Influence of the Medium Composition and the Culture Conditions on Surfactin Biosynthesis by a Native Bacillus subtilis natto BS19 Strain
Abstract
1. Introduction
2. Results
2.1. Synthetic Carbon Sources
2.2. Synthetic Nitrogen Sources
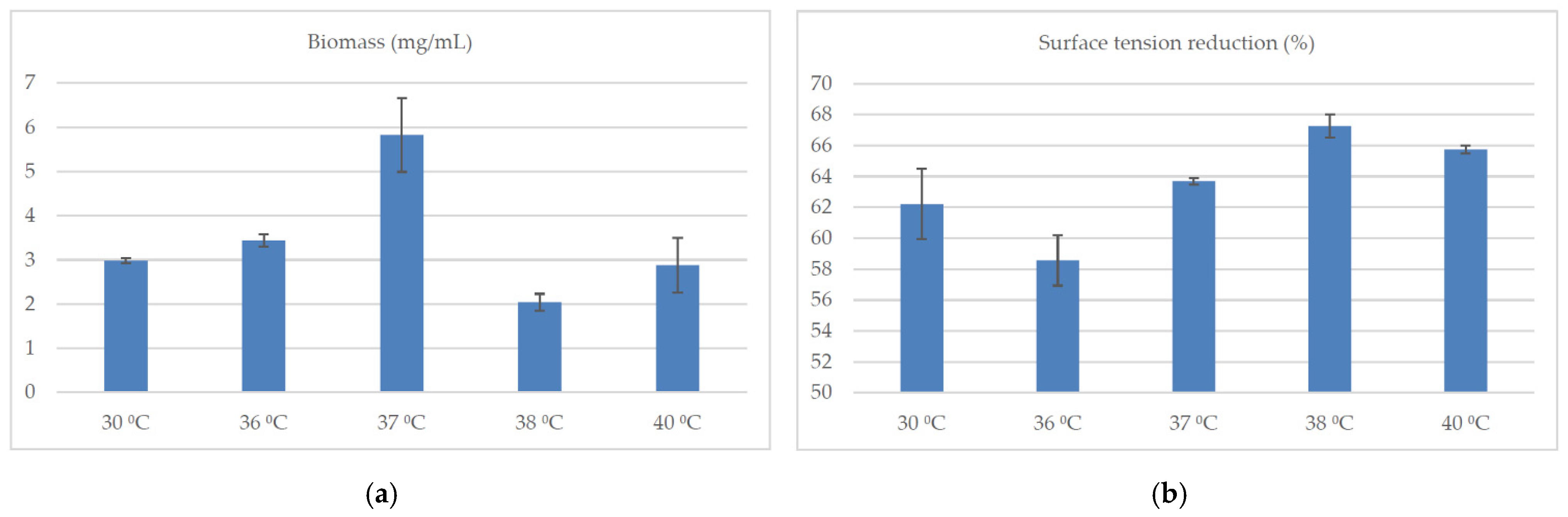
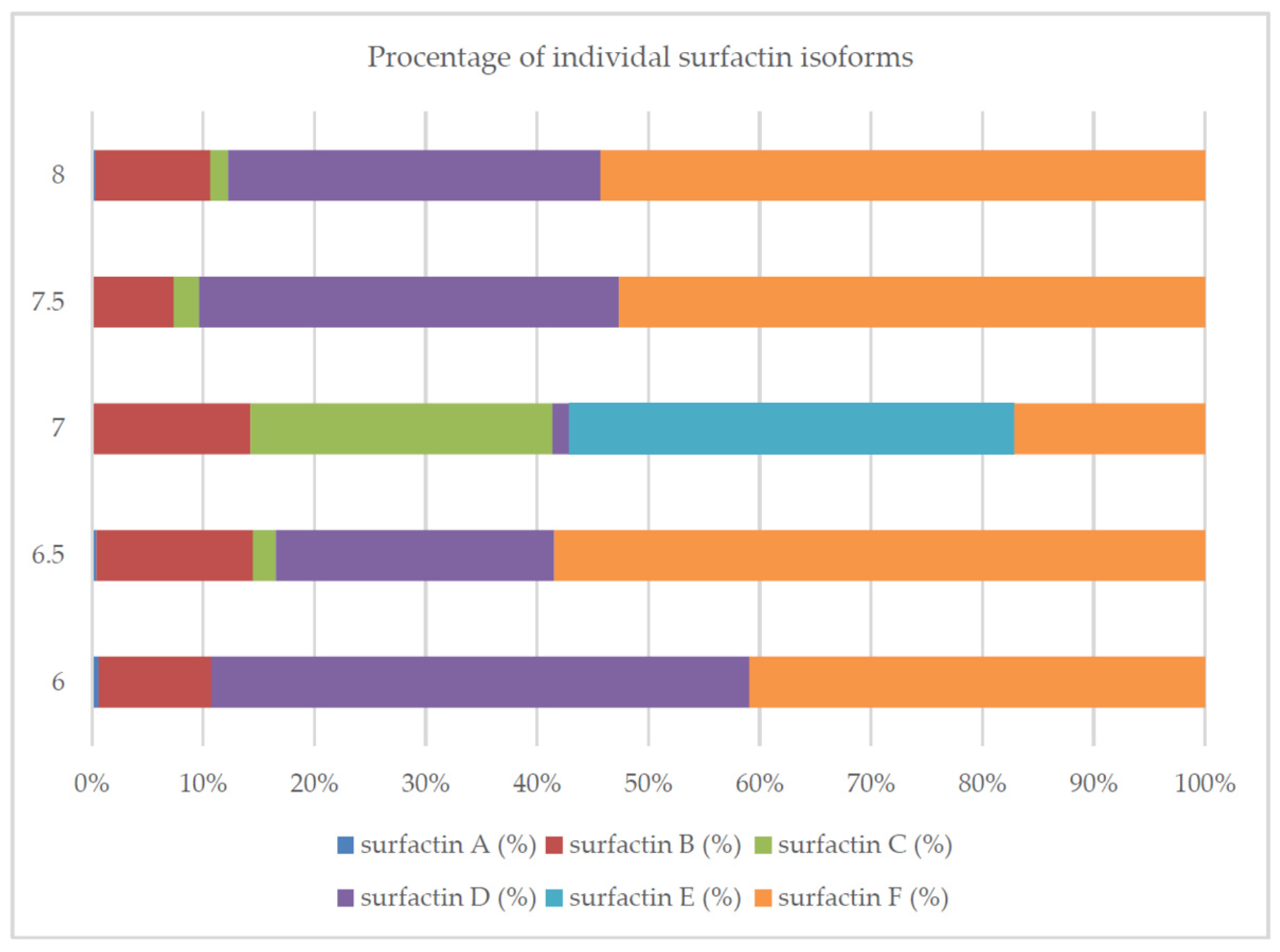
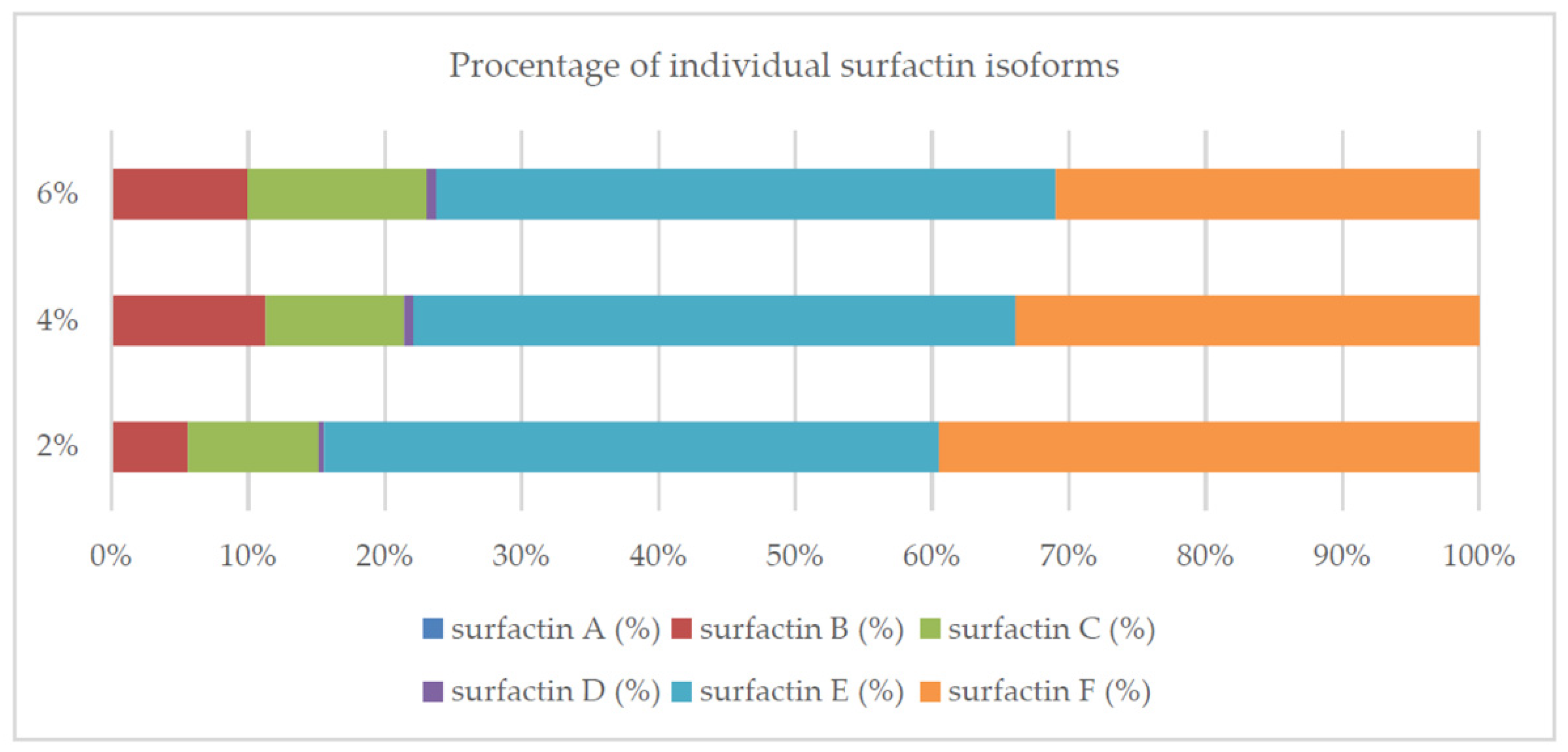
2.3. Culture Conditions
2.4. Potato Peelings as an Alternative Carbon Source
3. Discussion
4. Materials and Methods
4.1. Microorganism
4.2. The Media and Culture Conditions
4.3. Determination of Bacillus Subtilis Natto BS19 Biomass Concentration
4.4. Reduction of the Surface Tension of the Culture Medium
4.5. Surfactin Extraction and Determination of Its Concentration
4.6. Measurement of the Hydrolytic Activity of Microorganisms
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Nitschke, M.; Costa, S.G.V.A.O. Biosurfactants in food industry. Trends Food Sci. Technol. 2007, 18, 252–259. [Google Scholar] [CrossRef]
- Vedaraman, N.; Venkatesh, N. Production of surfactin by Bacillus subtilis MTCC 2423 from waste frying oils. Braz. J. Chem. Eng. 2011, 28, 175–180. [Google Scholar] [CrossRef]
- Vollenbroich, D.; Özel, M.; Vater, J.; Kamp, R.M.; Pauli, G. Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from Bacillus subtilis. Biologicals 1997, 25, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Seydlová, G.; Svobodová, J. Review of surfactin chemical properties and the potential biomedical applications. Cent. Eur. J. Med. 2008, 3, 123–133. [Google Scholar] [CrossRef]
- de Faria, A.F.; Teodoro-Martinez, D.S.; De Oliveira Barbosa, G.N.; Vaz, B.G.; Silva, I.S.; Garcia, J.S.; Totola, M.R.; Eberlin, M.N.; Grossman, M.; Alves, O.L.; et al. Production and structural characterization of surfactin (C 14/Leu7) produced by Bacillus subtilis isolate LSFM-05 grown on raw glycerol from the biodiesel industry. Process Biochem. 2011, 46, 1951–1957. [Google Scholar] [CrossRef]
- Campos, J.M.; Stamford, T.L.; Sarubbo, L.A.; de Luna, J.M.; Rufino, R.D.; Banat, I.M. Microbial biosurfactants as additives for food industries. Biotechnol. Prog. 2013, 29, 1097–1108. [Google Scholar] [CrossRef]
- Willenbacher, J.; Zwick, M.; Mohr, T.; Schmid, F.; Syldatk, C.; Hausmann, R. Evaluation of different Bacillus strains in respect of their ability to produce surfactin in a model fermentation process with integrated foam fractionation. Appl. Microbiol. Biotechnol. 2014, 98, 9623–9632. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.A.; Etchegaray, A.; Machini, M.T. Design, synthesis and valued properties of surfactin oversimplifed analogues. Amino Acids 2020, 52, 25–33. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, S.Z.; Mu, B.Z. Quantitative analyses of the isoforms of surfactin produced by Bacillus subtilis HSO 121 using GC-MS. Anal. Sci. 2012, 28, 789–793. [Google Scholar] [CrossRef]
- Jajor, P.; Piłakowska-Pietras, D.; Krasowska, A.; Łukaszewicz, M. Surfactin analogues produced by Bacillus subtilis strains grown on rapeseed cake. J. Mol. Struct. 2016, 1126, 141–146. [Google Scholar] [CrossRef]
- Meena, K.R.; Kanwar, S.S. Lipopeptides as the antifungal and antibacterial agents: Applications in food safety and therapeutics. BioMed Res. Int. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Paraszkiewicz, K.; Bernat, P.; Kuśmierska, A.; Chojniak, J.; Płaza, G. Structural identification of lipopeptide biosurfactants produced by Bacillus subtilis strains grown on the media obtained from renewable natural resources. J. Environ. Manag. 2018, 209, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.G.; Macdonald, C.R.; Duff, S.J.B.; Kosaric, N. Enhanced production of surfactin from Bacillus subtilis by continuous product removal and metal cation additions. Appl. Env. Microbiol. 1981, 42, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Akpa, E.; Jacques, P.; Wathelet, B.; Paquot, M.; Fuchs, R.; Budzikiewicz, H.; Thonart, P. Influence of culture conditions on lipopeptide production by Bacillus subtilis. Appl. Biochem. Biotechnol. 2001, 9193, 551–561. [Google Scholar] [CrossRef]
- Arima, K.; Kakinuma, A.; Tamura, G. Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: Isolation, characterization and its inhibition of fibrin clot formation. Biochem. Biophys. Res. Commun. 1968, 31, 488–494. [Google Scholar] [CrossRef]
- Sandrin, C.; Peypoux, F.; Michel, G. Coproduction of surfactin and iturin A, lipopeptides with surfactant and antifungal properties, by Bacillus subtilis. Biotechnol. Appl. Biochem. 1990, 12, 370–375. [Google Scholar]
- Davis, D.A.; Lynch, H.C.; Varley, J. The production of surfactin in batch culture by Bacillus subtilis ATCC 21332 is strongly influenced by the conditions of nitrogen metabolism. Enzym. Microb. Technol. 1999, 25, 322–329. [Google Scholar] [CrossRef]
- Abushady, H.M.; Bashandy, A.S.; Aziz, N.H.; Ibrahim, H.M.M. Molecular Characterization of Bacillus subtilis surfactin producing strain and the factors affecting its production. Int. J. Agric. Biol. 2005, 7, 337–344. [Google Scholar]
- Cheng, Y.H.; Zhang, N.; Han, J.C.; Chang, C.W.; Hsiao, F.S.; Yu, Y.H. Optimization of surfactin production from Bacillus subtilis in fermentation and its effects on Clostridium perfringens-induced necrotic enteritis and growth performance in broilers. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1232–1244. [Google Scholar] [CrossRef]
- Chen, W.C.; Juang, R.S.; Wei, Y.H. Applications of a lipopeptide biosurfactant, surfactin, produced by microorganisms. Biochem. Eng. J. 2015, 103, 158–169. [Google Scholar] [CrossRef]
- Das, K.; Mukherjee, A.K. Comparison of lipopeptide biosurfactants production by Bacillus subtilis strains in submerged and solid state fermentation systems using a cheap carbon source: Some industrial applications of biosurfactants. Process Biochem. 2007, 2, 1191–1199. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Fernandes, E.C.; Rodrigues, A.I.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactant production by Bacillus subtilis using corn steep liquor as culture medium. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Hmidet, N.; Ben Ayed, H.; Jacques, P.; Nasri, M. Enhancement of surfactin and fengycin production by Bacillus mojavensis A21: Application for diesel biodegradation. BioMed Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Pastore, G.M. Production and properties of a surfactant obtained from Bacillus subtilis grown on cassava wastewater. Bioresour. Technol. 2006, 97, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Rautela, R.; Cameotra, S.S. Substrate dependent in vitro antifungal activity of Bacillus sp. strain AR2. Microb. Cell Factories 2014, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.S.; Cameotra, S.S. Utilization of molasses for biosurfactant production by two Bacillus strains at thermophilic conditions. J. Am. Oil Chem. Soc. 1997, 74, 887–889. [Google Scholar] [CrossRef]
- Ghribi, D.; Ellouze-Chaabouni, S. Enhancement of Bacillus subtilis lipopeptide biosurfactants production through optimization of medium composition and adequate control of aeration. Biotechnol. Res. Int. 2011, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mawgoud, A.M.; Aboulwafa, M.M.; Hassouna, N.A.-H. Characterization of surfactin produced by Bacillus subtilis Isolate BS5. Appl. Biochem. Biotechnol. 2008, 150, 289–303. [Google Scholar] [CrossRef]
- Jacques, P. Surfactin and Other Lipopeptides from Bacillus spp. In Biosurfactants; Springer: Berlin/Heidelberg, Germany, 2011; Volume 20, pp. 57–91. [Google Scholar]
- Shaligram, N.S.; Singhal, R.S. Surfactin—A review on biosynthesis, fermentation, purification and applications. Food Technol. Biotechnol. 2010, 48, 119–134. [Google Scholar]
- Joshi, P.A.; Shekhawat, D.B. Screening and isolation of biosurfactant producing bacteria from petroleum contaminated soil. Eur. J. Exp. Biol. 2014, 4, 164–169. [Google Scholar]
- Rane, A.N.; Baikar, V.V.; Ravi Kumar, V.; Deopurkar, R.L. Agro-industrial wastes for production of biosurfactant by Bacillus subtilis ANR 88 and its application in synthesis of silver and gold nanoparticles. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Makkar, R.S.; Cameotra, S.S. An update on the use of unconventional substrates for biosurfactant production and their new applications. Appl. Microbiol. Biotechnol. 2002, 58, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Al-Bahry, S.N.; Al-Wahaibi, Y.M.; Elshafie, A.E.; Al-Bemani, A.S.; Joshi, S.J.; AlMakhmari, H.S.; Al-Sulaimani, H.S. Biosurfactant production by Bacillus subtilis B20 using date molasses and its possible application in enhanced oil recovery. Int. Biodeterior. Biodegrad. 2013, 8, 141–146. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, F.; Wei, Z.; Ran, W.; Shen, Q. The usage of rice straw as a major substrate for the production of surfactin by Bacillus amyloliquefaciens XZ-173 in solid-state fermentation. J. Environ. Manag. 2013, 127, 96–102. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.W.F.; França, I.W.L.; Félix, A.K.N.; Martins, J.J.L.; Giro, M.E.A.; Melo, V.M.M.; Goncalves, L.R.B. Kinetic study of biosurfactant production by Bacillus subtilis LAMI005 grown in clarified cashew apple juice. Colloid Surf. B 2013, 101, 34–43. [Google Scholar] [CrossRef]
- Bartal, A.; Vigneshwari, A.; Bóka, B.; Vörös, M.; Takács, I.; Kredics, L.; Manczinger, L.; Varga, M.; Vágvölgyi, C.; Szekeres, A. Effects of different cultivation parameters on the production of surfactin variants by a Bacillus subtilis strain. Molecules 2018, 23, 2675. [Google Scholar] [CrossRef]
- Ing-Lung, S.; Chia-Yu, K.; Feng-Chia, H.; Suey-Sheng, K.; Chienyan, H. Use of surface response methodology to optimize culture conditions for iturin A production by Bacillus subtilis in solid-state fermentation. J. Chin. Inst. Chem. Eng. 2008, 39, 635–643. [Google Scholar] [CrossRef]
- Mizumoto, S.; Hirai, M.; Shoda, M. Production of lipopeptide antibiotic iturin A using soybean curd residue cultivated with Bacillus subtilis in solid-state fermentation. Appl. Microbiol. Biotechnol. 2006, 72, 869–875. [Google Scholar] [CrossRef]
- Liu, J.F.; Yang, J.; Yang, S.Z.; Ye, R.Q.; Mu, B.Z. Effects of different amino acids in culture media on surfactin variants produced by Bacillus subtilis TD7. Appl. Biochem. Biotechnol. 2012, 166, 2091–2100. [Google Scholar] [CrossRef]
- Konsula, Z.; Liakopoulou-Kyriakides, M. Hydrolysis of starches by the action of an -amylase from Bacillus subtilis. Process Biochem. 2004, 39, 1745–1749. [Google Scholar] [CrossRef]
- Tavallaire, S.; Khomeiri, M.; Mousivand, M.; Maghsoudlou, Y.; Hashemi, M. Starches from different sources hydrolysis using a new thermo-tolerant amylase complex produced by Bacillus subtilis T41a: Characterization and efficiency evaluation. LWT 2019, 112, 108218. [Google Scholar] [CrossRef]
- Clarke, K.G. 2-Microbiology. Bioprocess Engineering, An Introductory Engineering and Life Sciences Approach; Woodhead Publishing: Cambridge, UK, 2013; pp. 7–24. [Google Scholar]
- Reissbrodt, R.; Beer, W.; Muller, R.; Claus, H. Characterization of casein peptones by HPLC profiles and microbiological growth parameters. Acta Biotechnologica 1995, 15, 223–232. [Google Scholar] [CrossRef]
- Tofalo, R.; Suzzi, G. Yeasts. In The Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; Volume 1, pp. 593–599. [Google Scholar] [CrossRef]
- Heron, J.R. Some observations on commercial malt extracts. J. Inst. Brew. 1966, 72, 452–457. [Google Scholar] [CrossRef]
- Pearson, A.M. MEAT/Extracts. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: London, UK, 2003; pp. 3812–3817. [Google Scholar]
- Sen, R.; Swaminathan, T. Application of response-surface methodology to evaluate the optimum environmental conditions for the enhanced production of surfactin. Appl. Microbiol. Biotechnol. 1997, 47, 358–363. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, S.; Peng, J.; Li, Y.; Yang, Q. Synthetic surfactin analogues have improved anti-PEDV properties. PLoS ONE 2019, 14, e0215227. [Google Scholar] [CrossRef]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2014, 5, 697. [Google Scholar] [CrossRef]
- Dzwonkowski, W. Evolution of potato production in Poland and the EU. Zeszyty Naukowe Szkoły Głównej Gospodarstwa Wiejskiego w Warszawie-Problemy Rolnictwa Światowego 2017, 17, 71–80. [Google Scholar] [CrossRef]
- Sampaio, S.L.; Petropoulos, S.A.; Alexopoulos, A.; Heleno, S.A.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Potato peels as sources of functional compounds for the food industry: A review. Trends Food Sci. Technol. 2020, 103, 118–129. [Google Scholar] [CrossRef]
- Sharma, D.; Ansari, M.J.; Gupta, S.; Al Ghamdi, A.; Pruthi, P.; Pruthi, V. Structural characterization and antimicrobial activity of a biosurfactant obtained from Bacillus pumilus DSVP18 grown on potato peels. Jundishapur J. Microbiol. 2015, 8, e21257. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.; Saldaña, M.D.A. Potato peels: A source of nutritionally and pharmacologically interesting compounds—A review. Food 2009, 23–29. [Google Scholar] [CrossRef]
- Pande, V.; Patel, V.; Salunke, P.; Patel, U. Biosynthesis and development of novel method for commercial production of biosurfactant utilizing waste potato peels. Indian Drugs 2019, 56, 12. [Google Scholar]
- Fox, S.L.; Bala, G.A. Production of surfactant from Bacillus subtilis ATCC 21332 using potato substrates. Bioresour. Technol. 2000, 75, 235–240. [Google Scholar] [CrossRef]
- Mukherjee, S.; Das, P.; Sen, R. Towards commercial production of microbial surfactants. Trends Biotechnol. 2006, 24, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.S.; Cameotra, S.S.; Banat, I.M. Advances in utilization of renewable substrates for biosurfactant production. AMB Express 2011, 1, 5. [Google Scholar] [CrossRef]
- Ansari, F.A.; Hussain, S.; Ahmed, B.; Akhter, J.; Shoeb, E. Use of patato peel as cheap carbon source for the bacterial production of biosurfactants. Int. J. Biol. Res. 2014, 2, 27–31. [Google Scholar]
- Koim-Puchowska, B.; Kłosowski, G.; Mikulski, D.; Menka, A. Evaluation of various methods of selection of B. subtilis strains capable of secreting surface-active compounds. PLoS ONE 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.O.; Kanekar, P.P.; Jog, J.P.; Sarnaik, S.S.; Nilegaonkar, S.S. Production of copolymer, poly (hydroxybutyrate-co-hydroxyvalerate) by Halomonas campisalis MCM B-1027 using agro-wastes. Int. J. Biol. Macromol. 2015, 72, 784–789. [Google Scholar] [CrossRef]
- Walter, V.; Syldatk, C.; Hausmann, R. Screening concepts for the isolation of biosurfactant producing microorganisms. Biosurfactants 2010, 672, 1–13. [Google Scholar]
- Ahmed, I.; Zia, M.A.; Iftikhar, T.; Iqbal, H.M.N. Characterization and detergent compatibility of purified protease produced from Aspergillus niger by utilizng of agro wastes. BioResources 2011, 6, 4505–4522. [Google Scholar]

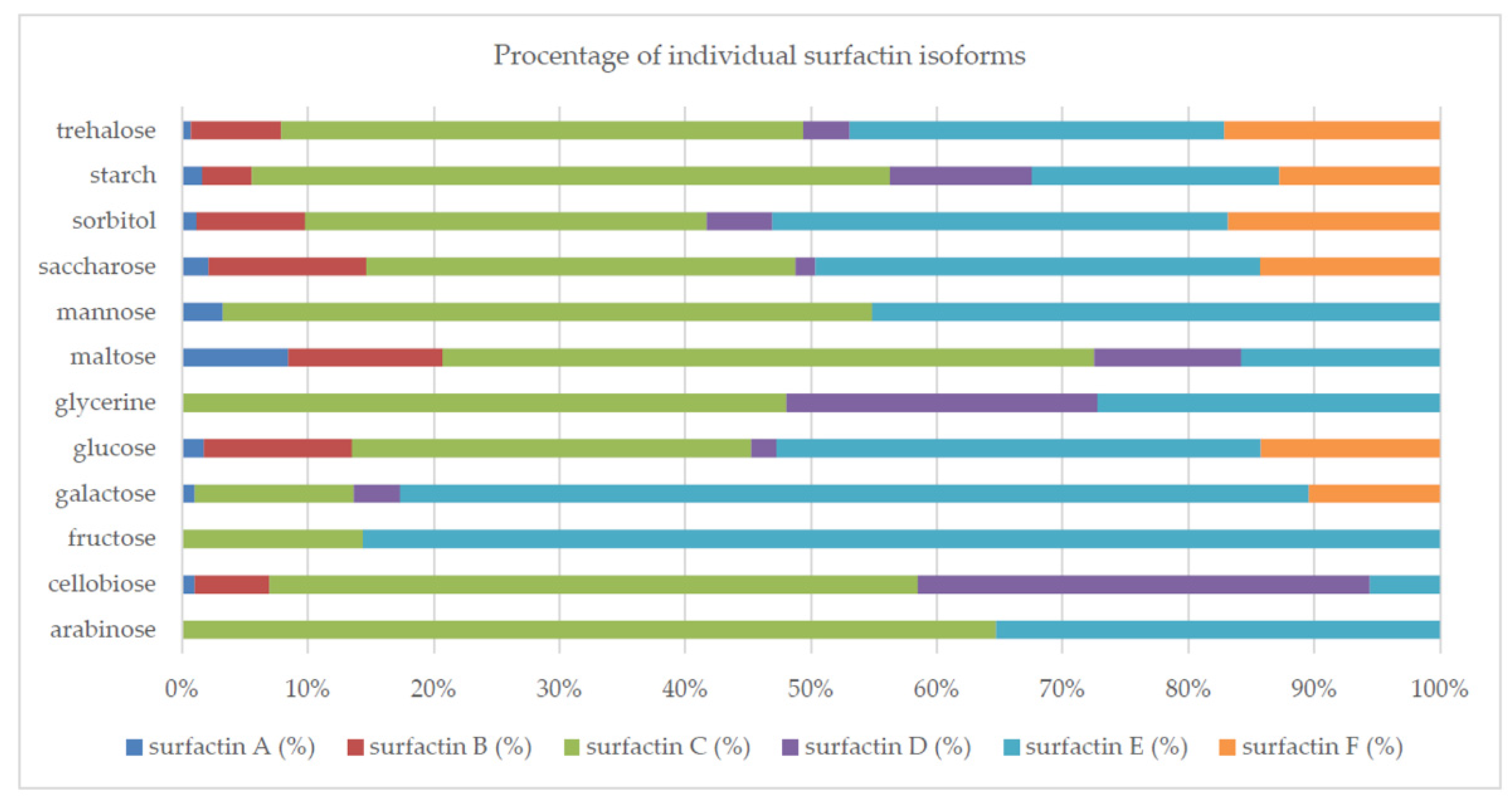
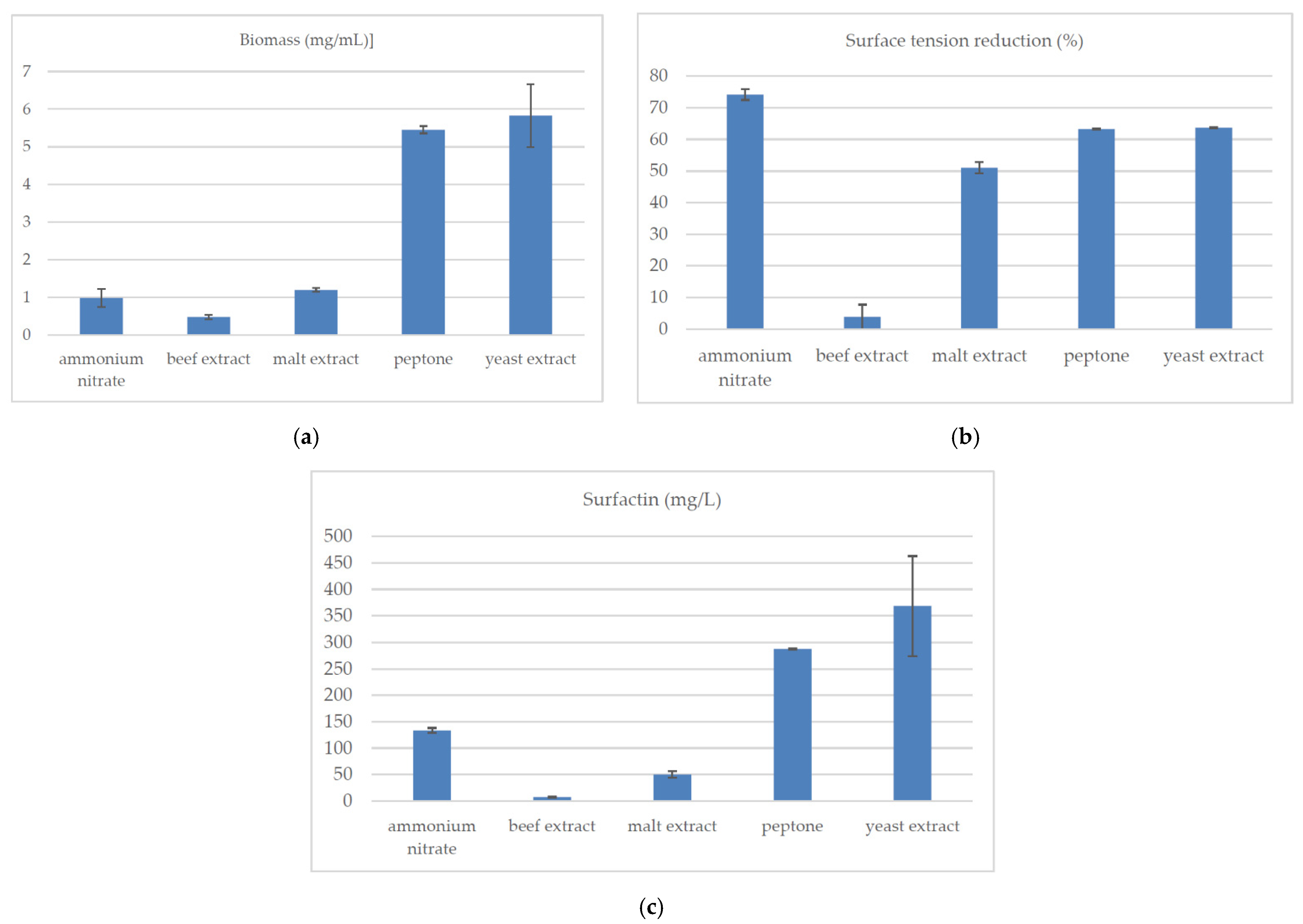
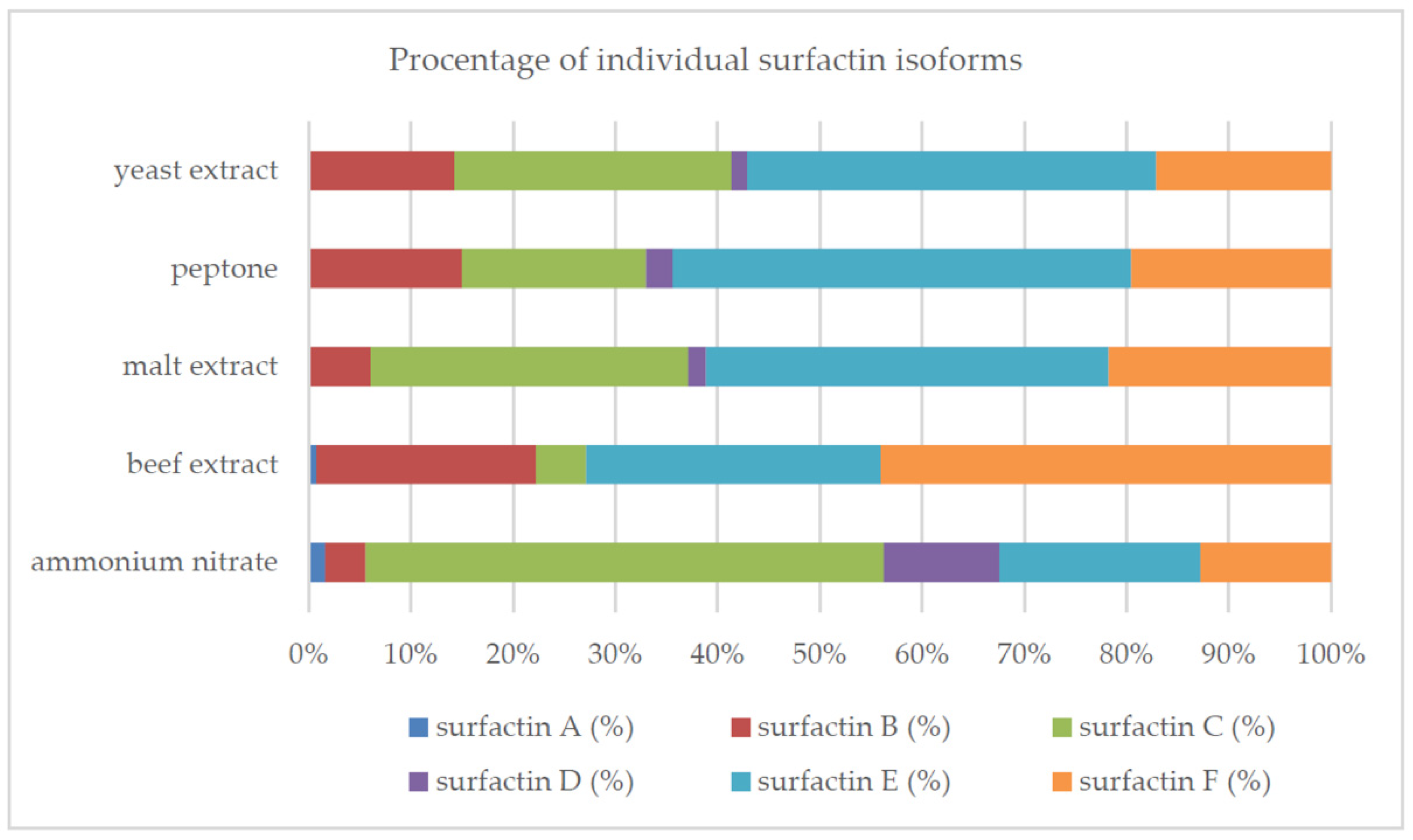
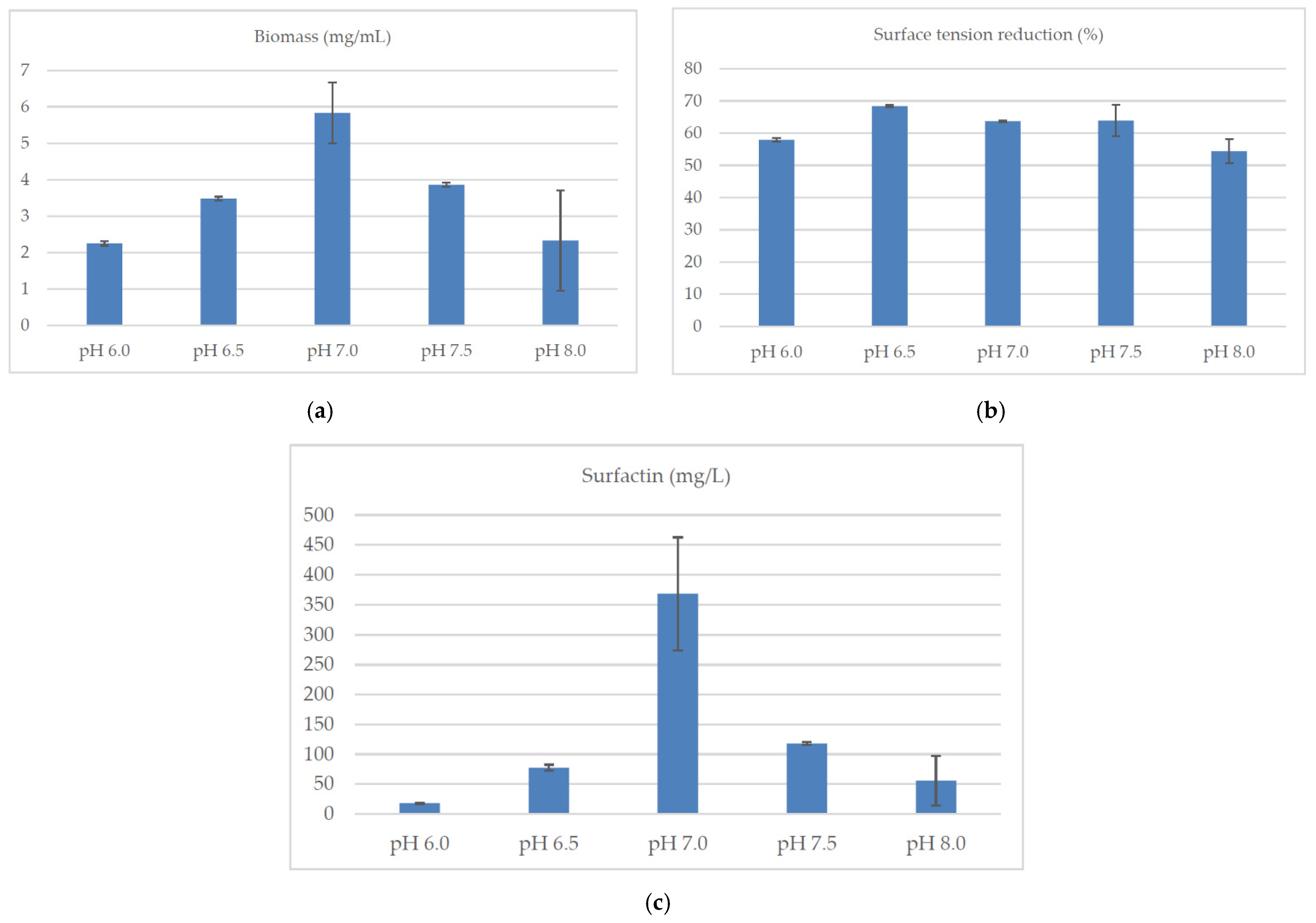



| Source of Carbon | Biomass (mg/mL) | Surface Tension Reduction (%) | Surfactin (mg/L) |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| arabinose | 0.42abdfjlmn ± 0.12 | 2.23e ± 2.23 | 0.29d ± 0.01 |
| cellobiose | 1.19abcdehij ± 0.02 | 25.06dg ± 1.29 | 4.09ad ± 0.34 |
| fructose | 0.68abcde ± 0.03 | 17.30c ± 0.78 | 0.77d ± 0.21 |
| galactose | 0.58abcdfijl ± 0.04 | 29.85afgh ± 3.64 | 8.80acd ± 1.34 |
| glucose | 0.84abcde ± 0.08 | 44.03b ± 1.64 | 3.68ad ± 1.81 |
| glycerine | 1.7ace ± 0.90 | 31.70ad ± 1.43 | 1.17d ± 0.92 |
| lactose | 0.1bdf ± 0.00 | 4.27e ± 4.27 | 0.00d ± 0.00 |
| maltose | 1.21abcde ± 0.10 | 36.37a ± 1.54 | 1.00d ± 0.25 |
| mannose | 1.2abcdehi ± 0.06 | 20.22cf ± 4.04 | 0.87d ± 0.31 |
| saccharose | 1.47abceh ± 0.07 | 30.09ad ± 0.22 | 1.88ad ± 1.41 |
| sorbitol | 1.03abcde ± 0.17 | 35.37a ± 0.11 | 12.71ac ± 9.04 |
| starch | 0.98abcdehijkln ± 0.24 | 74.15b ± 1.70 | 133.38b ± 4.83 |
| trehalose | 1.56abcehijk ± 0.14 | 33.20ah ± 2.73 | 17.98ac ± 4.71 |
| xylose | 0.05dflm ± 0.01 | 0.00e ± 0.00 | 0.00d ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koim-Puchowska, B.; Kłosowski, G.; Dróżdż-Afelt, J.M.; Mikulski, D.; Zielińska, A. Influence of the Medium Composition and the Culture Conditions on Surfactin Biosynthesis by a Native Bacillus subtilis natto BS19 Strain. Molecules 2021, 26, 2985. https://doi.org/10.3390/molecules26102985
Koim-Puchowska B, Kłosowski G, Dróżdż-Afelt JM, Mikulski D, Zielińska A. Influence of the Medium Composition and the Culture Conditions on Surfactin Biosynthesis by a Native Bacillus subtilis natto BS19 Strain. Molecules. 2021; 26(10):2985. https://doi.org/10.3390/molecules26102985
Chicago/Turabian StyleKoim-Puchowska, Beata, Grzegorz Kłosowski, Joanna Maria Dróżdż-Afelt, Dawid Mikulski, and Alicja Zielińska. 2021. "Influence of the Medium Composition and the Culture Conditions on Surfactin Biosynthesis by a Native Bacillus subtilis natto BS19 Strain" Molecules 26, no. 10: 2985. https://doi.org/10.3390/molecules26102985
APA StyleKoim-Puchowska, B., Kłosowski, G., Dróżdż-Afelt, J. M., Mikulski, D., & Zielińska, A. (2021). Influence of the Medium Composition and the Culture Conditions on Surfactin Biosynthesis by a Native Bacillus subtilis natto BS19 Strain. Molecules, 26(10), 2985. https://doi.org/10.3390/molecules26102985








