Effects of Nifedipine on Renal and Cardiovascular Responses to Neuropeptide Y in Anesthetized Rats
Abstract
:1. Introduction
2. Results
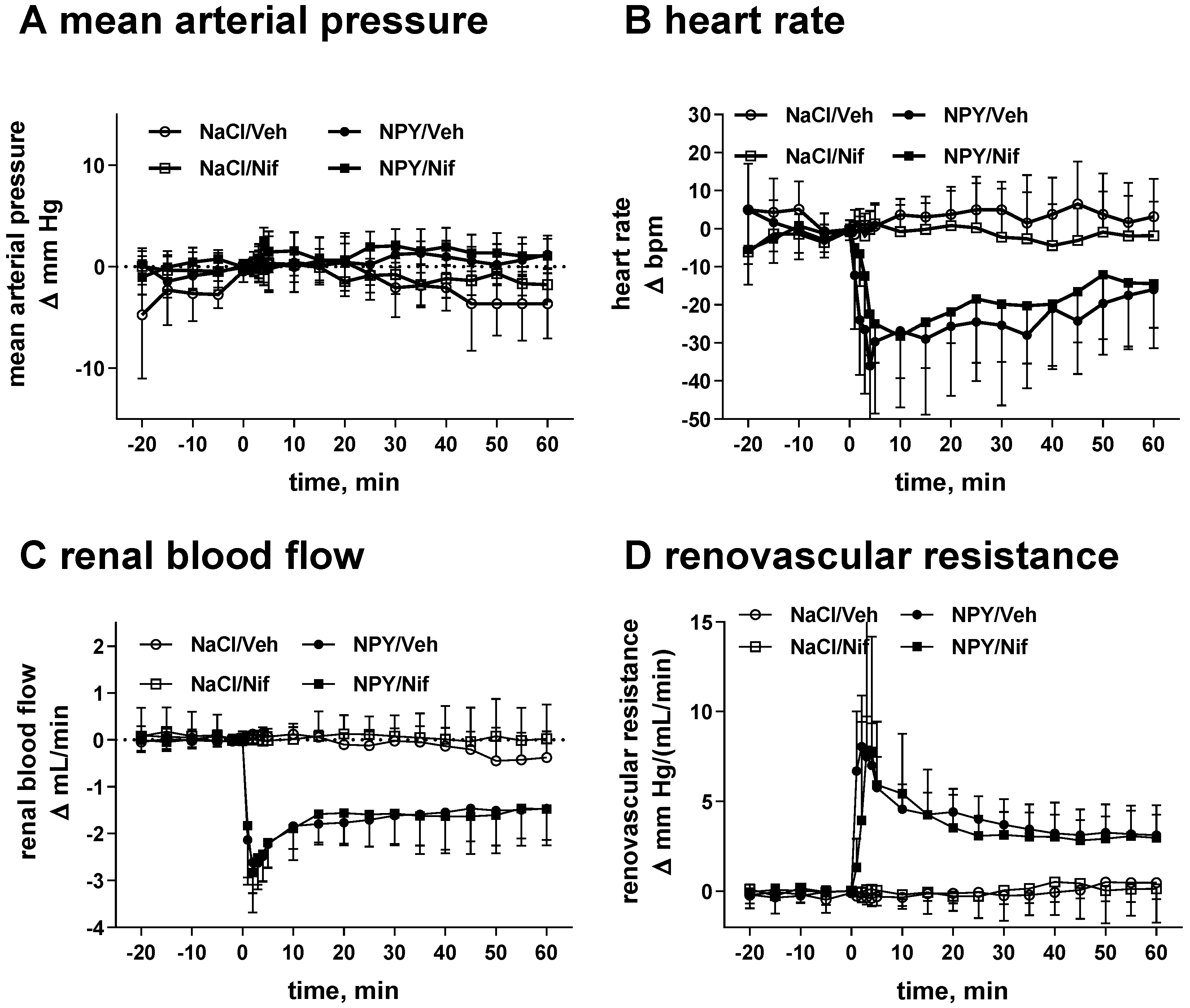
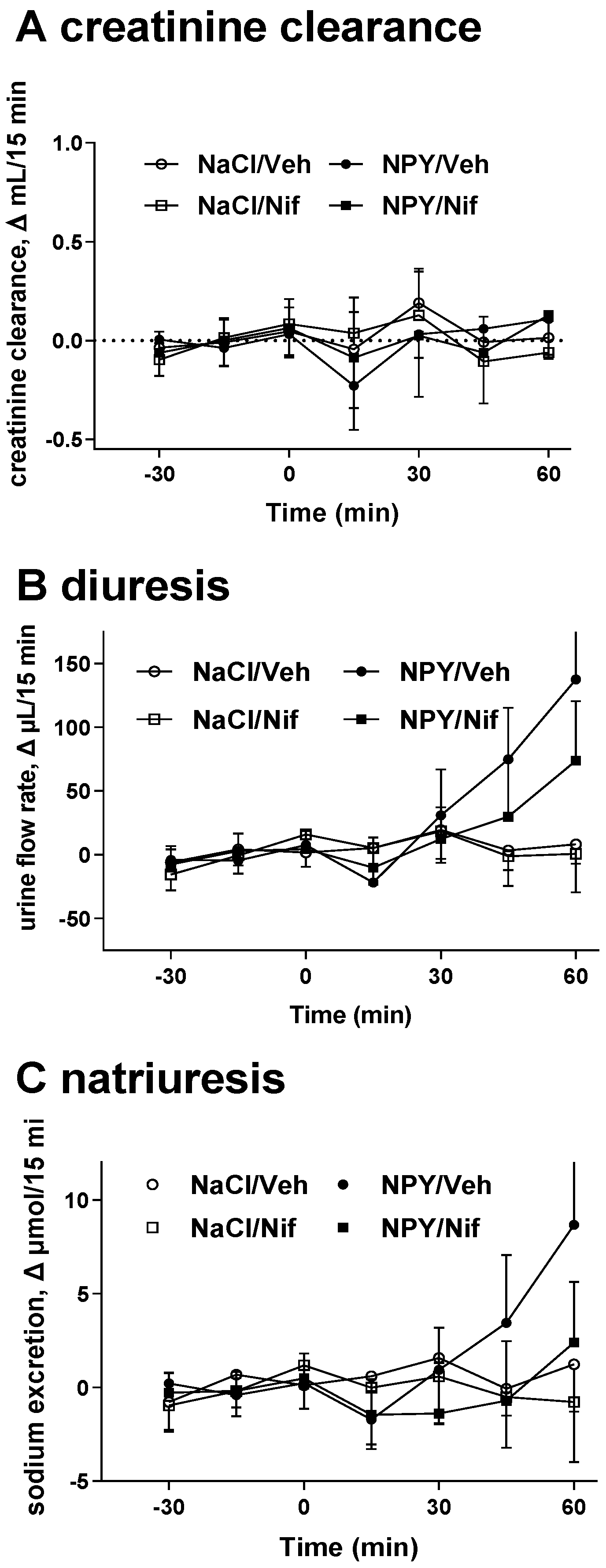
2.1. Study I (NPY Infusion)
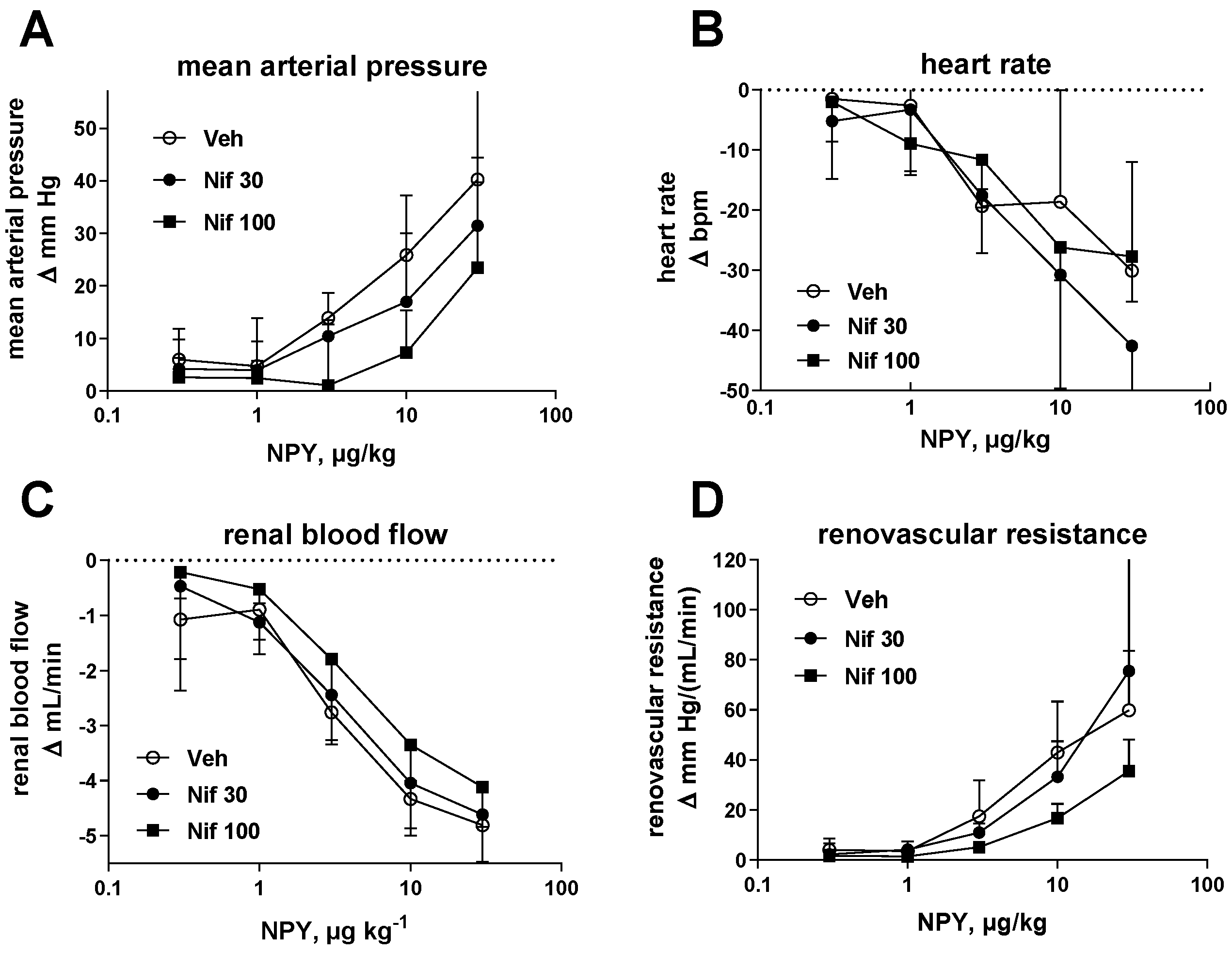
2.2. Study II (NPY Bolus Injection)
3. Discussion
4. Materials and Methods
4.1. Study I (NPY Infusion)
4.2. Study II (NPY Bolus Injections)
4.3. Chemicals
4.4. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Grundemar, L.; Hakanson, R. Multiple neuropeptide Y receptors are involved in cardiovascular regulation. Peripheral and central mechanisms. Gen. Pharmacol. 1993, 24, 785–796. [Google Scholar] [CrossRef]
- Franco-Cereceda, A.; Liska, J. Neuropeptide Y Y1 receptors in vascular pharmacology. Eur. J. Pharmacol. 1998, 349, 1–14. [Google Scholar] [CrossRef]
- Bischoff, A.; Michel, M.C. Renal effects of neuropeptide Y. Pflügers Arch. Eur. J. Physiol. 1998, 435, 443–453. [Google Scholar] [CrossRef]
- Michel, M.C.; Beck-Sickinger, A.G.; Cox, H.; Doods, H.N.; Herzog, H.; Larhammar, D.; Quirion, R.; Schwartz, T.W.; Westfall, T.X.V.I. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY and pancreatic polypeptide receptors. Pharmacol. Rev. 1998, 50, 143–150. [Google Scholar]
- Chen, H.; Fetscher, C.; Schäfers, R.F.; Wambach, G.; Philipp, T.; Michel, M.C. Effects of noradrenaline and neuropeptide Y on rat mesenteric microvessel contraction. Naunyn Schmiedeberg’s Arch. Pharmacol. 1996, 353, 314–323. [Google Scholar]
- Prieto, D.; Buus, C.L.; Mulvany, M.J.; Nilsson, H. Neuropeptide Y regulates intracellular calcium through different signalling pathways linked to a Y 1 -receptor in rat mesenteric small arteries. Br. J. Pharmacol. 2000, 129, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.P.; Chiba, S. Effects of a selective neuropeptide Y Y1 receptor antagonist BIBP 3226 on double peaked vasoconstrictor responses to periarterial nerve stimulation in canine splenic arteries. Br. J. Pharmacol. 2000, 130, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, A.; Stickan-Verfürth, M.; Michel, M.C. Renovascular and tubular effects of neuropeptide Y are discriminated by PP56 (D-myo-inositol 1,2,6-triphosphate) in anaesthetized rats. Pflügers Arch. Eur. J. Physiol. 1997, 434, 57–62. [Google Scholar] [CrossRef]
- Chen, H.; Bischoff, A.; Schäfers, R.F.; Wambach, G.; Philipp, T.; Michel, M.C. Vasoconstriction of rat renal interlobar arteries by noradrenaline and neuropeptide Y. J. Auton. Pharmacol. 1997, 17, 137–146. [Google Scholar] [CrossRef]
- Modin, A.; Malmström, R.E.; Meister, B. Vascular neuropeptide Y Y1-receptors in the rat kidney: Vasoconstrictor effects and expression of Y1-receptor mRNA. Neuropeptides 1999, 33, 253–259. [Google Scholar] [CrossRef]
- Oberhauser, V.; Vonend, O.; Rump, L.C. Neuropeptide Y and ATP interact to control renovascular resistance in the rat. J. Am. Soc. Nephrol. 1999, 10, 1179–1185. [Google Scholar] [CrossRef]
- Cnrkovic, S.; Egemnazarov, B.; Jain, P.; Seay, U.; Gattinger, N.; Marsh, L.M.; Balint, Z.; Kovacs, G.; Ghanim, B.; Klepetko, W.; et al. NPY/Y1 receptor-mediated vasoconstrictory and prolfierative effects in pulmonary hypertension. Br. J. Pharmacol. 2014, 171, 3895–3907. [Google Scholar] [CrossRef] [Green Version]
- Michel, M.C.; Schlicker, E.; Fink, K.; Boublik, J.H.; Göthert, M.; Willette, R.N.; Daly, R.N.; Hieble, J.P.; Rivier, J.E.; Motulsky, H.J. Distinction of NPY receptors in vitro and in vivo. I. NPY-(18-36) discriminates NPY receptor subtypes in vitro. Am. J. Physiol. 1990, 259, E131–E139. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, A.; Freund, A.; Michel, M.C. The Y1 antagonist BIBP 3226 inhibits potentiation of methoxamine-induced vasoconstriction by neuropeptide Y. Naunyn Schmiedeberg’s Arch. Pharmacol. 1997, 356, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Montelongo, M.d.C.; Fountain, S.J. Neuropeptide Y facilitates P2X1 receptor-dependent vasoconstriction via Y1 receptor activation in small mesenteric arteries during sympathetic neurogenic responses. Vasc. Pharmacol. 2021, 136, 106810. [Google Scholar] [CrossRef] [PubMed]
- Malmström, R.E.; Lundberg, J.M.; Weitzberg, E. Effects of the neuropeptide Y Y2 antagonist BIIE0246 on sympathetic transmitter release in the pig in vivo. Naunyn Schmiedeberg’s Arch. Pharmacol. 2002, 365, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Rump, L.C.; Riess, M.; Schwertfeger, E.; Michel, M.C.; Bohmann, C.; Schollmeyer, P. Prejunctional neuropeptide Y receptors in human kidney and atrium. J. Cardiovasc. Pharmacol. 1997, 29, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Schwertfeger, E.; Klein, T.; Vonend, O.; Oberhauser, V.; Stegbauer, J.; Rump, L.C. Neuropeptide Y inhibits acetylcholine release in human heart atrium by activation of Y2-receptors. Naunyn Schmiedeberg’s Arch. Pharmacol. 2004, 369, 455–461. [Google Scholar] [CrossRef]
- Martire, M.; Pistritto, G.; Mores, N.; Agnati, L.F.; Fuxe, K. Region-specific inhibition of potassium-evoked [3H]noradrenaline release from rat brain synaptosomes by neuropeptide Y-(13-36). Involvement of NPY receptors of the Y2 type. Eur. J. Pharmacol. 1993, 230, 231–234. [Google Scholar] [CrossRef]
- Mashiko, S.; Ishihara, A.; Iwaasa, H.; Sano, H.; Ito, J.; Gomori, A.; Oda, Z.; Moriya, R.; Matushita, H.; Jitsuoka, M.; et al. A pair-feeding study reveals that a Y5 antagonist casus weight loss in diet-induced obese mice by modulating food intake and energy expenditure. Mol. Pharmacol. 2007, 71, 602–608. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, A.; Michel, M.C. Neuropeptide Y lowers blood glucose in anaesthetized rats via a Y5 receptor subtype. Endocrinology 1998, 139, 3018–3021. [Google Scholar] [CrossRef]
- Feth, F.; Rascher, W.; Michel, M.C. G-Protein coupling and signalling of Y1-like neuropeptide Y receptors in SK-N-MC cells. Naunyn Schmiedeberg’s Arch. Pharmacol. 1991, 344, 1–7. [Google Scholar] [CrossRef]
- Mihara, S.I.; Shigeri, Y.; Fujimoto, M. Neuropeptide Y-induced intracellular Ca2+ increases in vascular smooth muscle. FEBS Lett. 1989, 259, 79–82. [Google Scholar] [CrossRef] [Green Version]
- Motulsky, H.J.; Michel, M.C. Neuropeptide Y mobilizes Ca++ and inhibits adenylate cyclase in human erythroleukemia cells. Am. J. Physiol. 1988, 255, E880–E885. [Google Scholar]
- Jacques, D.; Sader, S.; El-Bizri, N.; Chouffani, S.; Hassan, G.; Shabklo, H. Neuropeptide Y induced increase of cytosolic and nuclear Ca2+ in heart and vascular smooth muscle. Can. J. Physiol. Pharmacol. 2000, 78, 162–172. [Google Scholar] [CrossRef] [Green Version]
- Prieto, D.; Simonsen, U.; Nyborg, N.C.B. Regional involvement of an endothelium-derived contractile factor in the vasoactive actions of neuropeptide Y in bovine isolated retinal arteries. Br. J. Pharmacol. 1995, 116, 2729–2737. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, M.; Aizawa, Y.; Satott, M.; Suzuki, K.; Shibata, A. Effect of nifedipine on neuropeptide Y-induced vasoconstriction in anesthetized dogs. Jpn. Heart J. 1986, 27, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Hegde, S.S.; Bonhaus, D.W.; Stanley, W.; Eglen, R.M.; Moy, T.M.; Loeb, M.; Shetty, S.G.; Desouza, A.; Krstenansky, J. Pharmacological evaluation of 1229U91, a novel high-affinity and selective neuropeptide Y-Y 1 receptor antagonist. J. Pharmacol. Exp. Ther. 1995, 275, 1261–1266. [Google Scholar]
- Ohtomo, Y.; Ono, S.; Zettergren, E.; Sahlgren, B. Neuropeptide Y regulates rat renal tubular Na,K-ATPase through several signaling pathways. Acta Physiol. Scand. 1996, 158, 97–105. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, I.; Brecha, N.C. Y2 receptor expression and inhibition of voltage-dependent Ca2+ influx into rod bipolar cell terminals. Neuroscience 2004, 125, 1039–1049. [Google Scholar] [CrossRef]
- Silva, A.P.; Carvalho, A.P.; Carvalho, C.M.; Malva, J.O. Functional interaction between neuropeptide Y receptors and modulation of calcium channels in the rat hippocampus. Neuropharmacology 2003, 44, 282–292. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, A.; Püttmann, K.; Kötting, A.; Moser, C.; Buschauer, A.; Michel, M.C. Limited signal transduction repertoire of human Y5 neuropeptide Y receptors expressed in HEC-1B cells. Peptides 2001, 22, 387–395. [Google Scholar] [CrossRef]
- Bischoff, A.; Avramidis, P.; Erdbrügger, W.; Münter, K.; Michel, M.C. Receptor subtypes Y1 and Y5 are involved in the renal effects of neuropeptide Y. Br. J. Pharmacol. 1997, 120, 1335–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischoff, A.; Erdbrügger, W.; Smits, J.; Michel, M.C. Neuropeptide Y-enhanced diuresis and natriuresis in anaesthetized rats is independent from renal blood flow reduction. J. Physiol. 1996, 495, 525–534. [Google Scholar] [CrossRef]
- Bischoff, A.; Gerbracht, A.; Michel, M.C. Gender and hypertension interact to regulate neuropeptide Y responsiveness. Naunyn Schmiedeberg’s Arch. Pharmacol. 2000, 361, 173–180. [Google Scholar] [CrossRef]
- Bischoff, A.; Limmroth, V.; Michel, M.C. Indomethacin inhibits the natriuretic effects of neuropeptide Y in anesthetized rats. J. Pharmacol. Exp. Ther. 1998, 286, 704–708. [Google Scholar]
- Bischoff, A.; Rascher, W.; Michel, M.C. Bradykinin may be involved in neuropeptide Y-induced diuresis, natriuresis, and calciuresis. Am. J. Physiol. 1998, 275, F502–F509. [Google Scholar] [CrossRef]
- Malmström, R.E.; Balmer, K.C.; Weilitz, J.; Nordlander, M.; Sjölander, M. Pharmacology of H394/84, a dihydropyridine neuropeptide Y Y1 receptor antagonist, in vivo. Eur. J. Pharmacol. 2001, 418, 95–104. [Google Scholar] [CrossRef]
- Campo, C.; Garcia-Vallejo, O.; Barrios, V.; Lahera, V.; Manero, M.; Esteban, E.; Rodicio, J.L.; Ruilope, L.M. The natriuretic effect of nifedipine gastrointestinal therapeutic system remains despite the presence of mild-to-moderate renal failure. J. Hypertens. 1997, 15, 1803–1808. [Google Scholar] [CrossRef]
- Schneider, T.; Hein, P.; Michel, M.C. Signal transduction underlying carbachol-induced contraction of rat urinary bladder. I. Phospholipases and Ca2+ sources. J. Pharmacol. Exp. Ther. 2004, 308, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Schneider, T.; Fetscher, C.; Krege, S.; Michel, M.C. Signal transduction underlying carbachol-induced contraction of human urinary bladder. J. Pharmacol. Exp. Ther. 2004, 309, 1148–1153. [Google Scholar] [CrossRef]
- Bischoff, A.; Michel, M.C. Neuropeptide Y enhances potassium excretion by mechanisms distinct from those contolling sodium excretion. Can. J. Physiol. Pharmacol. 2000, 78, 93–99. [Google Scholar] [CrossRef]
- Bischoff, A.; Finger, J.; Michel, M.C. Nifedipine inhibits sphingosine-1-phosphate-induced renovascular contraction in vitro and in vivo. Naunyn Schmiedeberg’s Arch. Pharmacol. 2001, 364, 179–182. [Google Scholar] [CrossRef]
- Michel, M.C.; Murphy, T.J.; Motulsky, H.J. New author guidelines for displaying data and reporting data analysis and statistical methods in experimental biology. Mol. Pharmacol. 2020, 97, 49–60. [Google Scholar] [CrossRef]
- Amrhein, V.; Greenland, S.; McShane, B. Scientists rise up against statistical significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Vehicle | Nifedipine | Difference | |
|---|---|---|---|
| MAP | 115 ± 13 | 121 ± 24 | 6 [−7; 17] |
| HR | 321 ± 42 | 368 ± 38 | 47 [21; 73] |
| RBF | 8.8 ± 1.7 | 7.9 ± 1.9 | −0.9 [−2.1; 0.3] |
| CCR | 1.2 ± 0.1 | 1.3 ± 0.7 | 0.1 [−1.5; 1.7] |
| Diuresis | 123 ± 20 | 362 ± 0.148 | 0.177 [0.035; 0.319] |
| Natriuresis | 1.2 ± 0.4 | 2.0 ± 0.1 | 0.8 [0.38; 1.22] |
| Vehicle | Nifedipine 30 | Nifedipine 100 | |
|---|---|---|---|
| MAP | 110 ± 4 | 106 ± 9 | 106 ± 20 |
| HR | 367 ± 24 | 328 ± 46 | 336 ± 35 |
| RBF | 5.6 ± 0.9 | 5.2 ± 0.9 | 5.3 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bischoff, A.; Stickan-Verfürth, M.; Michel, M.C. Effects of Nifedipine on Renal and Cardiovascular Responses to Neuropeptide Y in Anesthetized Rats. Molecules 2021, 26, 4460. https://doi.org/10.3390/molecules26154460
Bischoff A, Stickan-Verfürth M, Michel MC. Effects of Nifedipine on Renal and Cardiovascular Responses to Neuropeptide Y in Anesthetized Rats. Molecules. 2021; 26(15):4460. https://doi.org/10.3390/molecules26154460
Chicago/Turabian StyleBischoff, Angela, Martina Stickan-Verfürth, and Martin C. Michel. 2021. "Effects of Nifedipine on Renal and Cardiovascular Responses to Neuropeptide Y in Anesthetized Rats" Molecules 26, no. 15: 4460. https://doi.org/10.3390/molecules26154460
APA StyleBischoff, A., Stickan-Verfürth, M., & Michel, M. C. (2021). Effects of Nifedipine on Renal and Cardiovascular Responses to Neuropeptide Y in Anesthetized Rats. Molecules, 26(15), 4460. https://doi.org/10.3390/molecules26154460







