Relationship between Volatile Composition and Bioactive Potential of Vegetables and Fruits of Regular Consumption—An Integrative Approach
Abstract
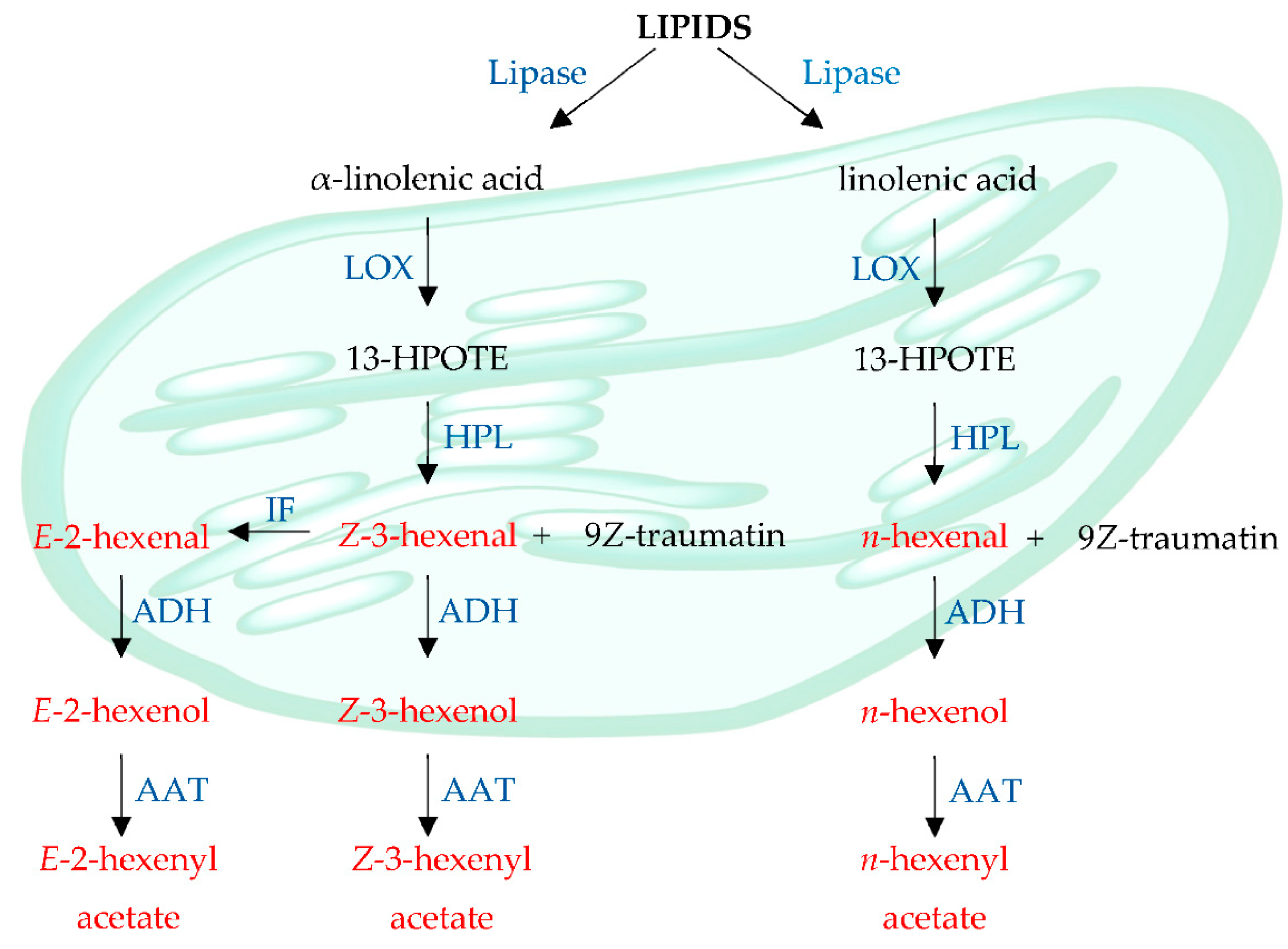
1. Introduction
2. Results and Discussion
2.1. Volatile Composition of Investigated Fruits and Vegetables
2.1.1. Beetroot
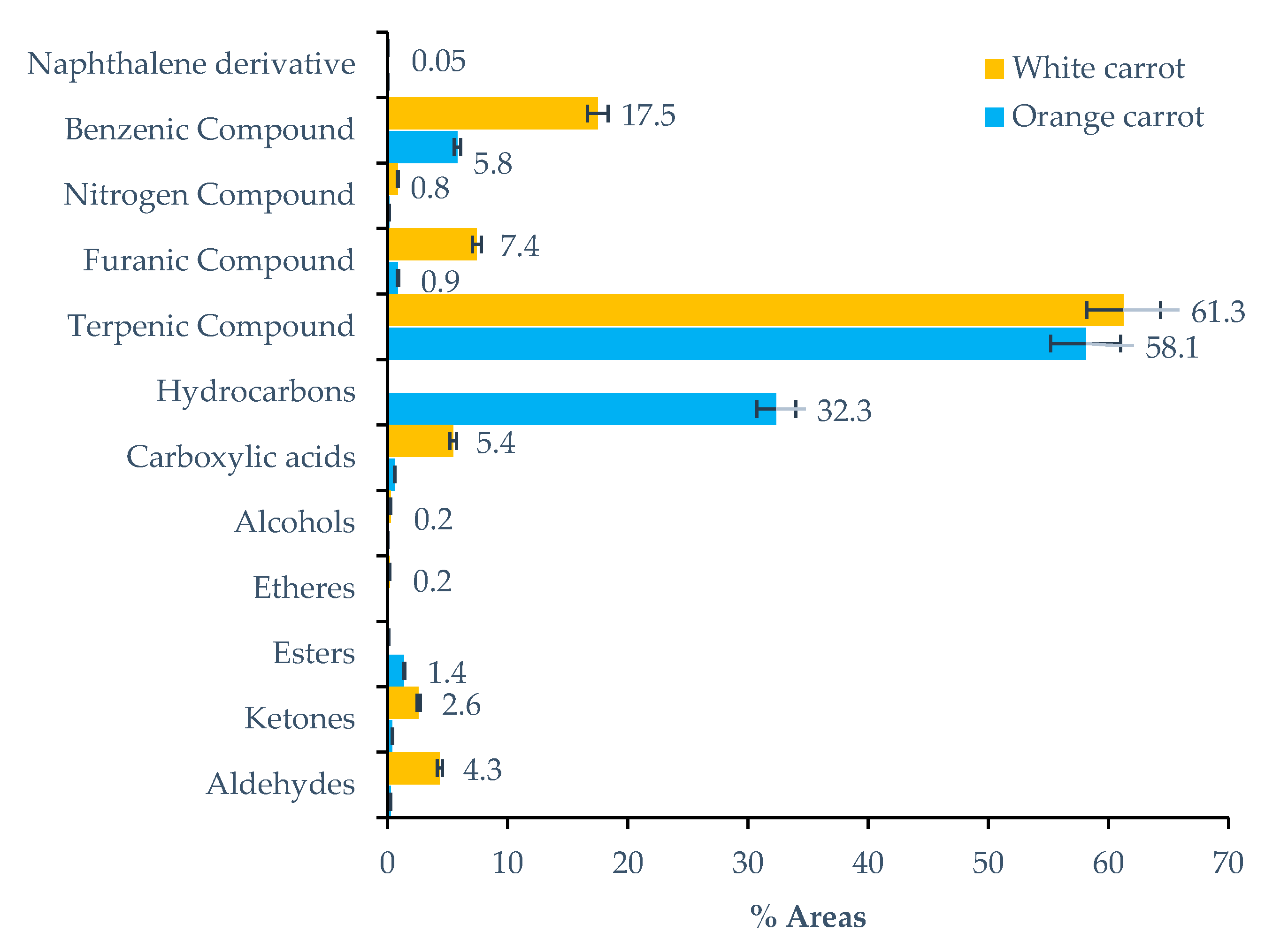
2.1.2. Carrot
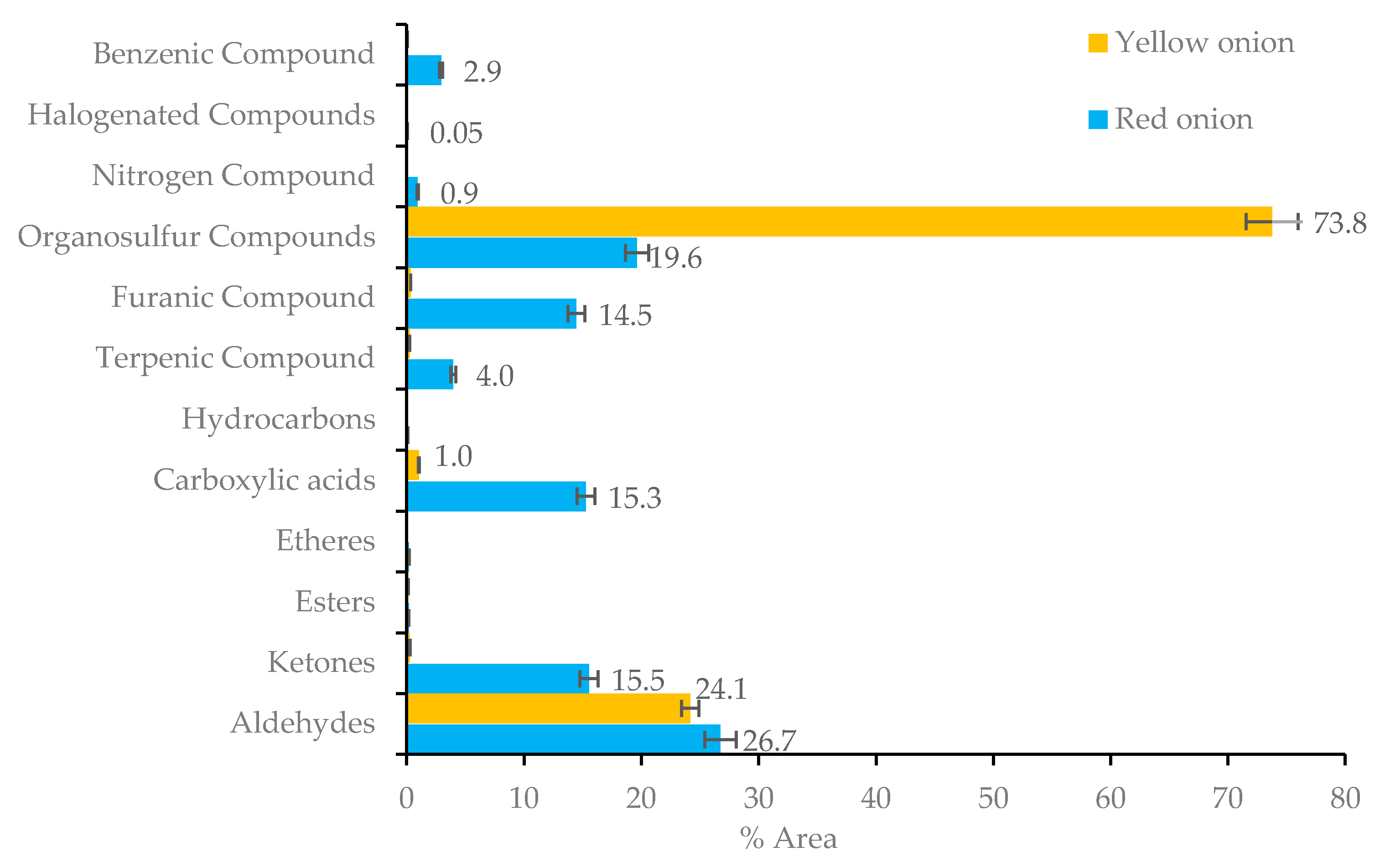
2.1.3. Onion
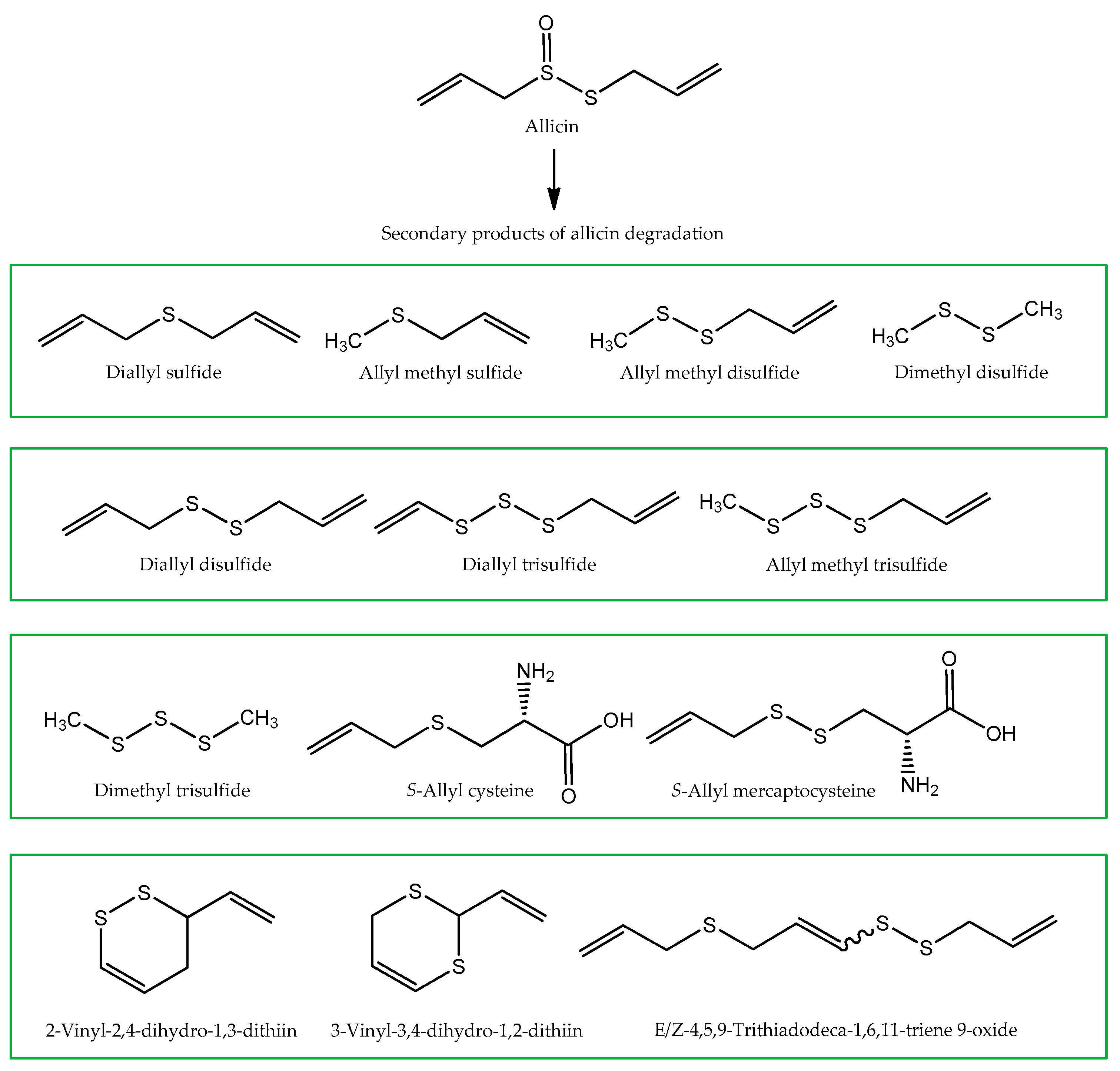
2.1.4. Garlic
2.1.5. Broccoli
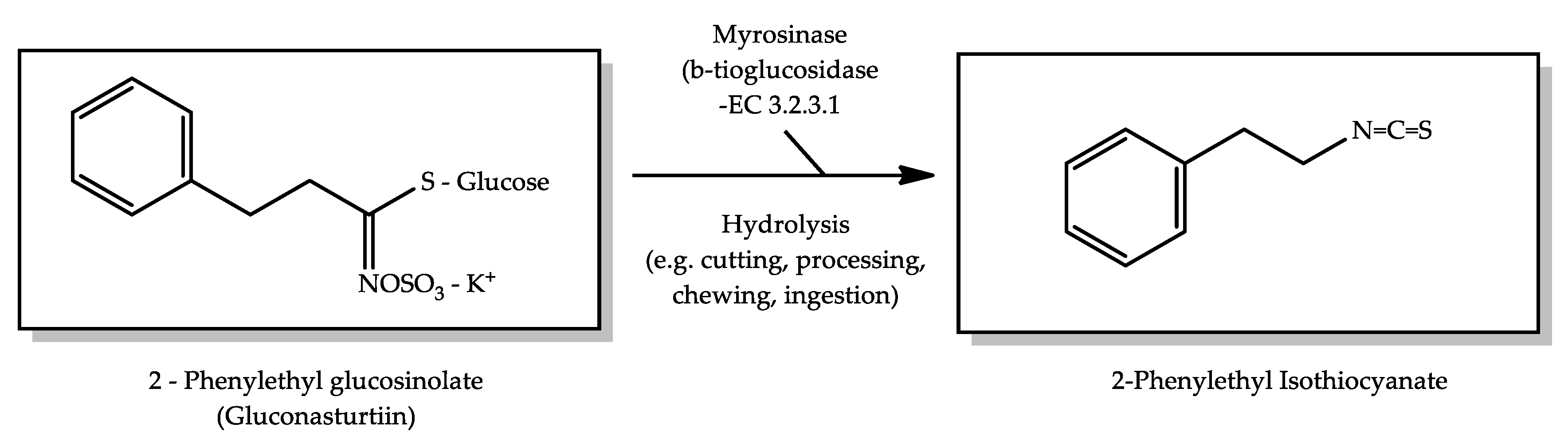
2.1.6. Watercress
2.1.7. Spinach
2.1.8. Tomato
2.1.9. Tamarillo
2.2. Potential Bioactive Properties of Volatile Metabolites Present in Studied Vegetables and Fruits
3. Materials and Methods
3.1. Reagents and Materials
3.2. Samples
3.3. HS-SPME Procedure
3.4. GC-MS Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mariutti, L.R.B.; Rodrigues, E.; Chisté, R.C.; Fernandes, E.; Mercadante, A.Z. The Amazonian fruit Byrsonima crassifolia effectively scavenges reactive oxygen and nitrogen species and protects human erythrocytes against oxidative damage. Food Res. Int. 2014, 64, 618–625. [Google Scholar] [CrossRef]
- Picinelli Lobo, A.; García, Y.D.; Sánchez, J.M.; Madrera, R.R.; Valles, B.S. Phenolic and antioxidant composition of cider. J. Food Compos. Anal. 2009, 22, 644–648. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Negi, P.S.; Jena, B.S.; Mohan Rao, L.J. Antioxidant and antimutagenic activities of Cinnamomum zeylanicum fruit extracts. J. Food Compos. Anal. 2007, 20, 330–336. [Google Scholar] [CrossRef]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Plant. Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Crozier, A., Clifford, M., Ashihara, H., Eds.; Blackwell: Oxford, UK, 2006; pp. 1–24. [Google Scholar]
- Demirci, F. Natural Products Isolation; American Chemical Society (ACS): Totowa, NJ, USA, 2007; Volume 70. [Google Scholar]
- Vivaldo, G.; Masi, E.; Taiti, C.; Caldarelli, G.; Mancuso, S. The network of plants volatile organic compounds. Sci. Rep. 2017, 7, 1–18. [Google Scholar] [CrossRef]
- Herrmann, A. The Chemistry and Biology of Volatiles; Herrmann, A., Ed.; Wiley: Chichester, UK, 2010. [Google Scholar]
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F. The role of volatiles in plant communication. Plant J. 2019, 100, 892–907. [Google Scholar] [CrossRef]
- Smith, E. Plant secondary metabolites: Occurrence, structure and role in the human diet. Phyther. Res. 2007, 21, 904. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger Ian Max Møller, E.; Murphy, A. Fisiologia e Desenvolvimento Vegetal—6a Edição; Artmed Editora: São Paulo, Brasil, 2017. [Google Scholar]
- Hamzalioğlu, A.; Gökmen, V. Interaction between Bioactive Carbonyl Compounds and Asparagine and Impact on Acrylamide. In Acrylamide in Food: Analysis, Content and Potential Health Effects; Gökmen, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 355–376. [Google Scholar]
- Aguiar, J.; Gonçalves, J.L.; Alves, V.L.; Câmara, J.S. Chemical fingerprint of free polyphenols and antioxidant activity in dietary fruits and vegetables using a non-targeted approach based on QuEChERS ultrasound-assisted extraction combined with UHPLC-PDA. Antioxidants 2020, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Taglienti, A.; Tiberini, A.; Ciampa, A.; Piscopo, A.; Zappia, A.; Tomassoli, L.; Poiana, M.; Dell’Abate, M.T. Metabolites response to onion yellow dwarf virus (OYDV) infection in ‘Rossa di Tropea’ onion during storage: A 1H HR-MAS NMR study. J. Sci. Food Agric. 2020, 100, 3418–3427. [Google Scholar] [CrossRef]
- Sharma, K.; Assefa, A.D.; Ko, E.Y.; Lee, E.T.; Park, S.W. Quantitative analysis of flavonoids, sugars, phenylalanine and tryptophan in onion scales during storage under ambient conditions. J. Food Sci. Technol. 2015, 52, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Rao, V. Phytochemicals: A Global Perspective of Their Role in Nutrition and Health; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar]
- De Cássia Da Silveira, E.; Sá, R.; Andrade, L.N.; Pergentino De Sousa, D. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Sagdic, O.; Tornuk, F. Antimicrobial properties of organosulfur compounds. In Dietary Phytochemicals and Microbes; Springer: Dordrecht, The Netherlands, 2012; pp. 127–156. ISBN 9789400739260. [Google Scholar]
- Tamemoto, K.; Takaishi, Y.; Chen, B.; Kawazoe, K.; Shibata, H.; Higuti, T.; Honda, G.; Ito, M.; Takeda, Y.; Kodzhimatov, O.K.; et al. Sesquiterpenoids from the fruits of Ferula kuhistanica and antibacterial activity of the constituents of F. kuhistanica. Phytochemistry 2001, 58, 763–767. [Google Scholar] [CrossRef]
- Gould, M.N. Cancer chemoprevention and therapy by monoterpenes. Environ. Health Perspect. 1997, 105, 977–979. [Google Scholar]
- Huang, M.; Lu, J.J.; Huang, M.Q.; Bao, J.L.; Chen, X.P.; Wang, Y.T. Terpenoids: Natural products for cancer therapy. Expert Opin. Investig. Drugs 2012, 21, 1801–1818. [Google Scholar] [CrossRef]
- Omar, S.H.; Al-Wabel, N.A. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm. J. 2010, 18, 51–58. [Google Scholar] [CrossRef]
- Perestrelo, R.; Barros, A.S.; Rocha, S.M.; Câmara, J.S. Optimisation of solid-phase microextraction combined with gas chromatography–mass spectrometry based methodology to establish the global volatile signature in pulp and skin of Vitis vinifera L. grape varieties. Talanta 2011, 85, 1483–1493. [Google Scholar] [CrossRef]
- Gouda, M.; Ma, M.; Sheng, L.; Xiang, X. SPME-GC-MS & metal oxide E-Nose 18 sensors to validate the possible interactions between bio-active terpenes and egg yolk volatiles. Food Res. Int. 2019, 125, 108611. [Google Scholar]
- Figueira, J.; Câmara, H.; Pereira, J.; Câmara, J.S. Evaluation of volatile metabolites as markers in Lycopersicon esculentum L. cultivars discrimination by multivariate analysis of headspace solid phase microextraction and mass spectrometry data. Food Chem. 2014, 145, 653–663. [Google Scholar] [CrossRef]
- Guijarro-Real, C.; Rodríguez-Burruezo, A.; Prohens, J.; Raigón, M.D.; Fita, A. HS-SPME analysis of the volatiles profile of water celery (Apium nodiflorum), a wild vegetable with increasing culinary interest. Food Res. Int. 2019, 121, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Liang, L.; Wang, Y. Volatile composition changes in navel orange at different growth stages by HS-SPME–GC–MS. Food Res. Int. 2020, 136, 109333. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Ruiz, C.; Calderon, R.; Marcelo, M.; Rojas, R. Characterisation of volatile profiles in 50 native Peruvian chili pepper using solid phase microextraction–gas chromatography mass spectrometry (SPME–GCMS). Food Res. Int. 2016, 89, 471–475. [Google Scholar] [CrossRef]
- Medina, S.; Perestrelo, R.; Santos, R.; Pereira, R.; Câmara, J.S. Differential volatile organic compounds signatures of apple juices from Madeira Island according to variety and geographical origin. Microchem. J. 2019, 150, 104094. [Google Scholar] [CrossRef]
- Stoewsand, G.S. Bioactive organosulfur phytochemicals in Brassica oleracea vegetables-a review. Food Chem. Toxicol. 1995, 33, 537–543. [Google Scholar] [CrossRef]
- Ayseli, M.T.; Ipek Ayseli, Y. Flavors of the future: Health benefits of flavor precursors and volatile compounds in plant foods. Trends Food Sci. Technol. 2016, 48, 69–77. [Google Scholar] [CrossRef]
- Lu, G.; Fellman, J.K.; Edwards, C.G.; Mattinson, D.S.; Navazio, J. Quantitative determination of geosmin in red beets (Beta vulgaris L.) using headspace solid-phase microextraction. J. Agric. Food Chem. 2003, 51, 1021–1025. [Google Scholar] [CrossRef]
- Koubaier, H.B.H.; Snoussi, A.; Essaidi, I.; Chaabouni, M.M.; Thonart, P.; Bouzouita, N. Betalain and phenolic compositions, antioxidant activity of Tunisian Red Beet (Beta vulgaris L. conditiva) roots and stems extracts. Int. J. Food Prop. 2014, 17, 1934–1945. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability 1,2. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Graßmann, J. Terpenoids as plant antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar] [PubMed]
- Güler, Z.; Karaca, F.; Yetisir, H. Identification of volatile organic compounds (VOCs) in different colour carrot (Daucus carota L.) cultivars using static headspace/gas chromatography/mass spectrometry. Cogent Food Agric. 2015, 1, 1117275. [Google Scholar] [CrossRef]
- Cecchi, L.; Ieri, F.; Vignolini, P.; Mulinacci, N.; Romani, A. Characterization of volatile and flavonoid composition of different cuts of dried onion (Allium cepa L.) by HS-SPME-GC-MS, HS-SPME-GC×GC-TOF and HPLC-DAD. Molecules 2020, 25, 408. [Google Scholar] [CrossRef] [PubMed]
- Järvenpää, E.P.; Zhang, Z.; Huopalahti, R.; King, J.W. Determination of fresh onion (Allium cepa L.) volatiles by solid phase microextraction combined with gas chromatography-mass spectrometry. Eur. Food Res. Technol. 1998, 207, 39–43. [Google Scholar] [CrossRef]
- Islam, M.S.; Choi, H.; Loots, D.T. Effects of dietary onion (Allium cepa L.) in a high-fat diet streptozotocin-induced diabetes rodent model. Ann. Nutr. Metab. 2008, 53, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Colina-Coca, C.; González-Peña, D.; Vega, E.; De Ancos, B.; Sánchez-Moreno, C. Novel approach for the determination of volatile compounds in processed onion by headspace gas chromatography-mass spectrometry (HS GC-MS). Talanta 2013, 103, 137–144. [Google Scholar] [CrossRef]
- Vazquez-Armenta, F.J.; Ayala-Zavala, J.F.; Olivas, G.I.; Molina-Corral, F.J.; Silva-Espinoza, B.A. Antibrowning and antimicrobial effects of onion essential oil to preserve the quality of cut potatoes. Acta Aliment. 2014, 43, 640–649. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, D.J.; Kim, J.Y.; Lim, S.T. Volatile composition and sensory characteristics of onion powders prepared by convective drying. Food Chem. 2017, 231, 386–392. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef]
- Martins, N.; Petropoulos, S.; Ferreira, I.C.F.R. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: A review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Miron, T.; Shin, I.; Feigenblat, G.; Weiner, L.; Mirelman, D.; Wilchek, M.; Rabinkov, A. A spectrophotometric assay for allicin, alliin, and alliinase (alliin lyase) with a chromogenic thiol: Reaction of 4-mercaptopyridine with thiosulfinates. Anal. Biochem. 2002, 307, 76–83. [Google Scholar] [CrossRef]
- Dasgupta, A. Antiinflammatory herbal supplements. In Translational Inflammation; Elsevier: Houston, TX, USA, 2018; pp. 69–91. ISBN 9780128138335. [Google Scholar]
- Schäfer, G.; Kaschula, C. The immunomodulation and anti-inflammatory effects of garlic organosulfur compounds in cancer chemoprevention. Anticancer Agents Med. Chem. 2014, 14, 233–240. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive compounds and biological functions of garlic (allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Yun, H.M.; Ban, J.O.; Park, K.R.; Lee, C.K.; Jeong, H.S.; Han, S.B.; Hong, J.T. Potential therapeutic effects of functionally active compounds isolated from garlic. Pharmacol. Ther. 2014, 2, 183–195. [Google Scholar] [CrossRef]
- Seki, T.; Hosono, T.; Hosono-Fukao, T.; Inada, K.; Tanaka, R.; Ogihara, J.; Ariga, T. Anticancer effects of diallyl trisulfide derived from garlic. Asia Pac. J. Clin. Nutr. 2008, 17, 249–252. [Google Scholar]
- Nagini, S. Cancer chemoprevention by garlic and its organosulfur compounds-panacea or promise? Anticancer Agents Med. Chem. 2008, 8, 313–321. [Google Scholar] [CrossRef]
- De Pinho, P.G.; Valentão, P.; Gonçalves, R.F.; Sousa, C.; Andrade, P.B. Volatile composition of Brassica oleracea L. var. costata DC leaves using solid-phase microextraction and gas chromatography/ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 2292–2300. [Google Scholar] [CrossRef]
- Berger, R.G. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin, Germany, 2007. [Google Scholar]
- Kebede, B.T.; Grauwet, T.; Tabilo-Munizaga, G.; Palmers, S.; Vervoort, L.; Hendrickx, M.; Van Loey, A. Headspace components that discriminate between thermal and high pressure high temperature treated green vegetables: Identification and linkage to possible process-induced chemical changes. Food Chem. 2013, 141, 1603–1613. [Google Scholar] [CrossRef] [PubMed]
- Conaway, C.C.; Getahun, S.M.; Liebes, L.L.; Pusateri, D.J.; Topham, D.K.; Botero-Omary, M.; Chung, F.L. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr. Cancer 2000, 38, 168–178. [Google Scholar] [CrossRef]
- Freitas, E.; Aires, A.; Rosa, E.A.d.S.; Saavedra, M.J. Antibacterial activity and synergistic effect between watercress extracts, 2-phenylethyl isothiocyanate and antibiotics against 11 isolates of Escherichia coli from clinical and animal source. Lett. Appl. Microbiol. 2013, 57, 266–273. [Google Scholar] [CrossRef]
- Gupta, P.; Wright, S.E.; Kim, S.H.; Srivastava, S.K. Phenethyl isothiocyanate: A comprehensive review of anti-cancer mechanisms. Biochim. Biophys. Acta 2014, 1846, 405–424. [Google Scholar] [CrossRef]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green Leaf Volatiles: A plant’s multifunctional weapon against herbivores and pathogens. OPEN ACCESS Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef]
- Vincenti, S.; Mariani, M.; Alberti, J.C.; Jacopini, S.; de Caraffa, V.B.B.; Berti, L.; Maury, J. Biocatalytic synthesis of natural green leaf volatiles using the lipoxygenase metabolic pathway. Catalysts 2019, 9, 873. [Google Scholar] [CrossRef]
- Deng, W.; Hamilton-Kemp, T.R.; Nielsen, M.T.; Andersen, R.A.; Collins, G.B.; Hildebrand, D.F. Effects of six-carbon aldehydes and alcohols on bacterial proliferation. J. Agric. Food Chem. 1993, 41, 506–510. [Google Scholar] [CrossRef]
- Tandon, K.S.; Baldwin, E.A.; Shewfelt, R.L. Aroma perception of individual volatile compounds in fresh tomatoes (Lycopersicon esculentum, Mill.) as affected by the medium of evaluation. Postharvest Biol. Technol. 2000, 20, 261–268. [Google Scholar] [CrossRef]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances useful in human healthcare. Arch. Immunol. Ther. Exp. (Warsz). 2007, 55, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and onions: Their cancer prevention properties. Cancer Prev. Res. 2015, 8, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.S. Therapeutic potential of volatile terpenes and terpenoids from forests for inflammatory diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef]
- Nazaruk, J.; Borzym-Kluczyk, M. The role of triterpenes in the management of diabetes mellitus and its complications. Phytochem. Rev. 2015, 14, 675–690. [Google Scholar] [CrossRef]
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2010, 62, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Brahmkshatriya, P.P.; Brahmkshatriya, P.S. Terpenes: Chemistry, biological role, and therapeutic applications. In Natural Products; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2665–2691. [Google Scholar]
- Suhail, M.M.; Wu, W.; Cao, A.; Mondalek, F.G.; Fung, K.M.; Shih, P.T.; Fang, Y.T.; Woolley, C.; Young, G.; Lin, H.K. Boswellia sacra essential oil induces tumor cell-specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells. BMC Complement. Altern. Med. 2011, 11, 129. [Google Scholar] [CrossRef]
- Ciftci, O.; Ozdemir, I.; Tanyildizi, S.; Yildiz, S.; Oguzturk, H. Antioxidative effects of curcumin, β-myrcene and 1,8-cineole against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced oxidative stress in rats liver. Toxicol. Ind. Health 2011, 27, 447–453. [Google Scholar] [CrossRef]
- Singh, B.K.; Tripathi, M.; Chaudhari, B.P.; Pandey, P.K.; Kakkar, P. Natural terpenes prevent mitochondrial dysfunction, oxidative stress and release of apoptotic proteins during nimesulide-hepatotoxicity in rats. PLoS ONE 2012, 7, e34200. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.-H.; Elmadfa, I. Biological relevance of terpenoids. Ann. Nutr. Metab. 2003, 47, 95–106. [Google Scholar] [CrossRef]
- Morales-Serna, F.N.; Caña-Bozada, V.H.; López-Moreno, D.G.; Medina-Guerrero, R.M.; Morales-Serna, J.A.; Fajer-Ávila, E.J. In vitro efficacy of two terpenes against ancyrocephalid monogeneans from Nile tilapia. J. Parasit. Dis. 2019, 43, 739–742. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Grando, T.H.; Souza, C.F.; Gressler, L.T.; Stefani, L.M.; da Silva, A.S.; Monteiro, S.G. In vitro and in vivo action of terpinen-4-ol, γ-terpinene, and α-terpinene against Trypanosoma evansi. Exp. Parasitol. 2016, 162, 43–48. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Cho, K.S.; Lim, Y.R.; Lee, K.; Lee, J.; Lee, J.H.; Lee, I.S. Terpenes from forests and human health. Toxicol. Res. 2017, 33, 97–106. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Nigam, P.S.; Ashraf, M.; Gilani, A.H. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four mentha species. J. Sci. Food Agric. 2010, 90, 1827–1836. [Google Scholar] [CrossRef]
- Formighieri, C.; Melis, A. Regulation of β-phellandrene synthase gene expression, recombinant protein accumulation, and monoterpene hydrocarbons production in Synechocystis transformants. Planta 2014, 240, 309–324. [Google Scholar] [CrossRef]
- Mander, L.; Liu, H.W. Comprehensive Natural Products II: Chemistry and Biology, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2010; Volume 1. [Google Scholar]
- Rathore, S.S.; Saxena, S.N.; Singh, B. Potential health benefits of major seed spices. Int. J. Seed Spices 2013, 3, 1–12. [Google Scholar]
- Miller, J.A.; Lang, J.E.; Ley, M.; Nagle, R.; Hsu, C.H.; Thompson, P.A.; Cordova, C.; Waer, A.; Chow, H.H.S. Human breast tissue disposition and bioactivity of limonene in women with early-stage breast cancer. Cancer Prev. Res. 2013, 6, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.M.; Lin, J.Y. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013, 141, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, M.T.; de Conti, A.; Ong, T.P.; Scolastici, C.; Purgatto, E.; Horst, M.A.; Bassoli, B.K.; Moreno, F.S. Chemopreventive effects of β-ionone and geraniol during rat hepatocarcinogenesis promotion: Distinct actions on cell proliferation, apoptosis, HMGCoA reductase, and RhoA. J. Nutr. Biochem. 2011, 22, 130–135. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α-and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Vespermann, K.A.C.; Paulino, B.N.; Barcelos, M.C.S.; Pessôa, M.G.; Pastore, G.M.; Molina, G. Biotransformation of α- and β-pinene into flavor compounds. Appl. Microbiol. Biotechnol. 2017, 101, 1805–1817. [Google Scholar] [CrossRef]
- Kang, G.-Q.; Duan, W.-G.; Lin, G.-S.; Yu, Y.-P.; Wang, X.-Y.; Lu, S.-Z. Synthesis of bioactive compounds from 3-carene (II): Synthesis, antifungal activity and 3D-QSAR study of (Z)- and (E)-3-caren-5-one oxime sulfonates. Molecules 2019, 24, 477. [Google Scholar] [CrossRef]
- Wu, Y.-R.; Li, L.; Sun, X.-C.; Wang, J.; Ma, C.-Y.; Zhang, Y.; Qu, H.-L.; Xu, R.-X.; Li, J.-J. Diallyl disulfide improves lipid metabolism by inhibiting PCSK9 expression and increasing LDL uptake via PI3K/Akt-SREBP2 pathway in HepG2 cells. Nutr. Metab. Cardiovasc. Dis. 2020, 31, 322–332. [Google Scholar] [CrossRef]
- Mathan Kumar, M.; Tamizhselvi, R. Protective effect of diallyl disulfide against cerulein-induced acute pancreatitis and associated lung injury in mice. Int. Immunopharmacol. 2020, 80, 106136. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; El-Sherbiny, M.; Zaitone, S.A. Diallyl trisulfide potentiates chemotherapeutic efficacy of doxorubicin in experimentally induced mammary carcinoma: Role of Notch signaling. Pathol. Res. Pract. 2020, 216, 153139. [Google Scholar] [CrossRef]
- Liu, C.T.; Hse, H.; Lii, C.K.; Chen, P.S.; Sheen, L.Y. Effects of garlic oil and diallyl trisulfide on glycemic control in diabetic rats. Eur. J. Pharmacol. 2005, 516, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Jos, A.; Llana Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Bermúdez, J.M.; Aucejo, S.; Cameán, A.M. Cytotoxicity study in the human cell line Caco-2 of dipropyl sulfide and dipropyl disulfide from garlic essential oil. Toxicol. Lett. 2013, 221, S118. [Google Scholar] [CrossRef]
- Kormányos, E.; Für, G.; Totunji, A.; Balla, Z.; Bálint, E.R.; Hegyi, P.; Rakonczay, Z.; Kiss, L. Dimethyl-trisulfide: New approach to ameliorate experimental acute pancreatitis. Pancreatology 2019, 19, S103. [Google Scholar] [CrossRef]
- Barillari, J.; Gueyrard, D.; Rollin, P.; Iori, R. Barbarea verna as a source of 2-phenylethyl glucosinolate, precursor of cancer chemopreventive phenylethyl isothiocyanate. Fitoterapia 2001, 72, 760–764. [Google Scholar] [CrossRef]
- Ansari, I.A.; Akhtar, M.S. Current insights on the role of terpenoids as anticancer agents: A perspective on cancer prevention and treatment. In Natural Bio-Active Compounds: Chemistry, Pharmacology and Health Care Practices; Springer: Singapore, 2019; pp. 53–80. ISBN 9789811372056. [Google Scholar]
- Putnik, P.; Gabrić, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Lorenzo, J.M.; Kovacević, D.B. Bioavailability and food production of organosulfur compounds from edible allium species. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Elsevier: Cambridge, UK, 2019; pp. 293–308. ISBN 9780128141748. [Google Scholar]
- Rai, S.K.; Sharma, M.; Tiwari, M. Inhibitory effect of novel diallyldisulfide analogs on HMG-CoA reductase expression in hypercholesterolemic rats: CREB as a potential upstream target. Life Sci. 2009, 85, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Sela, U.; Ganor, S.; Hecht, I.; Brill, A.; Miron, T.; Rabinkov, A.; Wilchek, M.; Mirelman, D.; Lider, O.; Hershkoviz, R. Allicin inhibits SDF-1α-induced T cell interactions with fibronectin and endothelial cells by down-regulating cytoskeleton rearrangement, Pyk-2 phosphorylation and VLA-4 expression. Immunology 2004, 111, 391–399. [Google Scholar] [CrossRef]
- Augusti, K.T. Studies on the effect of allicin (diallyl disulphide-oxide) on alloxan diabetes. Experientia 1975, 31, 1263–1265. [Google Scholar] [CrossRef]
- Lee, D.Y.; Li, H.; Lim, H.J.; Lee, H.J.; Jeon, R.; Ryu, J.H. Anti-inflammatory activity of sulfur-containing compounds from garlic. J. Med. Food 2012, 15, 992–999. [Google Scholar] [CrossRef]
- Mikaili, P.; Maadirad, S.; Moloudizargari, M.; Aghajanshakeri, S.; Sarahroodi, S. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iran. J. Basic Med. Sci. 2013, 16, 1031–1048. [Google Scholar] [PubMed]
- Oosthuizen, C.B.; Reid, A.M.; Lall, N. Garlic (allium sativum) and its associated molecules, as medicine. In Medicinal Plants for Holistic Health and Well-Being; Elsevier: London, UK, 2017; pp. 277–295. ISBN 9780128124758. [Google Scholar]
- Druesne, N.; Pagniez, A.; Mayeur, C.; Thomas, M.; Cherbuy, C.; Duée, P.H.; Martel, P.; Chaumontet, C. Diallyl disulfide (DADS) increases histone acetylation and p21waf1/cip1 expression in human colon tumor cell lines. Carcinogenesis 2004, 25, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
| Volatile Metabolites | Biological Effects | Sample | Reference |
|---|---|---|---|
| β-Myrcene | Analgesic, anti-inflammatory, antibiotic, anticancer, antioxidant | Beetroot, orange carrot, spinach, | [73,74] |
| Camphene | Antimicrobial, antioxidant | Beetroot, red onion, orange and white carrot, spinach | [75,76] |
| α-Terpinene | Antimicrobial | Beetroot, orange and white carrot, tomato, tamarillo | [77,78] |
| Terpinolene | Antioxidant | Beetroot, red onion, orange and white carrot | [79] |
| γ-terpinene | Anti-inflammatory, antioxidant | Beetroot, red and yellow onion, orange and white carrot, tomato, tamarillo | [39,80] |
| β-Phellandrene | Antibacterial, anticancer | Beetroot, orange and white carrot | [81,82] |
| β-Pinene | Antitumor, anti-inflammatory, antimicrobial, antioxidant, antineoplastic, chemoprotective | Beetroot, orange and white carrot, spinach, tamarillo | [80,83,84] |
| D-Limonene | Antimutagenic, antitumor, antioxidant, antimicrobial, antiproliferative, chemoprotective | Beetroot, orange and white carrot, yellow onion, broccoli, spinach, tomato, tamarillo | [80,84,85] |
| Eucalyptol | Anti-inflammatory | Broccoli, tomato | [86] |
| Geraniol | Chemopreventive activity, antimutagenic, anti-inflammatory | Tomato | [86,87] |
| Eugenol | Anticancer, antimicrobial, antioxidant | Tomato, tamarillo | [79] |
| β-ionone | Chemopreventive activity | Orange carrot, broccoli, watercress, spinach, tomato, tamarillo | [87] |
| α-Pinene | Antimicrobial, anticancer, anti-inflammatory, antiallergic | Beetroot, red onion, orange and white carrot, broccoli, watercress, spinach, tomato, tamarillo | [88,89] |
| 3-Carene | Antimicrobial, antioxidant, anticancer, | Orange and white carrot, spinach, tamarillo | [90], |
| Diallyl disulfide | Anticancer, antioxidant, anti-inflammatory | Garlic | [91,92] |
| Diallyl trisulfide | Anticancer, antidiabetic | Garlic | [93,94] |
| Dipropyl disulfide | Anticancer | Yellow onion | [95] |
| Dimethyl trisulfide | Reduction in acute pancreatitis | Yellow and red onion | [96] |
| 2-Phenylethyl glucosinolate | Chemopreventive | Watercress | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguiar, J.; Gonçalves, J.L.; Alves, V.L.; Câmara, J.S. Relationship between Volatile Composition and Bioactive Potential of Vegetables and Fruits of Regular Consumption—An Integrative Approach. Molecules 2021, 26, 3653. https://doi.org/10.3390/molecules26123653
Aguiar J, Gonçalves JL, Alves VL, Câmara JS. Relationship between Volatile Composition and Bioactive Potential of Vegetables and Fruits of Regular Consumption—An Integrative Approach. Molecules. 2021; 26(12):3653. https://doi.org/10.3390/molecules26123653
Chicago/Turabian StyleAguiar, Joselin, João L. Gonçalves, Vera L. Alves, and José S. Câmara. 2021. "Relationship between Volatile Composition and Bioactive Potential of Vegetables and Fruits of Regular Consumption—An Integrative Approach" Molecules 26, no. 12: 3653. https://doi.org/10.3390/molecules26123653
APA StyleAguiar, J., Gonçalves, J. L., Alves, V. L., & Câmara, J. S. (2021). Relationship between Volatile Composition and Bioactive Potential of Vegetables and Fruits of Regular Consumption—An Integrative Approach. Molecules, 26(12), 3653. https://doi.org/10.3390/molecules26123653






