Inhibitory Effects of Cucurbitane-Type Triterpenoids from Momordica charantia Fruit on Lipopolysaccharide-Stimulated Pro-Inflammatory Cytokine Production in Bone Marrow-Derived Dendritic Cells
Abstract
:1. Introduction
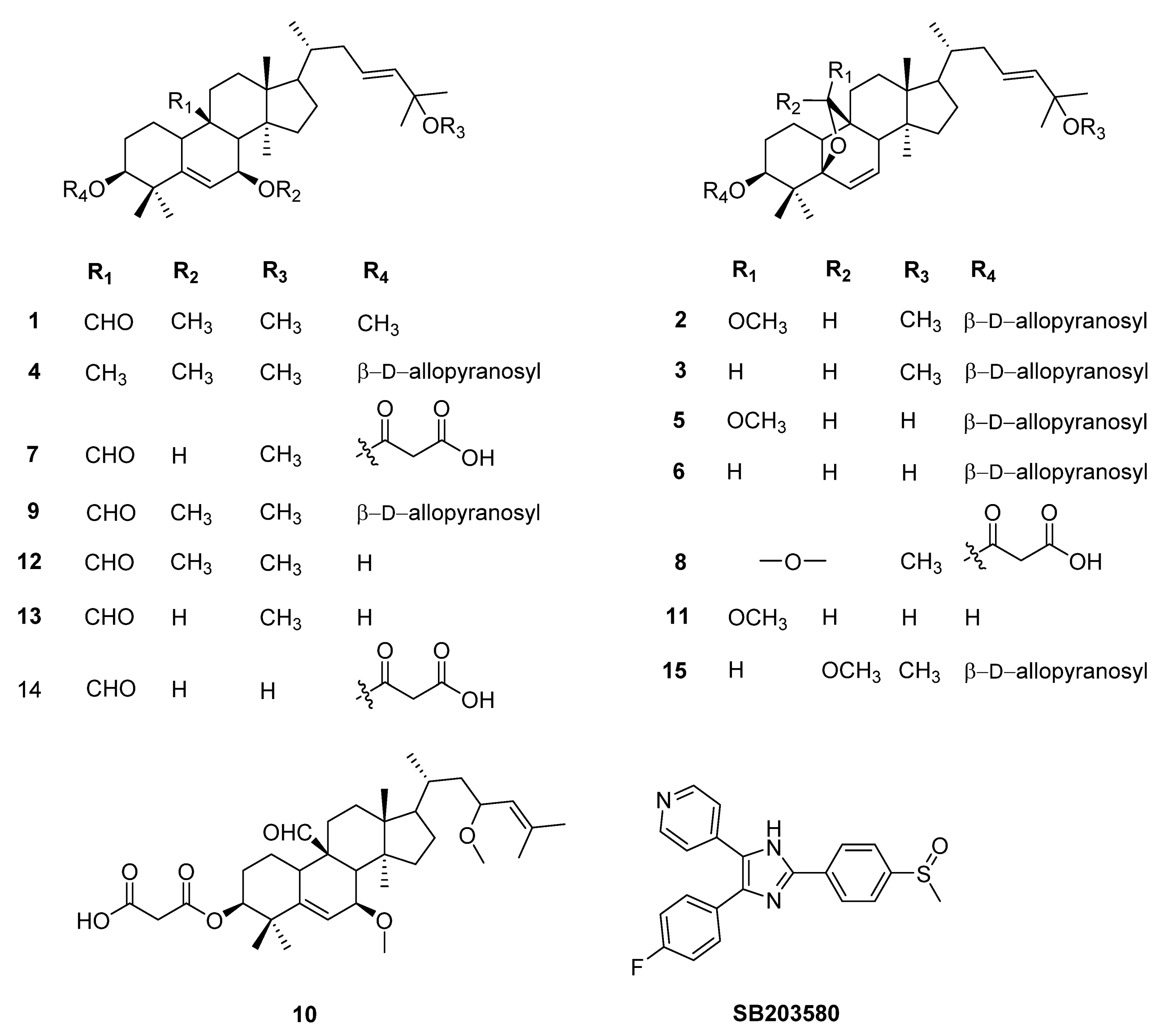
2. Results and Discussion
2.1. Inhibition of IL-6 Expression
2.2. Inhibition of IL-12 Expression
2.3. Inhibition of TNF-α Production
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
Physical and the Key of Spectroscopic Data of New Natural Compound
3.4. Cell Culture and Reagents
3.5. Cytokine Production Measurements
3.6. Cell Viability Assay
3.7. Statistical Analysis
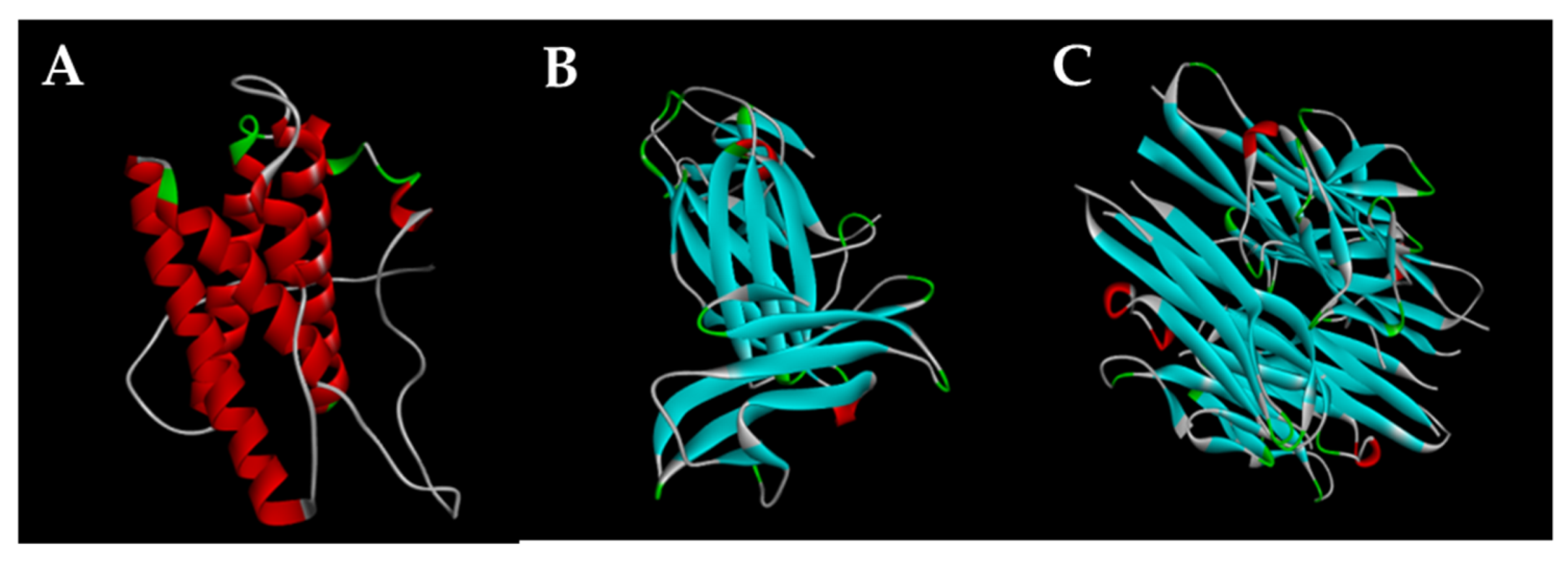
3.8. Preparation of Structures of Proteins Molecular Docking
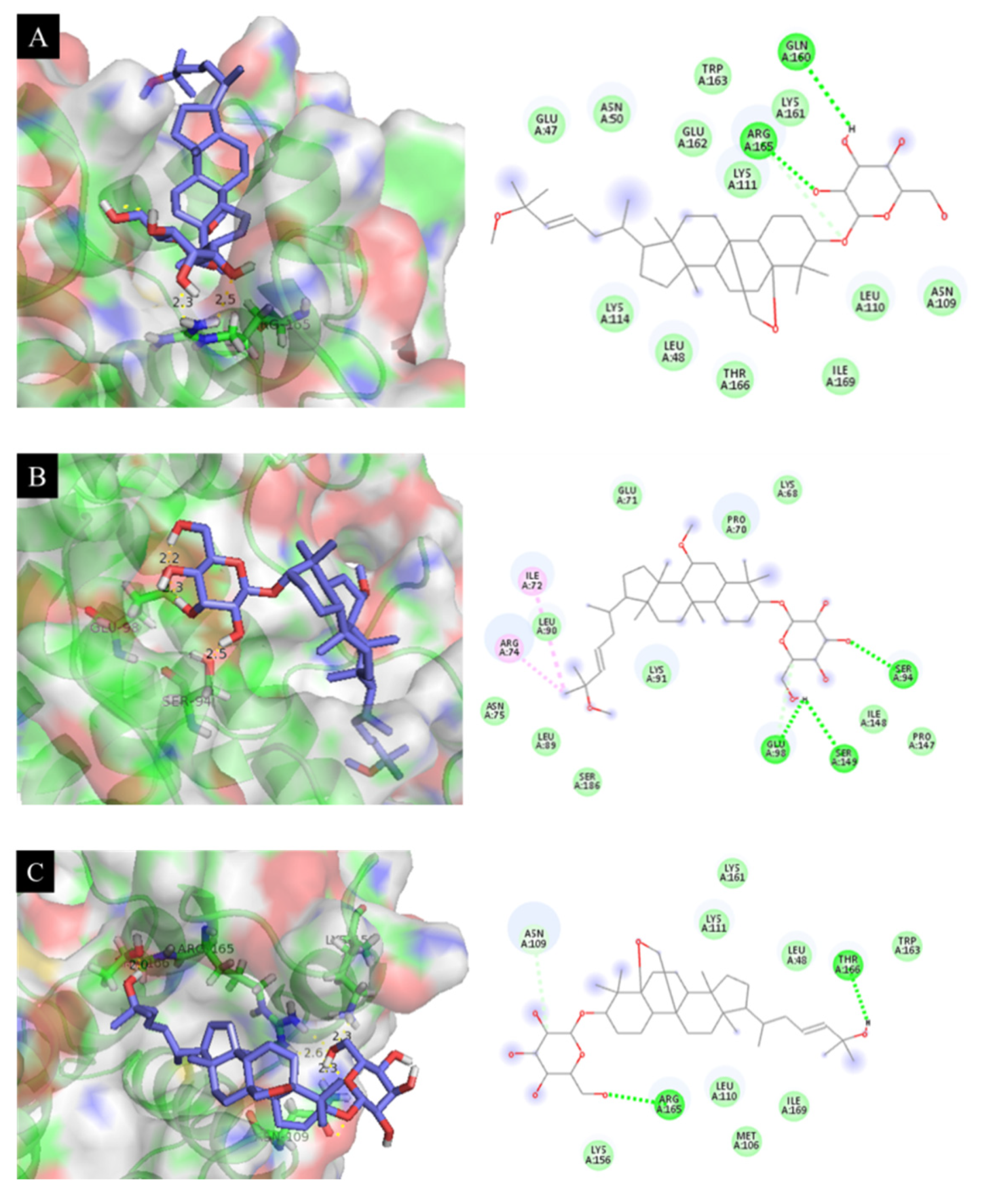
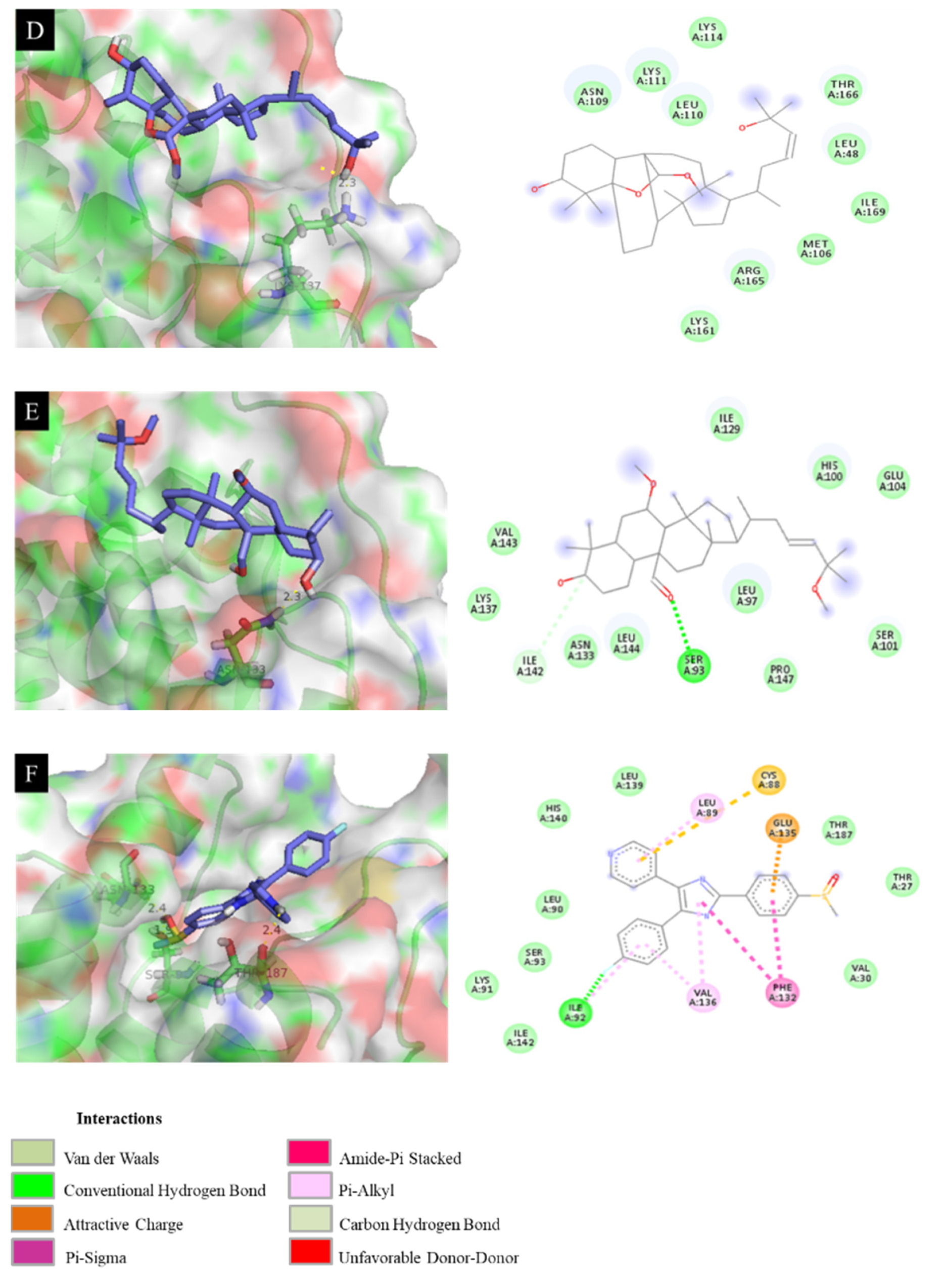
3.9. Molecular Docking of Isolated Compounds with IL-6, IL-12 p40, and TNF-α Proteins
3.10. Docking Results Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Brooks-Worrell, B.; Palmer, J. Immunology in the Clinic Review Series; focus on metabolic diseases: Development of islet autoimmune disease in type 2 diabetes patients: Potential sequelae of chronic inflammation. Clin. Exp. Immunol. 2012, 167, 40–46. [Google Scholar] [CrossRef]
- Cao, T.Q.; Tran, M.H.; Kim, J.A.; Tran, P.T.; Lee, J.-H.; Woo, M.H.; Lee, H.-K.; Min, B.S. Inhibitory effects of compounds from Styrax obassia on NO production. Bioorg. Med. Chem. Lett. 2015, 25, 5087–5091. [Google Scholar] [CrossRef]
- Wang, R.X.; Zhou, M.; Ma, H.L.; Qiao, Y.B.; Li, Q.S. The Role of Chronic Inflammation in Various Diseases and Anti-inflammatory Therapies Containing Natural Products. ChemMedChem 2021, 16, 1576–1592. [Google Scholar] [CrossRef]
- Subramaniam, S.; Selvaduray, K.R.; Radhakrishnan, A.K. Bioactive compounds: Natural defense against cancer? Biomolecules 2019, 9, 758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mushtaq, S.; Abbasi, B.H.; Uzair, B.; Abbasi, R. Natural products as reservoirs of novel therapeutic agents. EXCLI J. 2018, 17, 420. [Google Scholar]
- Raman, A.; Lau, C. Anti-diabetic properties and phytochemistry of Momordica charantia L. (Cucurbitaceae). Phytomedicine 1996, 2, 349–362. [Google Scholar] [CrossRef]
- Perera, W.H.; Shivanagoudra, S.R.; Pérez, J.L.; Kim, D.M.; Sun, Y.; Jayaprakasha, G.K.; Patil, B.S. Anti-Inflammatory, antidiabetic properties and in silico modeling of cucurbitane-type triterpene glycosides from fruits of an indian cultivar of Momordica charantia L. Molecules 2021, 26, 1038. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008, 110, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Higo, N.; Tokuda, H.; Ukiya, M.; Akazawa, H.; Tochigi, Y.; Kimura, Y.; Suzuki, T.; Nishino, H. Cucurbitane-type triterpenoids from the fruits of Momordica charantia and their cancer chemopreventive effects. J. Nat. Prod. 2007, 70, 1233–1239. [Google Scholar] [CrossRef]
- Alam, S.; Asad, M.; Asdaq, S.M.B.; Prasad, V.S. Antiulcer activity of methanolic extract of Momordica charantia L. in rats. J. Ethnopharmacol. 2009, 123, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yang, L.; Guo, X.-Y.; Zhang, Y.-Y.; Zhang, Y.; Liu, X.-W. Novel cucurbitane-type triterpene saponins from Hemsleya amabilis. J. Asian Nat. Prod. Res. 2020, 22, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Sahranavard, S.; Naghibi, F.; Siems, K.; Jenett-Siems, K. New cucurbitane-type triterpenoids from Bryonia aspera. Planta Med. 2010, 76, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Qiang, S.; Lou, L.; Zhao, W. Cucurbitane-type triterpenoids from the stems of Cucumis melo. J. Nat. Prod. 2009, 72, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Sun, Z.; Hu, M.; Li, Y.; Zhang, D.; Wu, H.; Tian, Y.; Li, P.; Yang, J.; Ma, G. Cucurbitane-type triterpenes from the tubers of Hemsleya penxianensis and their bioactive activity. Phytochemistry 2018, 147, 49–56. [Google Scholar] [CrossRef]
- Chen, J.-C.; Zhang, G.-H.; Zhang, Z.-Q.; Qiu, M.-H.; Zheng, Y.-T.; Yang, L.-M.; Yu, K.-B. Octanorcucurbitane and cucurbitane triterpenoids from the tubers of Hemsleya endecaphylla with HIV-1 inhibitory activity. J. Nat. Prod. 2008, 71, 153–155. [Google Scholar] [CrossRef]
- Huang, H.-T.; Zhang, L.-J.; Huang, H.-C.; Hwang, S.-Y.; Wu, C.-L.; Lin, Y.-C.; Liaw, C.-C.; Cheng, Y.-Y.; Morris-Natschke, S.L.; Huang, C.-Y. Cucurbitane-Type Triterpenoids from the Vines of Momordica charantia and Their Anti-inflammatory Activities. J. Nat. Prod. 2020, 83, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Harinantenaina, L.; Tanaka, M.; Takaoka, S.; Oda, M.; Mogami, O.; Uchida, M.; Asakawa, Y. Momordica charantia constituents and antidiabetic screening of the isolated major compounds. Chem. Pharm. Bull. 2006, 54, 1017–1021. [Google Scholar] [CrossRef] [Green Version]
- Murakami, T.; Emoto, A.; Matsuda, H.; Yoshikawa, M. Medicinal foodstuffs. XXI. Structures of new cucurbitane-type triterpene glycosides, goyaglycosides-a,-b,-c,-d,-e,-f,-g, and-h, and new oleanane-type triterpene saponins, goyasaponins I, II, and III, from the fresh fruit of Japanese Momordica charantia L. Chem. Pharm. Bull. 2001, 49, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Okabe, H.; Miyahara, Y.; Yamauchi, T. Studies on the constituents of Momordica charantia L. III. Characterization of new cucurbitacin glycosides of the immature fruits. Structures of momordicosides G, F1, F2 and I. Chem. Pharm. Bull. 1982, 30, 3977–3986. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.-Q.; Chen, J.-C.; Wang, C.-F.; Qiu, M.-H. New cucurbitane triterpenoids and steroidal glycoside from Momordica charantia. Molecules 2009, 14, 4804–4813. [Google Scholar] [CrossRef] [PubMed]
- Tuan, N.Q.; Lee, D.-H.; Oh, J.; Kim, C.S.; Heo, K.-S.; Myung, C.-S.; Na, M. Inhibition of proliferation of vascular smooth muscle cells by cucurbitanes from Momordica charantia. J. Nat. Prod. 2017, 80, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Sun, Y.; Xu, J.; Cao, J.; Chen, G.; Zhang, H.; Zhang, X.; Zhao, Y. Cucurbitane triterpenoids from the fruit of Momordica charantia L. and their anti-hepatic fibrosis and anti-hepatoma activities. Phytochemistry 2019, 157, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, K.; Nishimoto, N.; Mihara, M.; Kishimoto, T. Therapy of Rheumatoid Arthritis by Blocking IL-6 Signal Transduction with a Humanized Anti-IL-6 Receptor Antibody. Springer Semin. Immunopathol. 1998, 20, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Hennigan, S.; Kavanaugh, A. Interleukin-6 inhibitors in the treatment of rheumatoid arthritis. Ther. Clin. Risk Manag. 2008, 4, 767. [Google Scholar]
- Kudo, O.; Sabokbar, A.; Pocock, A.; Itonaga, I.; Fujikawa, Y.; Athanasou, N. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone 2003, 32, 1–7. [Google Scholar] [CrossRef]
- Zundler, S.; Neurath, M.F. Interleukin-12: Functional activities and implications for disease. Cytokine Growth Factor. Rev. 2015, 26, 559–568. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- Kim, T.S.; Kang, B.; Lee, M.; Choe, Y.K.; Hwang, S. Inhibition of interleukin-12 production by auranofin, an anti-rheumatic gold compound, deviates CD4+ T cells from the Th1 to the Th2 pathway. Br. J. Pharmacol. 2001, 134, 571–578. [Google Scholar] [CrossRef]
- Romanowska-Próchnicka, K.; Felis-Giemza, A.; Olesińska, M.; Wojdasiewicz, P.; Paradowska-Gorycka, A.; Szukiewicz, D. The Role of TNF-α and Anti-TNF-α Agents during Preconception, Pregnancy, and Breastfeeding. Int. J. Mol. Sci. 2021, 22, 2922. [Google Scholar] [CrossRef]
- Ting, H.-K.; Chen, C.-L.; Meng, E.; Cherng, J.-H.; Chang, S.-J.; Kao, C.-C.; Yang, M.-H.; Leung, F.-S.; Wu, S.-T. Inflammatory Regulation by TNF-α-Activated Adipose-Derived Stem Cells in the Human Bladder Cancer Microenvironment. Int. J. Mol. Sci. 2021, 22, 3987. [Google Scholar] [CrossRef]
- Vinh, L.B.; Heo, M.; Phong, N.V.; Ali, I.; Koh, Y.S.; Kim, Y.H.; Yang, S.Y. Bioactive Compounds from Polygala tenuifolia and Their Inhibitory Effects on Lipopolysaccharide-Stimulated Pro-inflammatory Cytokine Production in Bone Marrow-Derived Dendritic Cells. Plants 2020, 9, 1240. [Google Scholar] [CrossRef]
- Vinh, L.B.; Phong, N.V.; Ali, I.; Dan, G.; Koh, Y.S.; Anh, H.L.T.; Yang, S.Y.; Kim, Y.H. Identification of potential anti-inflammatory and melanoma cytotoxic compounds from Aegiceras corniculatum. Med. Chem. Res. 2020, 29, 2020–2027. [Google Scholar] [CrossRef]
- Ali, I.; Manzoor, Z.; Koo, J.-E.; Kim, J.-E.; Byeon, S.-H.; Yoo, E.-S.; Kang, H.-K.; Hyun, J.-W.; Lee, N.-H.; Koh, Y.-S. 3-Hydroxy-4, 7-megastigmadien-9-one, isolated from Ulva pertusa, attenuates TLR9-mediated inflammatory response by down-regulating mitogen-activated protein kinase and NF-κB pathways. Pharm. Biol. 2017, 55, 435–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Compounds | IC50 (µM) | ||
|---|---|---|---|
| IL-6 | IL-12 p40 | TNF-α | |
| 1 | 0.886 ± 0.118 | 1.192 ± 0.104 | 2.809 ± 0.264 |
| 2 | 1.616 ± 0.211 | 0.733 ± 0.041 | 2.616 ± 0.181 |
| 3 | 0.245 ± 0.008 | 0.495 ± 0.003 | 4.357 ± 0.037 |
| 4 | 0.363 ± 0.015 | 0.031 ± 0.001 | 0.810 ± 0.068 |
| 5 | 0.582 ± 0.024 | 0.063 ± 0.017 | 2.144 ± 0.151 |
| 6 | 0.381 ± 0.039 | 0.012 ± 0.000 | 0.043 ± 0.006 |
| 7 | 1.299 ± 0.222 | 0.551 ± 0.048 | 3.888 ± 0.149 |
| 8 | 1.348 ± 0.052 | 0.085 ± 0.005 | 1.568 ± 0.209 |
| 9 | 0.568 ± 0.004 | 0.052 ± 0.005 | 0.087 ± 0.033 |
| 10 | 0.858 ± 0.002 | 0.553 ± 0.010 | 2.143 ± 0.003 |
| 11 | 0.157 ± 0.011 | 0.073 ± 0.086 | 0.033 ± 0.002 |
| 12 | 0.028 ± 0.005 | 0.045 ± 0.026 | 0.142 ± 0.009 |
| 13 | 1.962 ± 0.096 | 0.795 ± 0.086 | 0.388 ± 0.015 |
| 14 | 0.751 ± 0.038 | 0.299 ± 0.002 | 0.811 ± 0.005 |
| 15 | 1.553 ± 0.245 | 1.360 ± 0.101 | 3.716 ± 0.011 |
| SB203580a | 5.000 ± 0.080 | 3.500 ± 0.080 | 7.200 ± 0.060 |
| Compounds | DS (kcal·mol−1) | RMSD (Å) | Interaction with Amino Acid |
|---|---|---|---|
| 3 | −7.18 | 18.64 | Arg 165 (2.3 Å) |
| 4 | −7.48 | 17.07 | Ser 94 (2.5 Å), Glu 98 (2.2 Å) |
| 6 | −7.26 | 21.27 | Lys 156 (2.3 Å), Thr 166 (2.0 Å), Arg 165 (2.6 Å), Asn 109 (2.3 Å) |
| 11 | −6.99 | 18.85 | Lys 137 (2.3 Å) |
| 12 | −6.41 | 11.79 | Asn 133 (2.3 Å) |
| SB203580 a | −7.96 | 7.02 | Asn 133 (2.4 Å), Ser 93 (1.9 Å), Thr 187 (2.4 Å) |
| Compounds | DS (kcal·mol−1) | RMSD (Å) | Interaction with Amino Acid |
|---|---|---|---|
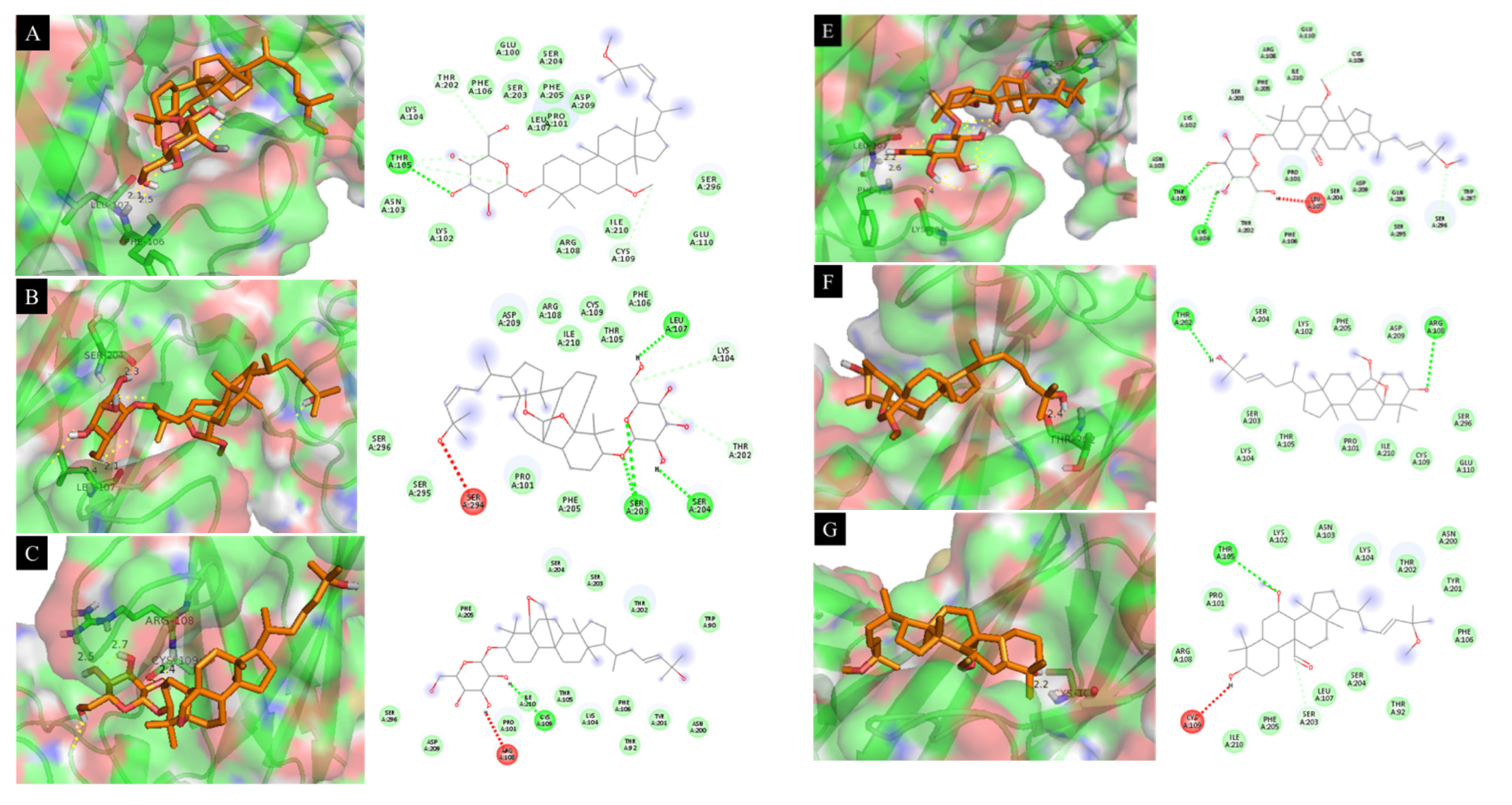
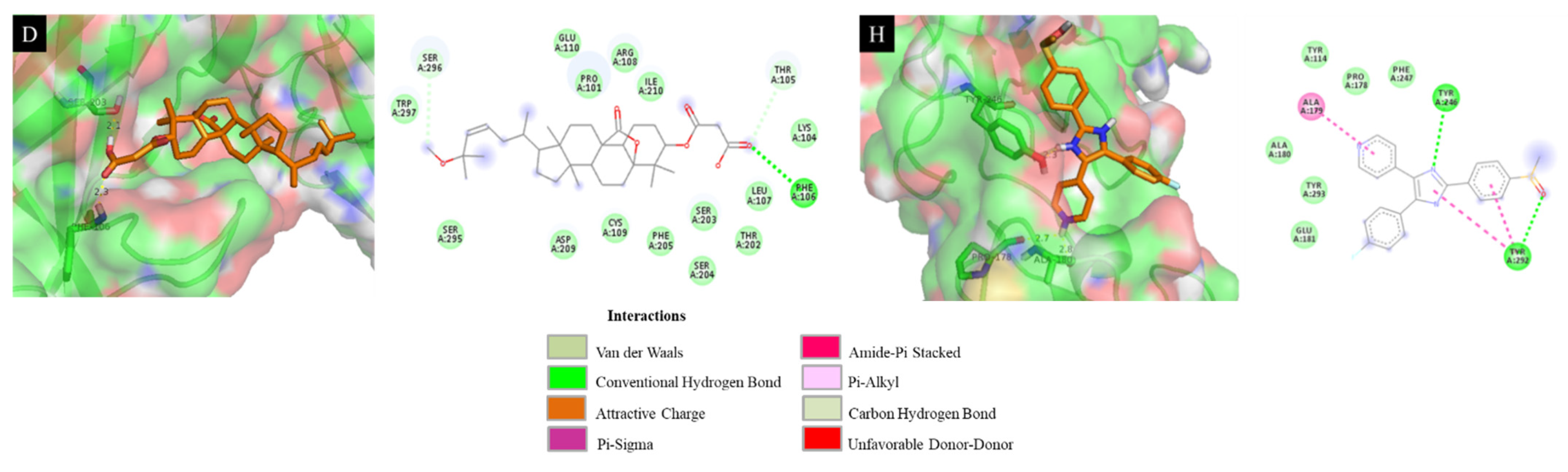
| 4 | −6.93 | 12.15 | Leu 107 (2.1 Å), Phe 106 (2.5 Å) |
| 5 | −7.28 | 5.67 | Ser 204 (2.3 Å), Leu 107 (2.1 Å) |
| 6 | −7.34 | 13.21 | Arg 108 (2.5 Å), Cys 109 (2.4 Å) |
| 8 | −6.98 | 11.93 | Ser 203 (2.1 Å), Phe 106 (2.3 Å) |
| 9 | −7.02 | 13.86 | Trp 297 (2.3 Å), Leu 107 (2.2 Å), Phe 106 (2.6 Å), Lys 104 (2.4 Å) |
| 11 | −6.66 | 16.38 | Thr 202 (2.4 Å) |
| 12 | −6.32 | 10.39 | Cys 109 (2.2 Å) |
| SB203580 a | −7.41 | 11.14 | Tyr 246 (2.3 Å), Pro 178 (2.7 Å), Glu 181 (2.8 Å) |
| Compounds | DS (kcal·mol−1) | RMSD (Å) | Interaction with Amino Acid |
|---|---|---|---|
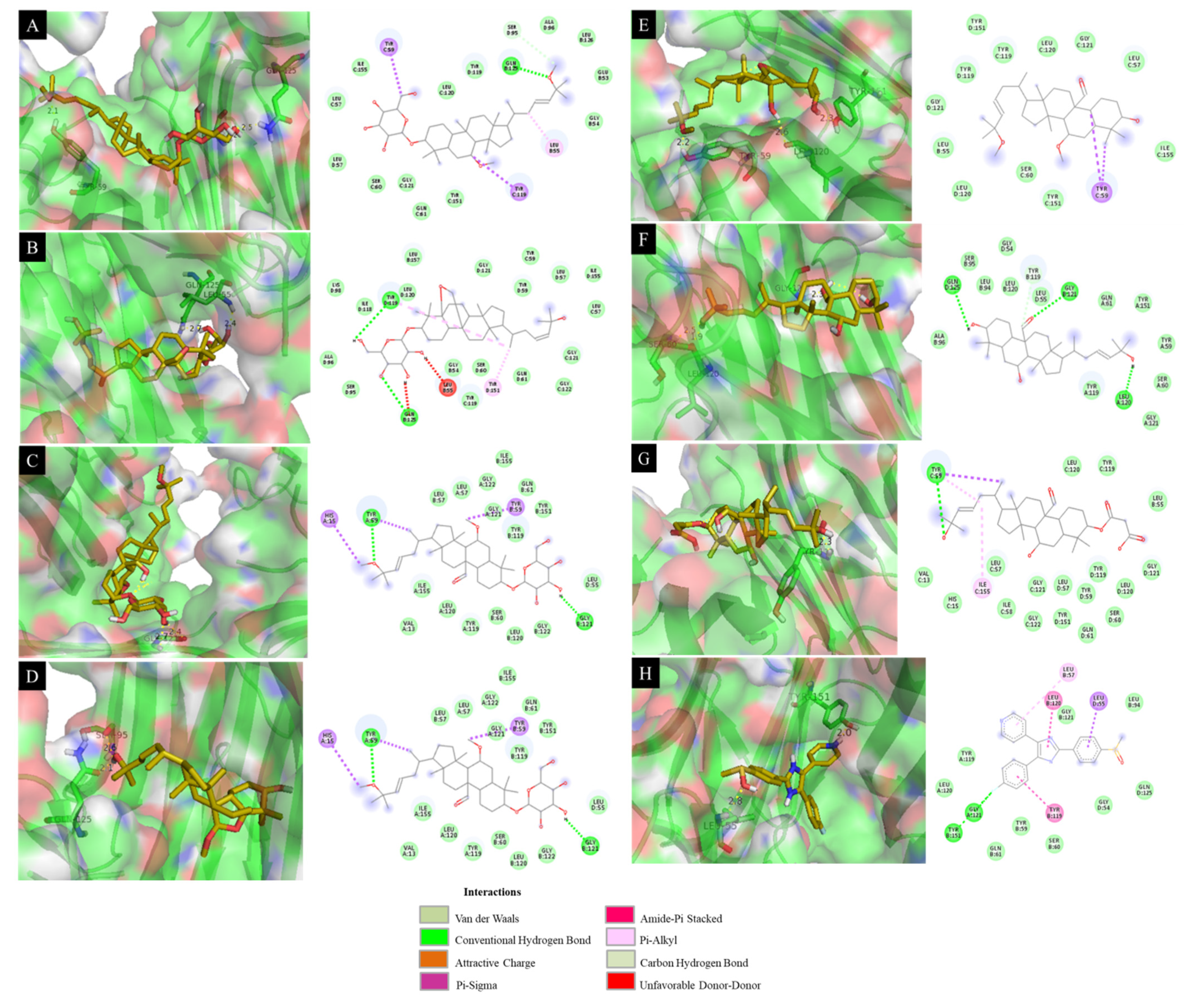
| 4 | −8.23 | 10.86 | Tyr 59 (2.1 Å), Gln 125 (2.5 Å), Gly 121 (2.2 Å) |
| 6 | −8.40 | 12.39 | Leu 55 (2.4 Å), Gln 125 (2.7 Å) |
| 9 | −8.22 | 9.15 | Gly 121 (2.4 Å) |
| 11 | −8.72 | 15.28 | Ser 95 (2.6 Å), Gln 125 (2.1 Å) |
| 12 | −7.83 | 14.34 | Tyr 151 (2.3 Å), Leu 120 (2.6 Å), Tyr 59 (2.2 Å) |
| 13 | −8.39 | 9.48 | Gly 121 (2.3 Å), Leu 120 (1.9 Å), Ser 60 (2.5 Å) |
| 14 | −8.10 | 13.16 | Tyr 119 (2.3 Å) |
| SB203580 a | −8.22 | 14.53 | Leu 55 (2.8 Å), Tyr 151 (2.0 Å) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, T.Q.; Phong, N.V.; Kim, J.H.; Gao, D.; Anh, H.L.T.; Ngo, V.-D.; Vinh, L.B.; Koh, Y.S.; Yang, S.Y. Inhibitory Effects of Cucurbitane-Type Triterpenoids from Momordica charantia Fruit on Lipopolysaccharide-Stimulated Pro-Inflammatory Cytokine Production in Bone Marrow-Derived Dendritic Cells. Molecules 2021, 26, 4444. https://doi.org/10.3390/molecules26154444
Cao TQ, Phong NV, Kim JH, Gao D, Anh HLT, Ngo V-D, Vinh LB, Koh YS, Yang SY. Inhibitory Effects of Cucurbitane-Type Triterpenoids from Momordica charantia Fruit on Lipopolysaccharide-Stimulated Pro-Inflammatory Cytokine Production in Bone Marrow-Derived Dendritic Cells. Molecules. 2021; 26(15):4444. https://doi.org/10.3390/molecules26154444
Chicago/Turabian StyleCao, Thao Quyen, Nguyen Viet Phong, Jang Hoon Kim, Dan Gao, Hoang Le Tuan Anh, Viet-Duc Ngo, Le Ba Vinh, Young Sang Koh, and Seo Young Yang. 2021. "Inhibitory Effects of Cucurbitane-Type Triterpenoids from Momordica charantia Fruit on Lipopolysaccharide-Stimulated Pro-Inflammatory Cytokine Production in Bone Marrow-Derived Dendritic Cells" Molecules 26, no. 15: 4444. https://doi.org/10.3390/molecules26154444
APA StyleCao, T. Q., Phong, N. V., Kim, J. H., Gao, D., Anh, H. L. T., Ngo, V.-D., Vinh, L. B., Koh, Y. S., & Yang, S. Y. (2021). Inhibitory Effects of Cucurbitane-Type Triterpenoids from Momordica charantia Fruit on Lipopolysaccharide-Stimulated Pro-Inflammatory Cytokine Production in Bone Marrow-Derived Dendritic Cells. Molecules, 26(15), 4444. https://doi.org/10.3390/molecules26154444






