Phytochemical Profile, Antioxidant Capacity, α-Amylase and α-Glucosidase Inhibitory Potential of Wild Moroccan Inula viscosa (L.) Aiton Leaves
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of Total Phenolics and Total Flavonoids Contents
2.2. Antioxidant Activity
2.3. Enzyme Inhibitory Activities
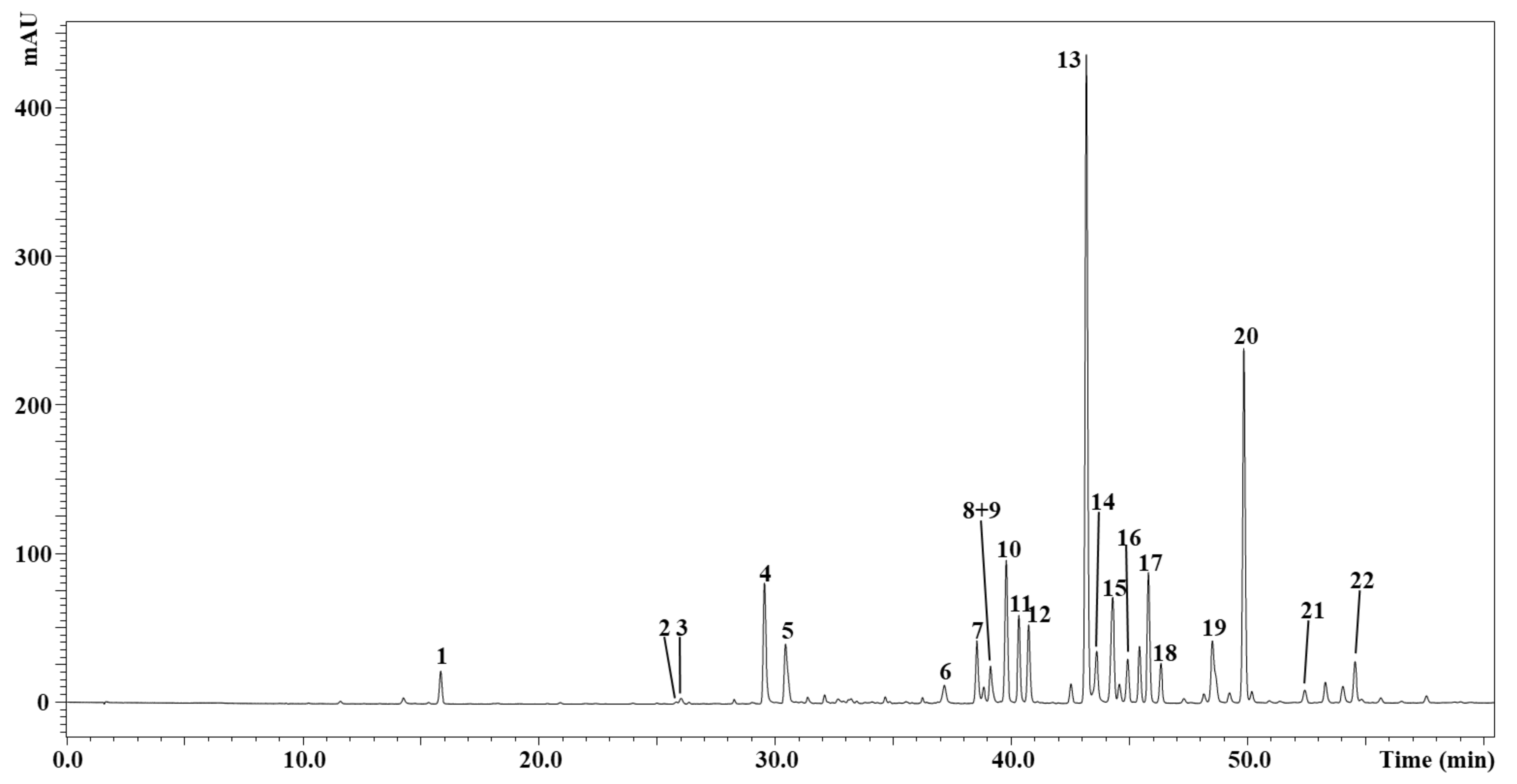
2.4. Phytochemical Profile of I. viscosa by GC-MS and HPLC-DAD/ESI-MS
2.5. Statistical Analysis
3. Materials and Methods
3.1. Plant Material
3.2. Chemical Reagents and Solvents
3.3. Preparation of Crude Extracts
3.4. Analysis and Quantification of Phenolic Contents
3.5. Determination of Antioxidant Capacity
3.5.1. Scavenging Capacity of DPPH Radical
3.5.2. Scavenging Capacity of ABTS Radical Cation
3.5.3. Total Reducing Power Assay Fe (III) to Fe (II)
3.6. Enzyme Inhibitory Activities
3.6.1. α-Glucosidase Inhibition Assay
3.6.2. α-Amylase Inhibition Assay
3.7. GC-MS
3.8. HPLC-DAD/ESI-MS
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Neurol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Mohti, H.; Taviano, M.F.; Cacciola, F.; Dugo, P.; Mondello, L.; Marino, A.; Crisafi, G.; Benameur, Q.; Zaid, A.; Miceli, N. Inula viscosa (L.) Aiton leaves and flower buds: Effect of extraction solvent/technique on their antioxidant ability, antimicrobial properties and phenolic profile. Nat. Prod. Res. 2020, 34, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Suta, S.; Dall’Acqua, S.; Sinan, K.I.; Bene, K.; Kumar, G.; Mahomood, M.F.; Picot-Allain, C.; Zengin, G. Cola caricifolia (G.Don) K. Schum and Crotalaria retusa L. from Ivory Coast as sources of bioactive constituents. Ind. Crops Prod. 2020, 147, 112246. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. Change of phenolics, carotenoids, and antioxidant capacity following simulated gastrointestinal digestion and dialysis of selected edible green leaves. Food Chem. 2018, 245, 371–379. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Grigore, A.; Pinto, D.C.G.A.; Silva, A.M.S. The genus Inula and their metabolites: From ethnopharmacological to medicinal uses. J. Ethnopharmacol. 2014, 154, 286–310. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Abu Ghdeib, S.I. Antifungal activity of plant extracts against dermatophytes. Mycoses 1999, 42, 665–672. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Zhang, M.-L.; Shi, Q.-W.; Kiyota, H. Chemical constituents of plants from the genus. Chem. Biodivers. 2006, 7, 1198–1207. [Google Scholar] [CrossRef]
- Tahraoui, A.; El-Hilaly, J.; Israili, Z.H.; Lyoussi, B. Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south-eastern Morocco (Errachidia province). J. Ethnopharmacol. 2007, 110, 105–117. [Google Scholar] [CrossRef]
- Eddouks, M.; Maghrani, M.; Lemhadri, A.; Ouahidi, M.L.; Jouad, H. Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet). J. Ethnopharmacol. 2002, 82, 97–103. [Google Scholar] [CrossRef]
- Cafarchia, C.; De Laurentis, N.; Milillo, M.A.; Losacco, V.; Puccini, V. Antifungal activity of essential oils from leaves and flowers of Inula viscosa (Asteraceae) by Apulian region. Parassitologia 2002, 44, 153–156. [Google Scholar]
- Wafa Rhimi, C.C.; Salem, I.B.; Camardac, A.; Saidi, M.; Boulila, A.; Otranto, D. Chemical characterization and acaricidal activity of Drimia maritima (L) bulbs and Dittrichia viscosa leaves against Dermanyssus gallinae. Vet. Parasitol. 2019, 268, 61–66. [Google Scholar] [CrossRef]
- Zeggwagh, N.A.; Ouahidi, M.L.; Lemhadri, A.; Eddouks, M. Study of hypoglycaemic and hypolipidemic effects of Inula viscosa L. aqueous extract in normal and diabetic rats. J. Ethnopharmacol. 2006, 108, 223–227. [Google Scholar] [CrossRef]
- Kattouf, J.; Belmoukhtar, M.; Harnafi, H.; Mekhfi, H.; Ziyyat, A.; Aziz, M.; Bnouham, M.; Legssyer, A. Effet antihypertenseur des feuilles d’Inula viscosa. Phytotherapie 2009, 7, 309–312. [Google Scholar] [CrossRef]
- Talib, W.H.; Abu Zarga, M.H.; Mahasneh, A.M. Antiproliferative, antimicrobial and apoptosis inducing effects of compounds isolated from Inula viscosa. Molecules 2012, 17, 3291–3303. [Google Scholar] [CrossRef]
- Hernández, V.; Recio, M.C.; Máñez, S.; Giner, R.M.; Ríos, J.L. Effects of naturally occurring dihydroflavonols from Inula viscosa on inflammation and enzymes involved in the arachidonic acid metabolism. Life Sci. 2007, 81, 480–488. [Google Scholar] [CrossRef]
- Schinella, G.R.; Tournier, H.A.; Prieto, J.M.; Mordujovich De Buschiazzo, P.; Ríos, J.L. Antioxidant activity of anti-inflammatory plant extracts. Life Sci. 2002, 70, 1023–1033. [Google Scholar] [CrossRef]
- Danino, O.; Gottlieb, H.E.; Grossman, S.; Bergman, M. Antioxidant activity of 1,3-dicaffeoylquinic acid isolated from Inula viscosa. Food Res. Int. 2009, 42, 1273–1280. [Google Scholar] [CrossRef]
- Tavares, W.R.; Seca, A.M.L. Inula L. secondary metabolites against oxidative stress-related human diseases. Antioxidants 2019, 8, 122. [Google Scholar] [CrossRef]
- Garayev, E.; Di Giorgio, C.; Herbette, G.; Mabrouki, F.; Chiffolleau, F.; Roux, D.; Sallanon, H.; Ollivier, E.; Elias, R.; Baghdikian, B. Bioassay-guided isolation and UHPLC-DAD-ESI-MS/MS quantification of potential anti-inflammatory phenolic compounds from flowers of Inula montana L. J. Ethnopharmacol. 2018, 226, 176–184. [Google Scholar] [CrossRef]
- Trimech, I.; Weiss, E.K.; Chedea, V.S.; Marin, D.; Detsi, A.; Ioannou, E.; Roussis, V.; Kefalas, P. Evaluation of anti-oxidant and acetylcholinesterase activity and identification of polyphenolics of the invasive weed dittrichia viscosa. Phytochem. Anal. 2014, 25, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Rhimi, W.; Ben Salem, I.; Immediato, D.; Saidi, M.; Boulila, A.; Cafarchia, C. Chemical composition, antibacterial and antifungal activities of crude Dittrichia viscosa (L.) greuter leaf extracts. Molecules 2017, 22, 942. [Google Scholar] [CrossRef]
- Chahmi, N.; Anissi, J.; Jennan, S.; Farah, A.; Sendide, K.; El Hassouni, M. Antioxidant activities and total phenol content of Inula viscosa extracts selected from three regions of Morocco. Asian Pac. J. Trop. Biomed. 2015, 5, 228–233. [Google Scholar] [CrossRef]
- El Mansouri, F.; Palma Lovillo, M.; El Farissi, H.; Oufdou, H.; Brigui, J. Extraction, analysis of polyphenols and antioxidant properties of morrocan barley seed extracts (Hordeum vulgare L.). Mater. Today Proc. 2021, 43, 1896–1902. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic content, Flavonoid content and antioxidant potential of wild vegetables from western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, S.K.; Sati, B.; Faujdar, S.; Sharma, S. Antioxidant and antibacterial activities of various extracts of Inula cuspidata C.B. Clarke stem. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 97–105. [Google Scholar] [CrossRef]
- Shi, P.; Du, W.; Wang, Y.; Teng, X.; Chen, X.; Ye, L. Total phenolic, flavonoid content, and antioxidant activity of bulbs, leaves, and flowers made from Eleutherine bulbosa (Mill.) Urb. Food Sci. Nutr. 2019, 7, 148–154. [Google Scholar] [CrossRef]
- Asraoui, F.; Kounnoun, A.; El Cadi, H.; Cacciola, F.; Oulad El Majdoub, Y.; Alibrando, F.; Mandolfino, F.; Dugo, P.; Mondello, L.; Louajri, A. Phytochemical Investigation and Antioxidant Activity of Globularia alypum L. Molecules 2021, 26, 759. [Google Scholar] [CrossRef]
- Albano, S.M.; Miguel, M.G. Biological activities of extracts of plants grown in Portugal. Ind. Crops Prod. 2011, 33, 338–343. [Google Scholar] [CrossRef]
- Kasote, D.M.; Jayaprakasha, G.K.; Patil, B.S. Leaf Disc Assays for Rapid Measurement of Antioxidant Activity. Sci. Rep. 2019, 9, 1884. [Google Scholar] [CrossRef]
- Brahmi-Chendouh, N.; Brahmi-Chendouh, N.; Piccolella, S.; Crescente, G.; Pacifico, F.; Boulekbache, L.; Hamri-Zeghich, S.; Akkal, S.; Madani, K.; Pacifico, S. A nutraceutical extract from Inula viscosa leaves: UHPLC-HR-MS/MS based polyphenol profile, and antioxidant and cytotoxic activities. J. Food Drug Anal. 2019, 27, 692–702. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Hosni, K.; Zaouali, W.; Amri, I.; Zargouni, H.; Ben Hamida, N.; Kaddour, R.; Hamrouni, L.; Ben Nasri, M.; Ouerghi, Z. Comprehensive Phytochemical Analysis, Antioxidant and Antifungal Activities of Inula viscosa Aiton Leaves. J. Food Saf. 2016, 36, 77–88. [Google Scholar] [CrossRef]
- Trendafilova, A.; Ivanova, V.; Rangelov, M.; Todorova, M.; Ozek, G.; Yur, S.; Ozek, T.; Aneva, I.; Veleva, R.; Moskova-Doumanova, V.; et al. Caffeoylquinic Acids, Cytotoxic, Antioxidant, Acetylcholinesterase and Tyrosinase Enzyme Inhibitory Activities of Six Inula Species from Bulgaria. Chem. Biodivers. 2020, 17, e2000051. [Google Scholar] [CrossRef]
- Ceylan, R.; Zengin, G.; Mahomoodally, M.F.; Sinan, K.I.; Ak, G.; Jugreet, S.; Cakır, O.; Ouelbani, R.; Yavuz Paksoy, M.; Yılmaz, M.A. Enzyme inhibition and antioxidant functionality of eleven Inula species based on chemical components and chemometric insights. Biochem. Syst. Ecol. 2020, 95, 104225. [Google Scholar] [CrossRef]
- Trinh, D.H.; Phuong, T.; Trinh, B.T.D.; Nguyen, H.T.; Nguyen, H.D.; Ly, D.H.; Nguyen, L.-H. Coumarins and acridone alkaloids with α-glucosidase inhibitory and antioxidant activity from the roots of Paramignya trimera. Phytochem. Lett. 2019, 35, 94–98. [Google Scholar] [CrossRef]
- Kast, R.E. Acarbose related diarrhea: Increased butyrate upregulates prostaglandin E. Inflamm. Res. 2002, 51, 117–118. [Google Scholar] [CrossRef]
- Apostolidis, E.; Kwon, Y.I.; Shetty, K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov. Food Sci. Emerg. Technol. 2007, 8, 46–54. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Ohhira, M.; Miyokawa, N.; Penn, R.D.; Kroin, J.S.; Reinkensmeyer, A. Acarbose-induced hepatic injury Injection of GABA-agonist into globus pallidus in patient with Parkinson’s disease Chlamydia trachomatis detection and the menstrual cycle. Lancet 1998, 351, 340–341. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative Properties of Xanthan on the Autoxidation of Soybean Oil in Cyclodextrin Emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Mahomoodally, M.F. Traditional medicines in Africa: An appraisal of ten potent African medicinal plants. Evid. Based Complement. Altern. Med. 2013, 2013, 617459. [Google Scholar] [CrossRef]
- Lee, H.-S. Cuminaldehyde: Aldose Reductase and a-Glucosidase Inhibitor Derived from Cuminum cyminum L. Seeds. J. Agric. Food Chem. 2005, 53, 2446–2450. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, A. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Kheyar-Kraouche, N.; da Silva, A.B.; Serra, A.T.; Bedjou, F.; Bronze, M.R. Characterization by liquid chromatography-mass spectrometry and antioxidant activity of an ethanolic extract of Inula viscosa leaves. J. Pharm. Biomed. Anal. 2018, 156, 297–306. [Google Scholar] [CrossRef]
- Grauso, L.; Cesarano, G.; Zotti, M.; Ranesi, M.; Sun, W.; Bonanomi, G.; Lanzotti, V. Exploring Dittrichia Viscosa (L.) Greuter Phytochemical Diversity to Explain Its Antimicrobial, Nematicidal and Insecticidal Activity. Phytochem. Rev. 2020, 19, 659–689. [Google Scholar] [CrossRef]
- Yuan, X.; Gao, M.; Xiao, H.; Tan, C.; Du, Y. Free radical scavenging activities and bioactive substances of Jerusalem artichoke (Helianthus tuberosus L.) leaves. Food Chem. 2012, 133, 10–14. [Google Scholar] [CrossRef]
- Males, Z.; Hazler Pilepic, K.; Petrovic, L.; Bagaric, I. Quantitative analysis of phenolic compounds of Inula candida (L.). Cass. Period. Biol. 2010, 112, 307–310. [Google Scholar]
- Ali Asgar, M.D. Anti-diabetic potential of phenolic compounds: A review. Int. J. Food Prop. 2013, 16, 91–103. [Google Scholar] [CrossRef]
- Benavente-Garcıa, O.; Castillo, J.; Lorente, J.; Ortuno, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Braca, A.; Sortino, C.; Politi, M.; Morelli, I.; Mendez, J. Antioxidant activity of flavonoids from Licania licaniaeflora. J. Ethnopharmacol. 2002, 79, 379–381. [Google Scholar] [CrossRef]
- Fotie, J. The antiprotozoan potential of flavonoids. Pharm. Rev. 2008, 2, 6. [Google Scholar]
- Galati, G.; Obrien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Laphookhieo, S.; Maneerat, W.; Koysomboon, S. Antimalarial and cytotoxic phenolic compounds from Cratoxylum maingayi and Cratoxylum cochinchinense. Molecules 2009, 14, 1389–1395. [Google Scholar] [CrossRef]
- Spanos, G.A.; Wrolstad, R.E. Influence of Processing and Storage on the Phenolic Composition of Thompson Seedless Grape Juice. J. Agric. Food Chem. 1990, 38, 1565–1571. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Liu, R.H. Processed sweet corn has higher antioxidant activity. J. Agric. Food Chem. 2002, 50, 4959–4964. [Google Scholar] [CrossRef]
- Hatano, T.; Kagawa, H.; Yasuhara, T. NII-Electronic Library Service. Chem. Pharm. Bull. 1988, 43, 2091. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Kabach, I.; Mrid, R.B.E.N.; Bouchmaa, N.; Bouargalne, Y. Phytochemical screening, antioxidant and cytotoxic activities of M. vulgare. Int. J. Pharm. Res. 2019, 11, 338–345. [Google Scholar]
- Kee, K.T.; Koh, M.; Oong, L.X.; Ng, K. Screening culinary herbs for antioxidant and α-glucosidase inhibitory activities. Int. J. Food Sci. Technol. 2013, 48, 1884–1891. [Google Scholar] [CrossRef]
- Hashim, A.; Khan, M.S.; Khan, M.S.; Baig, M.H.; Ahmad, S. Antioxidant and α-amylase inhibitory property of phyllanthus virgatus L.: An in vitro and molecular interaction study. BioMed Res. Int. 2013, 2013, 729393. [Google Scholar] [CrossRef]
| Extracts | Polyphenols (mg GAE/g of Extract) | Flavonoids (mg CE/g of Extract) |
|---|---|---|
| EtOAc | 87.2 ± 0.50 | 78.6 ± 0.55 |
| Methanol | 65.3 ± 0.78 | 52.1 ± 0.80 |
| Chloroform | 34.0 ± 0.48 | 18.3 ± 0.40 |
| Antioxidant Properties (IC50 Value µg/mL ± Standard Deviation) | |||
|---|---|---|---|
| Plant Extracts | DPPH | ABTS | FRAP (mg EAA/g DW) |
| EtOAC | 0.6 ± 0.03 | 8.6 ± 0.08 | 634.8 ± 1.45 |
| Methanol | 8.2 ± 1.16 | 25.5 ± 0.45 | 552.1 ± 0.88 |
| Chloroform | 40.8 ± 0.88 | 81.6 ± 0.05 | 90.1 ± 0.66 |
| BHT | 0.3 ± 0.11 | - | - |
| Ascorbic acid | - | 16.9 ± 4.77 | - |
| Plant Extracts | IC50 (µg/mL) | Percentage of Inhibition (%) |
|---|---|---|
| α-Glucosidase Inhibition | α-Amylase Inhibition | |
| EtOAc | 29.9 ± 1.04 | 22% |
| Methanol | 22.3 ± 2.82 | 27% |
| Chloroform | 39.8 ± 0.76 | 17% |
| # | Compounds | Match | LRI Ref | LRI Exp | Library |
|---|---|---|---|---|---|
| 1 | Cuminaldehyde | 98 | 1243 | 1246 | FFNSC 4.0 |
| 2 | Phenylacetic acid | 95 | 1261 | 1251 | FFNSC 4.0 |
| 3 | α-Terpinen-7-al | 97 | 1287 | 1290 | FFNSC 4.0 |
| 4 | α-Cubebene | 96 | 1347 | 1348 | FFNSC 4.0 |
| 5 | Eugenol | 94 | 1357 | 1357 | FFNSC 4.0 |
| 6 | α-Copaene | 96 | 1375 | 1377 | FFNSC 4.0 |
| 7 | β-Cubebene | 93 | 1392 | 1389 | FFNSC 4.0 |
| 8 | (E)-Caryophyllene | 97 | 1424 | 1421 | FFNSC 4.0 |
| 9 | Germacrene D | 92 | 1478 | 1477 | FFNSC 4.0 |
| 10 | α-Curcumene | 93 | 1480 | 1482 | FFNSC 4.0 |
| 11 | β-Selinene | 91 | 1492 | 1491 | FFNSC 4.0 |
| 12 | α-Zingiberene | 92 | 1496 | 1496 | FFNSC 4.0 |
| 13 | α-Muurolene | 96 | 1497 | 1501 | FFNSC 4.0 |
| 14 | (E,E)-, α-Farnesene | 89 | 1504 | 1505 | FFNSC 4.0 |
| 15 | epi-Cubebol | 89 | 1498 | 1506 | FFNSC 4.0 |
| 16 | β-Bisabolene | 97 | 1508 | 1509 | FFNSC 4.0 |
| 17 | γ-Cadinene | 96 | 1512 | 1516 | FFNSC 4.0 |
| 18 | δ-Cadinene | 92 | 1518 | 1521 | FFNSC 4.0 |
| 19 | β-Sesquiphellandrene | 96 | 1523 | 1526 | FFNSC 4.0 |
| 20 | α-Cadinene | 94 | 1538 | 1540 | FFNSC 4.0 |
| 21 | Caryophyllene oxide | 94 | 1587 | 1586 | FFNSC 4.0 |
| 22 | Fokienol | 97 | 1596 | 1601 | FFNSC 4.0 |
| 23 | β-Oplopenone | 88 | 1606 | 1608 | FFNSC 4.0 |
| 24 | δ-Cadinol | 92 | 1641 | 1650 | FFNSC 4.0 |
| 25 | α-, epi-Muurolol | 93 | 1645 | 1654 | FFNSC 4.0 |
| 26 | Cadin-4-en-10-ol | 92 | 1659 | 1665 | FFNSC 4.0 |
| 27 | Oplopanone | 92 | 1738 | 1744 | FFNSC 4.0 |
| 28 | Neophytadiene | 95 | 1836 | 1837 | FFNSC 4.0 |
| 29 | Phytone | 92 | 1841 | 1843 | FFNSC 4.0 |
| 30 | n-Hexadecanoic acid | 94 | 1977 | 1977 | FFNSC 4.0 |
| 31 | (Z,Z)-9,12-Octadecadienoic acid | 92 | 2140 | 2142 | W11N17 |
| 32 | (Z,Z,Z)-9,12,15-Octadecatrienoic acid | 97 | 2154 | 2152 | W11N17 |
| 33 | n-Tricosane | 97 | 2300 | 2300 | FFNSC 4.0 |
| 34 | n-Tetracosane | 97 | 2400 | 2400 | FFNSC 4.0 |
| 35 | n-Pentacosane | 97 | 2500 | 2501 | FFNSC 4.0 |
| 36 | n-Hexacosane | 95 | 2600 | 2600 | FFNSC 4.0 |
| 37 | n-Heptacosane | 96 | 2700 | 2701 | FFNSC 4.0 |
| 38 | methyl-Tetracosanoate | 96 | 2732 | 2733 | FFNSC 4.0 |
| 39 | n-Octacosane | 94 | 2800 | 2800 | FFNSC 4.0 |
| 40 | 2-methyl-Octacosane | 95 | 2864 | 2863 | W11N17 |
| 41 | n-Nonacosane | 92 | 2900 | 2901 | FFNSC 4.0 |
| 42 | Methyl hexacosanoate | 93 | 2940 | 2935 | W11N17 |
| 43 | n-Triacontane | 93 | 3000 | 3000 | FFNSC 4.0 |
| 44 | n-Hentriacontane | 95 | 3100 | 3101 | FFNSC 4.0 |
| 45 | n-Dotriacontane | 94 | 3200 | 3200 | FFNSC 4.0 |
| 46 | n-Tritriacontane | 94 | 3300 | 3300 | FFNSC 4.0 |
| 47 | n-Tetratriacontane | 92 | 3400 | 3400 | FFNSC 4.0 |
| 48 | n-Pentatriacontane | 91 | 3500 | 3500 | FFNSC 4.0 |
| N | Compounds | tR (min) | UVmax (nm) | [M-H]- | Content (mg/kg) | Employed Standard for Quantification | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Caffeic acid | 15.83 | 322 | 179 | 157.7 ± 0.13 | Caffeic acid | [41] |
| 2 | Galloylquinic acid | 25.82 | 281 | 343 | 80.9 ± 0.18 | Gallic acid | [42] |
| 3 | Dihydroquercetin | 26.02 | 287 | 303 | 119.6 ± 0.12 | Quercetin | - |
| 4 | Di-O-Caffeoylquinic acid | 29.55 | 328 | 515 | 621.0 ± 1.53 | Caffeic acid | [42] |
| 5 | Di-O-Caffeoylquinic acid isomer | 30.45 | 326 | 515 | 374.8 ± 1.27 | Caffeic acid | [42] |
| 6 | Unknown | 37.18 | 288 | 181 | - | - | - |
| 7 | Quercetin | 38.55 | 359 | 301 | 384.7 ± 0.94 | Quercetin | [42] |
| 8 | Nepetin | 39.14 | 342 | 315 | 191.8 ± 0.14 | Luteolin | [42] |
| 9 | Padmatin | 39.22 | 290 | 317 | 573.5 ± 2.15 | Naringin | [42] |
| 10 | Unknown | 39.79 | 289 | 635, 317 | - | - | - |
| 11 | 3-O-methylquercetin | 40.33 | 355 | 315 | 486.5 ± 1.18 | Quercetin | [3] |
| 12 | Spinacetin | 40.76 | 291, 339 sh | 345 | 450.6 ± 1.44 | Apigenin | [42] |
| 13 | Diosmetin | 43.18 | 273 sh, 335 | 299 | 3365.2 ± 4.32 | Apigenin | [42] |
| 14 | Rhamnetin | 43.60 | 355 | 315 | 502.1 ± 1.77 | Luteolin | [42] |
| 15 | Hesperetin | 44.30 | 290 | 301 | 660.3 ± 0.36 | Naringin | [3,42] |
| 16 | Hispidulin | 44.92 | 348 | 299 | 281.4 ± 0.14 | Apigenin | [3,42,44] |
| 17 | Cirsiliol | 45.44 | 339 | 329 | 313.1 ± 0.29 | Apigenin | [42] |
| 18 | 3-O-Acetylpadmatin | 46.34 | 350 | 359 | 683.3 ± 0.36 | Naringin | [42] |
| 19 | Isorhamnetin | 48.52 | 367 | 315 | 820.3 ± 1.77 | Luteolin | [42] |
| 20 | Rosmarinic acid | 49.86 | 323 | 359 | 1529.5 ± 0.99 | Rosmarinic acid | [42] |
| 21 | Luteolin | 52.43 | 288 | 285 | 82.3 ± 0.30 | Luteolin | [3,43] |
| 22 | Unknown | 54.50 | 292 | 343 | - | - | - |
| Paired Difference Values | ||||||||
|---|---|---|---|---|---|---|---|---|
| Type of Analysis | Type of Solvent | Mean | Ecart Type | Variance | Std. Error | 95% Confidence Interval of Difference | Sig. (Bilateral) | |
| Lower Bound | Upper Bound | |||||||
| Polyphenols (mg GAE/g of extract) | EtOAc-Methanol | 21.900 | 0.100 | 0.010 | 0.057 | 21.651 | 22.148 | 0.000 S |
| EtOAc-Chloroform | 53.200 | 0.150 | 0.0225 | 0.086 | 52.827 | 53.572 | 0.001 | |
| Methanol-Chloroform | 31.300 | 0.050 | 0.0025 | 0.028 | 31.175 | 31.421 | 0.000 S | |
| Flavonoids (mg CE/g of extract) | EtOAc-Methanol | 26.500 | 0.100 | 0.01 | 0.057 | 26.251 | 26.748 | 0.001 S |
| EtOAc-Chloroform | 60.300 | 0.086 | 0.0073 | 0.050 | 60.084 | 60.515 | 0.000 S | |
| Methanol-Chloroform | 33.800 | 0.050 | 0.0025 | 0.028 | 33.675 | 33.924 | 0.000 S | |
| Type of Analysis | Solvant Type | Average | Ecart Type | 95% Confidence Interval | Test ANOVA | ||
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | Variance | Sig. | ||||
| ABTS assay (IC50 μg/mL) | EtOAc | 8.633 ± 0.088 | 0.152 | 8.254 | 9.013 | 0.023 | 0.001 S |
| Chloroform | 81.646 ± 0.057 | 0.100 | 81.3978 | 81.895 | 0.010 | ||
| Methanol | 25.223 ± 0.453 | 0.785 | 23.2711 | 27.175 | 0.618 | ||
| FRAP assay (mg/g) | EtOAc | 634.810 ± 1.452 | 2.516 | 628.558 | 641.062 | 6.333 | 0.000 S |
| Chloroform | 90.143 ± 0.666 | 1.154 | 87.275 | 93.0123 | 1.333 | ||
| Methanol | 552.143 ± 0.881 | 1.527 | 548.349 | 555.938 | 2.333 | ||
| DPPH assay (IC50 μg/mL) | EtOAc | 0.62 ± 0.037 | 0.064 | 0.46 | 0.78 | 0.004 | 0.000 S |
| Chloroform | 40.85 ± 0.887 | 1.536 | 37.03 | 44.66 | 2.360 | ||
| Methanol | 8.17 ± 1.165 | 2.018 | 3.16 | 13.19 | 4.073 | ||
| α-glucosidase inhibition assay (IC50 μg/mL) | EtOAc | 29.920 ± 1.049 | 1.817 | 25.405 | 34.436 | 3.305 | 0.000 S |
| Chloroform | 39.801 ± 0.768 | 1.330 | 36.497 | 43.106 | 1.770 | ||
| Methanol | 22.263 ± 2.825 | 4.894 | 10.104 | 34.422 | 23.957 | ||
| α-amylase inhibition assay (%) | EtOAc | 22.152 ± 0.387 | 0.670 | 20.486 | 23.819 | 0.450 | 0.000 S |
| Chloroform | 17.157 ± 0.634 | 1.099 | 14.426 | 19.887 | 1.208 | ||
| Methanol | 27.162 ± 1.623 | 2.811 | 20.178 | 34.146 | 7.904 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asraoui, F.; Kounnoun, A.; Cacciola, F.; El Mansouri, F.; Kabach, I.; Oulad El Majdoub, Y.; Alibrando, F.; Arena, K.; Trovato, E.; Mondello, L.; et al. Phytochemical Profile, Antioxidant Capacity, α-Amylase and α-Glucosidase Inhibitory Potential of Wild Moroccan Inula viscosa (L.) Aiton Leaves. Molecules 2021, 26, 3134. https://doi.org/10.3390/molecules26113134
Asraoui F, Kounnoun A, Cacciola F, El Mansouri F, Kabach I, Oulad El Majdoub Y, Alibrando F, Arena K, Trovato E, Mondello L, et al. Phytochemical Profile, Antioxidant Capacity, α-Amylase and α-Glucosidase Inhibitory Potential of Wild Moroccan Inula viscosa (L.) Aiton Leaves. Molecules. 2021; 26(11):3134. https://doi.org/10.3390/molecules26113134
Chicago/Turabian StyleAsraoui, Fadoua, Ayoub Kounnoun, Francesco Cacciola, Fouad El Mansouri, Imad Kabach, Yassine Oulad El Majdoub, Filippo Alibrando, Katia Arena, Emanuela Trovato, Luigi Mondello, and et al. 2021. "Phytochemical Profile, Antioxidant Capacity, α-Amylase and α-Glucosidase Inhibitory Potential of Wild Moroccan Inula viscosa (L.) Aiton Leaves" Molecules 26, no. 11: 3134. https://doi.org/10.3390/molecules26113134
APA StyleAsraoui, F., Kounnoun, A., Cacciola, F., El Mansouri, F., Kabach, I., Oulad El Majdoub, Y., Alibrando, F., Arena, K., Trovato, E., Mondello, L., & Louajri, A. (2021). Phytochemical Profile, Antioxidant Capacity, α-Amylase and α-Glucosidase Inhibitory Potential of Wild Moroccan Inula viscosa (L.) Aiton Leaves. Molecules, 26(11), 3134. https://doi.org/10.3390/molecules26113134









