A Novel Calcium-Dependent Protein Kinase 1 Inhibitor Potently Prevents Toxoplasma gondii Transmission to Foetuses in Mouse
Abstract
:1. Introduction
2. Results
2.1. Chemical Synthesis of SP230 and Validation of Its Therapeutic Index
2.2. Establishment of Specific Standard Curves for qPCR
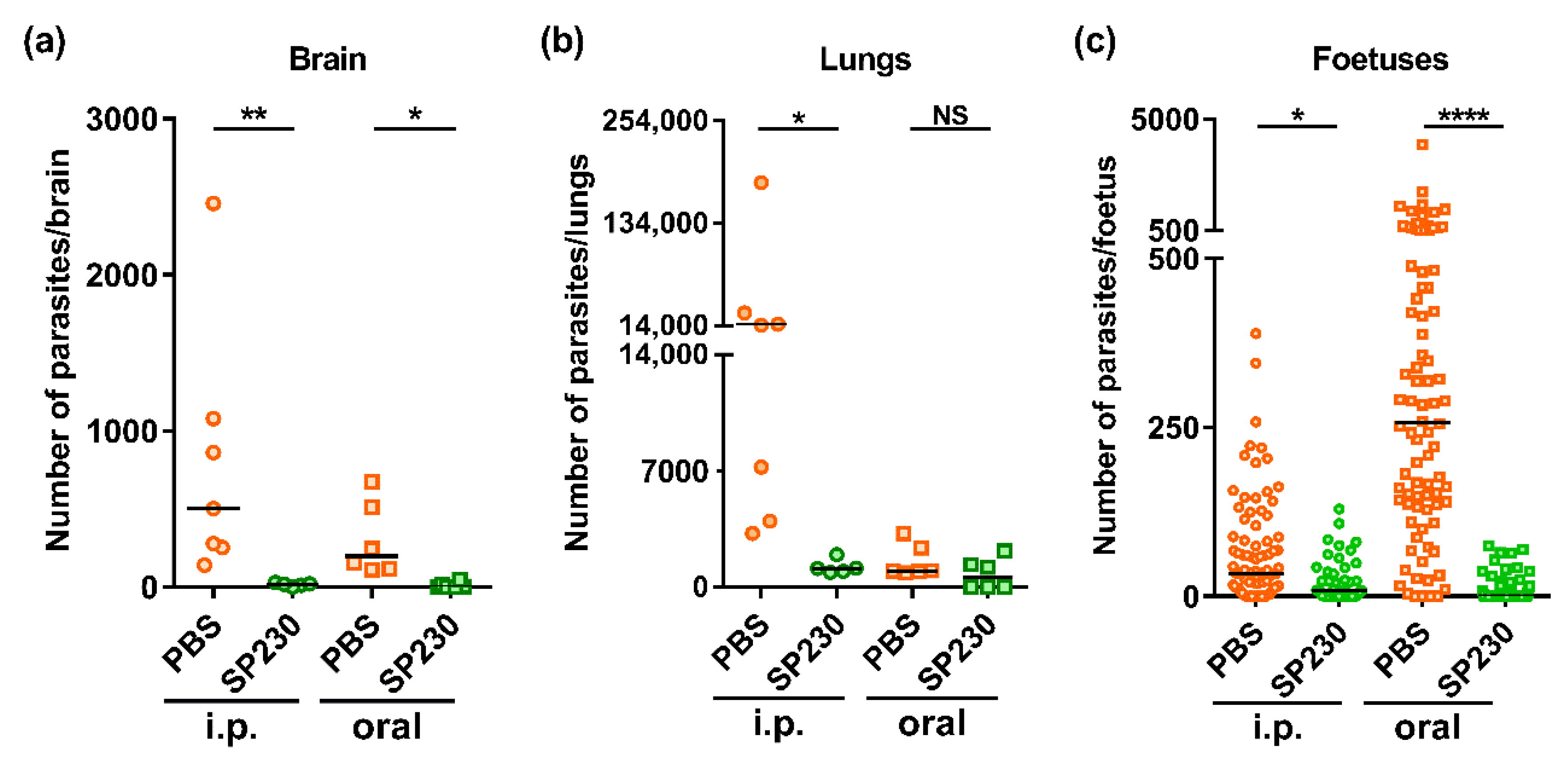
2.3. SP230-Treatment of Pregnant Mice Infected with T. gondii
2.4. SP230-Treatment of Pregnant Non-Infected Mice
3. Discussion
4. Materials and Methods
4.1. Chemical Synthesis of SP230
4.2. Functional Characterization of SP230
4.3. Treatment of T. gondii-Infected Mice with SP230
4.4. Quantitation of Parasite Loads by qPCR
4.5. Survival of Offspring Born from Mice Treated with SP230
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Khan, K.; Khan, W. Congenital toxoplasmosis: An overview of the neurological and ocular manifestations. Parasitol. Int. 2018, 67, 715–721. [Google Scholar] [CrossRef]
- Picone, O.; Fuchs, F.; Benoist, G.; Binquet, C.; Kieffer, F.; Wallon, M.; Wehbe, K.; Mandelbrot, L.; Villena, I. Toxoplasmosis screening during pregnancy in France: Opinion of an expert panel for the CNGOF. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101814. [Google Scholar] [CrossRef]
- Peyron, F.; L’ollivier, C.; Mandelbrot, L.; Wallon, M.; Piarroux, R.; Kieffer, F.; Hadjadj, E.; Paris, L.; Garcia-Meric, P. Maternal and Congenital Toxoplasmosis: Diagnosis and Treatment Recommendations of a French Multidisciplinary Working Group. Pathogens 2019, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.-X.; Wei, S.-S.; Lindsay, D.S.; Peng, H.-J. A Systematic Review and Meta-Analysis of the Efficacy of Anti-Toxoplasma gondii Medicines in Humans. PLoS ONE 2015, 10, e0138204. [Google Scholar] [CrossRef]
- Teil, J.; Dupont, D.; Charpiat, B.; Corvaisier, S.; Vial, T.; Leboucher, G.; Wallon, M.; Peyron, F. Treatment of Congenital Toxoplasmosis: Safety of the Sulfadoxine-Pyrimethamine Combination in Children Based on a Method of Causality Assessment. Pediatr. Infect. Dis. J. 2016, 35, 634–638. [Google Scholar] [CrossRef]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Shahdin, S.; Daryani, A. Activities of anti-Toxoplasma drugs and compounds against tissue cysts in the last three decades (1987 to 2017), a systematic review. Parasitol. Res. 2018, 117, 3045–3057. [Google Scholar] [CrossRef]
- Moine, E.; Moiré, N.; Dimier-Poisson, I.; Brunet, K.; Couet, W.; Colas, C.; Van Langendonck, N.; Enguehard-Gueiffier, C.; Gueiffier, A.; Héraut, B.; et al. Imidazo[1,2-b]pyridazines targeting Toxoplasma gondii calcium-dependent protein kinase 1 decrease the parasite burden in mice with acute toxoplasmosis. Int. J. Parasitol. 2018, 48, 561–568. [Google Scholar] [CrossRef]
- Müller, J.; Aguado-Martínez, A.; Ortega-Mora, L.-M.; Moreno-Gonzalo, J.; Ferre, I.; Hulverson, M.A.; Choi, R.; McCloskey, M.C.; Barrett, L.K.; Maly, D.J.; et al. Development of a murine vertical transmission model for Toxoplasma gondii oocyst infection and studies on the efficacy of bumped kinase inhibitor (BKI)-1294 and the naphthoquinone buparvaquone against congenital toxoplasmosis. J. Antimicrob. Chemother. 2017, 72, 2334–2341. [Google Scholar] [CrossRef] [Green Version]
- Hulverson, M.A.; Bruzual, I.; McConnell, E.V.; Huang, W.; Vidadala, R.S.R.; Choi, R.; Arnold, S.L.M.; Whitman, G.R.; McCloskey, M.C.; Barrett, L.K.; et al. Pharmacokinetics and In Vivo Efficacy of Pyrazolopyrimidine, Pyrrolopyrimidine, and 5-Aminopyrazole-4-Carboxamide Bumped Kinase Inhibitors against Toxoplasmosis. J. Infect. Dis. 2019, 219, 1464–1473. [Google Scholar] [CrossRef]
- Paquet, C.; Yudin, M.H. Society of Obstetricians and Gynaecologists of Canada Toxoplasmosis in pregnancy: Prevention, screening, and treatment. J Obstet Gynaecol Can 2013, 35, 78–81. [Google Scholar] [CrossRef]
- Maldonado, Y.A.; Read, J.S. COMMITTEE ON INFECTIOUS DISEASES Diagnosis, Treatment, and Prevention of Congenital Toxoplasmosis in the United States. Pediatrics 2017, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Bissati, K.; Levigne, P.; Lykins, J.; Adlaoui, E.B.; Barkat, A.; Berraho, A.; Laboudi, M.; El Mansouri, B.; Ibrahimi, A.; Rhajaoui, M.; et al. Global initiative for congenital toxoplasmosis: An observational and international comparative clinical analysis. Emerg. Microbes. Infect. 2018, 7, 165. [Google Scholar] [CrossRef] [Green Version]
- Montoya, J.G. Systematic screening and treatment of toxoplasmosis during pregnancy: Is the glass half full or half empty? Am. J. Obstet. Gynecol. 2018, 219, 315–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belk, K.; Connolly, M.P.; Schlesinger, L.; Ben-Harari, R.R. Patient and treatment pathways for toxoplasmosis in the United States: Data analysis of the Vizient Health Systems Data from 2011 to 2017. Pathog. Glob. Health 2018, 112, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Ben-Harari, R.R.; Connolly, M.P. High burden and low awareness of toxoplasmosis in the United States. Postgrad. Med. 2019, 131, 103–108. [Google Scholar] [CrossRef]
- Wallon, M.; Peyron, F. Effect of Antenatal Treatment on the Severity of Congenital Toxoplasmosis. Clin. Infect. Dis. 2016, 62, 811–812. [Google Scholar] [CrossRef] [Green Version]
- Peyron, F.; Mc Leod, R.; Ajzenberg, D.; Contopoulos-Ioannidis, D.; Kieffer, F.; Mandelbrot, L.; Sibley, L.D.; Pelloux, H.; Villena, I.; Wallon, M.; et al. Congenital Toxoplasmosis in France and the United States: One Parasite, Two Diverging Approaches. PLoS Negl. Trop. Dis. 2017, 11, e0005222. [Google Scholar] [CrossRef]
- Olariu, T.R.; Press, C.; Talucod, J.; Olson, K.; Montoya, J.G. Congenital toxoplasmosis in the United States: Clinical and serologic findings in infants born to mothers treated during pregnancy. Parasite 2019, 26, 13. [Google Scholar] [CrossRef]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug Resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar] [CrossRef] [Green Version]
- Ben-Harari, R.R.; Goodwin, E.; Casoy, J. Adverse Event Profile of Pyrimethamine-Based Therapy in Toxoplasmosis: A Systematic Review. Drugs R D 2017, 17, 523–544. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Sánchez, R.; Ferre, I.; Re, M.; Ramos, J.J.; Regidor-Cerrillo, J.; Pizarro Díaz, M.; González-Huecas, M.; Tabanera, E.; Benavides, J.; Hemphill, A.; et al. Treatment with Bumped Kinase Inhibitor 1294 Is Safe and Leads to Significant Protection against Abortion and Vertical Transmission in Sheep Experimentally Infected with Toxoplasma gondii during Pregnancy. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [Green Version]
| Treatment of Dams | Number of Offspring | Weight of Female | Weight of Male |
|---|---|---|---|
| PBS i.p. (n = 9) | 4 ± 2 | 17.4 ± 1.6 | 19.6 ± 1.2 |
| SP230 i.p. (n = 9) | 3.89 ± 1 ns | 18.6 ± 2.3 a | 21.6 ± 2.4 b |
| PBS oral (n = 9) | 5.22 ± 2 | 17.4 ± 2.4 | 20.5 ± 1.5 |
| SP230 oral (n = 9) | 4.11 ± 2 ns | 16.7 ± 2.8 ns | 21.9 ± 2.3 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Débare, H.; Moiré, N.; Baron, F.; Lantier, L.; Héraut, B.; Van Langendonck, N.; Denevault-Sabourin, C.; Dimier-Poisson, I.; Debierre-Grockiego, F. A Novel Calcium-Dependent Protein Kinase 1 Inhibitor Potently Prevents Toxoplasma gondii Transmission to Foetuses in Mouse. Molecules 2021, 26, 4203. https://doi.org/10.3390/molecules26144203
Débare H, Moiré N, Baron F, Lantier L, Héraut B, Van Langendonck N, Denevault-Sabourin C, Dimier-Poisson I, Debierre-Grockiego F. A Novel Calcium-Dependent Protein Kinase 1 Inhibitor Potently Prevents Toxoplasma gondii Transmission to Foetuses in Mouse. Molecules. 2021; 26(14):4203. https://doi.org/10.3390/molecules26144203
Chicago/Turabian StyleDébare, Héloïse, Nathalie Moiré, Firmin Baron, Louis Lantier, Bruno Héraut, Nathalie Van Langendonck, Caroline Denevault-Sabourin, Isabelle Dimier-Poisson, and Françoise Debierre-Grockiego. 2021. "A Novel Calcium-Dependent Protein Kinase 1 Inhibitor Potently Prevents Toxoplasma gondii Transmission to Foetuses in Mouse" Molecules 26, no. 14: 4203. https://doi.org/10.3390/molecules26144203
APA StyleDébare, H., Moiré, N., Baron, F., Lantier, L., Héraut, B., Van Langendonck, N., Denevault-Sabourin, C., Dimier-Poisson, I., & Debierre-Grockiego, F. (2021). A Novel Calcium-Dependent Protein Kinase 1 Inhibitor Potently Prevents Toxoplasma gondii Transmission to Foetuses in Mouse. Molecules, 26(14), 4203. https://doi.org/10.3390/molecules26144203








