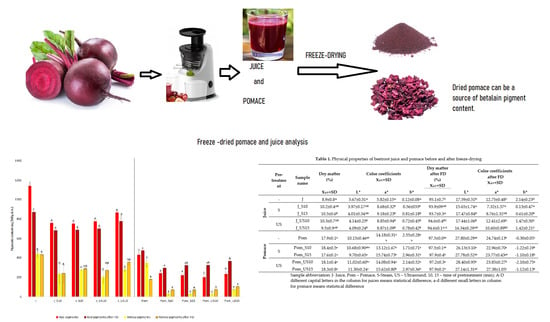
The Influence of Different Pretreatment Methods on Color and Pigment Change in Beetroot Products
Abstract
1. Introduction
2. Results and Discussion
2.1. Overall Physical Properties of Juice and Pomace
2.2. Color Analysis
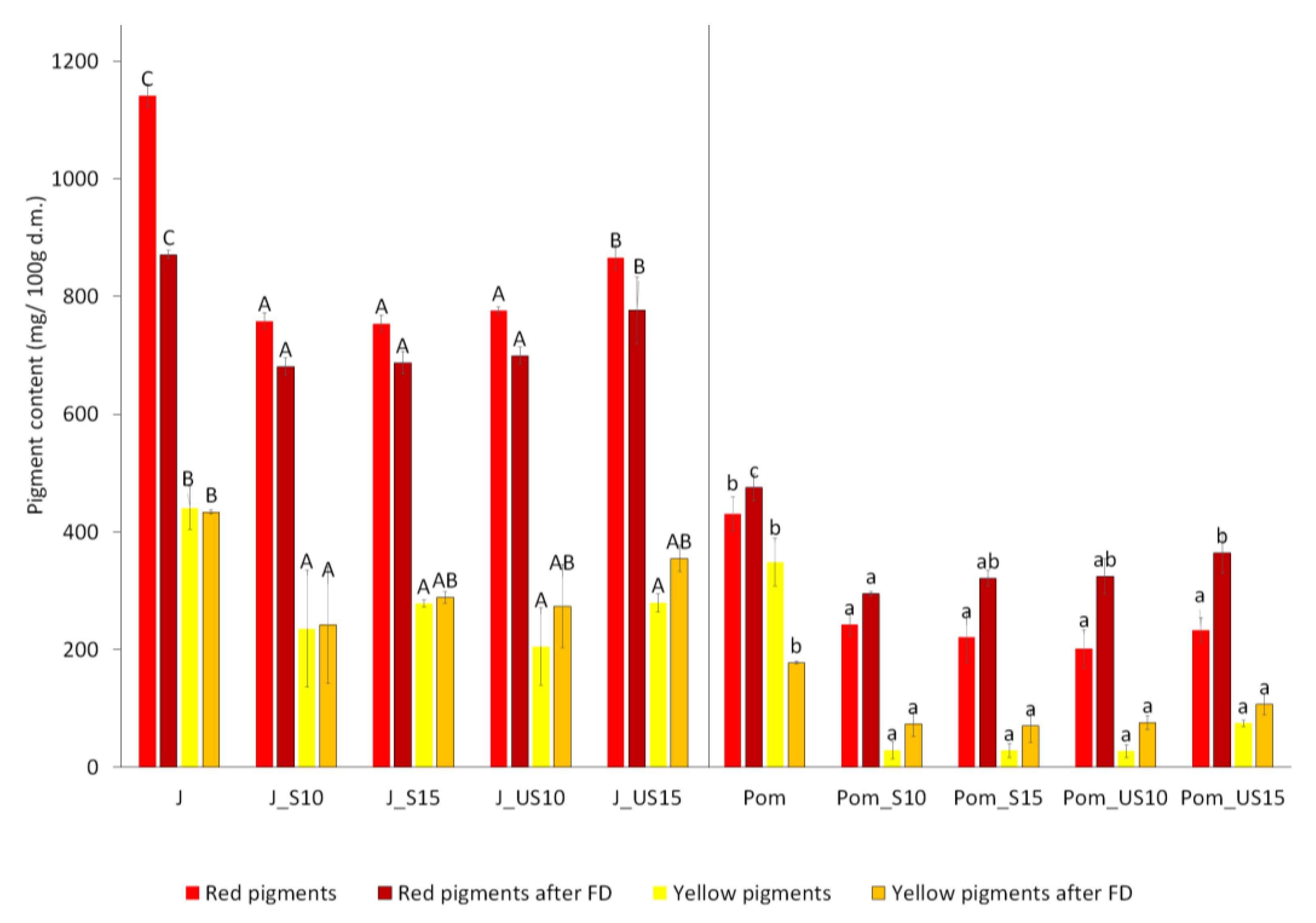
2.3. Pigment Content Analysis
3. Materials and Methods
3.1. Materials
3.2. Technological Treatment
3.2.1. Pretreatment
Steam Pretreatment
Ultrasound
3.2.2. Juice Pressing
3.2.3. Freeze-Drying
3.3. Analytical Method
3.3.1. Dry Matter
3.3.2. Color Analysis
3.3.3. Betalain Analysis
3.4. Statistical Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Seremet, L.; Nistor, O.V.; Andronoiu, D.G.; Mocanu, G.D.; Barbu, V.V.; Maidan, A.; Rudi, L.; Botez, E. Development of several hybrid drying methods used to obtain red beetroot powder. Food Chem. 2020, 310, 125637. [Google Scholar] [CrossRef]
- Dalla Costa, A.P.; Hermes, V.S.; de Oliveira Rios, A.; Flôres, S.H. Minimally processed beetroot waste as an alternative source to obtain functional ingredients. J. Food Sci. Technol. 2017, 54, 2050–2058. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Klewicka, E. Betacyjaniny–biodostępność i biologiczna aktywność. Żywność Nauka Technol. Jakość 2012, 2, 5–21. [Google Scholar]
- Stintzing, F.C.; Carle, R. Betalains—Emerging prospects for food scientists. Trends Food Sci. Technol. 2007, 18, 514–525. [Google Scholar] [CrossRef]
- Kohajdová, Z.; Karovičová, J.; Kuchtová, V.; Lauková, M. Utilisation of beetroot powder for bakery applications. Chem. Pap. 2018, 72, 1507–1515. [Google Scholar] [CrossRef]
- Komolka, P.; Górecka, D. Wpływ obróbki cieplnej na strukturę wybranych warzyw i owoców®. Postępy Tech. Przetwórstwa Spożywczego 2017, 2, 67–73. [Google Scholar]
- Witrowa-Rajchert, D.; Wiktor, A.; Sledz, M.; Nowacka, M. Selected emerging technologies to enhance the drying process: A review. Dry. Technol. 2014, 32, 1386–1396. [Google Scholar] [CrossRef]
- Onwude, D.I.; Hashim, N.; Janius, R.; Abdan, K.; Chen, G.; Oladejo, A.O. Non-thermal hybrid drying of fruits and vegetables: A review of current technologies. Innov. Food Sci. Emerg. Technol. 2017, 43, 223–238. [Google Scholar] [CrossRef]
- Dadan, M.; Nowacka, M.; Wiktor, A.; Sobczynska, A.; Witrowa-Rajchert, D. Ultrasound to improve drying processes and prevent thermolabile nutrients degradation. In Design and Optimization of Innovative Food Processing Techniques Assisted by Ultrasound; Academic Press: Cambridge, MA, USA, 2021; pp. 55–110. [Google Scholar]
- Huang, D.; Men, K.; Li, D.; Wen, T.; Gong, Z.; Sunden, B.; Wu, Z. Application of ultrasound technology in the drying of food products. Ultrason. Sonochem. 2020, 63, 104950. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Dadan, M.; Tylewicz, U. Current applications of ultrasound in fruit and vegetables osmoticdehydration processes. Appl. Sci. 2021, 11, 1269. [Google Scholar] [CrossRef]
- Šeregelj, V.; Ćetković, G.; Čanadanović-Brunet, J.; Šaponjac, V.T.; Vulić, J.; Lević, S.; Nedović, V.; Brandolini, A.; Hidalgo, A. Encapsulation of carrot waste extract by freeze and spray drying techniques: An optimization study. LWT Food Sci. Technol. 2021, 138, 110696. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of food industry waste for high-value products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef]
- Kumar, V.; Kushwaha, R.; Goyal, A.; Tanwar, B.; Kaur, J. Process optimization for the preparation of antioxidant rich ginger candy using beetroot pomace extract. Food Chem. 2018, 245, 168–177. [Google Scholar] [CrossRef]
- Majerska, J.; Michalska, A.; Figiel, A. A review of new directions in managing fruit and vegetable processing by-products. Trends Food Sci. Technol. 2019, 88, 207–219. [Google Scholar] [CrossRef]
- Różyło, R. Recent trends in methods used to obtain natural food colorants by freeze-drying. Trends Food Sci. Technol. 2020, 102, 39–50. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. The freeze-drying of foods—The characteristic of the process course and the effect of its parameters on the physical properties of food materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef] [PubMed]
- Harnkarnsujarit, N.; Charoenrein, S. Effect of water activity on sugar crystallization and β-carotene stability of freeze-dried mango powder. J. Food Eng. 2011, 105, 592–598. [Google Scholar] [CrossRef]
- Fongin, S.; Kawai, K.; Harnkarnsujarit, N.; Hagura, Y. Effects of water and maltodextrin on the glass transition temperature of freeze-dried mango pulp and an empirical model to predict plasticizing effect of water on dried fruits. J. Food Eng. 2017, 210, 91–97. [Google Scholar] [CrossRef]
- Harnkarnsujarit, N.; Charoenrein, S. Influence of collapsed structure on stability of β-carotene in freeze-dried mangoes. Food Res. Int. 2011, 44, 3188–3194. [Google Scholar] [CrossRef]
- Janiszewska, E. Microencapsulated beetroot juice as a potential source of betalain. Powder Technol. 2014, 264, 190–196. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Witrowa-Rajchert, D. The influence of carrot pretreatment, type of carrier and disc speed on the physical and chemical properties of spray-dried carrot juice microcapsules. Dry. Technol. 2021, 39, 439–449. [Google Scholar] [CrossRef]
- Fijałkowska, A.; Nowacka, M.; Witrowa-Rajchert, D. Wpływ obróbki wstępnej ultradźwiękami na przebieg suszenia oraz barwę i zawartość betalain w buraku ćwikłowym. Zesz. Probl. Postępów Nauk. Rol. 2015, 581, 11–20. [Google Scholar]
- Fijałkowska, A.; Nowacka, M.; Witrowa-Rajchert, D. Effect of ultrasound waves on drying process and selected properties of beetroot tissue. Food Sci. Technol. Qual. 2015, 2, 138–149. [Google Scholar] [CrossRef]
- Nistor, O.V.; Seremet Ceclu, L.; Andronoiu, D.G.; Rudi, L.; Botez, E. Influence of different drying methods on the physicochemical properties of red beetroot (Beta vulgaris L. var. Cylindra). Food Chem. 2017, 236, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.E.; Kalemba, D.; Adamiec, J.; Piątkowski, M. Microencapsulation of Nigella sativa oleoresin by spray drying for food and nutraceutical applications. Food Chem. 2016, 204, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Ciurzyńska, A.; Falacińska, J.; Kowalska, H.; Kowalska, J.; Galus, S.; Marzec, A.; Domian, E. The effect of pre-treatment (blanching, ultrasound and freezing) on quality of freeze-dried red beets. Foods 2021, 10, 132. [Google Scholar] [CrossRef]
- Domian, E.; Brynda-Kopytowska, A.; Cieśla, J.; Górska, A. Effect of carbohydrate type on the DVS isotherm-induced phase transitions in spray-dried fat-filled pea protein-based powders. J. Food Eng. 2018, 222, 115–125. [Google Scholar] [CrossRef]
- Dadan, M.; Nowacka, M. The assessment of the possibility of using ethanol and ultrasound to design the properties of dried carrot tissue. Appl. Sci. 2021, 11, 689. [Google Scholar] [CrossRef]
- Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Parniakov, O.; Nowacka, M. The quality of red bell pepper subjected to freeze-drying preceded by traditional and novel pretreatment. Foods 2021, 10, 226. [Google Scholar] [CrossRef]
- Nowacka, M.; Wedzik, M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. Appl. Acoust. 2016, 103, 163–171. [Google Scholar] [CrossRef]
- Coy-Barrera, E. Chapter 17—Analysis of betalains (betacyanins and betaxanthins). In Recent Advances in Natural Products Analysis; Sanches Silva, A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 593–619. [Google Scholar]
- Wilska-Jeszka, J. Food colorants. In Chemical and Functional Properties of Food Components; Sikorski, Z.E., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 245–274. [Google Scholar]
- Klewicka, E.; Czyżowska, A. Biological stability of lactofermented beetroot juice during refrigerated storage. Pol. J. Food Nutr. Sci. 2011, 61, 251–256. [Google Scholar] [CrossRef]
- Czapski, J. The effect of heating conditions on losses and regeneration of betacyanins. Z. Lebensm. Unters. Forsch. 1985, 180, 21–25. [Google Scholar] [CrossRef]
- Czapski, J.; Walkowiak-Tomczak, D. Przewidywanie zmian barwy soku z buraka ćwikłowego podczas ogrzewania. Apar. Badaw. Dydakt. 2002, 7, 26–32. [Google Scholar]
- Rodriguez-Amaya, D.B. Update on natural food pigments—A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Tappi, S.; Wiktor, A.; Rybak, K.; Miszczykowska, A.; Czyzewski, J.; Drozdzal, K.; Witrowa-Rajchert, D.; Tylewicz, U. The Impact of Pulsed Electric Field on the Extraction of Bioactive Compounds from Beetroot. Foods 2019, 8, 244. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Fernández-Lledó, V.; Angosto, J.M. New insights into red plant pigments: More than just natural colorants. RSC Adv. 2020, 10, 24669–24682. [Google Scholar] [CrossRef]
- Flores-Mancha, M.A.; Ruíz-Gutiérrez, M.G.; Sánchez-Vega, R.; Santellano-Estrada, E.; Chávez-Martínez, A. Characterization of beet root extract (Beta vulgaris) encapsulated with maltodextrin and inulin. Molecules 2020, 25, 5498. [Google Scholar] [CrossRef]
- Zeece, M. Flavors. In Introduction to the Chemistry of Food; Academic Press: Cambridge, MA, USA, 2020; pp. 213–250. [Google Scholar]
- Bassama, J.; Tamba, A.; Ndong, M.; Sarr, K.D.D.; Cissé, M. Degradation Kinetics of Betacyanins during the Pasteurization and Storage of Cactus Pear (Opuntia dillenii Haw.) Juice Using the Arrhenius, Eyring, and Ball Models. Beverages 2021, 7, 2. [Google Scholar] [CrossRef]
- Gimenez, P.J.; Fernandez-Lopez, J.A.; Angosto, J.M.; Obon, J.M. Comparative thermal degradation patterns of natural yellow colorants used in foods. Plant Foods Hum. Nutr. 2015, 70, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, V.; Anna, J.L.; Vijayeeswarri, J.; Swaminathan, G. Ultrasound assisted enhancement in natural dye extraction from beetroot for industrial applications and natural dyeing of leather. Ultrason. Sonochem. 2009, 16, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Rybak, K.; Parniakov, O.; Samborska, K.; Wiktor, A.; Witrowa-Rajchert, D.; Nowacka, M. Energy and quality aspects of freeze-drying preceded by traditional and novel pre-treatment methods as exemplified by red bell pepper. Sustainability 2021, 13, 2035. [Google Scholar] [CrossRef]
- Association of Official Analytical Collaboration International. Official Methods of Analysis of AOAC International, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Janiszewska, E.; Włodarczyk, J. Influence of spray drying conditions on the beetroot pigments retention after microencapsulation process. Acta Agrophys. 2013, 20, 343–356. [Google Scholar]
| Pre-Treatment | Sample Name | Dry Matter (%) | Color Coefficients XAV + SD | Dry Matter after FD (%) XAV + SD | Color Coefficients after FD XAV + SD | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| XAV + SD | L* | a* | b* | L* | a* | b* | ||||
| Juice | - | J | 8.9 ± 0.8 A | 3.67 ± 0.31 A | 5.82 ± 0.15 A | 0.12 ± 0.08 A | 95.1 ± 0.7 C | 17.59 ± 0.51 B | 12.75 ± 0.48 C | 2.14 ± 0.23 D |
| S | J_S10 | 10.2 ± 0.4 AB | 3.97 ± 0.17 AB | 8.68 ± 0.32 B | 0.56 ± 033 B | 93.9 ± 09 AB | 15.65 ± 1.74 A | 7.32 ± 1.57 A | 0.13 ± 0.47 A | |
| J_S15 | 10.5 ± 0.6 B | 4.01 ± 0.34 AB | 9.18 ± 0.23 B | 0.81 ± 0.18 B | 93.7 ± 0.3 A | 17.47 ± 0.84 B | 8.76 ± 1.32 AB | 0.61 ± 0.20 B | ||
| US | J_US10 | 10.3 ± 0.7 AB | 4.14 ± 0.25 B | 8.85 ± 0.94 B | 0.72 ± 0.45 B | 94.6 ± 0.4 BC | 17.44 ± 1.06 B | 12.41 ± 2.69 C | 1.47 ± 0.50 C | |
| J_US15 | 9.5 ± 0.9 AB | 4.09 ± 0.24 B | 8.87 ± 1.09 B | 0.78 ± 0.42 B | 94.6 ± 0.1 A–C | 16.34 ± 0.29 AB | 10.60 ± 0.89 BC | 1.42 ± 0.21 C | ||
| Pomace | - | Pom | 17.9 ± 0.1 a | 10.15 ± 0.46 ab | 14.18 ± 0.31 ab | 2.55 ± 0.28 ab | 97.5 ± 0.0 ab | 27.80 ± 0.29 ab | 24.74 ± 0.13 b | −0.30 ± 0.05 c |
| S | Pom_S10 | 18.4 ± 0.5 a | 10.68 ± 0.90 abc | 13.12 ± 1.67 a | 1.71 ± 0.71 a | 97.5 ± 0.1 ab | 26.13 ± 3.10 a | 22.96 ± 0.70 a | −1.22 ± 0.19 b | |
| Pom_S15 | 17.6 ± 0.1 a | 9.70 ± 0.65 a | 15.74 ± 0.73 b | 2.96 ± 0.31 b | 97.9 ± 0.4 b | 27.78 ± 0.52 ab | 23.77 ± 0.43 ab | −1.10 ± 0.18 b | ||
| US | Pom_US10 | 18.1 ± 0.4 a | 11.02 ± 0.60 bc | 14.08 ± 0.94 a | 2.14 ± 0.52 a | 97.2 ± 0.3 a | 28.40 ± 0.95 b | 23.85 ± 0.27 b | −2.10 ± 0.75 a | |
| Pom_US15 | 18.3 ± 0.8 a | 11.30 ± 0.24 c | 15.62 ± 0.80 b | 2.97 ± 0.36 b | 97.9 ± 0.1 b | 27.14 ± 1.31 ab | 27.38 ± 1.05 c | −1.12 ± 0.13 b | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janiszewska-Turak, E.; Rybak, K.; Grzybowska, E.; Konopka, E.; Witrowa-Rajchert, D. The Influence of Different Pretreatment Methods on Color and Pigment Change in Beetroot Products. Molecules 2021, 26, 3683. https://doi.org/10.3390/molecules26123683
Janiszewska-Turak E, Rybak K, Grzybowska E, Konopka E, Witrowa-Rajchert D. The Influence of Different Pretreatment Methods on Color and Pigment Change in Beetroot Products. Molecules. 2021; 26(12):3683. https://doi.org/10.3390/molecules26123683
Chicago/Turabian StyleJaniszewska-Turak, Emilia, Katarzyna Rybak, Ewelina Grzybowska, Ewelina Konopka, and Dorota Witrowa-Rajchert. 2021. "The Influence of Different Pretreatment Methods on Color and Pigment Change in Beetroot Products" Molecules 26, no. 12: 3683. https://doi.org/10.3390/molecules26123683
APA StyleJaniszewska-Turak, E., Rybak, K., Grzybowska, E., Konopka, E., & Witrowa-Rajchert, D. (2021). The Influence of Different Pretreatment Methods on Color and Pigment Change in Beetroot Products. Molecules, 26(12), 3683. https://doi.org/10.3390/molecules26123683