Transformation of Iodosulfuron-Methyl into Ionic Liquids Enables Elimination of Additional Surfactants in Commercial Formulations of Sulfonylureas
Abstract
1. Introduction
2. Results
2.1. Synthesis
2.2. Solubility
2.3. Octanol-Water Partition Coefficient
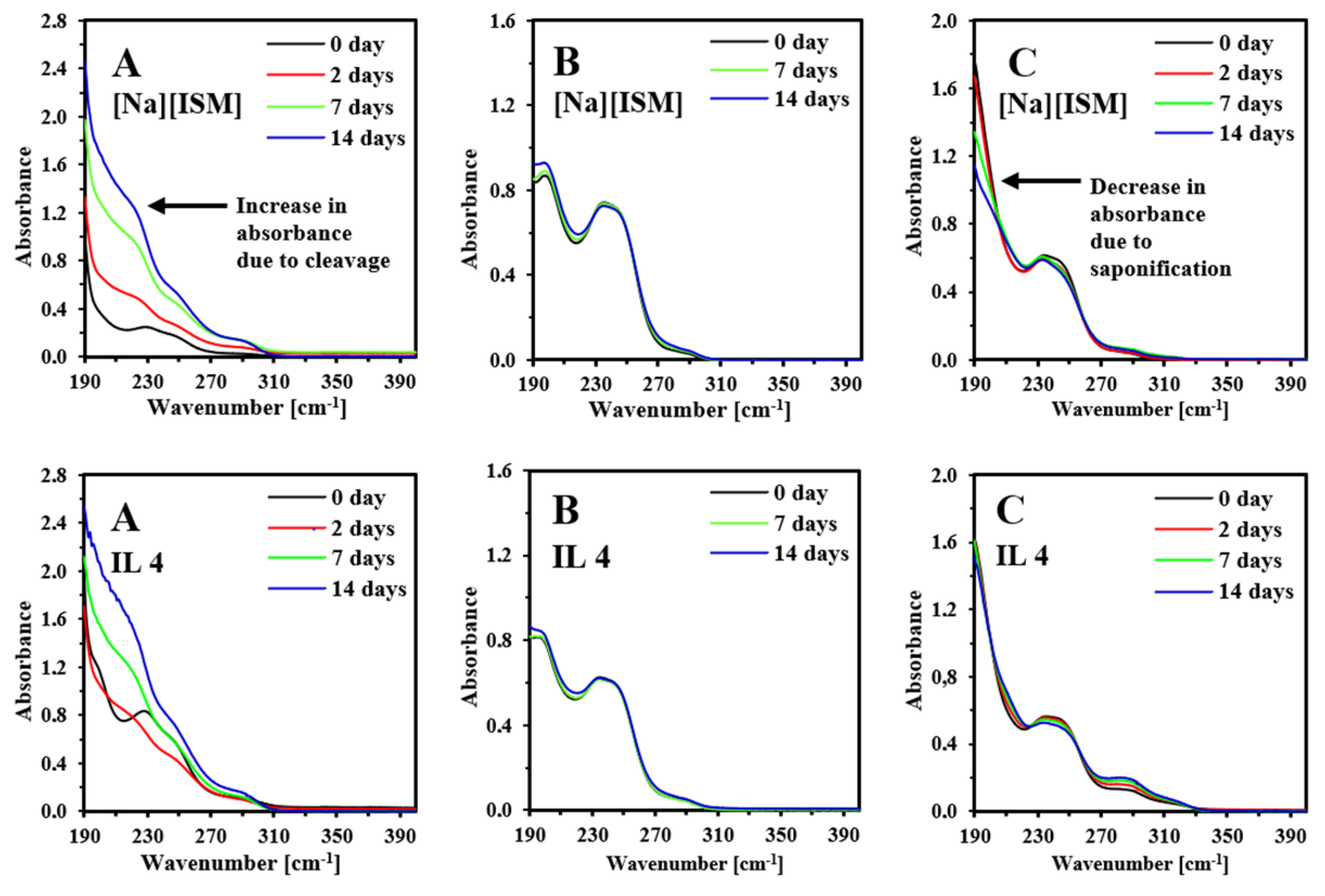
2.4. Hydrolysis of Anion in Aqueous Solution
2.5. Quantitative Estimation of Emission of Volatile Organic Compounds (VOC)
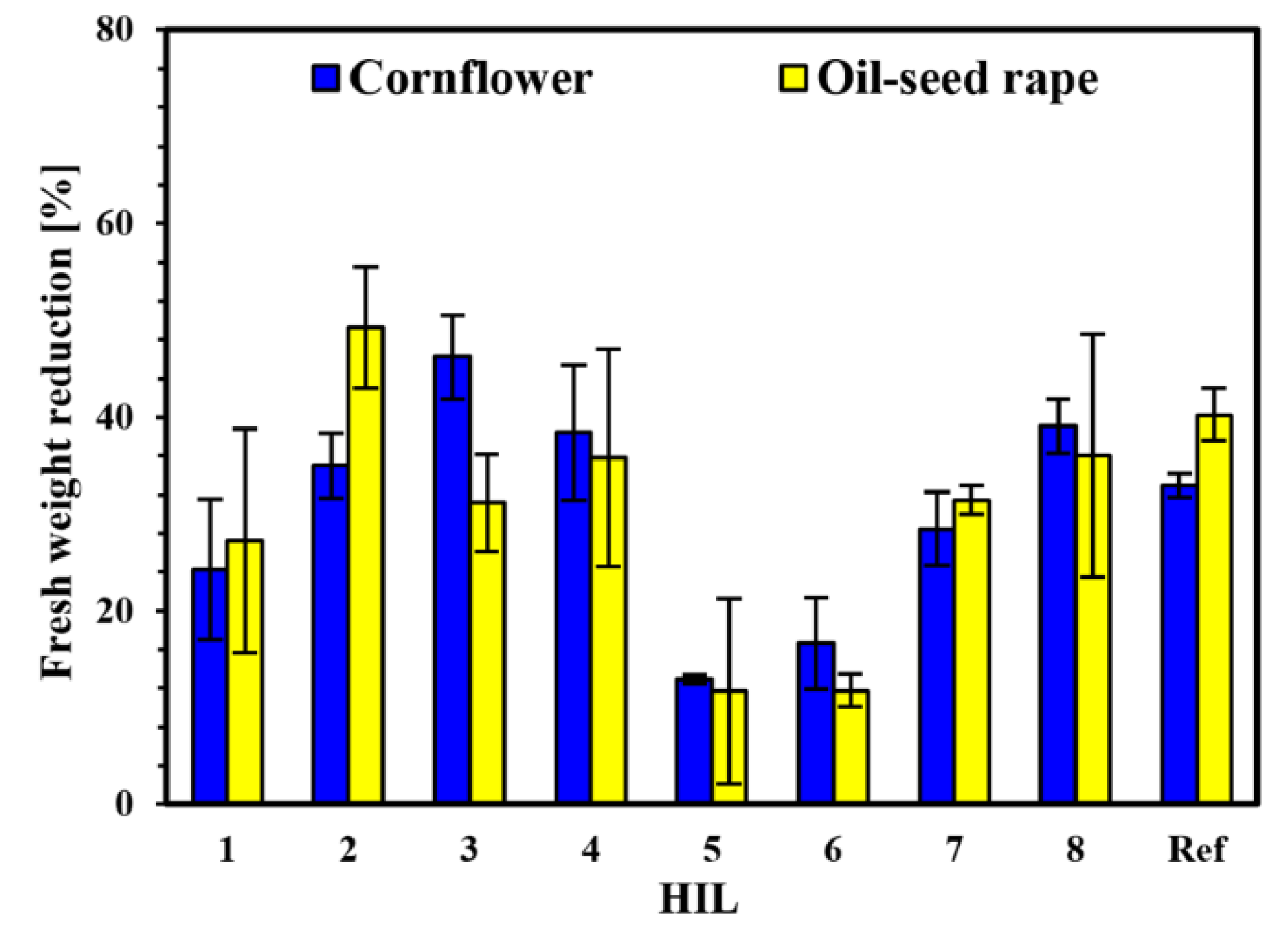
2.6. Herbicidal Activity
2.7. Determination of Antimicrobial Activity
2.8. Mobility in Soil
3. Materials and Methods
3.1. Materials
3.2. Syntheses
3.2.1. Classical Method of Synthesis of ILs
3.2.2. Simplified Method of Synthesis of ILs
3.3. Methods
3.3.1. Spectral Analysis
3.3.2. Melting Point
3.3.3. Solubility
Method 1
Method 2
3.3.4. Octanol-Water Partition Coefficients
3.3.5. Hydrolysis in Water
3.3.6. Structure Modeling
3.3.7. Quantitative Estimation of Emission of Volatile Organic Compounds (VOC)
3.3.8. Herbicidal Activity
3.3.9. Determination of Soil Mobility
3.3.10. Antimicrobials Activity Testing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sola, D.; Rossi, L.; Schianca, G.P.C.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Paolo Fra, G.; Bartoli, E.; Derosa, G. Sulfonylureas and their use in clinical practice. Arch. Med. Sci. 2015, 11, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, A.K.; Sabadie, J. Hydrolysis of sulfonylurea herbicides in soils and aqueous solutions: A review. J. Agric. Food Chem. 2002, 50, 6253–6265. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, Z.; Tang, J.; Tang, G.; Yang, J.; Wang, W.; Huo, H.; Jiang, N.; Li, J.; Cao, Y. Dicationic Ionic Liquids of Herbicide 2,4-Dichlorophenoxyacetic Acid with Reduced Negative Effects on Environment. J. Agric. Food Chem. 2018, 66, 10362–10368. [Google Scholar] [CrossRef] [PubMed]
- Pernak, J.; Syguda, A.; Janiszewska, D.; Materna, K.; Praczyk, T. Ionic liquids with herbicidal anions. Tetrahedron 2011, 67, 4838–4844. [Google Scholar] [CrossRef]
- Boldt, T.S.; Jacobsen, C.S. Different toxic effects of the sulfonylurea herbicides metsulfuron methyl, chlorsulfuron and thifensulfuron methyl on fluorescent pseudomonads isolated from an agricultural soil. FEMS Microbiol. Lett. 1998, 161, 29–35. [Google Scholar] [CrossRef]
- Boschin, G.; D’Agostina, A.; Antonioni, C.; Locati, D.; Arnoldi, A. Hydrolytic degradation of azimsulfuron, a sulfonylurea herbicide. Chemosphere 2007, 68, 1312–1317. [Google Scholar] [CrossRef]
- Niemczak, M.; Sobiech, Ł.; Grzanka, M. Iodosulfuron-Methyl-Based Herbicidal Ionic Liquids Comprising Alkyl Betainate Cation as Novel Active Ingredients with Reduced Environmental Impact and Excellent Efficacy. J. Agric. Food Chem. 2020, 68, 13661–13671. [Google Scholar] [CrossRef] [PubMed]
- Rosario, J.M.; Cruz-Hipolito, H.; Smeda, R.J.; De Prado, R. White mustard (Sinapis alba) resistance to ALS-inhibiting herbicides and alternative herbicides for control in Spain. Eur. J. Agron. 2011, 35, 57–62. [Google Scholar] [CrossRef]
- FAOSTAT Pesticides Use. Available online: http://www.fao.org/faostat/en/#data/RP (accessed on 16 February 2021).
- Rouchaud, J.; Moulard, C.; Eelen, H.; Bulcke, R. Persistence of the sulfonylurea herbicide iodosulfuron-methyl in the soil of winter wheat crops. Toxicol. Environ. Chem. 2003, 85, 103–120. [Google Scholar] [CrossRef]
- Roechling, A.; Akyuez, A. Chemical Stabilization of Iodosulfuron-Methyl Sodium Salt by Hydroxystearates. U.S. Patent 9,763,450, 19 September 2017. [Google Scholar]
- Deckwer, R.; Haase, D.; Krause, H.-P.; Schnabel, G. Oil Suspension Concentrate. International Application No. PCT/EP2003/013389, 1 July 2004. [Google Scholar]
- Reap, J.; Beestman, G. Liquid Sulfonylurea Herbicide Formulations. U.S. Patent Application 11/990,090, 21 May 2009. [Google Scholar]
- Wang, W.; Zhu, J.; Tang, G.; Huo, H.; Zhang, W.; Liang, Y.; Dong, H.; Yang, J.; Cao, Y. Novel herbicide ionic liquids based on nicosulfuron with increased efficacy. New J. Chem. 2019, 43, 827–833. [Google Scholar] [CrossRef]
- Pernak, J.; Niemczak, M.; Shamshina, J.L.; Gurau, G.; Głowacki, G.; Praczyk, T.; Marcinkowska, K.; Rogers, R.D. Metsulfuron-Methyl-Based Herbicidal Ionic Liquids. J. Agric. Food Chem. 2015, 63, 3357–3366. [Google Scholar] [CrossRef]
- Pernak, J.; Łęgosz, B.; Klejdysz, T.; Marcinkowska, K.; Rogowski, J.; Kurasiak-Popowska, D.; Stuper-Szablewska, K. Ammonium bio-ionic liquids based on camelina oil as potential novel agrochemicals. RSC Adv. 2018, 8, 28676–28683. [Google Scholar] [CrossRef]
- Green, J.M.; Cahill, W.R. Enhancing the biological activity of nicosulfuron with silicone adjuvants and pH adjusters. ASTM Spec. Tech. Publ. 2003, 115–124. [Google Scholar] [CrossRef]
- Berger, B.M.; Wolfe, N.L. Hydrolysis and biodegradation of sulfonylurea herbicides in aqueous buffers and anaerobic water-sediment systems: Assessing fate pathways using molecular descriptors. Environ. Toxicol. Chem. 1996, 15, 1500–1507. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Wesołowski, M.; Harasim, E.; Gawęda, D.; Drabowicz, M. The effect of reduced rates of crop protection agents and adjuvants on productivity, weed infestation and health of spring barley (Hordeum sativum L.). Acta Agrobot. 2013, 66, 103–112. [Google Scholar] [CrossRef][Green Version]
- Zajac, A.; Kukawka, R.; Pawlowska-Zygarowicz, A.; Stolarska, O.; Smiglak, M. Ionic liquids as bioactive chemical tools for use in agriculture and the preservation of agricultural products. Green Chem. 2018, 20, 4764–4789. [Google Scholar] [CrossRef]
- Mesnage, R.; Antoniou, M.N. Ignoring Adjuvant Toxicity Falsifies the Safety Profile of Commercial Pesticides. Front. Public Health 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Bradberry, S.M.; Proudfoot, A.T.; Vale, J.A. Glyphosate poisoning. Toxicol. Rev. 2004, 23, 159–167. [Google Scholar] [CrossRef]
- Huzar Activ 387 OD MSDS. Available online: https://www.agro.bayer.com.pl/produkty/srodki-ochrony-roslin/huzar-activ-387-od (accessed on 16 February 2021).
- Hussar® Selective Herbicide MSDS. Available online: https://resources.bayer.com.au/resources/uploads/msds/file7357.pdf (accessed on 16 February 2021).
- Huzar 05 WG label. Available online: https://gov.pl/documents/912055/913531/Huzar+05+WG.pdf (accessed on 16 February 2021).
- Tang, G.; Niu, J.; Tang, J.; Yang, J.; Zhou, Z.; Gao, Y.; Chen, X.; Tang, R.; Tian, Y.; Li, Y.; et al. Development of Poly(ionic liquids) Based on Mepiquat Chloride with Improved Rainfastness and Long-Lasting Activity on Growth Regulation of Cotton Plant. ACS Sustain. Chem. Eng. 2020, 8, 14996–15004. [Google Scholar] [CrossRef]
- Wilms, W.; Wozniak-Karczewska, M.; Syguda, A.; Niemczak, M.; Ławniczak, Ł.; Pernak, J.; Rogers, R.D.; Chrzanowski, Ł. Herbicidal ionic liquids: A promising future for old herbicides? Review on synthesis, toxicity, biodegradation, and efficacy studies. J. Agric. Food Chem. 2020, 68, 10456–10488. [Google Scholar] [CrossRef]
- Kaczmarek, D.K.; Rzemieniecki, T.; Gwiazdowska, D.; Kleiber, T.; Praczyk, T.; Pernak, J. Choline-based ionic liquids as adjuvants in pesticide formulation. J. Mol. Liq. 2020, 327, 114792. [Google Scholar] [CrossRef]
- Niemczak, M.; Rzemieniecki, T.; Biedziak, A.; Marcinkowska, K.; Pernak, J. Synthesis and Structure–Property Relationships in Herbicidal Ionic Liquids and their Double Salts. Chempluschem 2018, 83, 529–541. [Google Scholar] [CrossRef]
- Feder-Kubis, J.; Czerwoniec, P.; Lewandowski, P.; Pospieszny, H.; Smiglak, M. Ionic Liquids with Natural Origin Component: A Path to New Plant Protection Products. ACS Sustain. Chem. Eng. 2020, 8, 842–852. [Google Scholar] [CrossRef]
- Wilms, W.; Woźniak-Karczewska, M.; Niemczak, M.; Lisiecki, P.; Zgoła-Grześkowiak, A.; Ławniczak, Ł.; Framski, G.; Pernak, J.; Owsianiak, M.; Vogt, C.; et al. Quantifying the Mineralization of 13C-Labeled Cations and Anions Reveals Differences in Microbial Biodegradation of Herbicidal Ionic Liquids between Water and Soil. ACS Sustain. Chem. Eng. 2020, 8, 3412–3426. [Google Scholar] [CrossRef]
- Tang, G.; Niu, J.; Zhang, W.; Yang, J.; Tang, J.; Tang, R.; Zhou, Z.; Li, J.; Cao, Y. Preparation of Acifluorfen-Based Ionic Liquids with Fluorescent Properties for Enhancing Biological Activities and Reducing the Risk to the Aquatic Environment. J. Agric. Food Chem. 2020, 68, 6048–6057. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liang, Y.; Yang, J.; Tang, G.; Zhou, Z.; Tang, R.; Dong, H.; Li, J.; Cao, Y. Ionic Liquid Forms of Mesotrione with Enhanced Stability and Reduced Leaching Risk. ACS Sustain. Chem. Eng. 2019, 7, 16620–16628. [Google Scholar] [CrossRef]
- Hora, P.I.; Pati, S.G.; McNamara, P.J.; Arnold, W.A. Increased Use of Quaternary Ammonium Compounds during the SARS-CoV-2 Pandemic and Beyond: Consideration of Environmental Implications. Environ. Sci. Technol. Lett. 2020, 7, 622–631. [Google Scholar] [CrossRef]
- Schindler, W.D.; Hauser, P.J. 3—Softening finishes. In Chemical Finishing of Textiles; Woodhead Publishing Series in Textiles; Woodhead Publishing: Cambridge, UK, 2004; pp. 29–42. ISBN 978-1-85573-905-5. [Google Scholar]
- Pernak, J.; Giszter, R.; Biedziak, A.; Niemczak, M.; Olszewski, R.; Marcinkowska, K.; Praczyk, T. Alkyl(C16, C18, C22)trimethylammonium-Based Herbicidal Ionic Liquids. J. Agric. Food Chem. 2017, 65, 260–269. [Google Scholar] [CrossRef]
- Annex 5 List of Preservatives Allowed in Cosmetic Products. Available online: https://ec.europa.eu/growth/tools-databases/cosing/pdf/COSING_Annex V_v2.pdf (accessed on 16 December 2020).
- Zhu, J.; Ding, G.; Liu, Y.; Wang, B.; Zhang, W.; Guo, M.; Geng, Q.; Cao, Y. Ionic liquid forms of clopyralid with increased efficacy against weeds and reduced leaching from soils. Chem. Eng. J. 2015, 279, 472–477. [Google Scholar] [CrossRef]
- Pernak, J.; Czerniak, K.; Niemczak, M.; Ławniczak, Ł.; Kaczmarek, D.K.; Borkowski, A.; Praczyk, T. Bioherbicidal Ionic Liquids. ACS Sustain. Chem. Eng. 2018, 6, 2741–2750. [Google Scholar] [CrossRef]
- Prat, D.; Hayler, J.; Wells, A. A survey of solvent selection guides. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Rogers, R.D. Green Chemistry and Ionic Liquids: Synergies and Ironies. ACS Symp. Ser. 2002, 818, 2–14. [Google Scholar] [CrossRef]
- Ordikhani Seyedlar, A.; Stapf, S.; Mattea, C. Cation Dynamics in Supercooled and Solid Alkyl Methylimidazolium Bromide Ionic Liquids. J. Phys. Chem. B 2017, 121, 5363–5373. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed]
- Pernak, J.; Niemczak, M.; Materna, K.; Zelechowski, K.; Marcinkowska, K.; Praczyk, T. Synthesis, properties and evaluation of biological activity of herbicidal ionic liquids with 4-(4-chloro-2-methylphenoxy)butanoate anion. RSC Adv. 2016, 6, 7330–7338. [Google Scholar] [CrossRef]
- Jiang, Y.; Nadolny, H.; Käshammer, S.; Weibels, S.; Schröer, W.; Weingärtner, H. The ion speciation of ionic liquids in molecular solvents of low and medium polarity. Faraday Discuss. 2012, 154, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Suyal, A.; Srivastava, A.; Srivastava, P.C. Role of organic amendments in reducing leaching of sulfosulfuron through wheat crop cultivated soil. Emerg. Contam. 2019, 5, 4–8. [Google Scholar] [CrossRef]
- Hsieh, C.M.; Wang, S.; Lin, S.T.; Sandler, S.I. A predictive model for the solubility and octanol-water partition coefficient of pharmaceuticals. J. Chem. Eng. Data 2011, 56, 936–945. [Google Scholar] [CrossRef]
- Ropel, L.; Belvèze, L.S.; Aki, S.N.V.K.; Stadtherr, M.A.; Brennecke, J.F. Octanol-water partition coefficients of imidazolium-based ionic liquids. Green Chem. 2005, 7, 83–90. [Google Scholar] [CrossRef]
- Crookes, M.J.; Fisk, P. Evaluation of Using Mobility of Chemicals in the Environment to Fulfil Bioaccumulation Criteria of the Stockholm Convention; Peter Fisk Associates: Whitstable, UK, 2018. [Google Scholar]
- Green, J.M.; Cahill, W.R. Enhancing the Biological Activity of Nicosulfuron with pH Adjusters 1. Weed Technol. 2003, 17, 338–345. [Google Scholar] [CrossRef]
- Parus, A.; Wilms, W.; Verkhovetska, V.; Framski, G.; Woźniak-Karczewska, M.; Syguda, A.; Strzemiecka, B.; Borkowski, A.; Ławniczak, Ł.; Chrzanowski, Ł. Transformation of herbicides into dual function quaternary tropinium salts. New J. Chem. 2020, 44, 8869–8877. [Google Scholar] [CrossRef]
- Trigo, C.; Spokas, K.A.; Cox, L.; Koskinen, W.C. Influence of soil biochar aging on sorption of the herbicides MCPA, nicosulfuron, terbuthylazine, indaziflam, and fluoroethyldiaminotriazine. J. Agric. Food Chem. 2014, 62, 10855–10860. [Google Scholar] [CrossRef] [PubMed]
- Carles, L.; Joly, M.; Bonnemoy, F.; Leremboure, M.; Donnadieu, F.; Batisson, I.; Besse-Hoggan, P. Biodegradation and toxicity of a maize herbicide mixture: Mesotrione, nicosulfuron and S-metolachlor. J. Hazard. Mater. 2018, 354, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, W.; Xiao, J. Preconcentration and determination of nicosulfuron, thifensulfuron-methyl and metsulfuron-methyl in water samples using carbon nanotubes packed cartridge in combination with high performance liquid chromatography. Anal. Chim. Acta 2006, 559, 200–206. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Furlong, E.T.; Burkhardt, M.R.; Peter, C.J. Occurrence of sulfonylurea, sulfonamide, imidazolinone, and other herbicides in rivers, reservoirs and ground water in the Midwestern United States, 1998. Sci. Total Environ. 2000, 248, 123–133. [Google Scholar] [CrossRef]
- Vogel, A.I. Textbook of Practical Organic Chemistry; John Wiley & Sons: New York, NY, USA, 1991; ISBN 83-204-3152-2. [Google Scholar]
- OECD. Water Solubility No. 105; OECD: Paris, France, 1995; pp. 1–7. [Google Scholar] [CrossRef]
- OECD. Partition Coefficient (n-Octanol/Water), Shake Flask Method, No. 107; OECD: Paris, France, 1995; pp. 1–4. [Google Scholar] [CrossRef]
- Syguda, A.; Wojcieszak, M.; Materna, K.; Woźniak-Karczewska, M.; Parus, A.; Ławniczak, Ł.; Chrzanowski, Ł. Double-Action Herbicidal Ionic Liquids Based on Dicamba Esterquats with 4-CPA, 2,4-D, MCPA, MCPP, and Clopyralid Anions. ACS Sustain. Chem. Eng. 2020, 8, 14584–14594. [Google Scholar] [CrossRef]






| No. | Structure of Cation | R 1 | R 2 | Yield [%] | Melting Point [°C] |
|---|---|---|---|---|---|
| 1 |  | coco1 | CH3 | 88 | 67.0–69.5 |
| 2 | C14H29 | CH3 | 92 | 64.3–66.1 | |
| 3 | C16H33 | CH3 | 91 | 76.2–78.5 | |
| 4 | C18H37 | CH3 | 96 | 89.3–91.1 | |
| 5 | C22H45 | CH3 | 92 | 61.0–62.6 | |
| 6 | C8H17 | C8H17 | 85 | 40.4–42.5 | |
| 7 | C10H21 | C10H21 | 92 | 51.1–52.2 | |
| 8 | hydrogenated tallow2 | hydrogenated tallow | 95 | 49.0–52.9 |
| Salt | Water | Methanol | DMSO | Acetonitrile | Acetone | Chloroform | Isopropanol | Ethyl Acetate | Toluene | Hexane |
|---|---|---|---|---|---|---|---|---|---|---|
| 9.0 a | 6.6 | 6.5 | 6.2 | 5.4 | 4.4 | 4.3 | 4.3 | 2.3 | 0.0 | |
| 1 | + | + | + | + | + | + | + | + | + | – |
| 2 | − | + | + | + | + | + | + | + | + | − |
| 3 | − | + | + | + | + | + | + | + | ± | − |
| 4 | − | + | + | + | + | + | + | + | + | − |
| 5 | − | + | + | + | + | + | + | ± | + | − |
| 6 | − | + | + | + | + | + | + | + | ± | − |
| 7 | − | + | + | + | + | + | + | + | ± | – |
| 8 | − | + | + | + | + | + | + | + | + | ± |
| REF b | ± | + | + | ± | ± | − | − | − | − | − |
| EC50 ± Standard Error of the Mean [mg·dm−3] | ||||
|---|---|---|---|---|
| Salt | P. putida | Toxicity Classification | B. cereus | Toxicity Classification |
| 1 | 186.06 ± 3.09 | PH b | <50 | ST |
| 2 | <50 | ST | <50 | ST |
| 3 | >200 | PH | <50 | ST |
| 4 | >200 | PH | <50 | ST |
| 5 | >200 | PH | <50 | ST |
| 6 | <50 | ST c | <50 | ST |
| 7 | <50 | ST | <50 | ST |
| 8 | 186.32 ± 4.69 | PH | 53.54 ± 0.46 | ST |
| REF a | >200 | PH | >200 | PH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachowiak, W.; Szumski, R.; Homa, J.; Woźniak-Karczewska, M.; Parus, A.; Strzemiecka, B.; Chrzanowski, Ł.; Niemczak, M. Transformation of Iodosulfuron-Methyl into Ionic Liquids Enables Elimination of Additional Surfactants in Commercial Formulations of Sulfonylureas. Molecules 2021, 26, 4396. https://doi.org/10.3390/molecules26154396
Stachowiak W, Szumski R, Homa J, Woźniak-Karczewska M, Parus A, Strzemiecka B, Chrzanowski Ł, Niemczak M. Transformation of Iodosulfuron-Methyl into Ionic Liquids Enables Elimination of Additional Surfactants in Commercial Formulations of Sulfonylureas. Molecules. 2021; 26(15):4396. https://doi.org/10.3390/molecules26154396
Chicago/Turabian StyleStachowiak, Witold, Radosław Szumski, Jan Homa, Marta Woźniak-Karczewska, Anna Parus, Beata Strzemiecka, Łukasz Chrzanowski, and Michał Niemczak. 2021. "Transformation of Iodosulfuron-Methyl into Ionic Liquids Enables Elimination of Additional Surfactants in Commercial Formulations of Sulfonylureas" Molecules 26, no. 15: 4396. https://doi.org/10.3390/molecules26154396
APA StyleStachowiak, W., Szumski, R., Homa, J., Woźniak-Karczewska, M., Parus, A., Strzemiecka, B., Chrzanowski, Ł., & Niemczak, M. (2021). Transformation of Iodosulfuron-Methyl into Ionic Liquids Enables Elimination of Additional Surfactants in Commercial Formulations of Sulfonylureas. Molecules, 26(15), 4396. https://doi.org/10.3390/molecules26154396











