Response Surface Methodology to Optimize the Isolation of Dominant Volatile Compounds from Monofloral Greek Thyme Honey Using SPME-GC-MS
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical and Melissopalynological Analysis
2.2. Isolation of Volatile Compounds
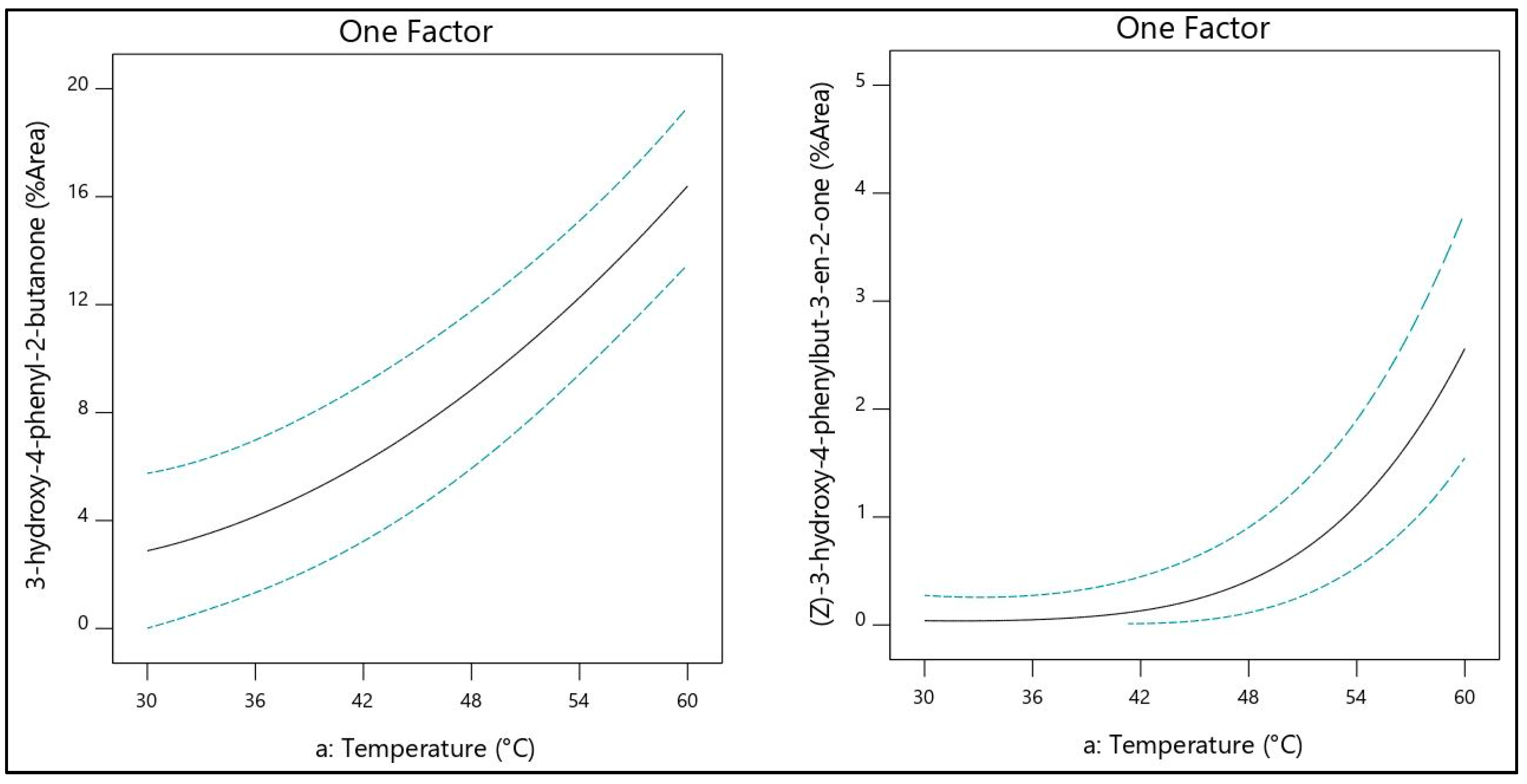
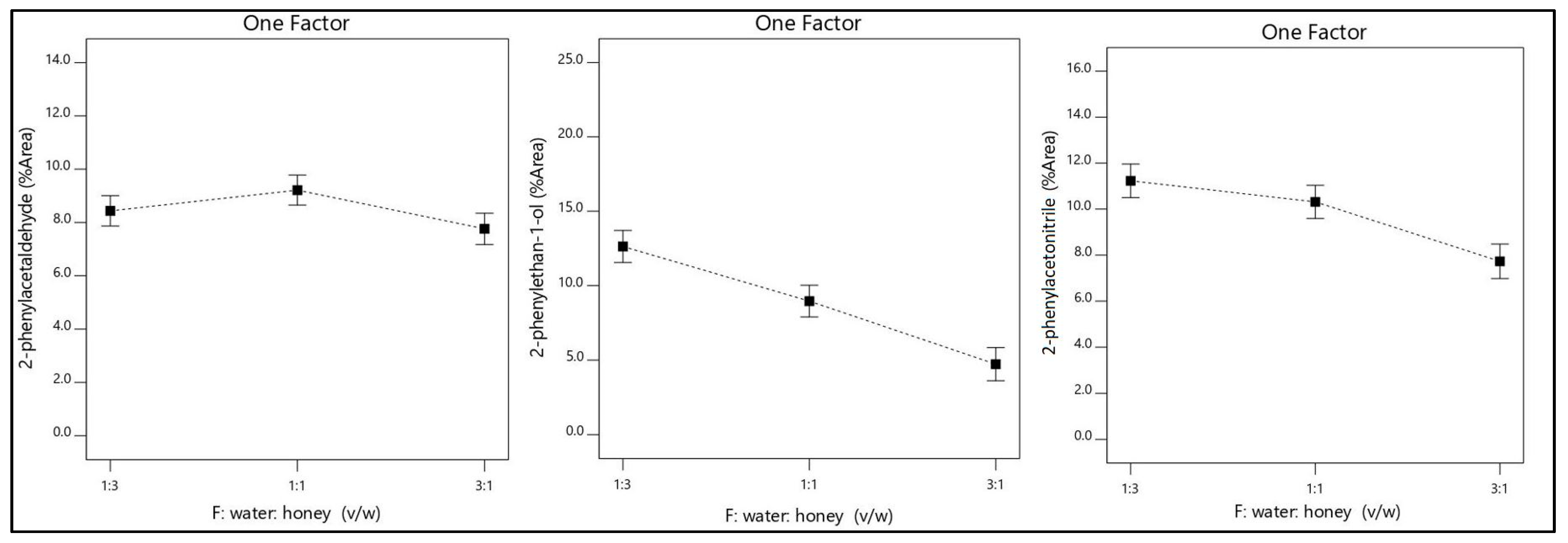
2.3. Evaluation of Factors
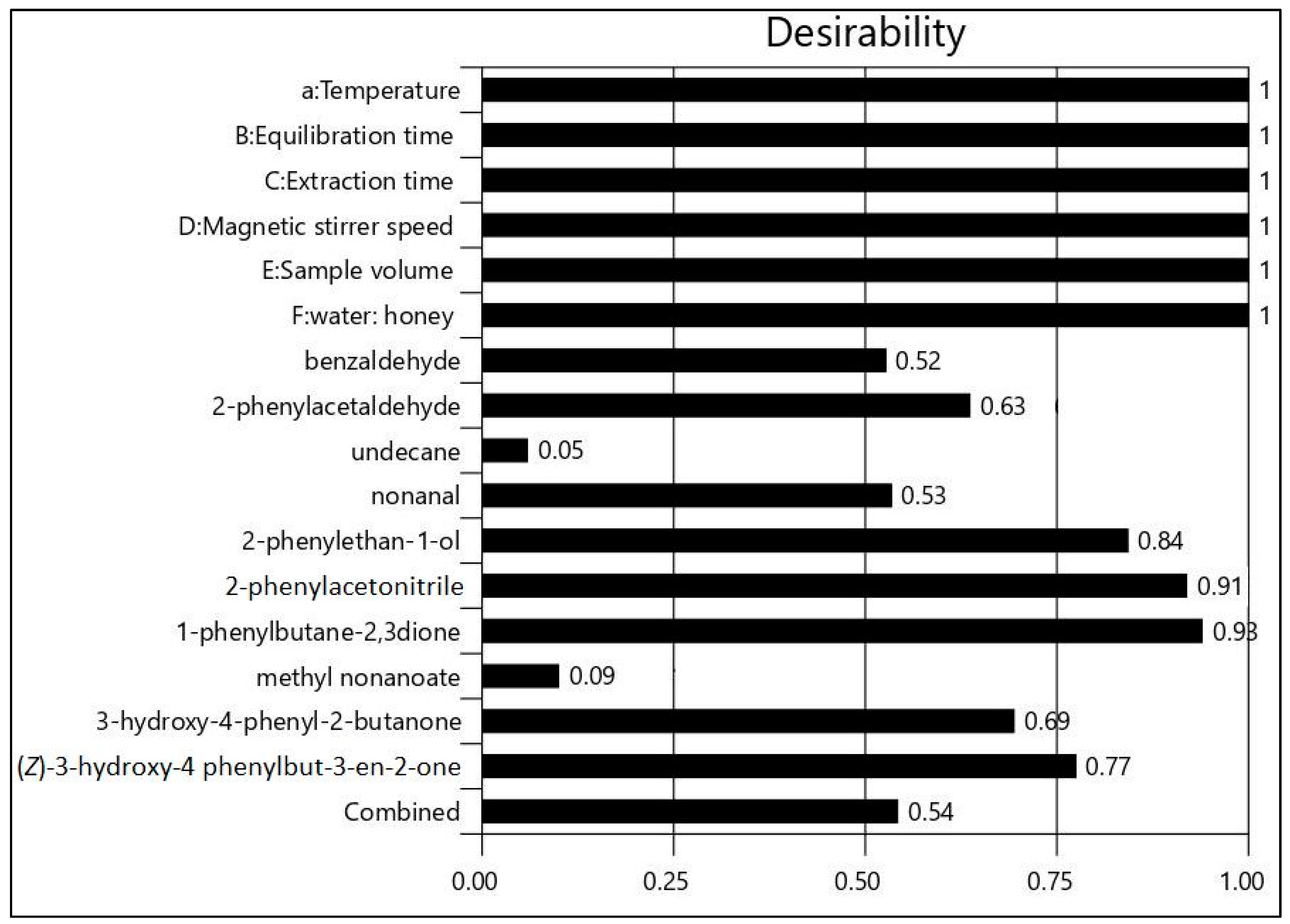
2.4. Evaluation and Optimization of Dominant Volatile Compounds
3. Materials and Methods
3.1. Honey Samples
3.2. Experimental Design
3.3. Isolation and Analysis of Volatile Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greek Statistical Authority. Available online: https://www.statistics.gr/el/statistics/agr (accessed on 1 February 2021).
- Pan-Hellenic Center for Export Research and Studies. Available online: http://www.pse.gr/node/145 (accessed on 1 February 2021).
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Samarghandian, S. Molecular Mechanism-Based Therapeutic Properties of Honey. Biomed. Pharmacother. 2020, 130, 110590. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vázquez, L.; Pérez-Coello, M.S.; Cabezudo, M.D. Analysis of Volatile Compounds of Rosemary Honey. Comparison of Different Extraction Techniques. Chromatographia 2003, 57, 227–233. [Google Scholar] [CrossRef]
- Cuevas-Glory, L.F.; Pino, J.A.; Santiago, L.S.; Sauri-Duch, E. A Review of Volatile Analytical Methods for Determining the Botanical Origin of Honey. Food Chem. 2007, 103, 1032–1043. [Google Scholar] [CrossRef]
- Bicchi, C.; Belliardo, F.; Frattini, C. Identification of the Volatile Components of Some Piedmontese Honeys. J. Apic. Res. 1983, 22, 130–136. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Tarantilis, P.A.; Harizanis, P.C.; Polissiou, M. Evaluation of Four Isolation Techniques for Honey Aroma Compounds. J. Sci. Food Agric. 2004, 85, 91–97. [Google Scholar] [CrossRef]
- Uckun, O.; Selli, S. Characterization of Key Aroma Compounds in a Representative Aromatic Extracts from Citrus and Astragalus Honeys Based on Aroma Extract Dilution Analyses. Food Meas. 2016, 11, 512–522. [Google Scholar] [CrossRef]
- Kuś, P.M.; Jerković, I. New Sample Preparation Method for Honey Volatiles Fingerprinting Based on Dehydration Homogeneous Liquid–Liquid Extraction (DHLLE). Molecules 2018, 23, 1769. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Makowicz, E.; Stanek, N. Chromatographic Fingerprint, Antioxidant Activity, and Colour Characteristic of Polish Goldenrod (Solidago virgaurea L.) Honey and Flower. Eur. Food. Res. Technol. 2018, 244, 1169–1184. [Google Scholar] [CrossRef]
- Bouseta, A.; Collin, S.; Dufour, J.-P. Characteristic Aroma Profiles of Unifloral Honeys Obtained with a Dynamic Headspace GC-MS System. J. Apic. Res. 1992, 31, 96–109. [Google Scholar] [CrossRef]
- Bianchi, F.; Mangia, A.; Mattarozzi, M.; Musci, M. Characterization of the Volatile Profile of Thistle Honey Using Headspace Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry. Food Chem. 2011, 129, 1030–1036. [Google Scholar] [CrossRef]
- Petretto, G.L.; Urgeghe, P.P.; Mascia, I.; Fadda, C.; Rourke, J.P.; Pintore, G. Stir Bar Sorptive Extraction Coupled with GC/MS Applied to Honey: Optimization of Method and Comparative Study with Headspace Extraction Techniques. Eur. Food Res. Technol. 2016, 243, 735–741. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Tarantilis, P.A.; Harizanis, P.C.; Polissiou, M. Comparison of the Volatile Composition in Thyme Honeys from Several Origins in Greece. J. Agric. Food Chem. 2007, 55, 8152–8157. [Google Scholar] [CrossRef] [PubMed]
- Xagoraris, M.; Revelou, P.-K.; Dedegkika, S.; Kanakis, C.D.; Papadopoulos, G.K.; Pappas, C.S.; Tarantilis, P.A. SPME-GC-MS and FTIR-ATR Spectroscopic Study as a Tool for Unifloral Common Greek Honeys’ Botanical Origin Identification. Appl. Sci. 2021, 11, 3159. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Daferera, D.; Tarantilis, P.A.; Polissiou, M.; Harizanis, P.C. Ultrasound-Assisted Extraction of Volatile Compounds from Citrus Flowers and Citrus Honey. Food Chem. 2003, 82, 575–582. [Google Scholar] [CrossRef]
- Soria, A.C.; Martínez-Castro, I.; Sanz, J. Analysis of Volatile Composition of Honey by Solid Phase Microextraction and Gas Chromatography-Mass Spectrometry. J. Sep. Sci. 2003, 26, 793–801. [Google Scholar] [CrossRef]
- Soria, A.C.; Sanz, J.; Martínez-Castro, I. SPME Followed by GC–MS: A Powerful Technique for Qualitative Analysis of Honey Volatiles. Eur. Food Res. Technol. 2008, 228, 579–590. [Google Scholar] [CrossRef]
- Plutowska, B.; Chmiel, T.; Dymerski, T.; Wardencki, W. A Headspace Solid-Phase Microextraction Method Development and Its Application in the Determination of Volatiles in Honeys by Gas Chromatography. Food Chem. 2011, 126, 1288–1298. [Google Scholar] [CrossRef]
- Bianchin, J.N.; Nardini, G.; Merib, J.; Dias, A.N.; Martendal, E.; Carasek, E. Screening of Volatile Compounds in Honey Using a New Sampling Strategy Combining Multiple Extraction Temperatures in a Single Assay by HS-SPME-GC-MS. Food Chem. 2014, 145, 1061–1065. [Google Scholar] [CrossRef]
- Robotti, E.; Campo, F.; Riviello, M.; Bobba, M.; Manfredi, M.; Mazzucco, E.; Gosetti, F.; Calabrese, G.; Sangiorgi, E.; Marengo, E. Optimization of the Extraction of the Volatile Fraction from Honey Samples by SPME-GC-MS, Experimental Design, and Multivariate Target Functions. J. Chem. 2017, 1–14. [Google Scholar] [CrossRef]
- da Costa, A.C.V.; Sousa, J.M.B.; Bezerra, T.K.A.; da Silva, F.L.H.; Pastore, G.M.; da Silva, M.A.A.P.; Madruga, M.S. Volatile Profile of Monofloral Honeys Produced in Brazilian Semiarid Region by Stingless Bees and Key Volatile Compounds. LWT 2018, 94, 198–207. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Government Gazette B-239/23-2-2005 Annex II Article 67 of Greek Food Code 2005. Available online: http://www.minagric.gr/images/stories/docs/agrotis/MeliMelissokomia/KYATaytopoiisi.pdf (accessed on 20 May 2021).
- Karabagias, I.K.; Badeka, A.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Characterization and Classification of Thymus capitatus (L.) Honey According to Geographical Origin Based on Volatile Compounds, Physicochemical Parameters and Chemometrics. Food Res. Int. 2014, 55, 363–372. [Google Scholar] [CrossRef]
- Karabagias, I.K. Volatile Metabolites or Pollen Characteristics as Regional Markers of Monofloral Thyme Honey? Sep. Sci. Plus 2018, 1, 83–92. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Ndip, R.N.; Clarke, A.M. Volatile Compounds in Honey: A Review on Their Involvement in Aroma, Botanical Origin Determination and Potential Biomedical Activities. Int. J. Mol. Sci. 2011, 12, 9514–9532. [Google Scholar] [CrossRef] [PubMed]
- Visser, F.R.; Allen, J.M.; Shaw, G.J. The Effect of Heat on the Volatile Flavour Fraction from a Unifloral Honey. J. Apic. Res. 1988, 27, 175–181. [Google Scholar] [CrossRef]
- Yang, X.; Peppard, T. Solid-Phase Microextraction for Flavor Analysis. J. Agric. Food Chem. 1994, 42, 1925–1930. [Google Scholar] [CrossRef]
- Zhang, Z.; Pawliszyn, J. Headspace Solid-Phase Microextraction. Anal. Chem. 1993, 65, 1843–1852. [Google Scholar] [CrossRef]
- Helrich, K. (Ed.) Official Methods of Analysis of Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; Volume 1. [Google Scholar]
- International Honey Commission. Harmonides Methods of the International Honey Commission; Bee Product Science; Bogdanov, S., Ed.; Swiss Bee Research Centre FAM: Bern, Switzerland, 2009; Available online: https://www.ihc-platform.net/ihcmethods2009.pdf (accessed on 20 May 2021).
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Xagoraris, M.; Lazarou, E.; Kaparakou, E.H.; Alissandrakis, E.; Tarantilis, P.A.; Pappas, C.S. Botanical origin discrimination of Greek honeys: Physicochemical parameters versus Raman spectroscopy. J. Sci. Food Agric. 2021, 101, 3319–3327. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.P.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. J. R. Stat. Soc. Ser. B Stat Methodol. 1951, 13, 1–38. [Google Scholar] [CrossRef]
- Al-Suod, H.; Ratiu, I.-A.; Górecki, R.; Buszewski, B. Pressurized liquid extraction of cyclitols and sugars: Optimization of extraction parameters and selective separation. J. Sep. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Al-Suod, H.; Ratiu, I.; Krakowska-Sieprawska, A.; Lahuta, L.; Górecki, R.; Buszewski, B. Supercritical fluid extraction in isolation of cyclitols and sugars from chamomile flowers. J. Sep. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Pub Corp: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]


| Aggregate Functions | Fructose + Glucose a (%w/w) | Sucrose b (%w/w) | Moisture c (%w/w) | Electrical Conductivity d (μS cm−1) | Diastase Activity e (Schade) | HMF f (mg kg−1) | Thymbra capitata L. Pollen g (%) |
|---|---|---|---|---|---|---|---|
| Min | 60.1 | 0.0 | 13.8 | 251 | 11.1 | 1.0 | 18.0 |
| Max | 86.4 | 2.1 | 17.9 | 600 | 51.1 | 14.7 | 77.9 |
| Average | 68.1 | 0.3 | 15.8 | 457 | 27.3 | 5.2 | 33.1 |
| No. | Volatile Compounds | RT a | RI b | Boiling Point (°C) | Min (mg kg−1) | Max (mg kg−1) | Average (mg kg−1) |
|---|---|---|---|---|---|---|---|
| Esters | |||||||
| 1 | methyl octanoate | 18.3 | 1123 | 190.6 | 0.00 | 2.72 | 0.46 |
| 2 | methyl nonanoate | 21.3 | 1222 | 213.5 | 0.09 | 11.63 | 1.41 |
| 3 | methyl decanoate | 24.3 | 1321 | 236.4 | 0.00 | 1.17 | 0.12 |
| 4 | methyl hexadecanoate | 39.7 | 1929 | 373.6 | 0.00 | 0.46 | 0.07 |
| Aldehydes | |||||||
| 5 | furan-2-carbaldehyde | 9.8 | 822 | 139.7 | 0.20 | 4.54 | 1.90 |
| 6 | benzaldehyde | 12.6 | 957 | 162.0 | 0.52 | 10.40 | 3.64 |
| 7 | 2-phenylacetaldehyde | 15.6 | 1041 | 184.8 | 0.39 | 3.56 | 1.78 |
| 8 | nonanal | 17.7 | 1104 | 181.0 | 0.00 | 1.45 | 0.31 |
| 9 | decanal | 20.8 | 1205 | 203.9 | 0.00 | 1.41 | 0.21 |
| 10 | 4-isopropylbenzaldehyde | 21.8 | 1240 | 235.1 | 0.00 | 0.20 | 0.05 |
| Alcohols | |||||||
| 11 | 2-phenylethan-1-ol | 17.9 | 1111 | 228.4 | 0.00 | 5.28 | 1.94 |
| 12 | 5-isopropyl-2-methylphenol (carvacrol) | 23.5 | 1299 | 267.1 | 0.00 | 0.26 | 0.06 |
| Ketones | |||||||
| 13 | 1-phenylbutane-2,3-dione | 20.9 | 1210 | 289.7 | 0.00 | 1.37 | 0.48 |
| 14 | 2-isopropyl-5-methylcyclohexa-2,5-diene-1,4-dione (thymoquinone) | 22.0 | 1247 | 323.0 | 0.00 | 0.59 | 0.08 |
| 15 | 3-hydroxy-4-phenyl-2-butanone | 24.9 | 1343 | 327.5 | 0.00 | 3.87 | 0.93 |
| 16 | (Z)-3-hydroxy-4-phenylbut-3-en-2-one | 27.7 | 1427 | 332.0 | 0.00 | 0.37 | 0.09 |
| Hydrocarbons | |||||||
| 17 | butane | 1.6 | <800 | 18.0 | 0.00 | 20.58 | 1.03 |
| 18 | heptane | 3.4 | <800 | 86.6 | 0.00 | 10.32 | 0.58 |
| 19 | octane | 5.9 | 800 | 109.5 | 0.00 | 5.83 | 0.45 |
| 20 | nonane | 10.3 | 896 | 132.4 | 0.00 | 1.68 | 0.20 |
| 21 | undecane | 17.6 | 1100 | 178.1 | 0.04 | 34.49 | 2.33 |
| Nitriles | |||||||
| 22 | isobutyronitrile | 2.2 | <800 | 119.6 | 0.00 | 11.66 | 0.92 |
| 23 | 2-methylbutanenitrile | 3.5 | <800 | 142.5 | 0.00 | 5.01 | 0.47 |
| 24 | 3-methylbutanenitrile | 3.7 | <800 | 142.5 | 0.05 | 59.16 | 7.01 |
| 25 | 2-phenylacetonitrile | 18.8 | 1136 | 215.4 | 0.25 | 5.35 | 1.93 |
| Terpenoids | |||||||
| 26 | methylbenzene | 4.7 | <800 | 113.3 | 0.00 | 3.38 | 0.34 |
| 27 | 1-isopropyl-4-methylbenzene (p-cymene) | 14.9 | 1022 | 186.5 | 0.00 | 2.62 | 0.39 |
| 28 | 1-methyl-4-(prop-1-en-2-yl)benzene | 17.2 | 1088 | 183.5 | 0.00 | 1.14 | 0.17 |
| Others | |||||||
| 29 | 2,5-diethyltetrahydrofuran | 10.0 | 890 | 147.1 | 0.00 | 7.22 | 1.35 |
| 30 | methyl 2-oxo-2-phenylacetate | 22.7 | 1271 | 271.1 | 0.00 | 0.54 | 0.18 |
| 31 | 1,1,5-trimethyl-1,2-dihydronaphthalene | 25.2 | 1351 | 270.9 | 0.00 | 1.39 | 0.37 |
| Response | Volatile Compound | Min (%Area) | Max (%Area) | Mean (%Area) | Std. Dev. |
|---|---|---|---|---|---|
| R1 | benzaldehyde | 4.60 | 17.32 | 11.55 | 1.57 |
| R2 | 2-phenylacetaldehyde | 0.82 | 13.48 | 6.99 | 1.03 |
| R3 | undecane | 0.62 | 16.17 | 3.86 | 0.85 |
| R4 | nonanal | 0.00 | 2.62 | 0.99 | 0.38 |
| R5 | 2-phenylethan-1-ol | 0.00 | 16.72 | 8.12 | 1.94 |
| R6 | 2-phenylacetonitrile | 1.40 | 11.59 | 6.97 | 1.31 |
| R7 | 1-phenylbutane-2,3-dione | 0.00 | 6.49 | 1.22 | 0.52 |
| R8 | methyl nonanoate | 0.53 | 15.23 | 4.32 | 2.27 |
| R9 | 3-hydroxy-4-phenyl-2-butanone | 0.00 | 17.26 | 4.58 | 1.22 |
| R10 | (Z)-3-hydroxy-4-phenylbut-3-en-2-one | 0.00 | 1.64 | 0.45 | 0.11 |
| Response | Volatile Compound | A | B | C | D | E | F | R2 |
|---|---|---|---|---|---|---|---|---|
| R1 | benzaldehyde | 0.011 a | 0.198 | 0.101 | 0.259 | 0.439 | 0.007 | 0.965 |
| R2 | 2-phenylacetaldehyde | 0.000 | 0.265 | 0.657 | 0.898 | 0.104 | 0.059 | 0.988 |
| R3 | undecane | 0.000 | 0.319 | 0.001 | 0.010 | 0.013 | 0.001 | 0.991 |
| R4 | nonanal | 0.090 | 0.951 | 0.843 | 0.004 | 0.168 | 0.007 | 0.958 |
| R5 | 2-phenylethan-1-ol | 0.000 | 0.545 | 0.013 | 0.170 | 0.324 | 0.001 | 0.980 |
| R6 | 2-phenylacetonitrile | 0.002 | 0.587 | 0.018 | 0.724 | 0.161 | 0.005 | 0.965 |
| R7 | 1-phenylbutane-2,3-dione | 0.001 | 0.943 | 0.078 | 0.916 | 0.413 | 0.012 | 0.962 |
| R8 | methyl nonanoate | 0.179 | 0.697 | 0.364 | 0.246 | 0.026 | 0.031 | 0.932 |
| R9 | 3-hydroxy-4-phenyl-2-butanone | 0.000 | 0.656 | 0.001 | 0.175 | 0.542 | 0.000 | 0.991 |
| R10 | (Z)-3-hydroxy-4-phenylbut-3-en-2-one | 0.014 | 0.092 | 0.011 | 0.680 | 0.357 | 0.026 | 0.994 |
| Response | Volatile Compound | A | B | C | D | E | F | Desirability | Predicted Mean (%Area) |
|---|---|---|---|---|---|---|---|---|---|
| R1 | benzaldehyde | 60 | 5 | 15 | 700 | 6 | 1:1 | 0.810 | 14.9 ± 1.6 |
| R2 | 2-phenylacetaldehyde | 60 | 15 | 15 | 400 | 6 | 1:1 | 0.975 | 13.2 ± 1.0 |
| R3 | undecane | 30 | 15 | 15 | 400 | 2 | 3:1 | 0.940 | 15.2 ± 0.9 |
| R4 | nonanal | 45 | 15 | 30 | 700 | 4 | 3:1 | 0.933 | 2.4 ± 0.4 |
| R5 | 2-phenylethan-1-ol | 60 | 15 | 15 | 400 | 6 | 1:3 | 0.923 | 15.4 ± 1.9 |
| R6 | 2-phenylacetonitrile | 60 | 15 | 30 | 400 | 6 | 1:3 | 0.957 | 11.1 ± 1.3 |
| R7 | 1-phenylbutane-2.3dione | 60 | 30 | 30 | 700 | 6 | 1:3 | 0.966 | 6.3 ± 0.6 |
| R8 | methyl nonanoate | 60 | 30 | 30 | 400 | 2 | 3:1 | 0.814 | 12.5 ± 2.3 |
| R9 | 3-hydroxy-4-phenyl-2-butanone | 60 | 30 | 30 | 700 | 4 | 1:3 | 0.841 | 14.5 ± 1.2 |
| R10 | (Z)-3-hydroxy-4-phenylbut-3-en-2-one | 60 | 5 | 30 | 700 | 6 | 1:3 | 0.947 | 1.5 ± 0.3 |
| Factor | Name | Units | Minimum | Maximum | Coded Low | Coded High | Mean | Std. Dev. |
|---|---|---|---|---|---|---|---|---|
| A | Temperature | °C | 30.0 | 60.0 | −1 ↔ 30.0 | +1 ↔ 60.0 | 45.0 | 13.5 |
| B | Equilibration time | min | 5.0 | 30.0 | −1 ↔ 5.0 | +1 ↔ 30.0 | 17.7 | 11.6 |
| C | Extraction time | min | 15.0 | 60.0 | −1 ↔ 15.0 | +1 ↔ 60.0 | 36.7 | 20.8 |
| D | Magnetic stirrer speed | rpm | 100.0 | 700.0 | −1 ↔ 100.0 | +1 ↔ 700.0 | 407.8 | 283.2 |
| E | Sample volume | mL | 2.0 | 6.0 | −1 ↔ 2.0 | +1 ↔ 6.0 | 4.1 | 1.8 |
| F | Water:honey ratio | v/w | 1:3 | 3:1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xagoraris, M.; Skouria, A.; Revelou, P.-K.; Alissandrakis, E.; Tarantilis, P.A.; Pappas, C.S. Response Surface Methodology to Optimize the Isolation of Dominant Volatile Compounds from Monofloral Greek Thyme Honey Using SPME-GC-MS. Molecules 2021, 26, 3612. https://doi.org/10.3390/molecules26123612
Xagoraris M, Skouria A, Revelou P-K, Alissandrakis E, Tarantilis PA, Pappas CS. Response Surface Methodology to Optimize the Isolation of Dominant Volatile Compounds from Monofloral Greek Thyme Honey Using SPME-GC-MS. Molecules. 2021; 26(12):3612. https://doi.org/10.3390/molecules26123612
Chicago/Turabian StyleXagoraris, Marinos, Alexandra Skouria, Panagiota-Kyriaki Revelou, Eleftherios Alissandrakis, Petros A. Tarantilis, and Christos S. Pappas. 2021. "Response Surface Methodology to Optimize the Isolation of Dominant Volatile Compounds from Monofloral Greek Thyme Honey Using SPME-GC-MS" Molecules 26, no. 12: 3612. https://doi.org/10.3390/molecules26123612
APA StyleXagoraris, M., Skouria, A., Revelou, P.-K., Alissandrakis, E., Tarantilis, P. A., & Pappas, C. S. (2021). Response Surface Methodology to Optimize the Isolation of Dominant Volatile Compounds from Monofloral Greek Thyme Honey Using SPME-GC-MS. Molecules, 26(12), 3612. https://doi.org/10.3390/molecules26123612











