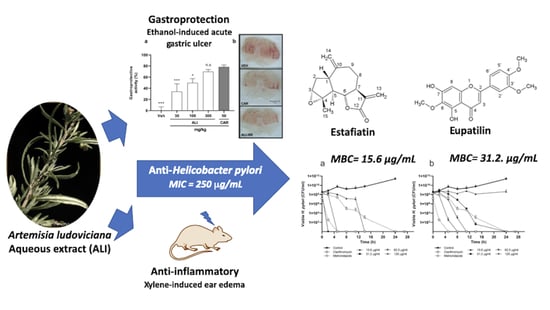
Anti-Helicobacter pylori Activity of Artemisia ludoviciana subsp. mexicana and Two of Its Bioactive Components, Estafiatin and Eupatilin
Abstract
1. Introduction
2. Results
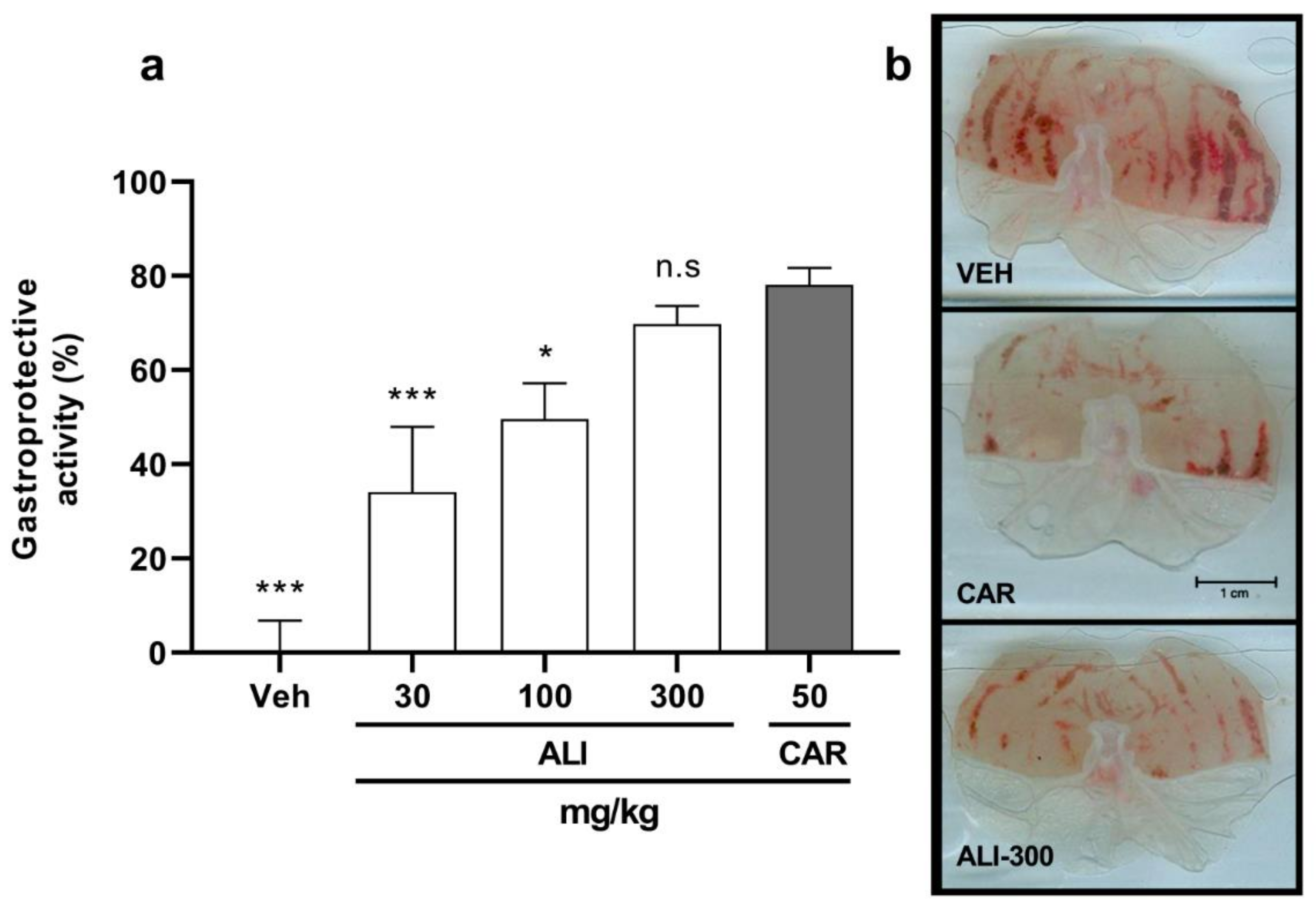
2.1. Biological Activities of Artemisia ludoviciana Infusion (ALI): Anti-H. pylori, Gastroprotective, and Anti-inflammatory Effects
2.2. Activity of ALI Primary Fractions
2.3. Eupatilin and Estafiatin as Anti-H. pylori Antibiotics
3. Discussion
4. Materials and Methods
4.1. Reagents and General Experimental Procedures
4.2. Plant Material
4.3. Extract Preparation and Primary Fractionation
4.4. Isolation and Identification of Compounds
4.4.1. Isolation and Identification of Estafiatin
4.4.2. Isolation and Identification of Eupatilin
4.5. Bacterial Strain and Culture Conditions
4.6. Anti-Helicobacter pylori Activity
4.7. Urease Inhibition Assay
4.8. Animals
4.9. Acute Oral Toxicity Assay
4.10. Gastric Antiulcerogenic Activity
4.11. Anti-Inflammatory Activity
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.H.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef]
- Atherton, J.C. The Pathogenesis of Helicobacter pylori–Induced Gastro-Duodenal Diseases. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 63–96. [Google Scholar] [CrossRef]
- Gravina, A.G.; Zagari, R.M.; De Musis, C.; Romano, L.; Loguercio, C.; Romano, M. Helicobacter pyloriand extragastric diseases: A review. World J. Gastroenterol. 2018, 24, 3204–3221. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Sheu, B.-S.; Wu, J.-J. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed. J. 2016, 39, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Clyne, M.; Rowland, M. The Role of Host Genetic Polymorphisms in Helicobacter pylori Mediated Disease Outcome. In Helicobacter pylori in Human Diseases, 1st ed.; Advances in Experimental Medicine and Biology 1149; Kamiya, S., Backert, S., Eds.; Springer: Cham, Switzerland, 2019; Volume 11, pp. 151–172. [Google Scholar] [CrossRef]
- Whitmire, J.M.; Merrell, D.S. Helicobacter pylori Genetic Polymorphisms in Gastric Disease Development. In Helicobacter pylori in Human Diseases, 1st ed.; Advances in Experimental Medicine and Biology 1149; Kamiya, S., Backert, S., Eds.; Springer: Cham, Switzerland, 2019; Volume 11, pp. 173–194. [Google Scholar] [CrossRef]
- Ansari, S.; Yamaoka, Y. Current understanding and management of Helicobacter pylori infection: An updated appraisal. F1000Research 2018, 7, 721. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.-N.; Tang, G.-Y.; Li, S.; Gan, R.-Y.; Li, H.-B. Natural Products for the Prevention and Management ofHelicobacter pyloriInfection. Compr. Rev. Food Sci. Food Saf. 2018, 17, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharopov, F.; Martorell, M.; Rajkovic, J.; Ademiluyi, A.O.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Phytochemicals in Helicobacter pylori Infections: What Are We Doing Now? Int. J. Mol. Sci. 2018, 19, 2361. [Google Scholar] [CrossRef] [PubMed]
- WHO Global Report on Traditional and Complementary Medicine 2019; World Health Organization: Geneva, Switzerland, 2019. Available online: https://www.who.int/publications/i/item/978924151536 (accessed on 15 April 2021).
- Castillo-Juárez, I.; González, V.; Jaime-Aguilar, H.; Martínez, G.; Linares, E.; Bye, R.; Romero, I. Anti-Helicobacter pylori activity of plants used in Mexican traditional medicine for gastrointestinal disorders. J. Ethnopharmacol. 2009, 122, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Hinojosa, W.I.; Del Carpio, J.D.; Espinosa, J.F.P.; Romero, I. Contribution to the ethnopharmacological and anti-Helicobacter pylori knowledge of Cyrtocarpa procera Kunth (Anacardiaceae). J. Ethnopharmacol. 2012, 143, 363–371. [Google Scholar] [CrossRef]
- Palacios-Espinosa, J.F.; Arroyo-García, O.; García-Valencia, G.; Linares, E.; Bye, R.; Romero, I. Evidence of the anti-Helicobacter pylori, gastroprotective and anti-inflammatory activities of Cuphea aequipetala infusion. J. Ethnopharmacol. 2014, 151, 990–998. [Google Scholar] [CrossRef] [PubMed]
- The Plant List. Version 1.1. 2013. Available online: http://www.theplantlist.org/tpl1.1/record/gcc-67684 (accessed on 23 March 2021).
- Argueta, A.; Cano, L.M.; Rodarte, M.E. Atlas de las Plantas de la Medicina Tradicional Mexicana, Tomo I-III, 1st ed.; Instituto Nacional Indigenista: Mexico City, Mexico, 1994. [Google Scholar]
- Esquivel-García, R.; Pérez-Calix, E.; Ochoa-Zarzosa, A.; García-Pérez, M.E. Ethnomedicinal plants used for the treatment of dermatological affections on the Purépecha Plateau, Michoacán, Mexico. Acta Bot. Mex. 2018, 125, 95–132. [Google Scholar] [CrossRef]
- Nicholson, M.S.; Arzeni, C.B. The market medicinal plants of Monterrey, Nuevo León, México. Econ. Bot. 1991, 47, 184–192. [Google Scholar] [CrossRef]
- Osuna-Torres, L.; Tapia Pérez, M.E.; Aguilar Contreras, A. Plantas Medicinales de la Medicina Tradicional Mexicana Para Tratar Afecciones Gastrointestinales: Estudio Etnobotánico, Fitoquímico y Farmacológico; Publicacions i Edicions de la Universitat de Barcelona: Barcelona, Spain, 2005; p. 173. [Google Scholar]
- Mata, R.; Figueroa, M.; Navarrete, A.; Rivero-Cruz, I. Chemistry and Biology of Selected Mexican Medicinal Plants. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A., Falk, H., Gibbons, S., Kobayashi, J., Asakawa, Y., Liu, J.K., Eds.; Springer: Cham, Switzerland, 2019; Volume 108, pp. 1–142. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Dominguez, F.; Maldonado-Miranda, J.J.; Pérez, L.J.C.; Carranza-Álvarez, C.; Solano, E.; Isiordia-Espinoza, M.A.; Juárez-Vázquez, M.D.C.; Zapata-Morales, J.R.; Argueta-Fuertes, M.A.; et al. Use of medicinal plants by health professionals in Mexico. J. Ethnopharmacol. 2017, 198, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Viesca, F.; Romo, J. Estafiatin, a new sesquiterpene lactone isolated from Artemisia mexicana (Willd). Tetrahedron 1963, 19, 1285–1291. [Google Scholar] [CrossRef]
- Lee, K.; Geissman, T. Sesquiterpene lactones of artemisia constituents of A. ludoviciana SSP. mexicana. Phytochemistry 1970, 9, 403–408. [Google Scholar] [CrossRef]
- Ruiz-Cancino, A.; Cano, A.E.; Delgado, G. Sesquiterpene lactones and flavonoids from Artemisia ludoviciana ssp. mexicana. Phytochemistry 1993, 33, 1113–1115. [Google Scholar] [CrossRef]
- Lopes-Lutz, D.; Alviano, D.S.; Alviano, C.S.; Kolodziejczyk, P.P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 2008, 69, 1732–1738. [Google Scholar] [CrossRef]
- Jimenez-Arellanes, A.; Meckes, M.; Ramírez, R.; Torres, J.; Luna-Herrera, J. Activity against multidrug-resistantMycobacterium tuberculosis in Mexican plants used to treat respiratory diseases. Phytotherapy Res. 2003, 17, 903–908. [Google Scholar] [CrossRef]
- Alanís, A.; Calzada, F.; Cervantes, J.; Torres, J.; Ceballos, G. Antibacterial properties of some plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. J. Ethnopharmacol. 2005, 100, 153–157. [Google Scholar] [CrossRef]
- Garcia, S.; Alarcón, G.; Rodríguez, C.; Heredia, N. Extracts of Acacia farnesiana and Artemisia ludoviciana inhibit growth, enterotoxin production and adhesion of Vibrio cholerae. World J. Microbiol. Biotechnol. 2006, 22, 669–674. [Google Scholar] [CrossRef]
- Castillo, S.L.; Heredia, N.; Contreras, J.F.; García, S. Extracts of Edible and Medicinal Plants in Inhibition of Growth, Adherence, and Cytotoxin Production of Campylobacter jejuni and Campylobacter coli. J. Food Sci. 2011, 76, M421–M426. [Google Scholar] [CrossRef]
- Calzada, F.; Yépez-Mulia, L.; Aguilar, A. In vitro susceptibility of Entamoeba histolytica and Giardia lamblia to plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. J. Ethnopharmacol. 2006, 108, 367–370. [Google Scholar] [CrossRef]
- Fernández, S.S.; Guerra, M.C.R.; Cárdenas, B.D.M.; Villarreal, J.V.; Treviño, L.V. In vitro antiprotozoal activity of the leaves of Artemisia ludoviciana. Fitoterapia 2005, 76, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Malagón, F.; Vázquez, J.; Delgado, G.; Ruiz, A. Antimalaric effect of an alcoholic extract of Artemisia ludoviciana mexicana in a rodent malaria model. Parassitologia 1997, 39, 3–7. [Google Scholar] [PubMed]
- Zavala-Sánchez, M.A.; Pérez-Gutiérrez, S.; Pérez-González, C.; Sánchez-Saldivar, D.; Arias-García, L. Antidiarrhoeal Activity of Nonanal, an Aldehyde Isolated from Artemisia ludoviciana. Pharm. Biol. 2002, 40, 263–268. [Google Scholar] [CrossRef]
- Estrada-Soto, S.; Sanchez-Recillas, A.; Navarrete-Vázquez, G.; Castillo-España, P.; Villalobos-Molina, R.; Ibarra-Barajas, M. Relaxant effects of Artemisia ludoviciana on isolated rat smooth muscle tissues. J. Ethnopharmacol. 2012, 139, 513–518. [Google Scholar] [CrossRef]
- Anaya-Eugenio, G.D.; Rivero-Cruz, I.; Bye, R.; Linares, E.; Mata, R. Antinociceptive activity of the essential oil from Artemisia ludoviciana. J. Ethnopharmacol. 2016, 179, 403–411. [Google Scholar] [CrossRef]
- Rivero-Cruz, I.; Anaya-Eugenio, G.D.; Pérez-Vásquez, A.; Martínez, A.L.; Mata, R. Quantitative Analysis and Pharmacological Effects of Artemisia ludoviciana Aqueous Extract and Compounds. Nat. Prod. Commun. 2017, 12, 1531–1534. [Google Scholar] [CrossRef]
- Anaya-Eugenio, G.D.; Rivero-Cruz, I.; Rivera-Chávez, J.; Mata, R. Hypoglycemic properties of some preparations and compounds from Artemisia ludoviciana Nutt. J. Ethnopharmacol. 2014, 155, 416–425. [Google Scholar] [CrossRef]
- Bucciarelli, A.; Skliar, M.I. Medicinal plants from Argentina with gastro protective activity. Ars Pharm. 2007, 48, 361–369. [Google Scholar]
- Adinortey, M.B.; Ansah, C.; Galyuon, I.K.; Nyarko, A. In Vivo Models Used for Evaluation of Potential Antigastroduodenal Ulcer Agents. Ulcers 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.-C. Medicinal plant activity onHelicobacter pylorirelated diseases. World J. Gastroenterol. 2014, 20, 10368–10382. [Google Scholar] [CrossRef] [PubMed]
- Eddouks, M.; Chattopadhyay, D.; Zeggwagh, N.A. Animal Models as Tools to Investigate Antidiabetic and Anti-Inflammatory Plants. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Bork, P.M.; Schmitz, M.; Kuhnt, M.; Escher, C.; Heinrich, M. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-κB. FEBS Lett. 1997, 402, 85–90. [Google Scholar] [CrossRef]
- Kaoudoune, C.; Benchikh, F.; Benabdallah, H.; Loucif, K.; Mehlous, S.; Amira, S. Gastroprotective effect and in vitro anti-oxidant activities of the aqueous extract from Artemisia absinthium L aerial parts. J. Drug Deliv. Ther. 2020, 10, 153–156. [Google Scholar] [CrossRef]
- Yeo, D.; Hwang, S.J.; Kim, W.J.; Youn, H.-J.; Lee, H.-J. The aqueous extract from Artemisia capillaris inhibits acute gastric mucosal injury by inhibition of ROS and NF-kB. Biomed. Pharmacother. 2018, 99, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Ferreira, M.L.; Maria-Ferreira, D.; Dallazen, J.L.; Silva, A.M.; Werner, M.F.D.P.; Petkowicz, C.L.D.O. Gastroprotective effects and structural characterization of a pectic fraction isolated from Artemisia campestris subsp maritima. Int. J. Biol. Macromol. 2018, 107, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Eidi, A.; Oryan, S.; Zaringhalam, J.; Rad, M. Antinociceptive and anti-inflammatory effects of the aerial parts ofArtemisia dracunculusin mice. Pharm. Biol. 2015, 54, 549–554. [Google Scholar] [CrossRef]
- Kwon, O.S.; Choi, J.S.; Islam, N.; Kim, Y.S.; Kim, H.P. Inhibition of 5-lipoxygenase and skin inflammation by the aerial parts of Artemisia capillaris and its constituents. Arch. Pharmacal Res. 2011, 34, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Oh, T.Y.; Kim, Y.S.; Sim, H.; Jang, E.J.; Park, J.S.; Baik, H.W.; Hahm, K.B. Artemisia asiatica extracts protect against ethanol-induced injury in gastric mucosa of rats. J. Gastroenterol. Hepatol. 2008, 23, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.; Maibach, H. Interindividual variation in transdermal and oral drug deliveries. J. Pharm. Sci. 2012, 101, 4293–4307. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Mabry, T. Flavonoids from Artemisia ludoviciana var. ludoviciana. Phytochemistry 1982, 21, 209–214. [Google Scholar] [CrossRef]
- Nageen, B.; Sarfraz, I.; Rasul, A.; Hussain, G.; Rukhsar, F.; Irshad, S.; Riaz, A.; Selamoglu, Z.; Ali, M. Eupatilin: A natural pharmacologically active flavone compound with its wide range applications. J. Asian Nat. Prod. Res. 2018, 22, 1–16. [Google Scholar] [CrossRef]
- Zater, H.; Huet, J.; Fontaine, V.; Benayache, S.; Stévigny, C.; Duez, P.; Benayache, F. Chemical constituents, cytotoxic, antifungal and antimicrobial properties of Centaurea diluta Ait. subsp. algeriensis (Coss. & Dur.) Maire. Asian Pac. J. Trop. Med. 2016, 9, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-Y.; Jeong, S.-J.; La Lee, H.; Park, K.-H.; Hwang, D.Y.; Park, S.-Y.; Lee, Y.G.; Moon, J.-H.; Ham, K.-S. Sesquiterpene lactones and scopoletins from Artemisia scoparia Waldst. & Kit. and their angiotensin I-converting enzyme inhibitory activities. Food Sci. Biotechnol. 2016, 25, 1701–1708. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Mitchell, P.T.; Kishkentaeva, А.S.; Shaimerdenova, Z.R.; Atazhanova, G.A.; Adekenov, S.M.; Quinn, M.T. The natural sesquiterpene lactones arglabin, grosheimin, agracin, parthenolide, and estafiatin inhibit T cell receptor (TCR) activation. Phytochemistry 2018, 146, 36–46. [Google Scholar] [CrossRef]
- Vega, A.; Wendel, G.; Maria, A.; Pelzer, L. Antimicrobial activity of Artemisia douglasiana and dehydroleucodine against Helicobacter pylori. J. Ethnopharmacol. 2009, 124, 653–655. [Google Scholar] [CrossRef]
- Goswami, S.; Bhakuni, R.S.; Chinniah, A.; Pal, A.; Kar, S.K.; Das, P.K. Anti-Helicobacter pylori Potential of Artemisinin and Its Derivatives. Antimicrob. Agents Chemother. 2012, 56, 4594–4607. [Google Scholar] [CrossRef]
- Missouri Botanical Garden, Tropicos: St. Louis, MO, USA. 2010. Available online: http://www.tropicos.org (accessed on 23 March 2021).
- Kolak, U.; Hacibekiroglu, I.; Öztürk, M.; Özagökce, F.; Topcu, G.; Ulubelen, A. Antioxidant and anticholinesterase con-stituents of Salvia poculata. Turk. J. Chem. 2009, 33, 813–823. [Google Scholar] [CrossRef]
- CLSI. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria, 3rd ed.; CLSI guideline M45; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Okoli, C.; Akah, P.; Nwafor, S.; Anisiobi, A.; Ibegbunam, I.; Erojikwe, O. Anti-inflammatory activity of hexane leaf extract of Aspilia africana C.D. Adams. J. Ethnopharmacol. 2007, 109, 219–225. [Google Scholar] [CrossRef]


| Activities | |||||
|---|---|---|---|---|---|
| Extract and Fractions | Yield (%) | Anti-H. pylori | Gastroprotective % | Anti-Inflammatory % | |
| MIC d (μg/mL) | Oral | Topical | Oral | ||
| 100 mg/kg | 0.5 mg/ear | 100 mg/kg | |||
| Aqueous (ALI) | 250 | 49.6 ± 7.5 | 38.8 ± 10.2 | 47.6 ± 12.4 | |
| Dichloromethane (ALI-1) | 7.9 | 62.5 | 61.0 ± 5.2 | 23.4 ± 6.6 | 20.7 ± 4.1 |
| Ethyl acetate (ALI-2) | 8.5 | 125 | 86.3 ± 3.9 | 29.4 ± 10.8 | 47.2 ± 5.3 |
| n-butanol (ALI-3) | 21.4 | >250 | 61.8 ± 11.4 | 31.8 ± 12.8 | 51.3 ± 6.7 |
| Residual (ALI-4) | 62.1 | >250 | 66.9 ± 10.6 | 0 ± 6.6 | 36.5 ± 6.6 |
| Metronidazole a | 300 | ||||
| Clarithromycin a | 0.01 | ||||
| Carbenoxolone b (50 mg/kg) | 78.2 ± 3.5 | ||||
| Indomethacin c (topical 0.5 mg/ear, oral 30 mg/kg) | 61.3 ± 8.1 | 54.8 ± 4.7 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios-Espinosa, J.F.; Núñez-Aragón, P.N.; Gomez-Chang, E.; Linares, E.; Bye, R.; Romero, I. Anti-Helicobacter pylori Activity of Artemisia ludoviciana subsp. mexicana and Two of Its Bioactive Components, Estafiatin and Eupatilin. Molecules 2021, 26, 3654. https://doi.org/10.3390/molecules26123654
Palacios-Espinosa JF, Núñez-Aragón PN, Gomez-Chang E, Linares E, Bye R, Romero I. Anti-Helicobacter pylori Activity of Artemisia ludoviciana subsp. mexicana and Two of Its Bioactive Components, Estafiatin and Eupatilin. Molecules. 2021; 26(12):3654. https://doi.org/10.3390/molecules26123654
Chicago/Turabian StylePalacios-Espinosa, Juan Francisco, Pablo Noé Núñez-Aragón, Erika Gomez-Chang, Edelmira Linares, Robert Bye, and Irma Romero. 2021. "Anti-Helicobacter pylori Activity of Artemisia ludoviciana subsp. mexicana and Two of Its Bioactive Components, Estafiatin and Eupatilin" Molecules 26, no. 12: 3654. https://doi.org/10.3390/molecules26123654
APA StylePalacios-Espinosa, J. F., Núñez-Aragón, P. N., Gomez-Chang, E., Linares, E., Bye, R., & Romero, I. (2021). Anti-Helicobacter pylori Activity of Artemisia ludoviciana subsp. mexicana and Two of Its Bioactive Components, Estafiatin and Eupatilin. Molecules, 26(12), 3654. https://doi.org/10.3390/molecules26123654