Setup of an Ultrasonic-Assisted Extraction to Obtain High Phenolic Recovery in Crataegus monogyna Leaves
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fitting the Model
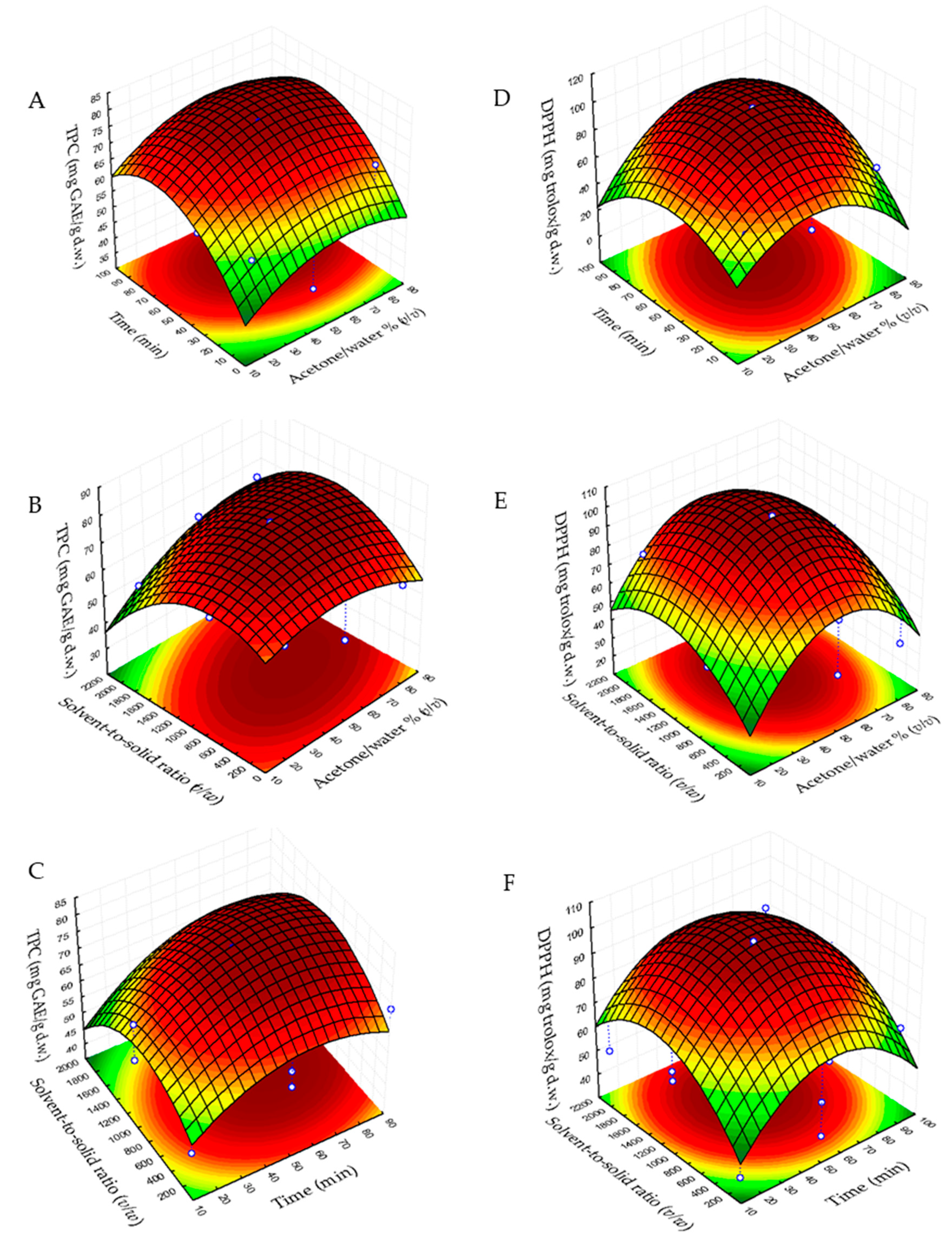
2.2. Analysis of Response Surfaces
2.3. Optimization of Ultrasonic-Assisted Extraction
2.4. Identification of Phenolic Compounds C. monogyna Leaf Extract at Optimum UAE conditions by HPLC-MS
2.5. Identification and Quantification of Proanthocyanididins in C. monogyna Optimum Leaf Extract by NP-HPLC-FLD-MS
2.6. Quantification of Phenolic Compounds in C. monogyna Optimum Leaf Extract by HPLC-MS
2.7. Quantification of Proanthocyanidins in C. monogyna Optimum Leaf Extract by HPLC-FLD
3. Materials and Methods
3.1. Samples
3.2. Chemicals
3.3. Experimental Design
3.4. Extraction of Phenolic Compounds in C. monogyna Leaves Ultrasonic-Assisted Extraction
3.5. Determination of Total Phenolic Content by Folin–Ciocalteu
3.6. Antioxidant Capacity
3.6.1. DPPH Radical Scavenging
3.6.2. ABTS Cation Radical Scavenging
3.6.3. Ferric Reducing Antioxidant Power (FRAP)
3.7. Determination of Phenolic Compounds in Crataegus monogyna Extracs by HPLC-ESI-TOF-MS Analysis
3.8. Determination of Procyanidins in Crataegus Leaf Extract by HPLC-FLD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ferioli, F.; Giambanelli, E.; D’Antuono, L.F. Application of different analytical methods for the determination of phenolics and antioxidant activity in hawthorn (Crataegus spp.) bud and sprout herbal extracts. J. Appl. Bot. Food Qual. 2020, 93, 1–10. [Google Scholar]
- Elsadig Karar, M.G.; Kuhnert, N. UPLC-ESI-Q-TOF-MS/MS Characterization of Phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) Leaves, Fruits and their Herbal Derived Drops (Crataegutt Tropfen). J. Chem. Biol. Ther. 2016, 1, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Christensen, K. Revision of Crataegus Sect. Crataegus and Nothosect. Crataeguineae (Rosaceae-Maloideae) in the Old World. Syst. Bot. Monogr. 1992, 35, 1–199. [Google Scholar] [CrossRef]
- Guinda, Á.; Rada, M.; Delgado, T.; Gutiérrez-Adánez, P.; Castellano, J.M. Pentacyclic Triterpenoids from Olive Fruit and Leaf. J. Agric. Food Chem. 2010, 58, 9685–9691. [Google Scholar] [CrossRef]
- Muñoz-Garmendia, F.; Navarro, C.; Aedo, C.; Crataegus, L. Plantas Vasculares de la Península Ibérica e Islas Baleares; Castroviejo, S., Ed.; Real Jardin Botanico: Madrid, Spain, 1998; p. 404. ISBN 8400062213. [Google Scholar]
- Alirezalu, A.; Ahmadi, N.; Salehi, P.; Sonboli, A.; Alirezalu, K.; Khaneghah, A.M.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M. Physicochemical characterization, antioxidant activity, and phenolic compounds of hawthorn (Crataegus spp.) fruits species for potential use in food applications. Foods 2020, 9, 436. [Google Scholar] [CrossRef] [Green Version]
- Tadić, V.M.; Dobrić, S.; Marković, G.M.; Dordević, S.M.; Arsić, I.A.; Menković, N.R.; Stević, T. Anti-inflammatory, Gastroprotective, Free-Radical-Scavenging, and Antimicrobial Activities of Hawthorn Berries Ethanol Extract. J. Agric. Food Chem. 2008, 56, 7700–7709. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Kallio, H.; Yang, B. Phenolic compounds in hawthorn (Crataegus grayana) fruits and leaves and changes during fruit ripening. J. Agric. Food Chem. 2011, 59, 11141–11149. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Calhelha, R.C.; Barreira, J.C.M.; Dueñas, M.; Carvalho, A.M.; Abreu, R.M.V.; Santos-Buelga, C.; Ferreira, I.C.F.R. Crataegus monogyna buds and fruits phenolic extracts: Growth inhibitory activity on human tumor cell lines and chemical characterization by HPLC-DAD-ESI/MS. Food Res. Int. 2012, 49, 516–523. [Google Scholar] [CrossRef]
- Yang, B.; Liu, P. Composition and health effects of phenolic compounds in hawthorn (Crataegus spp.) of different origins. J. Sci. Food Agric. 2012, 92, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, J.; Mitic, S.; Mitic, M.; Kocic, G.; Pavlovic, A.; Tosic, S. Variation in the phenolic compounds profi le and antioxidant activity in different parts of hawthorn (Crataegus pentagyna Willd.) during harvest periods. Polish J. Food Nutr. Sci. 2019, 69, 367–378. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Habtemariam, S.; Ahmed, T.; Sureda, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.M. Polyphenolic composition of Crataegus monogyna jacq.: From chemistry to medical applications. Nutrients 2015, 7, 7708–7728. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, J.J.C. Plants as source of new drugs. Pharm. Weekbl. 1991, 126, 305. [Google Scholar]
- Caliskan, O. Mediterranean Hawthorn Fruit (Crataegus) Species and Potential Usage. In The Mediterranean Diet: An Evidence-Based Approach; Elsevier: Amsterdam, The Netherlands, 2015; pp. 621–628. ISBN 9780124079427. [Google Scholar]
- European Directorate for the Quality of Medicines & HealthCare (EDQM); European Parlament, Council of Europe: Strasbourg, France, 2004.
- Ngoc, P.C.; Leclercq, L.; Rossi, J.C.; Desvignes, I.; Hertzog, J.; Fabiano-Tixier, A.S.; Chemat, F.; Schmitt-Kopplin, P.; Cottet, H. Optimizing water-based extraction of bioactive principles of hawthorn: From experimental laboratory research to homemade preparations. Molecules 2019, 24, 4420. [Google Scholar] [CrossRef] [Green Version]
- Hellenbrand, N.; Sendker, J.; Lechtenberg, M.; Petereit, F.; Hensel, A. Isolation and quantification of oligomeric and polymeric procyanidins in leaves and flowers of Hawthorn (Crataegus spp.). Fitoterapia 2015, 104, 14–22. [Google Scholar] [CrossRef]
- Verardo, V.; Cevoli, C.; Pasini, F.; MaríaGómez-Caravaca, A.; Marconi, E.; Fabbri, A.; Caboni, M.F. Analysis of oligomer proanthocyanidins in different barley genotypes using High-Performance Liquid Chromatography—Fluorescence Detection − Mass Spectrometry and Near-Infrared Methodologies. J. Agric. Food Chem. 2015, 63, 4130–4137. [Google Scholar] [CrossRef]
- Díaz-de-Cerio, E.; Pasini, F.; Verardo, V.; Fernández-Gutiérrez, A.; Segura-Carretero, A.; Caboni, M.F. Psidium guajava L. leaves as source of proanthocyanidins: Optimization of the extraction method by RSM and study of the degree of polymerization by NP-HPLC-FLD-ESI-MS. J. Pharm. Biomed. Anal. 2017, 133, 1–7. [Google Scholar] [CrossRef]
- Díaz-de-cerio, E.; Tylewicz, U.; Verardo, V.; Fernández-Gutiérrez, A.; Segura-Carretero, A.; Romani, S. Design of Sonotrode Ultrasound-Assisted Extraction of Phenolic Compounds from Psidium guajava L. Leaves. Food Anal. Methods 2017, 10, 2781–2791. [Google Scholar] [CrossRef]
- Martín-García, B.; Pasini, F.; Verardo, V.; Díaz-De-cerio, E.; Tylewicz, U.; Gómez-Caravaca, A.M.; Caboni, M.F. Optimization of sonotrode ultrasonic-assisted extraction of proanthocyanidins from brewers’ spent grains. Antioxidants 2019, 8, 282. [Google Scholar] [CrossRef] [Green Version]
- Le Man, H.; Behera, S.K.; Park, H.S. Optimization of operational parameters for ethanol production from korean food waste leachate. Int. J. Environ. Sci. Technol. 2010, 7, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Pan, G.; Yu, G.; Zhu, C.; Qiao, J. Optimization of ultrasound-assisted extraction (UAE) of flavonoids compounds (FC) from hawthorn seed (HS). Ultrason. Sonochem. 2012, 19, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Šavikin, K.P.; Krstić-Milošević, D.B.; Menković, N.R.; Beara, I.N.; Mrkonjić, Z.O.; Pljevljakušić, D.S. Crataegus orientalis leaves and berries: Phenolic profiles, antioxidant and anti-inflammatory activity. Nat. Prod. Commun. 2017, 12, 159–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alirezalu, A.; Salehi, P.; Ahmadi, N.; Sonboli, A.; Aceto, S.; Maleki, H.H.; Ayyari, M. Flavonoids profile and antioxidant activity in flowers and leaves of hawthorn species (Crataegus spp.) from different regions of iran. Int. J. Food Prop. 2018, 21, 452–470. [Google Scholar] [CrossRef] [Green Version]
- Żurek, N.; Karatsai, O.; Rędowicz, M.J.; Kapusta, I.T. Polyphenolic Compounds of Crataegus Berry, Leaf, and Flower Extracts Affect Viability and Invasive Potential of Human Glioblastoma Cells. Molecules 2021, 26, 2656. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Sun, J.; Chen, P.; Zhang, R.W.; Fan, X.E.; Li, L.W.; Harnly, J.M. Profiling of glucosinolates and flavonoids in rorippa indica (Linn.) Hiern. (cruciferae) by UHPLC-PDA-ESI/HRMSn. J. Agric. Food Chem. 2014, 62, 6118–6129. [Google Scholar] [CrossRef]
- Russo, D.; Kenny, O.; Smyth, T.J.; Milella, L.; Hossain, M.B.; Diop, M.S.; Rai, D.K.; Brunton, N.P. Profiling of Phytochemicals in Tissues from Sclerocarya birrea by HPLC-MS and Their Link with Antioxidant Activity. ISRN Chromatogr. 2013, 2013, 283462. [Google Scholar] [CrossRef] [Green Version]
- Dissanayake, A.A.; Zhang, C.R.; Gaber, M.K.A.; Nair, M.G. Salicylic glycosides in Salix mucronata with antioxidant and antiinflammatory activities. Nat. Prod. Commun. 2017, 12, 1755–1758. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Huélamo, M.; Vallverdú-Queralt, A.; Di Lecce, G.; Valderas-Martínez, P.; Tulipani, S.; Jáuregui, O.; Escribano-Ferrer, E.; Estruch, R.; Illan, M.; Lamuela-Raventós, R.M. Bioavailability of tomato polyphenols is enhanced by processing and fat addition: Evidence from a randomized feeding trial. Mol. Nutr. Food Res. 2016, 60, 1578–1589. [Google Scholar] [CrossRef]
- Verardo, V.; Gómez-Caravaca, A.M.; Marconi, E.; Caboni, M.F. Air classification of barley flours to produce phenolic enriched ingredients: Comparative study among MEKC-UV, RP-HPLC-DAD-MS and spectrophotometric determinations. LWT Food Sci. Technol. 2011, 44, 1555–1561. [Google Scholar] [CrossRef]
- Robbins, R.J.; Leonczak, J.; Johnson, J.C.; Li, J.; Kwik-Uribe, C.; Prior, R.L.; Gu, L. Method performance and multi-laboratory assessment of a normal phase high pressure liquid chromatography-fluorescence detection method for the quantitation of flavanols and procyanidins in cocoa and chocolate containing samples. J. Chromatogr. A 2009, 1216, 4831–4840. [Google Scholar] [CrossRef]
- Chai, W.M.; Chen, C.M.; Gao, Y.S.; Feng, H.L.; Ding, Y.M.; Shi, Y.; Zhou, H.T.; Chen, Q.X. Structural analysis of proanthocyanidins isolated from fruit stone of chinese hawthorn with potent antityrosinase and antioxidant activity. J. Agric. Food Chem. 2014, 62, 123–129. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, D.-W. Critical Reviews in Food Science and Nutrition Enhancement of Food Processes by Ultrasound: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 570–594. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Leb. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verni, M.; Pontonio, E.; Krona, A.; Jacob, S.; Pinto, D.; Rinaldi, F.; Verardo, V.; Díaz-de-Cerio, E.; Coda, R.; Rizzello, C.G. Bioprocessing of Brewers’ Spent Grain Enhances Its Antioxidant Activity: Characterization of Phenolic Compounds and Bioactive Peptides. Front. Microbiol. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]

| Run | Independent Factors | Dependent Factors | |||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | TPC | DPPH | ABTS | FRAP | |
| 1 | 20 | 10 | 1000 | 56.73 | 60.31 | 72.59 | 65.95 |
| 2 | 80 | 10 | 1000 | 67.84 | 64.22 | 69.26 | 79.15 |
| 3 | 20 | 90 | 1000 | 64.24 | 37.68 | 82.21 | 109.22 |
| 4 | 80 | 90 | 1000 | 67.11 | 75.2 | 45.71 | 87.51 |
| 5 | 20 | 50 | 20 | 62.12 | 54.92 | 54.87 | 77.07 |
| 6 | 80 | 50 | 20 | 57.29 | 41.05 | 37.61 | 54.58 |
| 7 | 20 | 50 | 1980 | 53.14 | 75.05 | 62.06 | 82.23 |
| 8 | 80 | 50 | 1980 | 69.31 | 73.4 | 70.89 | 91.89 |
| 9 | 50 | 10 | 20 | 50.3 | 40.53 | 40.41 | 49.62 |
| 10 | 50 | 90 | 20 | 68.27 | 69.74 | 70.45 | 90.46 |
| 11 | 50 | 10 | 1980 | 38.04 | 54.03 | 86.7 | 65.54 |
| 12 | 50 | 90 | 1980 | 66.48 | 85.11 | 90.67 | 117.16 |
| 13 | 50 | 50 | 1000 | 78.6 | 93.04 | 98.18 | 134.68 |
| 14 | 50 | 50 | 1000 | 75.44 | 99.44 | 104.3 | 134.02 |
| 15 | 50 | 50 | 1000 | 78.32 | 101.3 | 102.43 | 125.51 |
| TPC | DPPH | ABTS | FRAP | |
|---|---|---|---|---|
| β0 | 33.78210 * | −4.23490 | −24.5001 * | −31.9747 |
| Linear | ||||
| β1 | 0.49921 * | 1.86645 * | 2.6944 * | 2.7900 * |
| β2 | 0.75200 * | 0.95654 * | 1.1523 * | 2.0480 * |
| β3 | 0.01257 * | 0.03980 * | 0.0532 * | 0.0559 * |
| Cross product | ||||
| β12 | −0.00170 | 0.00700 ** | −0.0069 * | −0.0073 ** |
| β13 | 0.00018 * | 0.00010 | 0.0002 ** | 0.0003 ** |
| β23 | 0.00007 | 0.00001 | −0.0002 ** | 0.0001 |
| Quadratic | ||||
| β11 | −0.00487 * | −0.02212 * | −0.0277 * | −0.0279 * |
| β22 | −0.00567 * | −0.01167 * | −0.0058 * | −0.0130 * |
| β33 | −0.00001 * | −0.00002 * | −0.0000 * | −0.0000 * |
| R2 | 0.85971 | 0.80642 | 0.92192 | 0.97543 |
| R2adujsted | 0.84991 | 0.80004 | 0.91427 | 0.97271 |
| CV | 0.81067 | 0.56165 | 0.58919 | 0.19745 |
| p (Lack of fit) | 0.052959 | 0.076608 | 0.056799 | 0.326531 |
| Optimal Conditions | TPC | DPPH | ABTS | FRAP |
|---|---|---|---|---|
| Acetone/water % (v/v) | 50 | 50 | 50 | 50 |
| Time (min) | 55 | 55 | 55 | 55 |
| Solvent-to-solid ratio (v/w) | 1000 | 1000 | 1000 | 1000 |
| Predicted | 78 ± 4 | 98 ± 11 | 102 ± 8 | 133 ± 13 |
| Observed | 78.9 ± 0.4 | 101 ± 2 | 103 ± 2 | 135 ± 2 |
| Significant differences | N.S. | N.S. | N.S. | N.S. |
| Peak | RT | m/z Experimental | m/z Calculated | Tolerance (ppm) | Error (ppm) | Fit Conf % | In Source Fragments | Molecular Formula | Compound |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.93 | 315.0719 | 315.0716 | 20 | 1 | 99.02 | 153.0171, 109.0246 | C13H15O9 | Protocatechuic acid-glucoside |
| 2 | 2.41 | 299.0759 | 299.0767 | 20 | −2.7 | 61.83 | 137.0249 | C13H15O8 | Hydroxybenzoylhexose |
| 3 | 3.63 | 285.0606 | 285.061 | 20 | −1.4 | 99.99 | 152.0104, 153.0187, 108.0197, 109.0269 | C12H13O8 | Dihydroxy benzoic acid pentoside |
| 4 | 5.32 | 515.1407 | 515.1401 | 20 | 1.2 | 69.65 | 191.0552, 161.0229, 323.0756 | C22H27O14 | 5-O-(3′-O-Caffeoyl glucosyl) quinic acid |
| 5 | 5.42 | 401.1449 | 401.1448 | 20 | 0.2 | 97.62 | 269.1012 | C18H25O10 | Benzyl alcohol- hexose-pentose |
| 6 | 5.76 | 353.0873 | 353.0872 | 20 | −0.3 | 100 | 191.0550, 179.0339, 173.0447, 161.0231, 135.0439 | C16H17O9 | Chlorogenic acid |
| 7 | 6.15 | 371.0981 | 371.0978 | 20 | 0.8 | 99.59 | C16H19O10 | Hydroferulic acid glucuronide | |
| 8 | 7.25 | 337.0913 | 337.0865 | 20 | 4.2 | 96.47 | 191.0553, 163.0390, 119.0492 | C23H13O3 | Cis-3-O-p-coumaroylquinic acid |
| 9 | 7.91 | 433.1132 | 433.1135 | 20 | 0 | 94.42 | 313.0688, 343.0743 | C21H21O10 | Naringenin C-hexoside |
| 10 | 9.09 | 577.1561 | 577.1557 | 20 | 0.7 | 99.66 | 293.0446, 413.0870, 311.0551, 457.1120 | C27H29O14 | 2″-O-rhamnosyl-C-hexosyl-apigenin |
| 11 | 10.13 | 609.1456 | 609.1456 | 20 | 0 | 93.46 | 301.0325, 300.0263, 271.0242 | C27H29O16 | Quercetin 3-O-rutinoside (rutoside) isomer a |
| 12 | 10.32 | 609.1479 | 609.1456 | 20 | 3.8 | 85.12 | 301.0331, 300.0272, 271.0242 | C27H29O16 | Quercetin 3-O-rutinoside (rutoside) isomer b |
| 13 | 10.39 | 463.0879 | 463.0877 | 20 | 0.4 | 99.95 | 301.0335, 300.0274 | C21H19O12 | Quercetin-3-O-galactoside (Hyperoside) |
| 14 | 10.53 | 463.0881 | 463.0877 | 20 | 0.9 | 99.79 | 301.035 | C21H19O12 | Quercetin-3-O-glucoside (Isoquercetin) |
| 15 | 11.106 | 447.0918 | 447.0927 | 20 | −2 | 91.01 | 285.0378, 255.0285, 284.0304 | C21H19O11 | Kaempferol -3-O-glucoside (astragalin) |
| 16 | 11.22 | 433.076 | 433.0771 | 20 | −2.5 | 94.31 | 301.031 | C20H17O11 | Quercetin-pentoside |
| 17 | 11.43 | 505.0973 | 505.0982 | 20 | −1.8 | 99.42 | 463.0902, 301.0323 | C23H21O13 | Quercetin-O-acetyl hexoside |
| 18 | 11.7 | 451.1028 | 451.1029 | 20 | −0.2 | 98.85 | 341.0648, 289.0706, 217.0145 | C24H19O9 | Cinchonain Ia |
| 19 | 12.6 | 461.239 | 461.2387 | 20 | 0.7 | 87.27 | C22H37O10 | Methyl luteolin-C-hexoside |
| Phenolic Compounds | Concentration (mg/g d.w.) |
|---|---|
| Protocatechuic acid-glucoside | 7.5 ± 0.1 |
| Hydroxybenzoylhexose | 1.16 ± 0.03 |
| Dihydroxy benzoic acid pentoside | 7.94 ± 0.05 |
| 5-O-(3′-O-caffeoyl glucosyl)quinic acid | 1.28 ± 0.07 |
| Benzyl alcohol- hexose-pentose | 1.10 ± 0.04 |
| Chlorogenic acid (3-O-caffeoylquinic acid) | 6.51 ± 0.09 |
| Hydroferulic acid (HFA) glucuronide | 2.79 ± 0.02 |
| Cis-3-O-p-coumaroylquinic acid | 0.855 ± 0.006 |
| Naringenin C-hexoside | 0.198 ± 0.001 |
| 2″-O-rhamnosyl-C-hexosyl-apigenin | 8.911 ± 0.008 |
| Quercetin 3-O-rutinoside (rutoside) isomer a | 0.94 ± 0.02 |
| Quercetin 3-O-rutinoside (rutoside) isomer b | 0.37 ± 0.01 |
| Quercetin-3-galactoside (hyperoside) | 4.47 ± 0.04 |
| Quercetin-3-O-glucoside (isoquercetin) | 3.041 ± 0.02 |
| Kaempferol 3-O-glucoside (astragalin) | 0.0471 ± 0.0002 |
| Quercetin-pentoside | 0.0033 ± 0.0001 |
| Quercetin-O-acetyl hexoside | 1.31 ± 0.01 |
| Cinchonain Ia | 0.017 ± 0.002 |
| Methyl luteolin-C-hexoside | 0.0966 ± 0.0009 |
| Sum of Flavonols | 8.9 ± 0.1 |
| Sum of Flavones | 9.007 ± 0.009 |
| Sum of phenolic acid derivatives | 28.1 ± 0.1 |
| Sum of phenolic compounds | 48.6 ± 0.3 |
| Proanthocyanidins | (M−H)− | Concentration (mg/g d.w.) |
|---|---|---|
| Monomers | 289 | 8.7 ± 0.2 |
| dp2 | 577 | 3.7 ± 0.3 |
| dp3 | 865 | 2.09 ± 0.02 |
| dp4 | 1153 | 1.09 ± 0.08 |
| dp5 | 1441 | 0.565 ± 0.03 |
| dp6 | - | 0.258 ± 0.06 |
| dp7 | - | 0.174 ± 0.007 |
| dp8 | - | 0.0723 ± 0.0004 |
| dp9 | - | 0.034 ± 0.001 |
| dp10 | - | 0.0165 ± 0.0002 |
| Polymers | - | 0.76 ± 0.05 |
| Total | - | 17.5 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-García, B.; Razola-Díaz, M.d.C.; Gómez-Caravaca, A.M.; Benítez, G.; Verardo, V. Setup of an Ultrasonic-Assisted Extraction to Obtain High Phenolic Recovery in Crataegus monogyna Leaves. Molecules 2021, 26, 4536. https://doi.org/10.3390/molecules26154536
Martín-García B, Razola-Díaz MdC, Gómez-Caravaca AM, Benítez G, Verardo V. Setup of an Ultrasonic-Assisted Extraction to Obtain High Phenolic Recovery in Crataegus monogyna Leaves. Molecules. 2021; 26(15):4536. https://doi.org/10.3390/molecules26154536
Chicago/Turabian StyleMartín-García, Beatriz, María del Carmen Razola-Díaz, Ana María Gómez-Caravaca, Guillermo Benítez, and Vito Verardo. 2021. "Setup of an Ultrasonic-Assisted Extraction to Obtain High Phenolic Recovery in Crataegus monogyna Leaves" Molecules 26, no. 15: 4536. https://doi.org/10.3390/molecules26154536
APA StyleMartín-García, B., Razola-Díaz, M. d. C., Gómez-Caravaca, A. M., Benítez, G., & Verardo, V. (2021). Setup of an Ultrasonic-Assisted Extraction to Obtain High Phenolic Recovery in Crataegus monogyna Leaves. Molecules, 26(15), 4536. https://doi.org/10.3390/molecules26154536










