Influence of N-Methylation and Conformation on Almiramide Anti-Leishmanial Activity
Abstract
1. Introduction
2. Results and Discussion
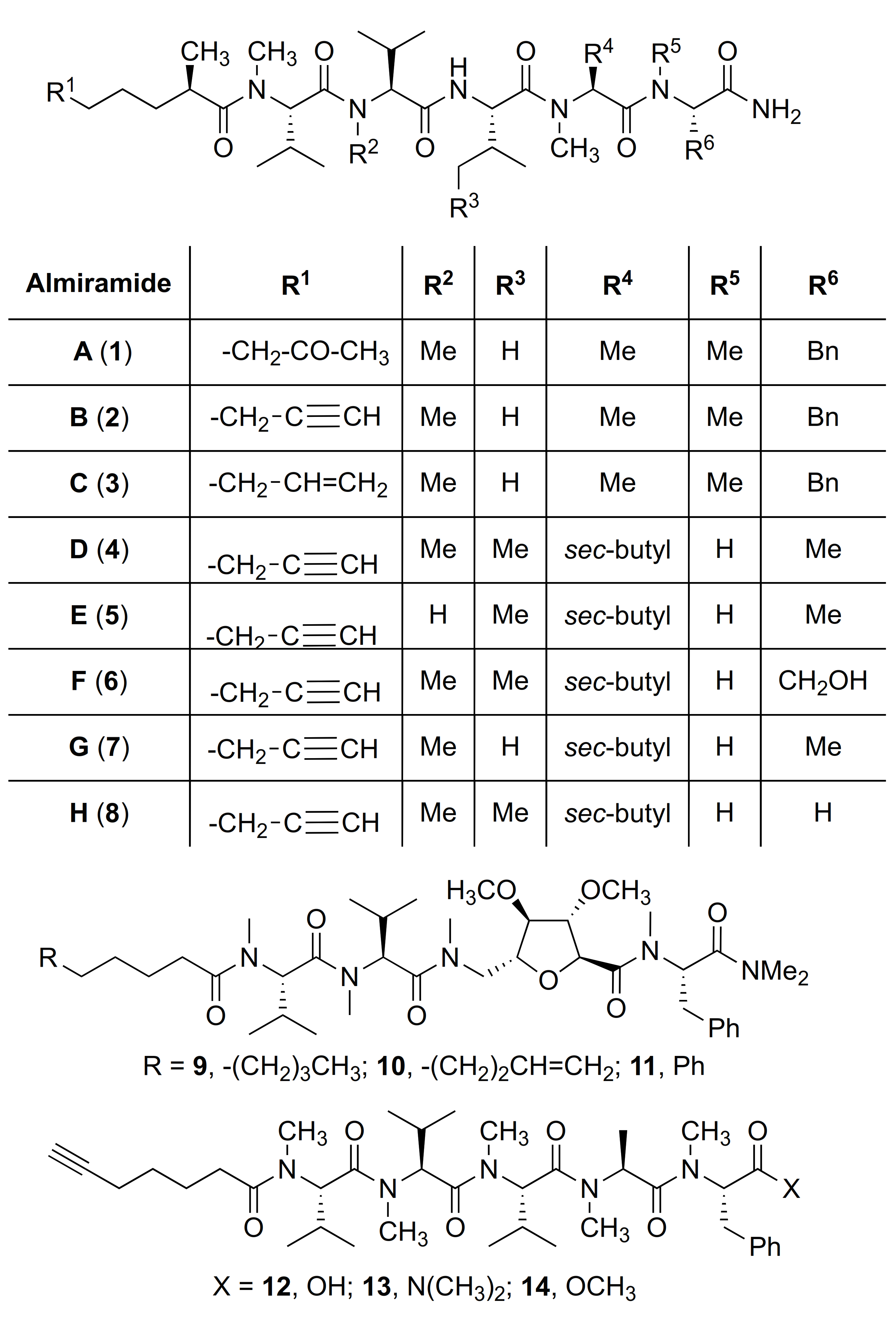
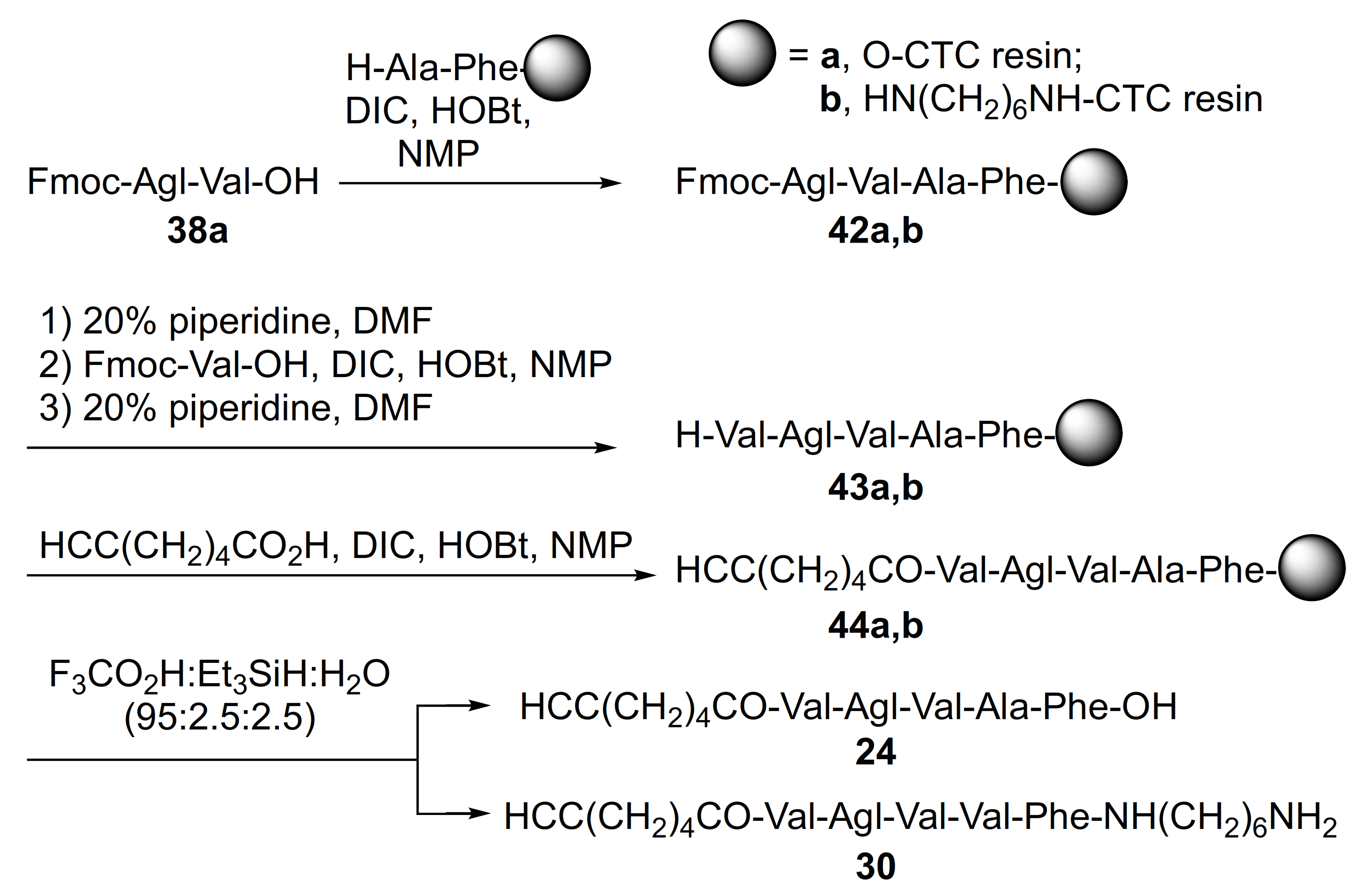
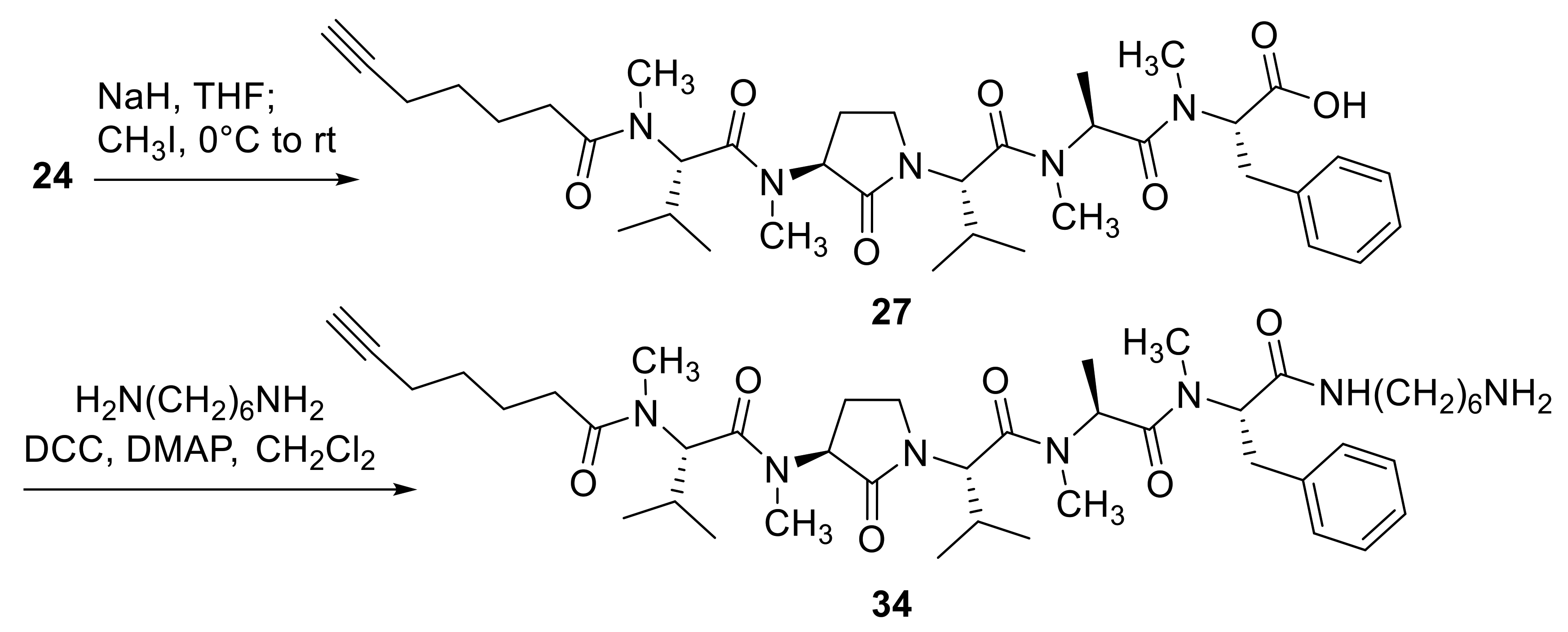
2.1. Chemistry
2.2. Bioactivity
3. Discussion
4. Materials and Methods
4.1. Experimental Section
4.1.1. Leishmania Cultures and Antileishmanial Activity Determination
4.1.2. LM-1 Macrophages and Cytotoxicity Determination
4.2. Materials







5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Andrews, K.T.; Fisher, G.; Skinner-Adams, T.S. Drug repurposing and human parasitic protozoan diseases. Int. J. Parasitol. Drugs Drug. Resist. 2014, 4, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Prada, C.; Douanne, N.; Minguez-Menendez, A.; Pena, J.; Tunes, L.G.; Pires, D.E.; Monte-Neto, R.L. Repurposed Molecules: A New Hope in Tackling Neglected Infectious Diseases. In In Silico Drug Design; Roy, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 119–160. [Google Scholar]
- Pramanik, P.K.; Alam, M.N.; Chowdhury, D.R.; Chakraborti, T. Drug resistance in protozoan parasites: An incessant wrestle for survival. J. Glob. Antimicrob. Resist. 2019, 18, 1–11. [Google Scholar] [CrossRef]
- Lupi, O.; Bartlett, B.L.; Haugen, R.N.; Dy, L.C.; Sethi, A.; Klaus, S.N.; Pinto, J.M.; Bravo, F.; Tyring, S.K. Tropical dermatology: Tropical diseases caused by protozoa. J. Am. Acad. Dermatol. 2009, 60, 897–925. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2019, 392, 951–970. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; Team, W.L.C. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Balaña-Fouce, R.; Pertejo, M.Y.P.; Domínguez-Asenjo, B.; Gutiérrez-Corbo, C.; Reguera, R.M. Walking a tightrope: Drug discovery in visceral leishmaniasis. Drug Discov. Today 2019, 24, 1209–1216. [Google Scholar] [CrossRef]
- Mehdi, D.S. The effect of visceral leishmaniasis on some liver enzyme and blood parameter. J. Thiqar Univ. 2008, 4, 2–5. [Google Scholar]
- Bilgic-Temel, A.; Murrell, D.F.; Uzun, S. Cutaneous leishmaniasis: A neglected disfiguring disease for women. Int. J. Womens Dermatol. 2019, 5, 158–165. [Google Scholar] [CrossRef]
- Strazzulla, A.; Cocuzza, S.; Pinzone, M.R.; Postorino, M.C.; Cosentino, S.; Serra, A.; Cacopardo, B.; Nunnari, G. Mucosal leishmaniasis: An underestimated presentation of a neglected disease. Biochem. Biophys. Res. Commun. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Lindoso, J.A.L.; Moreira, C.H.V.; Cunha, M.A.; Queiroz, I.T. Visceral leishmaniasis and HIV coinfection: Current perspectives. HIV AIDS 2018, 10, 193. [Google Scholar] [CrossRef]
- Uliana, S.R.B.; Trinconi, C.T.; Coelho, A.C. Chemotherapy of leishmaniasis: Present challenges. Parasitology 2018, 145, 464. [Google Scholar] [CrossRef]
- Sneader, W. Chemical Medicines. In Drug Discovery: A History; John Wiley and Sons: Hoboken, NJ, USA, 2005; pp. 57–59. [Google Scholar]
- Sundar, S.; More, D.K.; Singh, M.K.; Singh, V.P.; Sharma, S.; Makharia, A.; Kumar, P.C.; Murray, H.W. Failure of pentavalent antimony in visceral leishmaniasis in India: Report from the center of the Indian epidemic. Clin. Infect. Dis. 2000, 31, 1104–1107. [Google Scholar] [CrossRef]
- Sampaio, S.A.; Godoy, J.T.; Paiva, L.; Dillon, N.L.; Lacaz, C.D.S. The treatment of American (mucocutaneous) leishmaniasis with amphotericin B. Arch. Dermatol. 1960, 82, 627–635. [Google Scholar] [CrossRef]
- Sundar, S.; Jha, T.; Thakur, C.; Engel, J.; Sindermann, H.; Fischer, C.; Junge, K.; Bryceson, A.; Berman, J. Oral miltefosine for Indian visceral leishmaniasis. N. Eng. J. Med. 2002, 347, 1739–1746. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. An update on pharmacotherapy for leishmaniasis. Expert Opin. Pharmacother. 2015, 16, 237–252. [Google Scholar] [CrossRef]
- Sundar, S.; Jha, T.; Thakur, C.P.; Sinha, P.K.; Bhattacharya, S.K. Injectable paromomycin for visceral leishmaniasis in India. N. Eng. J. Med. 2007, 356, 2571–2581. [Google Scholar] [CrossRef]
- Hailu, A.; Musa, A.; Wasunna, M.; Balasegaram, M.; Yifru, S.; Mengistu, G.; Hurissa, Z.; Hailu, W.; Weldegebreal, T.; Tesfaye, S. Geographical variation in the response of visceral leishmaniasis to paromomycin in East Africa: A multicentre, open-label, randomized trial. PLoS Negl. Trop. Dis. 2010, 4, e709. [Google Scholar] [CrossRef]
- Brindha, J.; Balamurali, M.M.; Chanda, K. An Overview on the Therapeutics of Neglected Infectious Diseases—Leishmaniasis and Chagas Diseases. Front. Chem. 2021, 9, 1–19. [Google Scholar] [CrossRef]
- Silva, C.F.; Pinto, D.C.; Fernandes, P.A.; Silva, A.M. Evolution of chromone-like compounds as potential antileishmanial agents, through the 21st century. Expert Opin. Drug Discov. 2020, 15, 1425–1439. [Google Scholar] [CrossRef]
- Ortalli, M.; Varani, S.; Cimato, G.; Veronesi, R.; Quintavalla, A.; Lombardo, M.; Monari, M.; Trombini, C. Evaluation of the pharmacophoric role of the O–O bond in synthetic antileishmanial compounds: Comparison between 1, 2-dioxanes and tetrahydropyrans. J. Med. Chem. 2020, 63, 13140–13158. [Google Scholar] [CrossRef]
- Shalev, M.; Rozenberg, H.; Smolkin, B.; Nasereddin, A.; Kopelyanskiy, D.; Belakhov, V.; Schrepfer, T.; Schacht, J.; Jaffe, C.L.; Adir, N. Structural basis for selective targeting of leishmanial ribosomes: Aminoglycoside derivatives as promising therapeutics. Nucleic Acids Res. 2015, 43, 8601–8613. [Google Scholar] [CrossRef] [PubMed]
- Nagle, A.; Biggart, A.; Be, C.; Srinivas, H.; Hein, A.; Caridha, D.; Sciotti, R.J.; Pybus, B.; Kreishman-Deitrick, M.; Bursulaya, B. Discovery and characterization of clinical candidate LXE408 as a kinetoplastid-selective proteasome inhibitor for the treatment of leishmaniases. J. Med. Chem. 2020, 63, 10773–10781. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.M.; Lopez, D.; Vesely, B.A.; Della Togna, G.; Gerwick, W.H.; Kyle, D.E.; Linington, R.G. Almiramides A− C: Discovery and development of a new class of leishmaniasis lead compounds. J. Med. Chem. 2010, 53, 4187–4197. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.M.; Knudsen, G.M.; Helbig, C.; De Muylder, G.; Mascuch, S.M.; Mackey, Z.B.; Gerwick, L.; Clayton, C.; McKerrow, J.H.; Linington, R.G. Examination of the mode of action of the almiramide family of natural products against the kinetoplastid parasite Trypanosoma brucei. J. Nat. Prod. 2013, 76, 630–641. [Google Scholar] [CrossRef]
- Kalel, V.C.; Mäser, P.; Sattler, M.; Erdmann, R.; Popowicz, G.M. Come, sweet death: Targeting glycosomal protein import for antitrypanosomal drug development. Curr. Opin. Microbiol. 2018, 46, 116–122. [Google Scholar] [CrossRef]
- Michels, P.A.; Bringaud, F.; Herman, M.; Hannaert, V. Metabolic functions of glycosomes in trypanosomatids. Biochim. Biophys. Acta 2006, 1763, 1463–1477. [Google Scholar] [CrossRef]
- Dawidowski, M.; Emmanouilidis, L.; Kalel, V.; Tripsianes, K.; Schorpp, K.; Hadian, K.; Kaiser, M.; Mäser, P.; Kolonko, M.; Tanghe, S. Inhibitors of PEX14 disrupt protein import into glycosomes and kill Trypanosoma parasites. Science 2017, 355, 1416–1420. [Google Scholar] [CrossRef]
- Dawidowski, M.; Kalel, V.C.; Napolitano, V.; Fino, R.; Schorpp, K.; Emmanouilidis, L.; Lenhart, D.; Ostertag, M.; Kaiser, M.; Kolonko, M. Structure–activity relationship in pyrazolo [4, 3-c] pyridines, first inhibitors of PEX14–PEX5 protein–protein interaction with trypanocidal activity. J. Med. Chem. 2019, 63, 847–879. [Google Scholar] [CrossRef]
- Quintana, J.; Bayona, L.M.; Castellanos, L.; Puyana, M.; Camargo, P.; Aristizábal, F.; Edwards, C.; Tabudravu, J.N.; Jaspars, M.; Ramos, F.A. Almiramide D, cytotoxic peptide from the marine cyanobacterium Oscillatoria nigroviridis. Bioorg. Med. Chem. 2014, 22, 6789–6795. [Google Scholar] [CrossRef]
- Das, D.; Khan, H.P.; Shivahare, R.; Gupta, S.; Sarkar, J.; Siddiqui, M.I.; Ampapathi, R.S.; Chakraborty, T.K. Synthesis, SAR and biological studies of sugar amino acid-based almiramide analogues: N-methylation leads the way. Org. Biomol. Chem. 2017, 15, 3337–3352. [Google Scholar] [CrossRef]
- Freidinger, R.M.; Hinkle, J.S.; Perlow, D.S. Synthesis of 9-fluorenylmethyloxycarbonyl-protected N-alkyl amino acids by reduction of oxazolidinones. J. Org. Chem. 1983, 48, 77–81. [Google Scholar] [CrossRef]
- Freidinger, R.M.; Veber, D.F.; Perlow, D.S.; Saperstein, R. Bioactive conformation of luteinizing hormone-releasing hormone: Evidence from a conformationally constrained analog. Science 1980, 210, 656–658. [Google Scholar] [CrossRef]
- St-Cyr, D.J.; García-Ramos, Y.; Doan, N.-D.; Lubell, W.D. Aminolactam, N-aminoimidazolone, and N-aminoimdazolidinone peptide mimics. In Peptidomimetics I.; Lubell, W.D., Ed.; Springer: Berlin, Germany, 2017; pp. 125–175. [Google Scholar]
- Poupart, J.; Hamdane, Y.; Lubell, W.D. Synthesis of enantiomerically enriched 4, 5-disubstituted N-aminoimidazol-2-one (Nai) peptide turn mimics. Can. J. Chem. 2020, 98, 278–284. [Google Scholar] [CrossRef]
- Geranurimi, A.; Cheng, C.W.; Quiniou, C.; Zhu, T.; Hou, X.; Rivera, J.C.; St-Cyr, D.J.; Beauregard, K.; Bernard-Gauthier, V.; Chemtob, S.; et al. Probing anti-inflammatory properties independent of NF-κB through conformational constraint of peptide-based interleukin-1 receptor biased ligands. Front. Chem. 2019, 7, 23. [Google Scholar] [CrossRef]
- Hamdane, Y.; Chauhan, P.S.; Vutla, S.; Mulumba, M.; Ong, H.; Lubell, W.D. 5-Substituted N-aminoimidazolone peptide mimic synthesis by organocatalyzed reactions of azopeptides and use in conformational analysis of biologically active backbone and side chain topology. Org. Lett. 2021, 23, 3491–3495. [Google Scholar] [CrossRef]
- Lubell, W.D.; Blankenship, J.W.; Fridkin, G.; Kaul, R. Product Class 11: Peptides. In Three Carbon-Heteroatom Bonds: Amides and Derivatives, Peptides, Lactams; Weinreb, S., Ed.; Georg Thieme Verlag KG: New York, NY, USA, 2005; pp. 713–810. [Google Scholar]
- Miller, S.C.; Scanlan, T.S. Site-selective N-methylation of peptides on solid support. J. Am. Chem. Soc. 1997, 119, 2301–2302. [Google Scholar] [CrossRef]
- Quiñones, W.; Acosta, H.; Gonçalves, C.S.; Motta, M.C.M.; Gualdrón-López, M.; Michels, P.A. Structure, properties, and function of glycosomes in Trypanosoma cruzi. Front. Cell. Infect. Microbiol. 2020, 10, 25. [Google Scholar] [CrossRef]
- Gualdron-Lopez, M.; Brennand, A.; Avilan, L.; Michels, P.A. Translocation of solutes and proteins across the glycosomal membrane of trypanosomes; possibilities and limitations for targeting with trypanocidal drugs. Parasitology 2013, 140, 1. [Google Scholar] [CrossRef]
- Mohapatra, S. Drug resistance in leishmaniasis: Newer developments. Trop. Parasitol. 2014, 4, 4. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.-C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Mbongo, N.; Loiseau, P.M.; Billion, M.A.; Robert-Gero, M. Mechanism of amphotericin B resistance in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 1998, 42, 352–357. [Google Scholar] [CrossRef]
- Katakai, R.; Toda, F.; Uno, K.; Iwakura, Y.; Oya, M. Conformation of sequential polypeptides of L-valine in solution. Chem. Lett. 1973, 2, 763–768. [Google Scholar] [CrossRef]
- Teixidó, M.; Albericio, F.; Giralt, E. Solid-phase synthesis and characterization of N-methyl-rich peptides. J. Pept. Res. 2005, 65, 153–166. [Google Scholar] [CrossRef]
- Revilla-López, G.; Rodríguez-Ropero, F.; Curcó, D.; Torras, J.; Isabel Calaza, M.; Zanuy, D.; Jiménez, A.I.; Cativiela, C.; Nussinov, R.; Alemán, C. Integrating the intrinsic conformational preferences of noncoded α-amino acids modified at the peptide bond into the noncoded amino acids database. Proteins 2011, 79, 1841–1852. [Google Scholar] [CrossRef]
- Doedens, L.; Opperer, F.; Cai, M.; Beck, J.G.; Dedek, M.; Palmer, E.; Hruby, V.J.; Kessler, H. Multiple N-methylation of MT-II backbone amide bonds leads to melanocortin receptor subtype hMC1R selectivity: Pharmacological and conformational studies. J. Am. Chem. Soc. 2010, 132, 8115–8128. [Google Scholar] [CrossRef]
- Merlino, F.; Billard, É.; Yousif, A.M.; Di Maro, S.; Brancaccio, D.; Abate, L.; Carotenuto, A.; Bellavita, R.; d’Emmanuele di Villa Bianca, R.; Santicioli, P. Functional selectivity revealed by N-methylation scanning of human urotensin II and related peptides. J. Med. Chem. 2019, 62, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, M.G.; Moya, C.G.; Pérez, C.S.; Porzel, A.; Wessjohann, L.A.; Rivera, D.G. Improved stability and tunable functionalization of parallel β-sheets via multicomponent N-alkylation of the turn moiety. Angew. Chem. Int. Ed. 2020, 59, 259–263. [Google Scholar] [CrossRef] [PubMed]
- St-Cyr, D.J.; Maris, T.; Lubell, W.D. Crystal-state structure analysis of β-hydroxy-γ-lactam constrained Ser/Thr peptidomimetics. Heterocycles 2010, 82, 729–737. [Google Scholar]
- Leprohon, P.; Legare, D.; Raymond, F.; Madore, E.; Hardiman, G.; Corbeil, J.; Ouellette, M. Gene expression modulation is associated with gene amplification, supernumerary chromosomes and chromosome loss in antimony-resistant Leishmania infantum. Nucleic Acids Res. 2009, 37, 1387–1399. [Google Scholar] [CrossRef]
- Brotherton, M.-C.; Bourassa, S.; Leprohon, P.; Légaré, D.; Poirier, G.G.; Droit, A.; Ouellette, M. Proteomic and genomic analyses of antimony resistant Leishmania infantum mutant. PLoS ONE 2013, 8, e81899. [Google Scholar] [CrossRef]
- El Fadili, K.; Messier, N.; Leprohon, P.; Roy, G.; Guimond, C.; Trudel, N.; Saravia, N.G.; Papadopoulou, B.; Légaré, D.; Ouellette, M. Role of the ABC transporter MRPA (PGPA) in antimony resistance in Leishmania infantum axenic and intracellular amastigotes. Antimicrob. Agents Chemother. 2005, 49, 1988–1993. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, M.-C.; Bourassa, S.; Légaré, D.; Poirier, G.G.; Droit, A.; Ouellette, M. Quantitative proteomic analysis of amphotericin B resistance in Leishmania infantum. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Prada, C.; Vincent, I.M.; Brotherton, M.-C.; Roberts, M.; Roy, G.; Rivas, L.; Leprohon, P.; Smith, T.K.; Ouellette, M. Different mutations in a P-type ATPase transporter in Leishmania parasites are associated with cross-resistance to two leading drugs by distinct mechanisms. PLoS Negl. Trop. Dis. 2016, 10, e0005171. [Google Scholar] [CrossRef] [PubMed]
- Moreira, W.; Leprohon, P.; Ouellette, M. Tolerance to drug-induced cell death favours the acquisition of multidrug resistance in Leishmania. Cell Death Dis. 2011, 2, e201. [Google Scholar] [CrossRef]


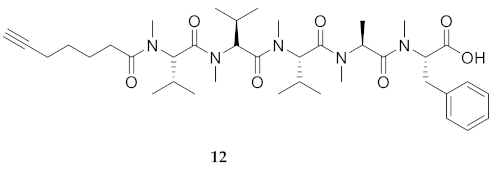
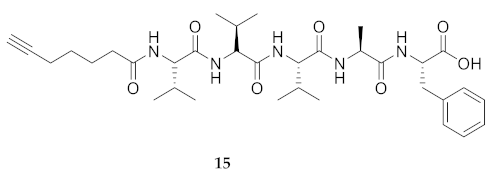
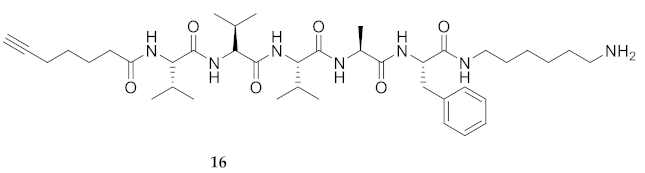
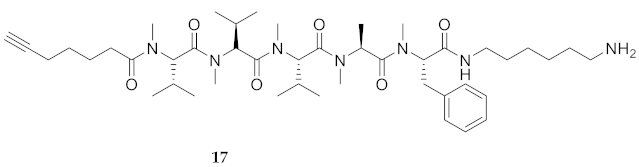
| # | Sequences (R- = HCC(CH2)4CO-) | RT | Purity at 214 nm | MS [M + 1] | ||
|---|---|---|---|---|---|---|
| CH3OH | CH3CN b | m/z (calcd) | m/z (obsd) | |||
| 12 | R-Val(Me)-Val(Me)-Val(Me)-Ala(Me)-Phe(Me)-OH | 6.83 a | 7.19 | >99 | 734.4463 | 734.4468 |
| 15 | R-Val-Val-Val-Ala-Phe-OH | 8.83 a | 7.44 | >99 | 642.3861 | 642.3865 |
| 16 | R-Val-Val-Val-Ala-Phe-NH(CH2)6NH2 | 7.33 a | 6.52 | >99 | 740.5067 | 740.5069 |
| 17 | R-Val(Me)-Val(Me)-Val(Me)-Ala(Me)-Phe(Me)-NH(CH2)6NH2 | 8.95 a | 7.81 | >99 | 810.5852 | 810.5851 |
| 18 | R-Val(Me)-Val-Val-Ala-Phe-NH(CH2)6NH2 | 8.42 a | 6.90 | >99 | 754.5225 | 754.5232 |
| 19 | R-Val-Val(Me)-Val-Ala-Phe-NH(CH2)6NH2 | 7.86 a | 6.15 | >99 | 754.5225 | 754.5235 |
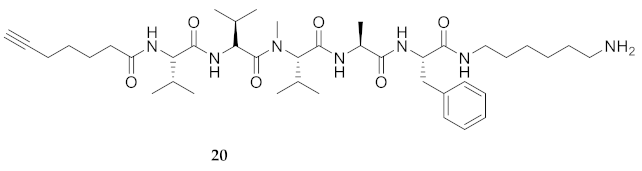
| 20 | R-Val-Val-Val(Me)-Ala-Phe-NH(CH2)6NH2 | 7.91 a | 6.57 | >99 | 754.5225 | 754.5229 |
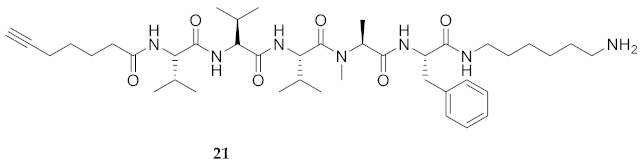
| 21 | R-Val-Val-Val-Ala(Me)-Phe-NH(CH2)6NH2 | 7.61 a | 6.44 | >99 | 754.5225 | 754.5225 |
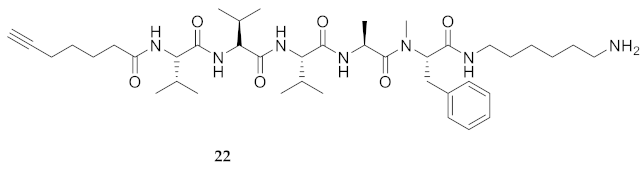
| 22 | R-Val-Val-Val-Ala-Phe(Me)-NH(CH2)6NH2 | 7.62 a | 6.62 | >99 | 754.5225 | 754.5225 |
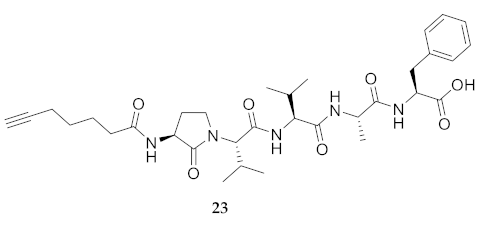
| 23 | R-Agl-Val-Val-Ala-Phe-OH | 7.11 c | 6.71 | >99 | 626.3548 | 626.3523 |
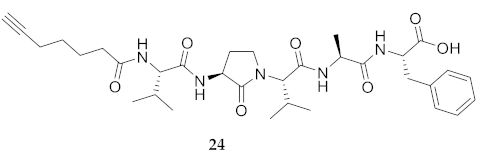
| 24 | R-Val-Agl-Val-Ala-Phe-OH | 7.55 c | 6.87 | >99 | 626.3548 | 626.3529 |
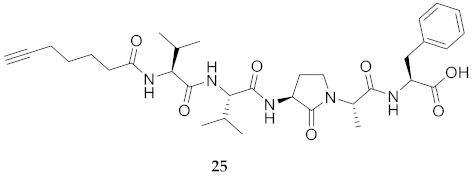
| 25 | R-Val-Val-Agl-Ala-Phe-OH | 7.94 c | 7.17 | >99 | 626.3548 | 626.3521 |
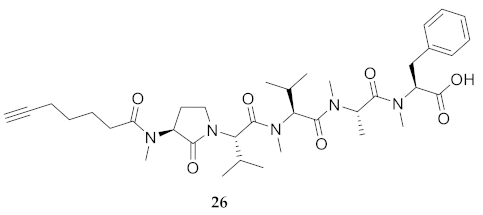
| 26 | R-Agl(Me)-Val-Val(Me)-Ala(Me)-Phe(Me)-OH | 8.69 c | 7.95 | >99 | 682.4174 | 682.4149 |
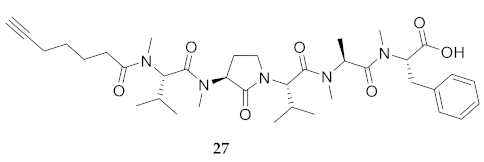
| 27 | R-Val(Me)-Agl(Me)-Val-Ala(Me)-Phe(Me)-OH | 9.12 c | 8.26 | >99 | 704.3993 d | 704.3999 d |
| 28 | R-Val(Me)-Val(Me)-Agl(Me)-Ala-Phe(Me)-OH | 9.01 c | 8.43 | >99 | 704.3993 d | 704.3964 d |
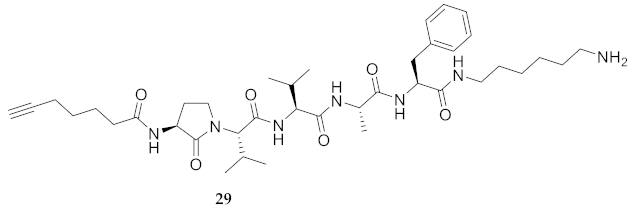
| 29 | R-Agl-Val-Val-Ala-Phe-NH(CH2)6NH2 | 6.72 a | 6.00 | >94 | 724.4756 | 724.4763 |
| 30 | R-Val-Agl-Val-Ala-Phe-NH(CH2)6NH2 | 6.83 a | 5.98 | >96 | 724.4756 | 724.4774 |
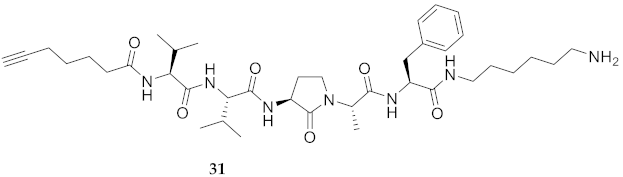
| 31 | R-Val-Val-Agl-Ala-Phe-NH(CH2)6NH2 | 7.50 c | 6.44 | >99 | 724.4756 | 724.4768 |
| 32 | R-Val-Val-Val-Agl-Phe-NH(CH2)6NH2 | 5.31 b | 6.42 | >92 | 752.5069 | 752.5082 |
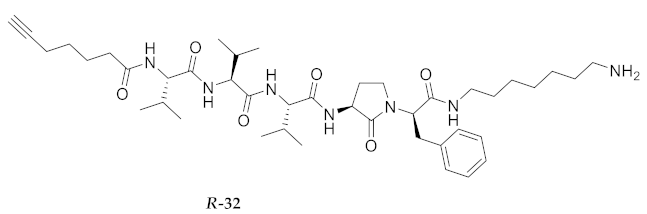
| R-32 | R-Val-Val-Val-Agl-D-Phe-NH(CH2)6NH2 | 5.07 b | 6.42 | >94 | 752.5069 | 752.5072 |
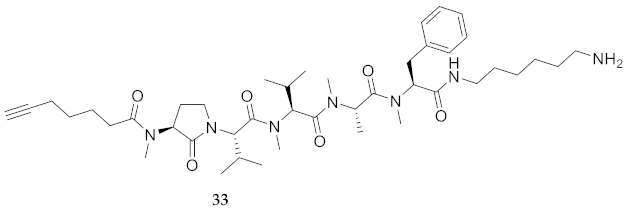
| 33 | R-Agl(Me)-Val-Val(Me)-Ala(Me)-Phe(Me)-NH(CH2)6NH2 | 6.72 c | 7.03 | >99 | 780.5382 | 780.5389 |
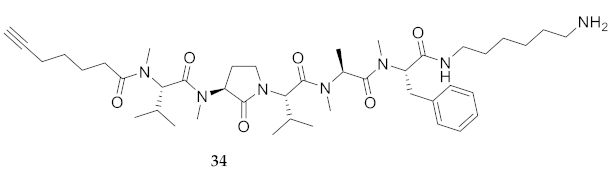
| 34 | R-Val(Me)-Agl(Me)-Val-Ala(Me)-Phe(Me)-NH(CH2)6NH2 | 6.86 c | 7.09 | >99 | 780.5382 | 780.5383 |
| 35 | R-Val(Me)-Val(Me)-Agl(Me)-Ala-Phe(Me)-NH(CH2)6NH2 | 6.80 c | 7.18 | >99 | 780.5382 | 780.5394 |
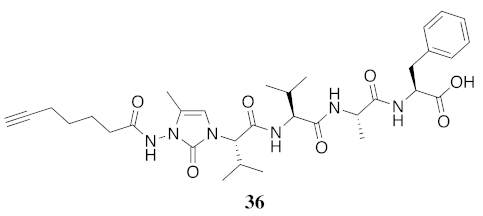
| 36 | R-(5-Me)Nai-Val-Val-Ala-Phe-OH | 5.34 c | 6.48 | >99 | 639.3501 | 639.3474 |
| 37 | R-Val-(5-Me)Nai-Val-Ala-Phe-OH | 4.41 c | 8.68 | >96 | 639.3501 | - |
| # | Sequence (R- = HCC(CH2)4CO-) | Ldi WT 1 (μM) | Ldi Sb-Res 2 (μM) | Ldi MF-Res 3 (μM) | Ldi AmB-Res 4 (μM) | CC50 (μM) | Selectivity Index |
|---|---|---|---|---|---|---|---|
| 12 | R-Val(Me)-Val(Me)-Val(Me)-Ala(Me)-Phe(Me)-OH | 68.33 [61.53, 75.00] | 70.69 [65.62, 75.89] | 75.60 [71.05, 79.15] | 79.59 [73.92, 85.46] | 281 | 4.1 |
| 15 | R-Val-Val-Val-Ala-Phe-OH | 44.69 [34.60, 58.13] | 60.26 [54.09, 66.33] | 50.77 [44.46, 57.93] | 46.38 [41.63, 51.59] | 316 | 7.0 |
| 16 | R-Val-Val-Val-Ala-Phe-NH(CH2)6NH2 | 23.38 [17.49, 32.79] | 30.34 [24.31, 38.73] | 18.09 [16.25, 20.15] | 20.76 [18.22, 23.64] | 446 | 19.0 |
| 17 | R-Val(Me)-Val(Me)-Val(Me)-Ala(Me)-Phe(Me)-NH(CH2)6NH2 | 48.42 [44.44, 52.60] | 60.18 [55.50, 65.25] | 25.46 [21.71, 29.97] | 5.60 [5.06, 6.16] | 338 | 6.9 |
| 18 | R-Val(Me)-Val-Val-Ala-Phe-NH(CH2)6NH2 | 36.70 [33.61, 40.00] | 39.65 [36.65, 42.83] | 12.12 [10.87, 13.58] | 5.03 [4.71, 5.34] | 177 | 4.8 |
| 19 | R-Val-Val(Me)-Val-Ala-Phe-NH(CH2)6NH2 | 81.17 [75.54, 87.30] | 78.91 [74.53, 83.48] | 89.23 [83.11, 95.96] | 70.26 [65.91, 75.02] | 316 | 3.9 |
| 20 | R-Val-Val-Val(Me)-Ala-Phe-NH(CH2)6NH2 | 53.86 [46.63, 62.51] | 40.25 [34.98, 46.35] | 60.64 [55.44, 66.22] | 57.18 [52.23, 62.35] | 281 | 5.2 |
| 21 | R-Val-Val-Val-Ala(Me)-Phe-NH(CH2)6NH2 | 75.83 [70.34, 81.57] | 79.78 [74.41, 85.68] | 63.53 [58.45, 69.01] | 65.66 [59.68, 72.35] | 1440 | 18.9 |
| 22 | R-Val-Val-Val-Ala-Phe(Me)-NH(CH2)6NH2 | 29.04 [24.49, 34.38] | 25.02 [20.45, 30.70] | 27.76 [23.00, 33.13] | 25.91 [21.00, 31.54] | 446 | 15.3 |
| 23 | R-Agl-Val-Val-Ala-Phe-OH | 225.1 [201.5, 261.6] | 240.6 [209.0, 299.3] | 231.5 [203.1, 280.7] | 211.7 [193.1, 238.8] | 363 | 1.61 |
| 24 | R-Val-Agl-Val-Ala-Phe-OH | 248.1 [231.3, 270.5] | 251.1 [233.4, 274.7] | 237.5 [218.6, 263.7] | 261.1 [233.1, 304.7] | 398 | 1.6 |
| 25 | R-Val-Val-Agl-Ala-Phe-OH | N.D. | N.D. | N.D. | N.D. | 416 | - |
| 26 | R-Agl(Me)-Val-Val(Me)-Ala(Me)-Phe(Me)-OH | 217.00 [204.5, 232.8] | 220.4 [209.9, 233.0] | 223.7 [211.1, 239.1] | 252.3 [229.1, 284.9] | 316 | 1.46 |
| 27 | R-Val(Me)-Agl(Me)-Val-Ala(Me)-Phe(Me)-OH | 283.5 [261.8, 314.1] | 295.8 [275.1, 324.4] | N.D. | N.D. | 295 | 1.0 |
| 28 | R-Val(Me)-Val(Me)-Agl(Me)-Ala-Phe(Me)-OH | 254.7 [238.4, 274.5] | 250.2 [232.1, 272.7] | 281.5 [269.8, 295.7] | N.D. | 245 | 0.9 |
| 29 | R-Agl-Val-Val-Ala-Phe-NH(CH2)6NH2 | 74.98 [69.52, 80.41] | 90.39 [86.16, 94.65] | 67.89 [64.58, 71.27] | 71.98 [69.28, 74.69] | 416 | 5.5 |
| 30 | R-Val-Agl-Val-Ala-Phe-NH(CH2)6NH2 | 47.43 [40.21, 55.05] | 37.65 [31.00, 45.19] | 129.3 [125.3, 133.6] | 114.4 [111.4, 117.5] | 407 | 8.6 |
| 31 | R-Val-Val-Agl-Ala-Phe-NH(CH2)6NH2 | 64.64 [59.94, 69.44] | 48.78 [41.26, 56.36] | 126.4 [122.8, 130.1] | 130.20 [128.1, 132.6] | 389 | 6.0 |
| 32 | R-Val-Val-Val-Agl-Phe-NH(CH2)6NH2 | 91.17 [88.95, 93.47] | 87.78 [85.60, 90.02] | 84.21 [82.07, 86.39] | 90.08 [88.22, 91.99] | 389 | 4.3 |
| R-32 | R-Val-Val-Val-Agl-D-Phe-NH(CH2)6NH2 | 92.56 [90.51, 94.66] | 110.8 [106.2, 115.5] | 123.1 [120.5, 125.8] | 117.6 [114.8, 120.4] | 338 | 3.6 |
| 33 | R-Agl(Me)-Val-Val(Me)-Ala(Me)-Phe(Me)-NH(CH2)6NH2 | 119.4 [116.2, 122.8] | 140.4 [138.4, 142.4] | 191.3 [185.1, 198.4] | 183.7 [177.1, 191.1] | 407 | 3.4 |
| 34 | R-Val(Me)-Agl(Me)-Val-Ala(Me)-Phe(Me)-NH(CH2)6NH2 | 33.22 [31.06, 35.49] | 35.75 33.73, 37.88] | 23.13 [20.72, 25.80] | 24.75 [22.12, 27.67] | 398 | 12.0 |
| 35 | R-Val(Me)-Val(Me)-Agl(Me)-Ala-Phe(Me)-NH(CH2)6NH2 | 78.56 [74.91, 82.31] | 81.52 [78.04, 85.14] | 85.30 [80.97, 89.88] | 92.04 [88.01, 96.27] | 371 | 4.7 |
| 36 | R-(5-Me)Nai-Val-Val-Ala-Phe-OH | N.D. | N.D. | N.D. | N.D. | 436 | - |
| 37 | R-Val-(5-Me)Nai-Val-Ala-Phe-OH | N.D. | N.D. | N.D. | N.D. | 407 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, A.M.T.; Brettell, S.; Douanne, N.; Duquette, C.; Corbeil, A.; Fajardo, E.F.; Olivier, M.; Fernandez-Prada, C.; Lubell, W.D. Influence of N-Methylation and Conformation on Almiramide Anti-Leishmanial Activity. Molecules 2021, 26, 3606. https://doi.org/10.3390/molecules26123606
Nguyen AMT, Brettell S, Douanne N, Duquette C, Corbeil A, Fajardo EF, Olivier M, Fernandez-Prada C, Lubell WD. Influence of N-Methylation and Conformation on Almiramide Anti-Leishmanial Activity. Molecules. 2021; 26(12):3606. https://doi.org/10.3390/molecules26123606
Chicago/Turabian StyleNguyen, Anh Minh Thao, Skye Brettell, Noélie Douanne, Claudia Duquette, Audrey Corbeil, Emanuella F. Fajardo, Martin Olivier, Christopher Fernandez-Prada, and William D. Lubell. 2021. "Influence of N-Methylation and Conformation on Almiramide Anti-Leishmanial Activity" Molecules 26, no. 12: 3606. https://doi.org/10.3390/molecules26123606
APA StyleNguyen, A. M. T., Brettell, S., Douanne, N., Duquette, C., Corbeil, A., Fajardo, E. F., Olivier, M., Fernandez-Prada, C., & Lubell, W. D. (2021). Influence of N-Methylation and Conformation on Almiramide Anti-Leishmanial Activity. Molecules, 26(12), 3606. https://doi.org/10.3390/molecules26123606








