Catalytic Antioxidant Activity of Bis-Aniline-Derived Diselenides as GPx Mimics
Abstract
:1. Introduction
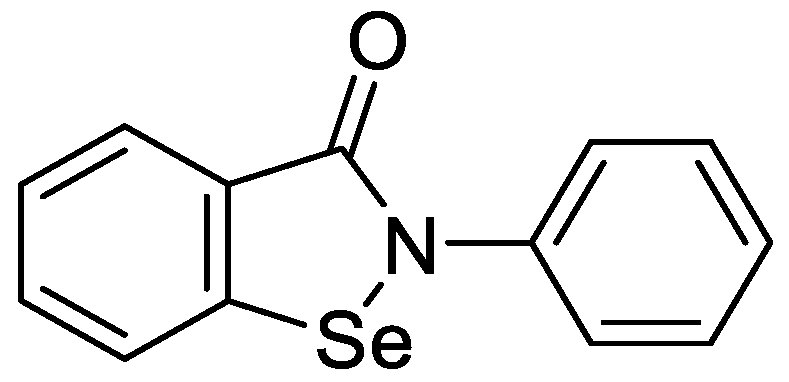
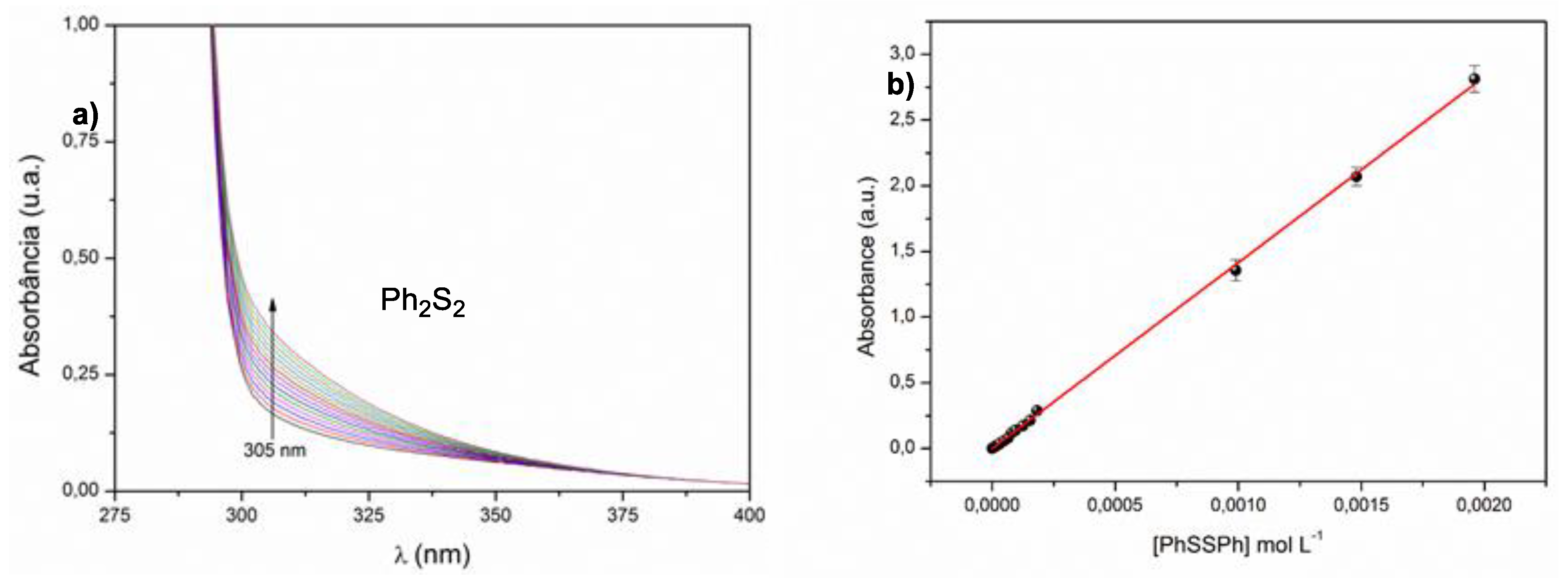
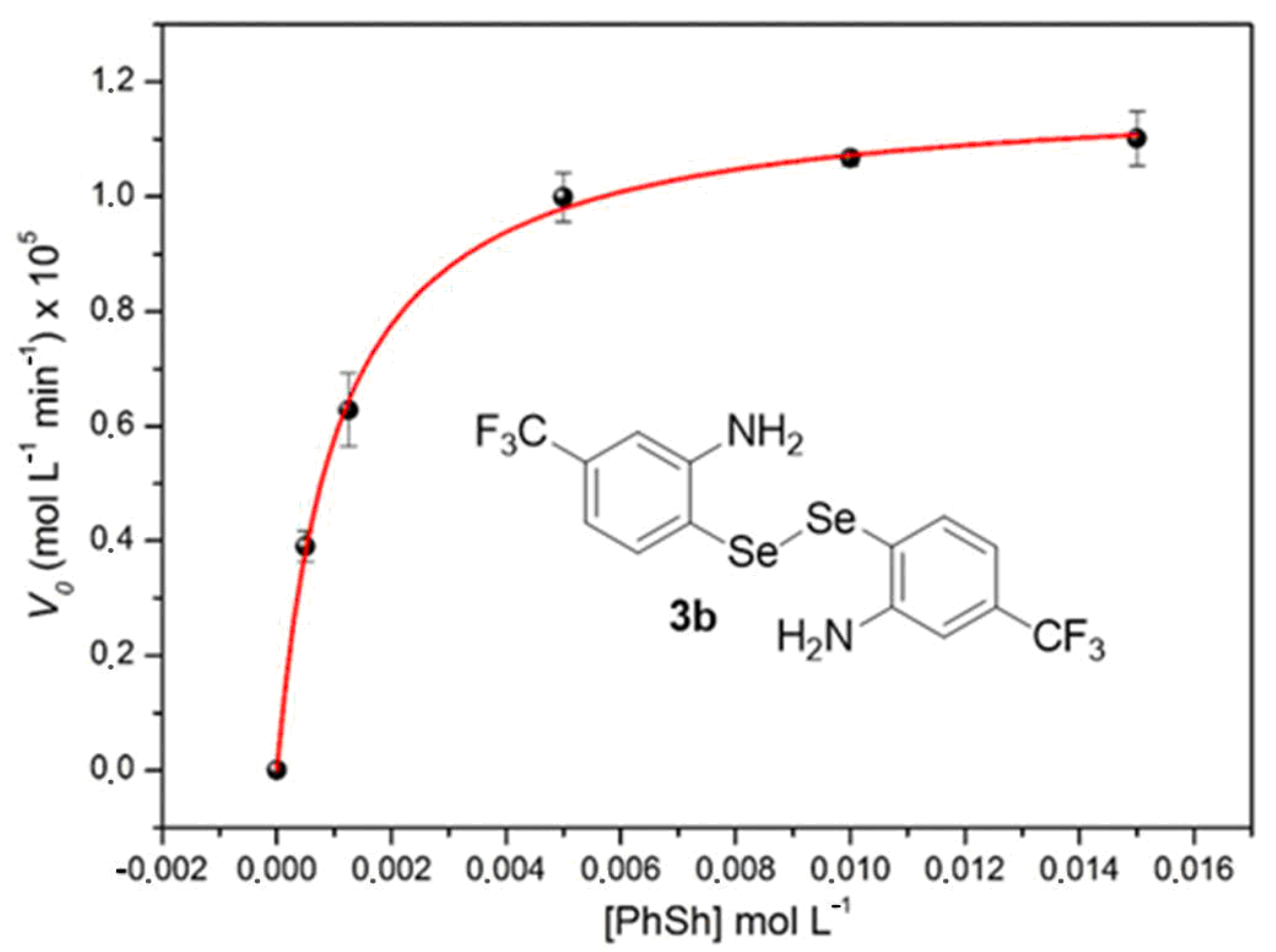
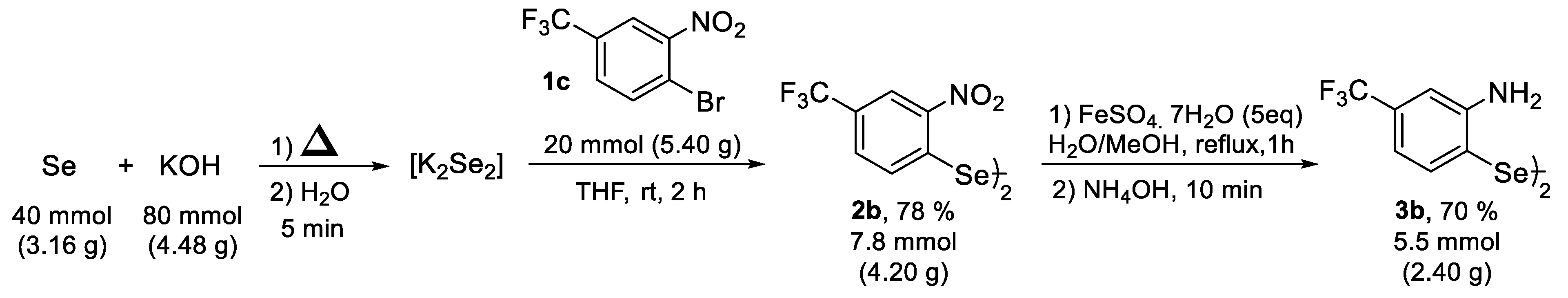
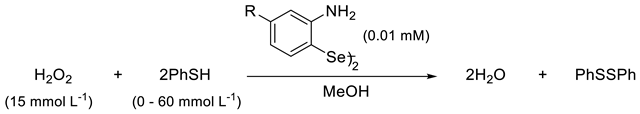
2. Results and Discussion
3. Materials and Methods
3.1. GPx-Like Experimental Procedure
3.2. Michaelis–Menten Equation
3.3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shao, L.; Li, Y.; Lu, J.; Jiang, X. Recent progress in selenium-catalyzed organic reactions. Org. Chem. Front. 2019, 6, 2999–3041. [Google Scholar] [CrossRef]
- Arora, A.; Sinhg, S.; Oswal, P.; Nautiyal, D.; Rao, G.K.; Kumar, S.; Kumar, A. Preformed molecular complexes of metals with organoselenium ligands: Syntheses and applications in catalysis. Coord. Chem. Rev. 2021, 438, 213885. [Google Scholar] [CrossRef]
- Rafique, J.; Rampoin, D.S.; Azeredo, J.B.; Coelho, F.L.; Schneider, P.H.; Braga, A.L. Light-mediated seleno-functionalization of organic molecules: Recent advances. Chem. Rec. 2021. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Y.; Chen, Z.; Pan, X.; Zhan, Z.; Zhu, J.; Zhu, X. Organoselenium chemistry-based polymer synthesis. Org. Chem. Front. 2020, 7, 2815–2841. [Google Scholar] [CrossRef]
- Xiao, X.; Shao, Z.; Yu, L. A perspective of the engineering applications of carbon-based selenium-containing materials. Chin. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Nogueria, C.W.; Barbos, N.V.; Rocha, J.B.T. Toxicology and pharmacology of synthetic organoselenium compounds: An update. Arch. Toxicol. 2021, 95, 1179–1226. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.L.; Rafique, J. Synthesis of biologically relevant small molecules containing selenium. Part A. Antioxidant compounds. In The Chemistry of Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; Wiley: Chichester, UK, 2013; Volume 4, pp. 989–1052. [Google Scholar]
- Barbosa, N.V.; Nogueira, C.W.; Nogara, P.A.; Bem, A.F.; Aschner, M.; Rocha, J.B. Organoselenium compounds as mimics of selenoproteins and thiol modifier agentes. Metallomics 2017, 9, 1703–1734. [Google Scholar] [CrossRef]
- Braga, A.L.; Rafique, J. Synthesis of biologically relevant small molecules containing selenium. Part C. Miscellaneous biological activities. In The Chemistry of Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; Wiley: Chichester, UK, 2013; Volume 4, pp. 1119–1174. [Google Scholar]
- Santi, C.; Bagnoli, L. Celebrating two centuries of research in selenium chemistry: State of the art and new prospective. Molecules 2017, 22, 2124. [Google Scholar] [CrossRef] [Green Version]
- Mugesh, G.; Singh, H.B. Synthetic organoselenium compounds as antioxidants: Glutathione peroxidase activity. Chem. Soc. Rev. 2000, 29, 347–357. [Google Scholar] [CrossRef]
- He, X.; Zhong, M.; Li, S.; Li, X.; Li, Y.; Li, Z.; Gao, Y.; Ding, F.; Wen, D.; Lei, Y.; et al. Synthesis and biological evaluation of organoselenium (NSAIDs-SeCN and SeCF3) derivatives as potential anticancer agents. Eur. J. Med. Chem. 2020, 208, 112864. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Nie, Y.; Zong, M.; Li, S.; Li, X.; Guo, Y.; Liu, Z.; Gao, Y.; Ding, F.; Wen, D.; et al. New organoselenides (NSAIDs-Se derivatives) as potential anticancer agents: Synthesis, biological evaluation and in silico calculations. Eur. J. Med. Chem. 2021, 218, 113384. [Google Scholar] [CrossRef]
- Sabir, S.; Yu, T.T.; Kuppusamy Almohaywi, B.; Iskander, G.; Das, T.; Willcox MD, P.; Black, D.S.; Kumar, N. Novel seleno- and thio-urea containing dihydropyrrol-2-one analogues as antibacterial agents. Antibiotics 2021, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Benedetti, R.; Handzlik, J.; Zwergel Battisetlli, C. The innovative potential of selenium-containing agents for fighting cancer and viral infections. Drug Discov. Today 2021, 26, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.M.; Rafique, J.; Saba, S.; Siminski, T.; Mota NS, R.S.; Filho, D.W.; Braga, A.L.; Pedrosa, R.C.; Ourique, F. Novel selenylated imidazo[1,2-a]pyridines for breast cancer chemotherapy: Inhibition of cell proliferation by Akt-mediated regulation, DNA cleavage and apoptosis. Biochem. Biophys. Res. Commun. 2018, 503, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lai, H.; Hou, L.; Chen, T. Rational design and action mechanisms of chemically innovative organoselenium in cancer therapy. Chem. Commun. 2020, 56, 179–196. [Google Scholar] [CrossRef]
- Santos, D.C.; Rafique, J.; Saba, S.; Almeida, G.M.; Siminski, T.; Padua, C.; Filho, D.W.; Zamoner, A.; Braga, A.L.; Pedrosa, R.C.; et al. Apoptosis oxidative damage-mediated and antiproliferative effect of selenylated imidazo[1,2-a]pyridines on hepatocellular carcinoma HepG2 cells and in vivo. J. Biochem. Mol. Toxicol. 2021, 35, e22663. [Google Scholar] [CrossRef]
- Spengler, G.; Gjdacs, M.; Marc, M.A.; Dominguez-Alvarez, E.; Sanmartin, C. Organoselenium compounds as novel adjuvants of chemotherapy drugs—A promising approach to fight cancer drug resistance. Molecules 2019, 24, 336. [Google Scholar] [CrossRef] [Green Version]
- Kumawat, A.; Raheem, S.; Ali, F.; Dar, T.A.; Chakrabarty, S.; Rizvi, M.A. Organoselenium compounds as acetylcholinesterase inhibitors: Evidence and mechanism of mixed inhibition. J. Phys. Chem. B 2021, 125, 1531–1541. [Google Scholar] [CrossRef]
- Rodrigures, J.; Saba, S.; Joussef, A.C.; Rafique, J.; Braga, A.L. KIO3-catalyzed C(sp2)-H bond selenylation/sulfenylation of (hetero)arenes: Synthesis of chalcogenated (hetero)arenes and their evaluation for anti-Alzheimer activity. Asian J. Org. Chem. 2018, 7, 1819–1824. [Google Scholar] [CrossRef]
- Kepp, K.P. Bioinorganic chemistry of Alzheimer’s disease. Chem. Rev. 2012, 112, 5193–5239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheide, M.R.; Schneider, A.R.; Jardin GA, M.; Martins, G.M.; Durigon, D.C.; Saba, S.; Rafique, J.; Braga, A.L. Electrochemical synthesis of selenyl-dihydrofurans via anodic selenofunctionalization of allyl-naphthol/phenol derivatives and their anti-Alzheimer activity. Org. Biomol. Chem. 2020, 18, 4916–4921. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Chen, H.; Lu, C.-J.; Li, X. Synthesis and evaluation of 8-hydroxyquinolin derivatives substituted with (benzo[d][1,2]selenazol-3(2H)-one) as effective inhibitor of metal-induced Aβ aggregation and antioxidant. Bioorg. Med. Chem. 2016, 24, 4741–4749. [Google Scholar] [CrossRef]
- Veloso, I.C.; Delangara, E.; Machado, A.E.; Braga, S.P.; Rosa, G.K.; Bem, A.F.; Rafique, J.; Saba, S.; Trindade, R.N.; Galetto, F.Z.; et al. A selanylimidazopyridine (3-SePh-IP) reverses the prodepressant- and anxiogenic-like effects of a high-fat/high-fructose diet in mice. J. Pharm. Pharmacol. 2021, 73, 673–681. [Google Scholar] [CrossRef]
- Gülçin, I.; Trofimov, B.; Kaya, R.; Taslimi, P.; Sobenina, L.; Schmidt, E.; Petrova, O.; Malysheva, S.; Gusarova, N.; Farzaliyev, V.; et al. Synthesis of nitrogen, phosphorus, selenium and sulfur-containing heterocyclic compounds—Determination of their carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase and α-glycosidase inhibition properties. Bioorg. Chem. 2021, 103, 104171. [Google Scholar] [CrossRef]
- Martín-Escolano, R.; Etxebeste-Mitxeltorena, M.; Martín-Escolano, J.; Plano, D.; Rosales, M.J.; Espuelas, S.; Moreno, E.; Sánchez-Moreno, M.; Sanmartín, C.; Marín, C. Selenium Derivatives as Promising Therapy for Chagas Disease: In Vitro and In Vivo Studies. ACS Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Galant, L.S.; Rafique, J.; Braga, A.L.; Braga, F.C.; Saba, S.; Radi, R.; Rocha JB, T.; Santi CMonsalve, M.; Farina, M.; Bem, A.F. The thiol-modifier effects of organoselenium compounds and their cytoprotective actions in neuronal cells. Neurochem. Res. 2021, 46, 120–130. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H.; Parnham, M.J. Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Radic. Biol. Med. 2020, 156, 107–112. [Google Scholar] [CrossRef]
- Sun, L.-Y.; Chen, C.; Su, J.; Li, J.-Q.; Jiang, Z.; Gao, H.; Ching, J.-Z.; Ding, H.-H.; Zhai, L.; Yang, K.-W. Ebsulfur and Ebselen as highly potent scaffolds for the development of potential SARS-CoV-2 antivirals. Bioorg. Chem. 2021, 112, 104889. [Google Scholar] [CrossRef] [PubMed]
- Masuda, R.; Kimura, R.; Karasaki, T.; Sase, S.; Goto, K. Modeling the catalytic cycle of glutathione peroxidase by nuclear magnetic resonance spectroscopic analysis of selenocysteine selenenic acids. J. Am. Chem. Soc. 2021, 143, 6345–6350. [Google Scholar] [CrossRef]
- Bortoli, M.; Madabeni, A.; Nogara, P.A.; Omage, F.B.; Ribaudo, G.; Zeppliil, D.; Rocha JB, T.; Orian, L. Chalcogen-nitrogen bond: Insights into a key chemical motif. Catalysts 2020, 11, 114. [Google Scholar] [CrossRef]
- Sudati, J.H.; Nogara, P.A.; Saraiva, R.A.; Wagner, C.; Alberto, A.E.; Braga, A.L.; Fachinetto, R.; Piquini, P.C.; Rocha, J.B.T. Diselenoamino acid derivatives as GPx mimics and as substrates of TrxR: In vitro and in silico studies. Org. Biomol. Chem. 2018, 16, 3777–3787. [Google Scholar] [CrossRef] [PubMed]
- Rafique, J.; Saba, S.; Canto RF, S.; Frizon TE, A.; Hassan, W.; Waczuk, E.P.; Jan, M.; Back, D.F.; Rocha JB, T.; Braga, A.L. Synthesis and biological evaluation of 2-picolylamide-based diselenides with non-bonded interactions. Molecules 2015, 20, 10095–10109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, C.A.; Fry, F.H.; Holme, A.L.; Yiakouvaki, A.; Al-Qenaei, A.; Pourzand, C.; Jacob, C. Towards multifunctional antioxidants: Synthesis, electrochemistry, in vitro and cell culture evaluation of compounds with ligand/catalytic properties. Org. Biomol. Chem. 2005, 3, 1541–1546. [Google Scholar] [CrossRef]
- Nowak, P.; Saluk-Juszczak, J.; Olas, B.; Kolodziejczyk, J.; Wachowics, B. The protective effects of selenoorganic compounds against peroxynitrite-induced changes in plasma proteins and lipids. Cell. Mol. Biol. Lett. 2006, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Saluk, J.; Bijak, M.; Nowak, P.; Wachowicz, B. Evaluating the antioxidative activity of diselenide containing compounds in human blood. Bioorg. Chem. 2013, 50, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kloc, K.; Mlochowski, L.; Osajda, K.; Sypeer, L.; Wójtowics, H. New heterocyclic selenenamides: 1,2,4-benzoselenadiazin-3(4H)-ones and 1,3,2-benzodiselenazoles. Tetrahedron Lett. 2002, 43, 4071–4074. [Google Scholar] [CrossRef]
- Viglianisi, C.; Simone, L.; Menichetti, S. Copper-Mediated One-Pot Transformation of 2-N-Sulfonyl- aminoaryl Diselenides into Benzo[b][1,4]selenazines. Adv. Synth. Catal. 2012, 354, 77–82. [Google Scholar] [CrossRef]
- Radatz, C.S.; Alves, D.; Schneider, P.H. Direct synthesis of 2-aryl-1,3-benzoselenazoles by reaction of bis(2-aminophenyl) diselenides with aryl aldehydes using sodium metabisulfite. Tetrahedron 2013, 69, 1316–1321. [Google Scholar] [CrossRef]
- Deobald, A.M.; Camargo LR, S.; Hörner, M.; Rodrigues OE, D.; Alves, D.; Braga, A.L. Synthesis of arylseleno-1,2,3-triazoles via copper-catalyzed 1,3-dipolar cycloaddition of azido arylselenides with alkynes. Synthesis 2011, 15, 2397–2406. [Google Scholar]
- Frizon TE, A.; Cararo, J.H.; Saba, S.; Dal-Pont, G.C.; Michels, M.; Braga, H.C.; Pimentel, T.; Dal-Pizzol, F.; Valvassori, S.S.; Rafique, J. Synthesis of novel selenocyanates and evaluation of their effect in cultured mouse neurons submitted to oxidative stress. Oxid. Med. Cell. Longev. 2020, 5417024. [Google Scholar] [CrossRef]
- Scheide, M.R.; Peterle, M.M.; Saba, S.; Neto JS, S.; Lenz, G.F.; Cezar, R.D.; Felix, J.F.; Botteselle, G.V.; Schneider, R.; Rafique, J.; et al. Borophosphate glass as an active media for CuO nanoparticle growth: An efficient catalyst for selenylation of oxadiazoles and application in redox reactions. Sci. Rep. 2020, 10, 15233. [Google Scholar] [CrossRef]
- Rafique, J.; Farias, G.; Saba, S.; Zapp, E.; Bellettini, I.C.; Salla, C.A.M.; Bechtold, I.H.; Scheide, M.R.; Neto JS, S.; Souza, D.M., Jr.; et al. Selenylated-oxadiazoles as promising DNA intercalators: Synthesis, electronic structure, DNA interaction and cleavage. Dyes Pigm. 2020, 180, 108519. [Google Scholar] [CrossRef]
- Peterle, M.M.; Scheide, M.R.; Silva, L.T.; Saba, S.; Rafique, J.; Braga, A.L. Copper-catalyzed three-component reaction of oxadiazoles, elemental Se/S and aryl iodides: Synthesis of chalcogenyl (Se/S)-oxadiazoles. Chemitryselect 2018, 3, 13191–13196. [Google Scholar] [CrossRef]
- Silva, L.T.; Azerefo, J.B.; Saba, S.; Rafique, J.; Bortoluzzi, A.J.; Braga, A.L. Solvent- and metal-free chalcogenation of bicyclic arenes Using I2/DMSO as non-metallic catalytic system. Eur. J. Org. Chem. 2017, 32, 4740–4748. [Google Scholar] [CrossRef]
- Rafique, J.; Saba, S.; Frizon TE, A.; Braga, A.L. Fe3O4 nanoparticles: A robust and magnetically recoverable catalyst for direct C-H bond selenylation and sulfenylation of benzothiazoles. Chemsitryselect 2018, 3, 328–334. [Google Scholar] [CrossRef]
- Rocah MS, T.; Rafique, J.; Saba Azerdo, J.B.; Back, D.; Godoi, M.; Braga, A.L. Regioselective hydrothiolation of terminal acetylene catalyzed by magnetite (Fe3O4) nanoparticles. Synth. Commun. 2017, 47, 291–298. [Google Scholar] [CrossRef]
- Rafique, J.; Saba, S.; Rosario, A.R.; Zeni, G.; Braga, A.L. K2CO3-mediated, direct C–H bond selenation and thiolation of 1,3,4-oxadiazoles in the absence of metal catalyst: An eco-friendly approach. RSC Adv. 2014, 4, 51648–51652. [Google Scholar] [CrossRef]
- Menichetti, S.; Capperucci Tanini, D.; Braga ALBotteselle, G.V.; Viglianisi, C. A one-pot access to benzo[b][1,4]selenazines from 2-aminoaryl diselenides. Euro. J. Org. Chem. 2016, 18, 3097–3102. [Google Scholar] [CrossRef]
- Sharma, U.; Verma, P.K.; Kumar, N.; Kumar, V.; Bala, M.; Singh, B. Phosphane-free green protocol for selective nitro reduction with an iron-based catalyst. Chem. Eur. J. 2011, 17, 5903–5907. [Google Scholar] [CrossRef]
- Iwaoka, M.; Tomoda, S. A model study on the effect of an amino group on the antioxidant activity of glutathione peroxidase. J. Am. Chem. Soc. 1994, 116, 2557–2561. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 8, e1327. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Landis, C.R.; Weinhold, F. NBO 6.0; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2013; Available online: http://nbo6.chem.wisc.edu/ (accessed on 12 July 2021).

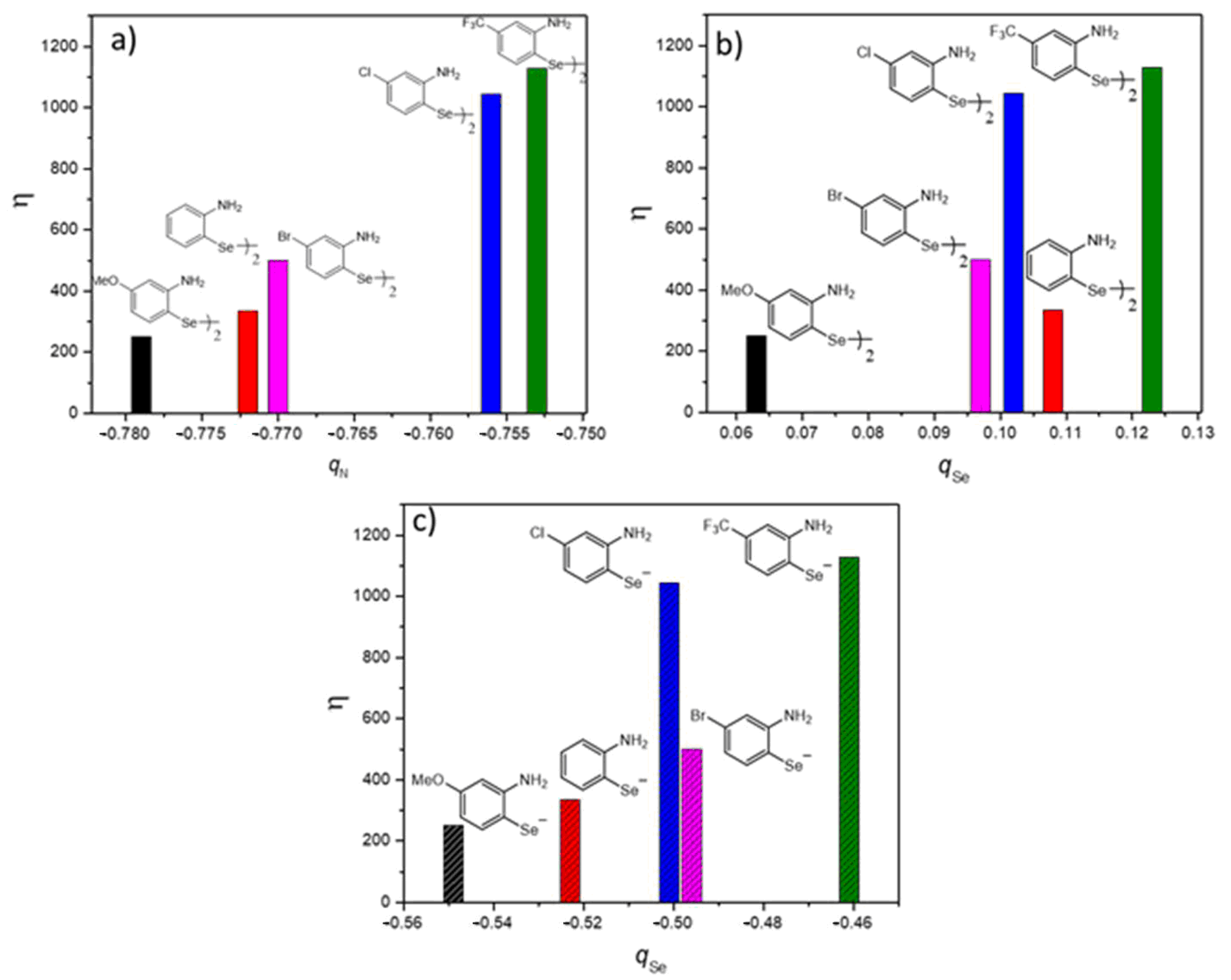

| Entry | Catalyst (1 × 10−5 mol L−1) | Km (mol L−1) | kcat (min−1) | η (L mol−1 min−1) |
|---|---|---|---|---|
| 1 |  | 0.00170 | 0.422 | 248.65 |
| 2 | 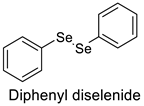 | 0.00114 | 0.601 | 527.78 |
| 3 | 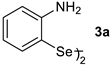 | 0.00134 | 0.446 | 333.40 |
| 4 | 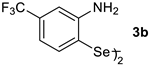 | 0.00105 | 1.185 | 1128.57 |
| 5 | 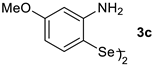 | 0.00187 | 0.470 | 251.51 |
| 6 |  | 0.00081 | 0.405 | 500.11 |
| 7 | 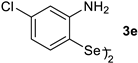 | 0.00088 | 0.918 | 1044.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botteselle, G.V.; Elias, W.C.; Bettanin, L.; Canto, R.F.S.; Salin, D.N.O.; Barbosa, F.A.R.; Saba, S.; Gallardo, H.; Ciancaleoni, G.; Domingos, J.B.; et al. Catalytic Antioxidant Activity of Bis-Aniline-Derived Diselenides as GPx Mimics. Molecules 2021, 26, 4446. https://doi.org/10.3390/molecules26154446
Botteselle GV, Elias WC, Bettanin L, Canto RFS, Salin DNO, Barbosa FAR, Saba S, Gallardo H, Ciancaleoni G, Domingos JB, et al. Catalytic Antioxidant Activity of Bis-Aniline-Derived Diselenides as GPx Mimics. Molecules. 2021; 26(15):4446. https://doi.org/10.3390/molecules26154446
Chicago/Turabian StyleBotteselle, Giancarlo V., Welman C. Elias, Luana Bettanin, Rômulo F. S. Canto, Drielly N. O. Salin, Flavio A. R. Barbosa, Sumbal Saba, Hugo Gallardo, Gianluca Ciancaleoni, Josiel B. Domingos, and et al. 2021. "Catalytic Antioxidant Activity of Bis-Aniline-Derived Diselenides as GPx Mimics" Molecules 26, no. 15: 4446. https://doi.org/10.3390/molecules26154446
APA StyleBotteselle, G. V., Elias, W. C., Bettanin, L., Canto, R. F. S., Salin, D. N. O., Barbosa, F. A. R., Saba, S., Gallardo, H., Ciancaleoni, G., Domingos, J. B., Rafique, J., & Braga, A. L. (2021). Catalytic Antioxidant Activity of Bis-Aniline-Derived Diselenides as GPx Mimics. Molecules, 26(15), 4446. https://doi.org/10.3390/molecules26154446









