abed, D.E. Facile synthesis of sulfonyl amidines by 1,3-dipolar cycloaddition between 1-morpholinocycloalkenes and sulfonyl azides without catalyst. Heterocycles 2016, 92, 1614–1628. [Google Scholar]
- Boyd, G.V. Reactions and Synthetic Uses of Amidines. In The Chemistry of Amidines and Imidates; Patai, S., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1991; pp. 67–424. [Google Scholar]
- Edwards, P.D.; Albert, J.S.; Sylvester, M.; Aharony, D.; Andisik, D.; Callaghan, O.; Campbell, J.B.; Carr, R.A.; Chessari, G.; Congreve, M.; et al. Application of fragment-based lead generation to the discovery of novel, cyclic amidine beta-secretase inhibitors with nanomolar potency, cellular activity, and high ligand efficiency. J. Med. Chem. 2007, 50, 5912–5925. [Google Scholar] [CrossRef] [PubMed]
- Peterlin-Masic, L.; Kikelj, D. Arginine mimetics. Tetrahedron 2001, 57, 7073–7105. [Google Scholar] [CrossRef]
- Iwakawa, T.; Tamura, H.; Masuko, M.; Murabayashi, A.; Hayase, Y. Synthesis and rice-blast control activity of sulfonylamidines. J. Pesticide Sci. 1992, 17, 131–135. [Google Scholar] [CrossRef]
- Gobis, K.; Foks, H.; Sławiński, J.; Sikorski, A.; Trzybiński, D.; Augustynowicz-Kopeć, E.; Napiórkowska, A.; Bojanowski, K. Synthesis, structure, and biological activity of novel heterocyclic sulfonyl-carboximidamides. Monatsh. Chem. 2013, 144, 647–658. [Google Scholar] [CrossRef]
- Chang, S.Y.; Bae, S.J.; Lee, M.Y.; Baek, S.H.; Chang, S.; Kim, S.H. Chemical affinity matrix-based identification of prohibitin as a binding protein to anti-resorptive sulfonyl amidine compounds. Bioorg. Med. Chem. Lett. 2011, 21, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Park, M.; Song, J.S.; Park, S.J.; Kim, S.H. Anti-resorptive activity and pharmacokinetic study of N1,N1-diisopropyl-N2-(diphenylphosphoryl)-2-(4-nitrophenyl)acetamidine. Bioorg. Med. Chem. Lett. 2011, 21, 4263–4266. [Google Scholar] [CrossRef]
- Lee, M.Y.; Kim, M.H.; Kim, J.; Kim, S.H.; Kim, B.T.; Jeong, I.H.; Chang, S.; Kim, S.H.; Chang, S.Y. Synthesis and SAR of sulfonyl- and phosphoryl amidine compounds as anti-resorptive agents. Bioorg. Med. Chem. Lett. 2010, 20, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Suja, T.D.; Divya, K.V.L.; Naik, L.V.; Kumar, A.R.; Kamal, A. Copper-catalyzed three-component synthesis of aminonaphthoquinone-sulfonylamidine conjugates and in vitro evaluation of their antiproliferative activity. Bioorg. Med. Chem. Lett. 2016, 26, 2072–2076. [Google Scholar] [CrossRef] [PubMed]
- Beryozkina, T.; Bakulev, V.; Dianova, L.; Berseneva, V.; Slepukhin, P.; Leban, J.; Kalaba, P.; Aher, N.Y.; Ilic, M.; Sitte, H.H.; et al. Organometallic routes to novel steroids containing heterocyclic c-17 side-chains. Synthesis 2016, 48, 48–56. [Google Scholar]
- Filimonov, V.O.; Dianova, L.N.; Galata, K.A.; Beryozkina, T.V.; Novikov, M.S.; Berseneva, V.S.; Eltsov, O.S.; Lebedev, A.T.; Slepukhin, P.A.; Bakulev, V.A. Switchable synthesis of 4,5-functionalized 1,2,3-thiadiazoles and 1,2,3-triazoles from 2-cyanothioacetamides under diazo group transfer conditions. J. Org. Chem. 2017, 82, 4056–4071. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.-L.; Chen, H.-L.; Wang, Y.-H.; Goto, M.; Gao, W.-J.; Cheng, P.-L.; Morris-Natschke, S.L.; Liu, Y.-Q.; Zhu, G.-X.; Wang, M.-J.; et al. Design and synthesis of novel PEG-conjugated 20(S)-camptothecin sulfonylamidine derivatives with potent in vitro antitumor activity via Cu-catalyzed three-component reaction. Bioorg. Med. Chem. Lett. 2015, 25, 2690–2693. [Google Scholar] [CrossRef] [PubMed]
- Ilkin, V.; Berseneva, V.; Beryozkina, T.; Glukhareva, T.; Dianova, L.; Dehaen, W.; Seliverstova, E.; Bakulev, V. Gastric cancer in young patients: A separate entity with aggressive features and poor prognosis. J. Org. Chem. 2020, 16, 2937–2947. [Google Scholar]
- Filimonov, V.O.; Dianova, L.N.; Beryozkina, T.V.; Mazur, D.; Beliaev, N.A.; Volkova, N.N.; Ilkin, V.G.; Dehaen, W.; Lebedev, A.T.; Bakulev, V.A. Water/Alkali-catalyzed reactions of azides with 2-cyanothioacetamides. eco-friendly synthesis of monocyclic and bicyclic 1,2,3-thiadiazole-4-carbimidamides and 5-amino-1,2,3-triazole-4-carbothioamides. J. Org. Chem. 2019, 84, 13430–13446. [Google Scholar] [CrossRef]
- Aswad, M.; Chiba, J.; Takenori, T.; Hatanaka, Y. Evaluation of dipole moment and electrophilicity on the nature of click-type coupling reaction between thioamide and sulfonyl azide. Tetrahedron Lett. 2016, 57, 1313–1316. [Google Scholar] [CrossRef]
- Fleury, L.M.; Wilson, E.E.; Vogt, M.; Fan, T.J.; Oliver, A.G.; Ashfeld, B.L. Amine-free approach toward N-toluenesulfonyl amidine construction: A phosphite-mediated Beckmann-like coupling of oximes and p-toluenesulfonyl azide. Angew. Chem. 2013, 52, 11589–11593. [Google Scholar] [CrossRef]
- Chandna, N.; Chandak, N.; Kumar, P.; Kapoor, J.K.; Sharma, P.K. Metal- and solvent-free synthesis of N-sulfonylformamidines. Green Chem. 2013, 15, 2294–2301. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Y.; Wan, X. Direct condensation of sulfonamide and formamide: NaI-catalyzed synthesis of N-sulfonyl formamidine using TBHP as oxidant. Org. Lett. 2011, 13, 6152–6155. [Google Scholar] [CrossRef] [PubMed]
- DeKorver, K.A.; Johnson, W.L.; Zhang, Y.; Hsung, R.P.; Dai, H.; Deng, J.; Lohse, A.G.; Zhang, Y.-S. N-allyl-N-sulfonyl ynamides as synthetic precursors to amidines and vinylogous amidines. An unexpected N-to-C 1,3-sulfonyl shift in nitrile synthesis. J. Org. Chem. 2011, 76, 5092–5103. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, S.H.; Choi, J.H.; Chang, S. Sulfonyl and phosphoryl azides: Going further beyond the click realm of alkyl and aryl azides. Chem. Asian. J. 2011, 6, 2618–2634. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhou, T.; Liao, M.; Hu, R.; Tang, B.Z. Multicomponent polymerizations of alkynes, sulfonyl azides, and 2-hydroxybenzonitrile/2-aminobenzonitrile toward multifunctional iminocoumarin/quinoline-containing poly(n-sulfonylimine)s. ACS Macro. Lett. 2019, 8, 101–106. [Google Scholar] [CrossRef]
- Yang, W.; Huang, D.; Zeng, X.; Zhang, J.; Wang, X.; Hu, Y. N-Sulfonyl acetylketenimine as a highly reactive intermediate for synthesis of N-Aroylsulfonamides. Tetrahedron 2019, 75, 381–386. [Google Scholar] [CrossRef]
- Yang, W.; Huang, D.; Zeng, X.; Luo, D.; Wang, X.; Hu, Y. N-Sulfonyl acetylketenimine as a highly reactive intermediate for the synthesis of N-sulfonyl amidines. Chem. Commun. 2018, 54, 8222–8225. [Google Scholar] [CrossRef] [PubMed]
- Nallagangula, M.; Namitharan, K. Copper-catalyzed sulfonyl azide-alkyne cycloaddition reactions: Simultaneous generation and trapping of copper-triazoles and -ketenimines for the synthesis of triazolopyrimidines. Org. Lett. 2017, 19, 3536–3539. [Google Scholar] [CrossRef]
- Reichart, B.; Cruz, G.G.D.L.; Zangger, K.; Kappe, C.O.; Glasnov, T. Copper/Nafion-catalyzed hydroarylation process involving ketenimine intermediates: A novel and synthetic approach to 4-sulfonamidoquinoline-2-ones and derivatives thereof. Adv. Synth. Catal. 2016, 358, 50–55. [Google Scholar] [CrossRef]
- Kumar, R.; Thorat, S.H.; Reddy, M.S. Cu-Catalyzed iminative hydroolefination of unactivated alkynes en route to 4-imino-tetrahydropyridines and 4-aminopyridines. Chem. Commun. 2016, 52, 13475–13478. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, D.; Pitchumani, K. Copper(I)-Y Zeolite-catalyzed regio- and stereoselective [2 + 2 + 2] cyclotrimerization cascade: An atom- and step-economical synthesis of pyrimido [1,6-a]quinolone. J. Org. Chem. 2015, 80, 10299–10308. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Cheng, B.; Wang, J.; Lu, P.; Wang, Y. Copper-catalyzed three-component synthesis of 3-aminopyrazoles and 4-iminopyrimidines via β-alkynyl-N-sulfonyl ketenimine intermediates. Org. Lett. 2014, 16, 4814–4817. [Google Scholar] [CrossRef]
- Yoo, E.J.; Ahlquist, M.; Bae, I.; Sharpless, K.B.; Fokin, V.V.; Chang, S. Mechanistic studies on the Cu-catalyzed three-component reactions of sulfonyl azides, 1-alkynes and amines, alcohols, or water: Dichotomy via a common pathway. J. Org. Chem. 2008, 73, 5520–5528. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Cho, S.H.; Chang, S. Comparison of phenolic compounds of rhubarbs in the section deserticola with Rheum palmatum by HPLC-DAD-ESI-MSn. Pure Appl. Chem. 2008, 80, 873–879. [Google Scholar] [CrossRef]
- Sedaghat, A.; Nematpour, M.; Bayanati, M.; Tabatabai, S.A. Synthesis of functionalized quinoline derivatives via intramolecular C–H activation reactions of N-sulfonylamidines and isocyanides. Monatsh. Chem. 2020, 151, 1591–1596. [Google Scholar] [CrossRef]
- Kumar, Y.K.; Kumar, G.R.; Reddy, T.J.; Sridhar, B.; Reddy, M.S. Synthesis of 3-Sulfonylamino Quinolines from 1-(2-Aminophenyl) Propargyl Alcohols through a Ag(I)-Catalyzed hydroamination, (2 + 3) cycloaddition, and an unusual strain-driven ring expansion. Org. Lett. 2015, 17, 2226–2229. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.L.; Shu, M.M.; Dong, B.X.; Gu, H.T.; Liang, R.; Bai, Q.X.; Yang, L.; Zhang, T.; Gao, G.X.; Chen, X.Q. Influence of CD117 expression on response of multiple myeloma patients to chemotherapy. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2015, 23, 1346–1351. [Google Scholar] [PubMed]
- Ghorai, S.; Lee, D. Selectivity for alkynyl or allenyl imidamides and imidates in copper-catalyzed reactions of terminal 1,3-diynes and azides. Org. Lett. 2021, 23, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, S.H.; Chang, S. Highly efficient synthesis of α-amino amidines from ynamides by the Cu-catalyzed three-component coupling reactions. Tetrahedron Lett. 2008, 49, 1745–1749. [Google Scholar] [CrossRef]
- Chauhan, D.P.; Varma, S.J.; Vijeta, A.; Banerjee, P.; Talukdar, P. A 1,3-amino group migration route to form acrylamidines. Chem. Commun. 2014, 50, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Yavari, I.; Ahmadian, S.; Ghazanfarpur-Darjani, M.; Solgi, Y. Formation of N-sulfonylamidines by copper-catalyzed coupling of sulfonyl azides, terminal alkynes, and trialkylamines. Tetrahedron Lett. 2011, 52, 668–670. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.Y.; Lee, J.; Do, Y.; Chang, S. Synthetic utility of ammonium salts in a Cu-catalyzed three-component reaction as a facile coupling partner. J. Org. Chem. 2008, 73, 9454–9457. [Google Scholar] [CrossRef]
- Cui, S.L.; Wang, J.; Wang, Y.J. Copper-catalyzed multicomponent reaction: Facile access to functionalized 5-arylidene-2-imino-3-pyrrolines. Org. Lett. 2007, 9, 5023–5025. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zou, S.; Wu, J. An efficient approach for the construction of benzazepine and benzoxepine derivatives. Chem. Asian J. 2012, 7, 2882–2887. [Google Scholar] [CrossRef] [PubMed]
- Massaro, N.P.; Chatterji, A.; Sharma, I. Three-component approach to pyridine-stabilized ketenimines for the synthesis of diverse heterocycles. J. Org. Chem. 2019, 84, 13676–13685. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Namirembe, S.; Lauchert, L.T.; Tsougranis, G.H.; Isaacs, A.K. Cu(I)-catalyzed synthesis of N-tosyl-4-iminoquinolizines. Tetrahedron Lett. 2015, 56, 4105–4108. [Google Scholar] [CrossRef]
- Tong, T.; Wu, X.; Li, E.; Kang, H.; Wang, X.; Lv, X. One-pot synthesis of 4-sulfonyliminotetrahydropyrimidin-2-one derivatives through a copper-catalyzed tandem reaction. J. Org. Chem. 2018, 83, 15533–15540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Nakamura, I.; Terada, M. Copper-catalyzed cascade transformation of o-propargylic oximes with sulfonyl azides to α,β-unsaturated N-acylamidines. Org. Lett. 2014, 16, 5184–5187. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Kim, J.; Ryu, T.; Kim, K.B.; Lee, P.H. Synthesis of N-Imidoyl and N-oxoimidoyl sulfoximines from 1-alkynes, N-sulfonyl azides, and sulfoximines. Org. Lett. 2015, 17, 3330–3333. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, D.; Pitchumani, K. Copper(I)-catalyzed one-pot synthesis of highly functionalized pyrrolidines from sulfonyl azides, alkynes, and dimethyl 2-(phenylamino)maleate. Eur. J. Org. Chem. 2015, 463–467. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, X.; Zhang, N.; Liang, Y.; Zhang, R.; Xin, X.; Dong, D. Copper-catalyzed three-component reaction: Solvent-controlled regioselective synthesis of 4-amino- and 6-amino-2-iminopyridines. Org. Lett. 2013, 15, 5786–5789. [Google Scholar] [CrossRef] [PubMed]

 | ||||||
|---|---|---|---|---|---|---|
| Entry | Cat. (10 mol%) | Base (10 mol%) | Solvent (10 mol%) | Temp. (°C) | Time (h) | Yield (%) b |
| entry 1 | CuI | Et3N | CH2Cl2 | rt | 1.0 | 78 |
| entry 2 | CuBr | Et3N | CH2Cl2 | rt | 1.0 | 76 |
| entry 3 | CuCl | Et3N | CH2Cl2 | rt | 1.0 | 72 |
| entry 4 | CuBr2 | Et3N | CH2Cl2 | rt | 1.0 | 64 |
| entry 5 | Cu(OAc)2 | Et3N | CH2Cl2 | rt | 1.0 | 52 |
| entry 6 | Cu(OTf)2 | Et3N | CH2Cl2 | rt | 1.0 | 21 |
| entry 7 | AgTFA | Et3N | CH2Cl2 | rt | 1.0 | 0 |
| entry 8 | CuI | DMAP | CH2Cl2 | rt | 1.0 | 26 |
| entry 9 | CuI | DIPEA | CH2Cl2 | rt | 1.0 | 75 |
| entry 10 | CuI | Pyridine | CH2Cl2 | rt | 1.0 | 32 |
| entry 11 | CuI | t-BuONa | CH2Cl2 | rt | 1.0 | 10 |
| entry 12 | CuI | K2CO3 | CH2Cl2 | rt | 1.0 | 8 |
| entry 13 | CuI | Et3N | CHCl3 | rt | 1.0 | 76 |
| entry 14 | CuI | Et3N | DCE | rt | 1.0 | 75 |
| entry 15 | CuI | Et3N | Toluene | rt | 1.0 | 84 |
| entry 16 | CuI | Et3N | MeCN | rt | 1.0 | 52 |
| entry 17 | CuI | Et3N | THF | rt | 1.0 | 80 |
| entry 18 | CuI | Et3N | DMSO | rt | 1.0 | 10 |
| entry 19 | CuI | Et3N | DMF | rt | 1.0 | 6 |
| entry 20 | CuI | Et3N | Toluene | 40 | 1.0 | 75 |
| entry 21 | CuI | Et3N | Toluene | rt | 0.5 | 80 |
| entry 22 | CuI | Et3N | Toluene | rt | 2.0 | 84 |
| entry 23 | CuI | Et3N | Toluene | rt | 3.0 | 84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhou, Z.; Chen, M.; Yang, W. Copper-Catalyzed One-Pot Synthesis of N-Sulfonyl Amidines from Sulfonyl Hydrazine, Terminal Alkynes and Sulfonyl Azides. Molecules 2021, 26, 3700. https://doi.org/10.3390/molecules26123700
Zhao Y, Zhou Z, Chen M, Yang W. Copper-Catalyzed One-Pot Synthesis of N-Sulfonyl Amidines from Sulfonyl Hydrazine, Terminal Alkynes and Sulfonyl Azides. Molecules. 2021; 26(12):3700. https://doi.org/10.3390/molecules26123700
Chicago/Turabian StyleZhao, Yu, Zitong Zhou, Man Chen, and Weiguang Yang. 2021. "Copper-Catalyzed One-Pot Synthesis of N-Sulfonyl Amidines from Sulfonyl Hydrazine, Terminal Alkynes and Sulfonyl Azides" Molecules 26, no. 12: 3700. https://doi.org/10.3390/molecules26123700
APA StyleZhao, Y., Zhou, Z., Chen, M., & Yang, W. (2021). Copper-Catalyzed One-Pot Synthesis of N-Sulfonyl Amidines from Sulfonyl Hydrazine, Terminal Alkynes and Sulfonyl Azides. Molecules, 26(12), 3700. https://doi.org/10.3390/molecules26123700






