Fluorescence Spectroscopy of Porphyrins and Phthalocyanines: Some Insights into Supramolecular Self-Assembly, Microencapsulation, and Imaging Microscopy
Abstract
1. Introduction
2. Supramolecular Self-Assembly
2.1. Luminescence of Porphyrins and Phthalocyanines Dyes
2.1.1. Porphyrinoids’ Self-Assembly by Different Templates
2.1.2. Porphyrinoids in Heterogeneous Assemblies
2.1.3. Porphyrinoids’ Peptide Conjugates
2.2. Microencapsulation
2.3. Imaging Microscopy
2.3.1. Polymer-Based Nanoparticles
2.3.2. Dendrimers
2.3.3. Carbon 2D Nanostructures
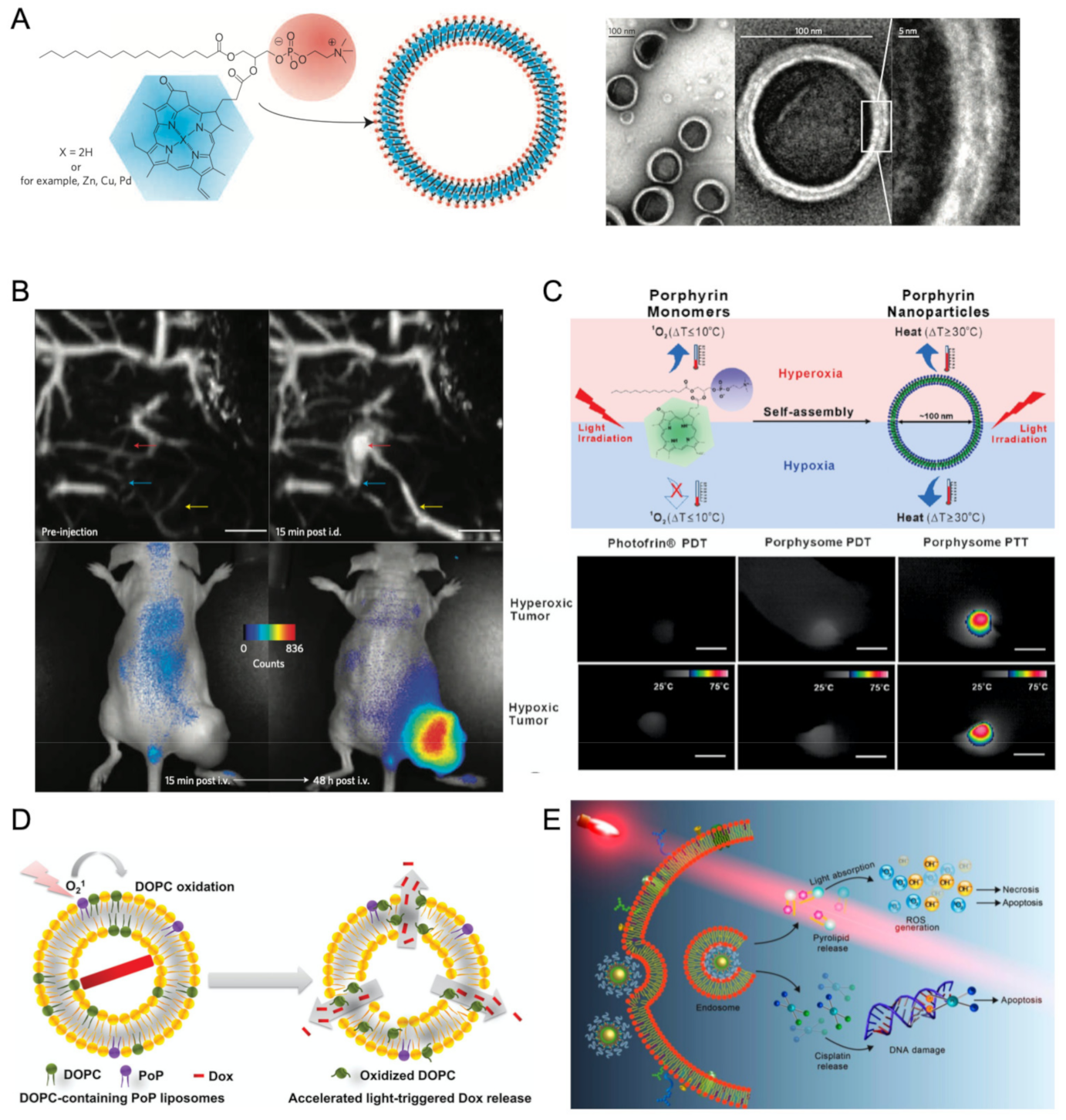
2.3.4. Liposome Nano-Assemblies
2.3.5. Metal Organic Frameworks and Silica Nanoparticles
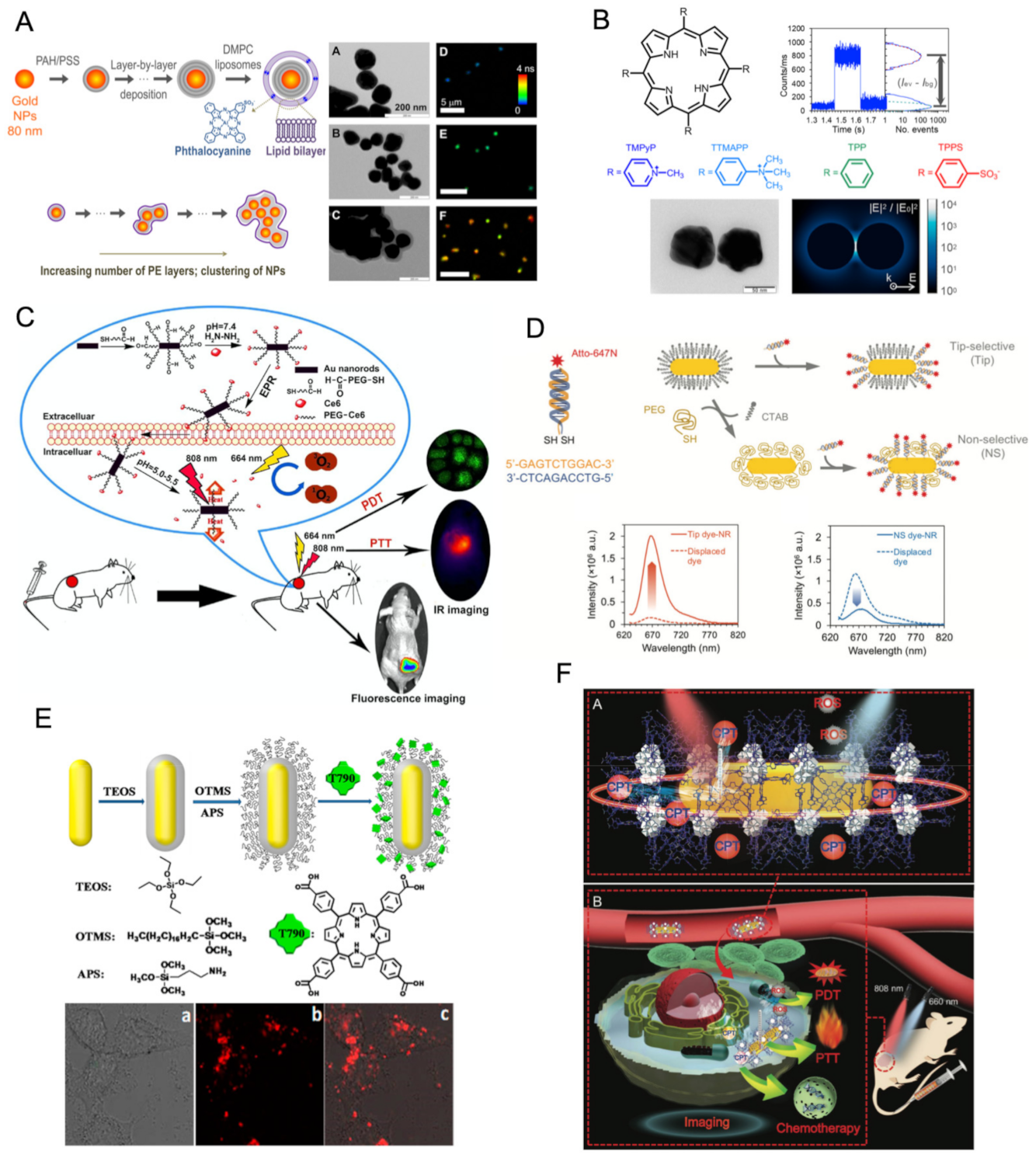
2.3.6. Core-Shell Metal Nanostructures
2.3.7. Methods and Applications in Imaging Microscopy
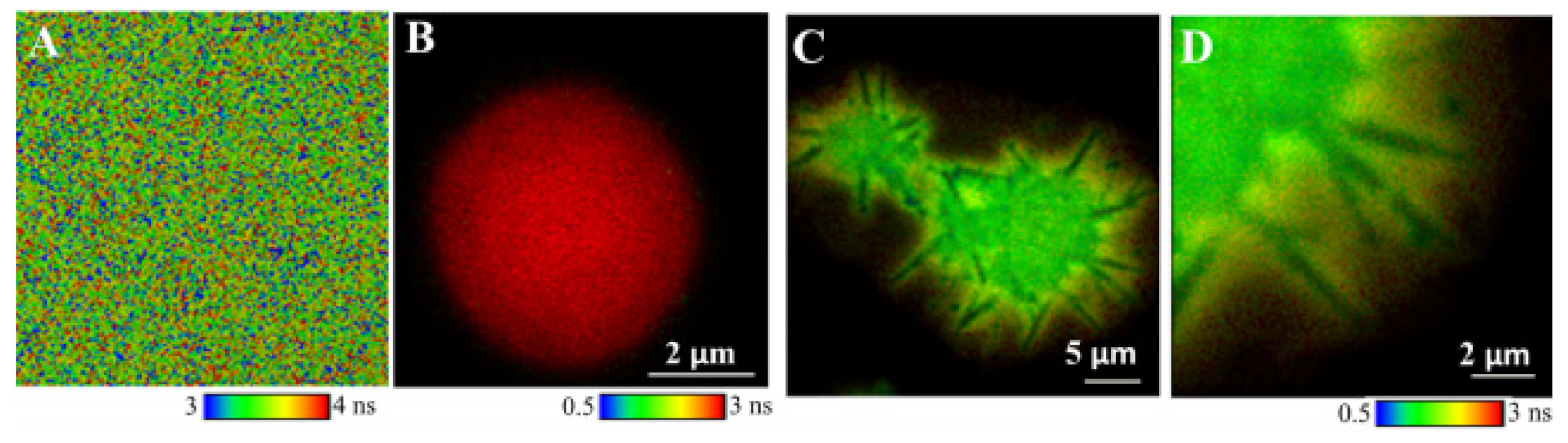
Fluorescence Lifetime Imaging Microscopy (FLIM)
Optical Sensors for Imaging Microscopy
3. Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis; Blackwell Science Ltd.: Oxford, UK, 2002; p. 4. [Google Scholar]
- Mirkovic, T.; Ostroumov, E.E.; Anna, J.M.; van Grondelle, R.; Govindjee; Scholes, G.D. Light Absorption and Energy Transfer in the Antenna Complexes of Photosynthetic Organisms. Chem. Rev. 2017, 117, 249–293. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef]
- Proppe, A.H.; Li, Y.C.; Aspuru-Guzik, A.; Berlinguette, C.P.; Chang, C.J.; Cogdell, R.; Doyle, A.G.; Flick, J.; Gabor, N.M.; van Grondelle, R.; et al. Bioinspiration in light harvesting and catalysis. Nat. Rev. Mater. 2020, 5, 828–846. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Fuhrhop, J.H. Porphyrin Assemblies and Their Scaffolds. Langmuir 2014, 30, 1–12. [Google Scholar] [CrossRef]
- Otsuki, J. Supramolecular Approach towards light-harvesting materials based on porphyrins and chlorophylls. J. Mater. Chem. A 2018, 6, 6710–6753. [Google Scholar] [CrossRef]
- Urbani, M.; Graẗzel, M.; Nazeeruddin, M.K.; Torres, T. Meso-Substituted Porphyrins for Dye-Sensitized Solar Cells. Chem. Rev. 2014, 114, 12330–12396. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.M. Supramolecular Chemistry: Concept and Perspectives; VCH: Weinheim, BC, Canada, 1995. [Google Scholar]
- Lehn, J.M. Supramolecular Chemistry—Scope and Perspectives Molecules, Supermolecules, and Molecular Devices (Nobel Lecture). Angew. Chem. Int. Ed. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Lehn, J.M. Perspectives in Supramolecular Chemistry—From Molecular Recognition towards Molecular Information Processing and Self-Organization. Angew. Chem. Int. Ed. 1990, 29, 1304–1319. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Bocheva, M. Beyond Molecules: Self-Assembly of Mesoscopic and Macroscopic Components. Proc. Natl. Acad. Sci. USA 2002, 99, 4769–4774. [Google Scholar] [CrossRef] [PubMed]
- Balzani, V.; Credi, A.; Venturi, M. Controlled Disassembling of Self-Assembling Systems: Toward Artificial Molecular-Level Devices and Machines. Proc. Natl. Acad. Sci. USA 2002, 99, 4814–4817. [Google Scholar] [CrossRef] [PubMed]
- Wasielewski, M.R. Self-Assembly Strategies for Integrating Light Harvesting and Charge Separation in Artificial Photosynthetic Systems. Acc. Chem. Res. 2009, 42, 1910–1921. [Google Scholar] [CrossRef]
- Mariani, G.; Moldenhauer, D.; Schweins, R.; Gröhn, F. Elucidating Electrostatic Self-Assembly: Molecular Parameters as Key to Thermodynamics and Nanoparticle Shape. J. Am. Chem. Soc. 2016, 138, 1280–1293. [Google Scholar] [CrossRef]
- Vantomme, G.; Meijer, E.W. The construction of supramolecular systems. Science 2019, 363, 1396–1397. [Google Scholar] [CrossRef]
- Freeman, R.; Han, M.; Álvarez, Z.; Lewis, J.A.; Wester, J.R.; Stephanopoulos, N.; McClendon, M.T.; Lynsky, C.; Godbe, J.M.; Sangji, H.; et al. Reversible self-assembly of superstructured networks. Science 2018, 362, 808–813. [Google Scholar] [CrossRef]
- Paulo, P.M.R.; Costa, S.M.B. Non-covalent dendrimer–porphyrin interactions: The intermediacy of H-aggregates? Photochem. Photobiol. Sci. 2003, 2, 597–604. [Google Scholar] [CrossRef]
- Paulo, P.M.R.; Gronheid, R.; De Schryver, F.C.; Costa, S.M.B. Porphyrin-Dendrimer Assemblies Studied by Electronic Absorption Spectra and Time-Resolved Fluorescence. Macromolecules 2003, 36, 9135–9144. [Google Scholar] [CrossRef]
- Kubát, P.; Lang, K.; Zelinger, Z. Interaction of porphyrins with PAMAM dendrimers in aqueous solution. J. Mol. Liq. 2007, 131-132, 200–205. [Google Scholar] [CrossRef]
- Paulo, P.M.R.; Costa, S.M.B. Single-Molecule Fluorescence of a Phthalocyanine in PAMAM Dendrimers Reveals Intensity−Lifetime Fluctuations from Quenching Dynamics. J. Phys. Chem. 2010, 114, 19035–19043. [Google Scholar] [CrossRef]
- Paulo, P.M.R.; Lopes, J.N.C.; Costa, S.M.B. Molecular Dynamics Simulations of Porphyrin−Dendrimer Systems: Toward Modeling Electron Transfer in Solution. J. Phys. Chem. 2008, 112, 14779–14792. [Google Scholar] [CrossRef] [PubMed]
- Paulo, P.M.R.; Costa, S.M.B. Photoinduced electron-transfer in supramolecular complex of zinc porphyrin with poly (amido amine) dendrimer donor. J. Photochem. Photobiol. Chem. 2012, 234, 66–74. [Google Scholar] [CrossRef]
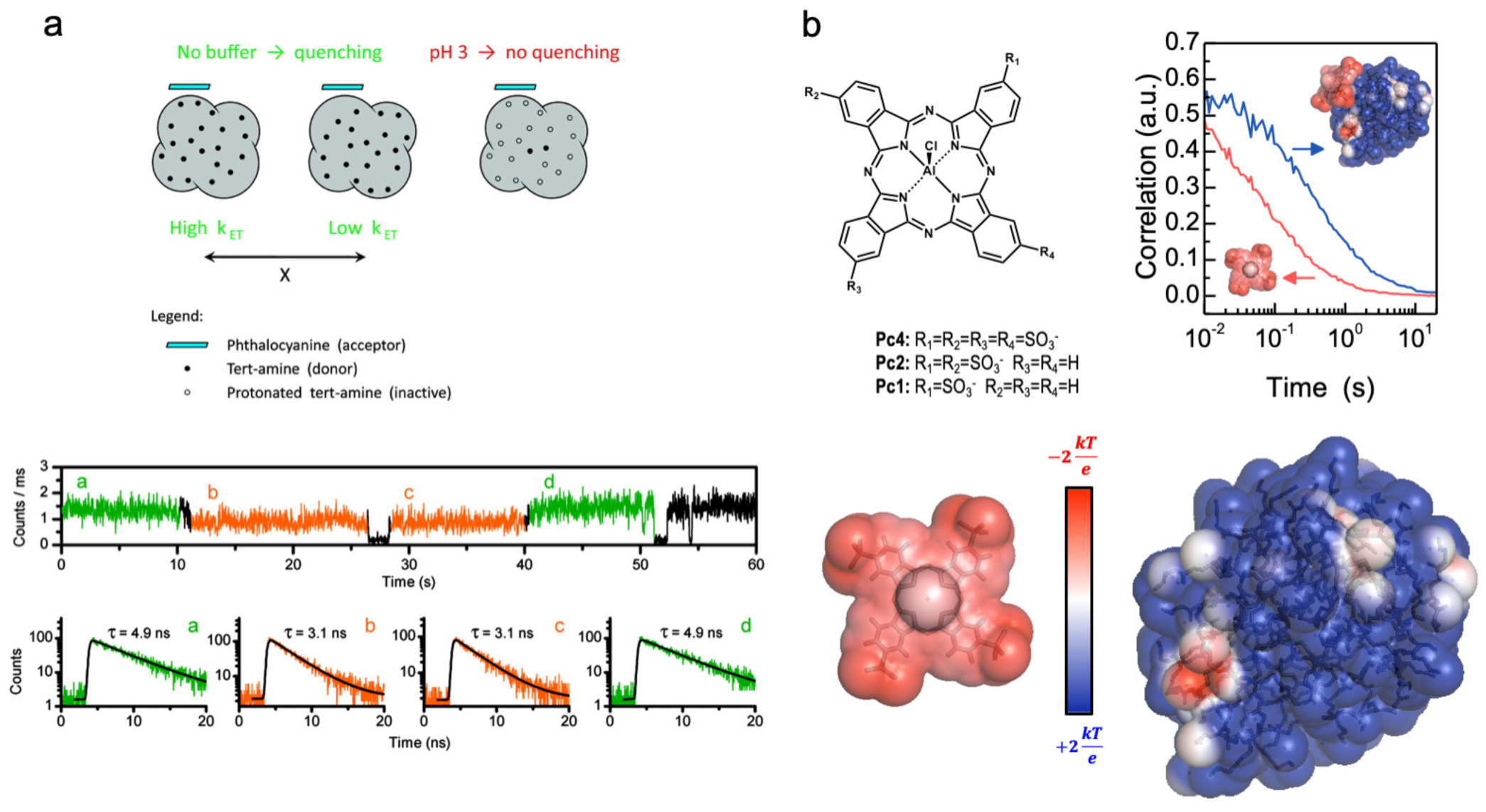
- Garcia-Fernandez, E.; Paulo, P.M.R.; Costa, S.M.B. Evaluation of electrostatic binding of PAMAM dendrimers and charged phthalocyanines by fluorescence correlation spectroscopy. Phys. Chem. Chem. Phys. 2015, 17, 4319–4327. [Google Scholar] [CrossRef] [PubMed]
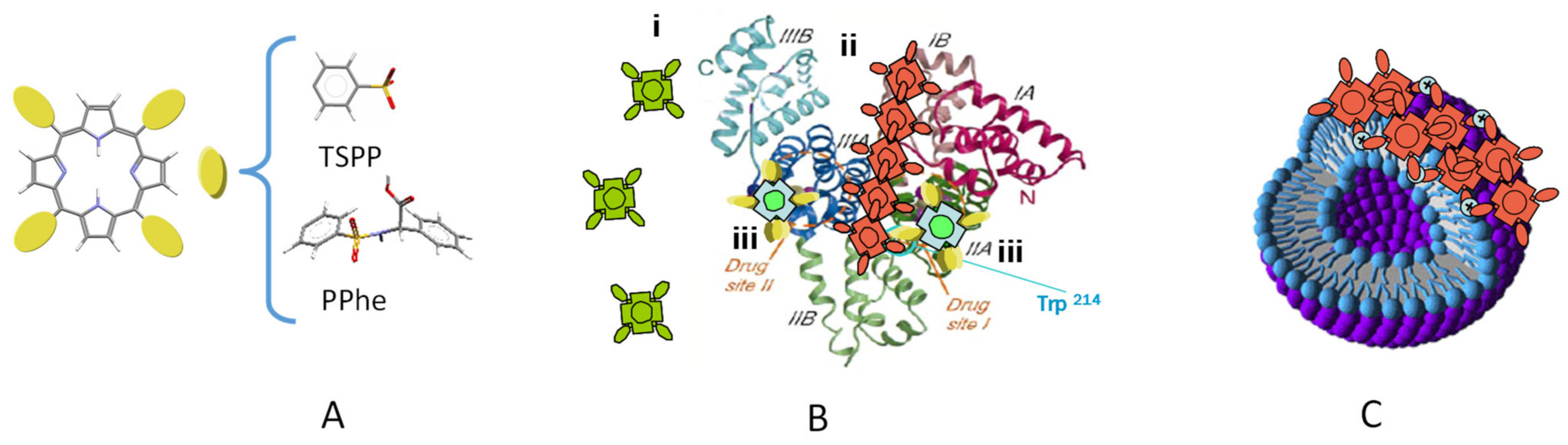
- Andrade, S.M.; Costa, S.M.B. Spectroscopic studies on the interaction of a water-soluble porphyrin and two drug carrier proteins. Biophys. J. 2002, 82, 1607–1619. [Google Scholar] [CrossRef]
- Andrade, S.M.; Costa, S.M.B.; Borst, J.W.; Van Hoek, A.; Visser, A.J.W.G. Translational and rotational motions of albumin sensed by a non-covalent associated porphyrin under physiological and acidic conditions: A fluorescence correlation spectroscopy and time resolved anisotropy study. J. Fluorescence 2008, 18, 601–610. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andrade, S.M.; Costa, S.M.B. Ordered Self-assembly of Protonated Porphyrin Induced by the Aqueous Environment of Biomimetic Systems. Ann. N. Y. Acad. Sci. 2008, 1130, 305–313. [Google Scholar] [CrossRef]
- Andrade, S.M.; Costa, S.M.B. tetrakis(4-sulfonatophenyl) porphyrin fluorescence as reporter of human serum albumin structural changes induced by guanidine hydrochloride. J. Photochem. Photobiol. Chem. 2011, 217, 125–135. [Google Scholar] [CrossRef]
- Andrade, S.M.; Teixeira, R.; Costa, S.M.B.; Sobral, A.J.F.N. Self-aggregation of free base porphyrins in aqueous solution and in DMPC vesicles. Biophys. Chem. 2008, 133, 1–10. [Google Scholar] [CrossRef]
- Choi, M.Y.; Pollard, J.A.; Webb, M.A.; McHale, J.L. Counterion-Dependent Excitonic Spectra of Tetra(p-carboxyphenyl) porphyrin Aggregates in Acidic Aqueous Solution. J. Am. Chem. Soc. 2003, 125, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Doan, S.C.; Shanmugham, S.; Aston, E.D.; McHale, J.L. Counterion Dependent Dye Aggregates: Nanorods and Nanorings of Tetra(p-carboxyphenyl)porphyrin. J. Am. Chem. Soc. 2005, 127, 5885–5892. [Google Scholar] [CrossRef]
- Hosomizu, K.; Oodoi, M.; Umeyama, T.; Matano, Y.; Yoshida, K.; Isoda, S.; Niemi, M.; Tkachenko, N.V.; Lemmetyinen, H.; Hiroshi, H. Substituent Effects of Porphyrins on Structures and Photophysical Properties of Amphiphilic Porphyrin Aggregates. J. Phys. Chem. 2008, 112, 16517–16524. [Google Scholar] [CrossRef] [PubMed]
- Sandanayaka, A.S.D.; Araki, Y.; Wada, T.; Hasobe, T. Structural and Photophysical Properties of Self-Assembled Porphyrin Nanoassemblies Organized by Ethylene Glycol Derivatives. J. Phys. Chem. 2008, 112, 19209–19216. [Google Scholar] [CrossRef]
- Serra, V.V.; Andrade, S.M.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Costa, S.M.B. J-aggregate formation in bis-(4-carboxyphenyl)porphyrins in water, pH and counterion dependence. New J. Chem. 2010, 34, 2757–2765. [Google Scholar] [CrossRef]
- Ferreira, J.A.B.; Serra, V.V.; Sánchez-Coronilla, A.; Pires, S.M.G.; Faustino, M.A.F.; Silva, A.M.S.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Costa, S.M.B. The Near-Mid-IR HOMO–LUMO gap in amide linked porphyrin–rhodamine dyads. Chem. Commun. 2013, 49, 8809–8811. [Google Scholar] [CrossRef]
- Scolaro, L.M.; Castriciano, M.; Romeo, A.; Patane, S.; Cefalì, E.; Allegroni, M. Aggregation Behavior of Protoporphyrin IX in Aqueous Solutions: Clear Evidence of Vesicle Formation. J. Phys. Chem. 2002, 106, 2453–2459. [Google Scholar] [CrossRef]
- Ericson, M.B.; Grapengiesser, S.; Gudmundson, F.; Wennberg, A.-M.; Larkö, O.; Moan, J.; Rosén, A. A spectroscopic study of the photobleaching of protoporphyrin IX in solution. Lasers Med. Sci. 2003, 18, 56–62. [Google Scholar] [CrossRef]
- Brancaleon, L.; Magennis, S.W.; Samuel, I.D.W.; Namdas, E.; Lesar, A.; Moseley, H. Characterization of the photoproducts of protoporphyrin IX bound to human serum albumin and immunoglobulin G. Biophys. Chem. 2004, 109, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.M.; Raja, P.; Saini, V.K.; Viana, A.S.; Serp, P.; Costa, S.M.B. Polyelectrolyte-Assisted Noncovalent Functionalization of Carbon Nanotubes with Ordered Self-Assemblies of a Water-Soluble Porphyrin. ChemPhysChem 2012, 13, 3622–3631. [Google Scholar] [CrossRef]
- Andrade, S.M.; Bueno-Alejo, C.J.; Serra, V.V.; Rodrigues, J.M.M.; Neves, M.G.P.M.S.; Viana, A.S.; Costa, S.M.B. Anchoring of Gold Nanoparticles on Graphene Oxide and Noncovalent Interactions with Porphyrinoids. ChemNanoMat 2015, 1, 502–510. [Google Scholar] [CrossRef]
- Belanger, S.S.; Hupp, J.T. Porphyrin-Based Thin-Film Molecular Materials with Highly Adjustable Nanoscale Porosity and Permeability Characteristics. Angew. Chem. Int. Ed. 1999, 38, 2222–2224. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Ma, P.; Lu, J.; Zhang, X.; Jiang, J. Morphology and chirality controlled self-assembled nanostructures of porphyrin–pentapeptide conjugate: Effect of the peptide secondary conformation. J. Mater. Chem. 2011, 21, 8057–8065. [Google Scholar] [CrossRef]
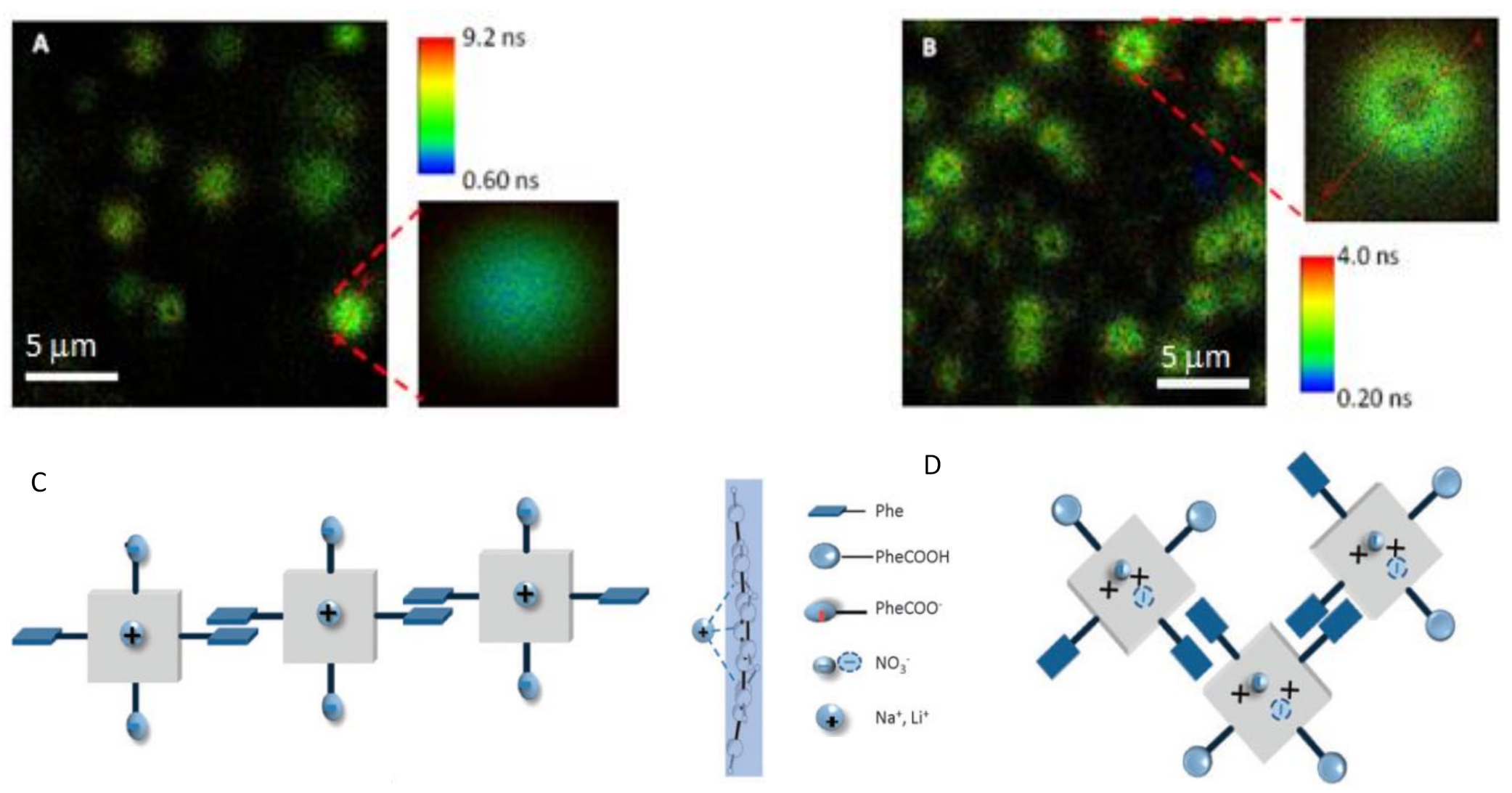
- Teixeira, R.; Andrade, S.M.; Vaz Serra, V.; Paulo, P.M.R.; Sánchez-Coronilla, A.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Costa, S.M.B. Reorganization of Self-Assembled Dipeptide Porphyrin J-Aggregates in Water-Ethanol Mixtures. J. Phys. Chem. 2012, 116, 2396–2404. [Google Scholar] [CrossRef]
- Meneghin, E.; Biscaglia, F.; Volpato, A.; Bolzonello, L.; Pedron, D.; Frezza, E.; Ferrarini, A.; Gobbo, M.; Collini, E. Biomimetic nanoarchitectures for light harvesting: Self-assembly of pyropheophorbide-peptide conjugates. J. Phys. Chem. Lett. 2020, 11, 7972–7980. [Google Scholar] [CrossRef]
- Dumele, O.; Chen, J.; Passarelli, J.V.; Stupp, S.I. Supramolecular Energy Materials. Adv. Mater. 2020, 32, 1907247. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.H.; Lee, J.S.; Park, C.B. Beta-Sheet-Forming, Self-Assembled Peptide Nanomaterials towards Optical, Energy, and Healthcare Applications. Small 2015, 11, 3623–3640. [Google Scholar] [CrossRef]
- Zou, Q.; Liu, K.; Abbas, M.; Yan, X. Peptide-Modulated Self-Assembly of Chromophores toward Biomimetic Light-Harvesting Nanoarchitectonics. Adv. Mater. 2016, 28, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Carnall, J.M.A.; Waudby, C.A.; Belenguer, A.M.; Stuart, M.C.A.; Peyralans, J.J.P.; Otto, S. Mechanosensitive Self-Replication Driven by Self-Organization. Science 2010, 327, 1502–1506. [Google Scholar] [CrossRef] [PubMed]
- Frederix, P.W.J.M.; Idé, J.; Altay, Y.; Schaeffer, G.; Surin, M.; Beljonne, D.; Bondarenko, A.S.; Jansen, T.L.C.; Otto, S.; Marrink, S.J. Structural and Spectroscopic Properties of Assemblies of Self-Replicating Peptide Macrocycles. ACS Nano 2017, 11, 7858–7868. [Google Scholar] [CrossRef] [PubMed]
- Gouterman, M. Spectra of Porphyrins. J. Mol. Spectrosc. 1961, 6, 138–163. [Google Scholar] [CrossRef]
- Sahin, T.; Harris, M.A.; Vairaprakash, P.; Niedzwiedzki, D.M.; Subramanian, V.; Shreve, A.P.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Self-Assembled Light-Harvesting System from Chromophores in Lipid Vesicles. J. Phys. Chem. 2015, 119, 10231–10243. [Google Scholar] [CrossRef]
- Gazit, E. Aromatic Dipeptides Light Up. Nat. Nanotechnol. 2016, 11, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Hilaire, M.R.; Ahmed, I.A.; Lin, C.W.; Jo, H.; DeGrado, W.F.; Gai, F. Blue Fluorescent Amino Acid for Biological Spectroscopy and Microscopy. Proc. Natl. Acad. Sci. USA 2017, 114, 6005. [Google Scholar] [CrossRef]
- Pansieri, J.; Josserand, V.; Lee, S.J.; Rongier, A.; Imbert, D.; Sallanon, M.M.; Kövari, E.; Dane, T.G.; Vendrely, C.; Chaix-Pluchery, O.; et al. Ultraviolet−Visible−Near—Infrared Optical Properties of Amyloid Fibrils Shed Light on Amyloidogenesis. Nat. Photonics 2019, 13, 473–479. [Google Scholar] [CrossRef]
- Gu, L.; Shi, H.; Bian, L.; Gu, M.; Ling, K.; Wang, X.; Ma, H.; Cai, S.; Ning, W.; Fu, L.; et al. Colour-Tunable Ultra-Long Organic Phosphorescence of a Single-Component Molecular Crystal. Nat. Photonics 2019, 13, 406–411. [Google Scholar] [CrossRef]
- Kim, D.; Yoo, J.M.; Hwang, H.; Lee, J.; Lee, S.H.; Yun, S.P.; Park, M.J.; Lee, M.; Choi, S.; Kwon, S.H.; et al. Graphene Quantum Dots Prevent α-Synucleinopathy in Parkinson’s Disease. Nat. Nanotechnol. 2018, 13, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Irimia-Vladu, M.; Głowacki, E.D.; Troshin, P.A.; Schwabegger, G.; Leonat, L.; Susarova, D.K.; Krystal, O.; Ullah, M.; Kanbur, Y.; Bodea, M.A.; et al. Indigo—A Natural Pigment for High Performance Ambipolar Organic Field Effect Transistors and Circuits. Adv. Mater. 2012, 24, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Hauser, C.A.E.; Zhang, S. Peptides as Biological Semi-conductors. Nature 2010, 468, 516. [Google Scholar] [CrossRef]
- Lee, H.E.; Ahn, H.Y.; Mun, J.; Lee, Y.Y.; Kim, M.; Cho, N.H.; Chang, K.; Kim, W.S.; Rho, J.; Nam, K.T. Amino-Acid- and Peptide-Directed Synthesis of Chiral Plasmonic Gold Nanoparticles. Nature 2018, 556, 360–365. [Google Scholar] [CrossRef]
- Fleming, S.; Ulijn, R.V. Design of Nanostructures Based on Aromatic Peptide Amphiphiles. Chem. Soc. Rev. 2014, 43, 8150–8177. [Google Scholar] [CrossRef]
- Stupp, S.I.; Palmer, L.C. Supramolecular Chemistry and Self-Assembly in Organic Materials Design. Chem. Mater. 2014, 26, 507–518. [Google Scholar] [CrossRef]
- Yan, X.; Zhu, P.; Li, J. Self-Assembly and Application of Diphenylalanine-Based Nanostructures. Chem. Soc. Rev. 2010, 39, 1877–1890. [Google Scholar] [CrossRef]
- Tao, K.; Makam, P.; Aizen, R.; Gazit, E. Self-Assembling Peptide Semiconductors. Science 2017, 358, eaam9756. [Google Scholar] [CrossRef]
- Berger, O.; Adler-Abramovich, L.; Levy-Sakin, M.; Grunwald, A.; Liebes-Peer, Y.; Bachar, M.; Buzhansky, L.; Mossou, E.; Forsyth, V.T.; Schwartz, T.; et al. Light-Emitting Self-Assembled Peptide Nucleic Acids Exhibit Both Stacking Interactions and Watson−Crick Base Pairing. Nat. Nanotechnol. 2015, 10, 353. [Google Scholar] [CrossRef]
- Bolisetty, S.; Mezzenga, R. Amyloid-Carbon Hybrid Membranes for Universal Water Purification. Nat. Nanotechnol. 2016, 11, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Lampel, A.; McPhee, S.A.; Park, H.-A.; Scott, G.G.; Humagain, S.; Hekstra, D.R.; Yoo, B.; Frederix, P.W.J.M.; Li, T.-D.; Abzalimov, R.R.; et al. Polymeric Peptide Pigments with Sequence-Encoded Properties. Science 2017, 356, 1064. [Google Scholar] [CrossRef]
- Jia, Y.; Li, J. Reconstitution of FoF1-ATPase-Based Biomimetic Systems. Nat. Rev. Chem. 2019, 3, 361–374. [Google Scholar] [CrossRef]
- Sun, B.; Tao, K.; Jia, Y.; Yan, X.; Zou, Q.; Gazit, E.; Li, J. Photoactive Properties of Supramolecular Assembled Short Peptides. Chem. Soc. Rev. 2019, 48, 4387–4400. [Google Scholar] [CrossRef]
- Xuan, M.; Shao, J.; Li, J. Cell Membrane-Covered Nano-particles as Biomaterials. Natl. Sci. Rev. 2019, 6, 551–561. [Google Scholar] [CrossRef]
- Li, X.; Fei, J.; Xu, Y.; Li, D.; Yuan, T.; Li, G.; Wang, C.; Li, J. A Photoinduced Reversible Phase Transition in a Dipeptide Supra-molecular Assembly. Angew. Chem. 2018, 130, 1921–1925. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, L.; Huang, Y.; Wang, Y.; Zhang, M. Bioinspired Fluorescent Dipeptide Nanoparticles for Targeted Cancer Cell Imaging and Real-Time Monitoring of Drug Release. Nat. Nanotechnol. 2016, 11, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Reches, M.; Gazit, E. Casting Metal Nanowires within Discrete Self-Assembled Peptide Nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Tamamis, P.; Adler-Abramovich, L.; Reches, M.; Marshall, K.; Sikorski, P.; Serpell, L.; Gazit, E.; Archontis, G. Self-Assembly of Phenylalanine Oligopeptides: Insights from Experiments and Simulations. Biophys. J. 2009, 96, 5020–5029. [Google Scholar] [CrossRef]
- Hong, Y.; Meng, L.; Chen, S.; Leung, C.W.T.; Da, L.-T.; Faisal, M.; Silva, D.-A.; Liu, J.; Lam, J.W.Y.; Huang, X.; et al. Monitoring and Inhibition of Insulin Fibrillation by a Small Organic Fluorogen with Aggregation-Induced Emission Characteristics. J. Am. Chem. Soc. 2012, 134, 1680–1689. [Google Scholar] [CrossRef]
- Chen, Y.; Orr, A.A.; Tao, K.; Wang, Z.; Ruggiero, A.; Shimon, L.J.W.; Schnaider, L.; Goodall, A.; Rencus-Lazar, S.; Gilead, S.; et al. High-Efficiency Fluorescence through Bioinspired Supramolecular Self-Assembly. ACS Nano 2020, 14, 2798–2807. [Google Scholar] [CrossRef]
- Barondeau, D.P.; Kassmann, C.J.; Tainer, J.A.; Getzoff, E.D. Structural Chemistry of a Green Fluorescent Protein Zn Biosensor. J. Am. Chem. Soc. 2002, 124, 3522–3524. [Google Scholar] [CrossRef]
- Ma, X.; Jia, J.; Cao, R.; Wang, X.; Fei, H. Histidine-Iridium(III) Coordination-Based Peptide Luminogenic Cyclization and Cyclo-RGD Peptides for Cancer-Cell Targeting. J. Am. Chem. Soc. 2014, 136, 17734–17737. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.D.; Buehler, L.K.; Ghadiri, M.R. Self-Assembling Cyclic β3-Peptide Nanotubes as Artificial Transmembrane Ion Channels. J. Am. Chem. Soc. 1998, 120, 651–656. [Google Scholar] [CrossRef]
- Bellezza, I.; Peirce, M.J.; Minelli, A. Cyclic Dipeptides: From Bugs to Brain. Trends Mol. Med. 2014, 20, 551–558. [Google Scholar] [CrossRef]
- Anderson, S.L.; Stylianou, K.C. Biologically Derived Metal Organic Frameworks. Coord. Chem. Rev. 2017, 349, 102–128. [Google Scholar] [CrossRef]
- Zou, R.; Wang, Q.; Wu, J.; Wu, J.; Schmuck, C.; Tian, H. Peptide Self-Assembly Triggered by Metal Ions. Chem. Soc. Rev. 2015, 44, 5200–5219. [Google Scholar] [CrossRef] [PubMed]
- Mannini, B.; Habchi, J.; Chia, S.; Ruggeri, F.S.; Perni, M.; Knowles, T.P.J.; Dobson, C.M.; Vendruscolo, M. Stabilization and Characterization of Cytotoxic Aβ40 Oligomers Isolated from an Aggregation Reaction in the Presence of Zinc Ions. ACS Chem. Neurosci. 2018, 9, 2959–2971. [Google Scholar] [CrossRef]
- Montenegro, J.; Ghadiri, M.R.; Granja, J.R. Ion Channel Models Based on Self-Assembling Cyclic Peptide Nanotubes. Acc. Chem. Res. 2013, 46, 2955–2965. [Google Scholar] [CrossRef]
- Mantion, A.; Massüger, L.; Rabu, P.; Palivan, C.; McCusker, L.B.; Taubert, A. Metal-Peptide Frameworks (MPFs): “Bioinspired” Metal Organic Frameworks. J. Am. Chem. Soc. 2008, 130, 2517–2526. [Google Scholar] [CrossRef]
- Otero, R.; Gallego, J.M.; De Parga, A.L.V.; Martín, N.; Miranda, R. Molecular Self-Assembly at Solid Surfaces. Adv. Mater. 2011, 23, 5148–5176. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.R.; Jeremy, G.; Ruggles, L.; Yu, A.; Gentle, I.R. Multilayer Nanostructured Porphyrin Arrays Constructed by Layer-by-Layer Self-Assembly. Langmuir 2009, 25, 9873–9878. [Google Scholar] [CrossRef]
- Ruthard, C.; Maskos, M.; Kolb, U.; Gröhn, F. Finite size networks from cylindrical polyelectrolyte brushes and porphyrins. Macromolecules 2009, 42, 830–840. [Google Scholar] [CrossRef]
- Ruthard, C.; Maskos, M.; Kolb, U.; Gröhn, F. Polystyrene sulfonate-porphyrin assemblies: Influence of polyelectrolyte and porphyrin structure. J. Phys. Chem. 2011, 115, 5716–5729. [Google Scholar] [CrossRef]
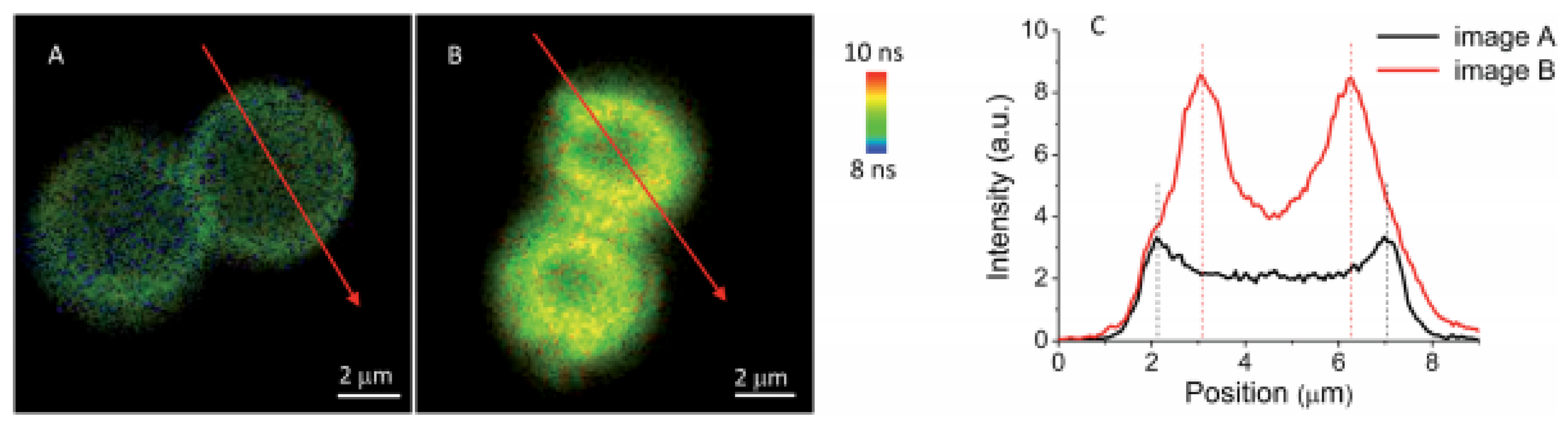
- Teixeira, R.; Serra, V.V.; Paulo, P.M.R.; Andrade, S.M.; Costa, S.M.B. Encapsulation of photoactive porphyrinoids in polyelectrolyte hollow microcapsules viewed by fluorescence lifetime imaging microscopy (FLIM). RSC Adv. 2015, 5, 79050–79060. [Google Scholar] [CrossRef]
- Serra, V.V.; Neto, N.G.B.; Andrade, S.M.; Costa, S.M.B. Core-Assisted Formation of Porphyrin J-Aggregates in PH-Sensitive Polyelectrolyte Microcapsules Followed by Fluorescence Lifetime Imaging Microscopy. Langmuir 2017, 33, 7680–7691. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.F.; Andrade, S.M.; Viseu, M.I. Aggregation and disaggregation of anionic aluminum phthalocyanines in cationic pre-micelle and micelle media: A fluorescence study. J. Photochem. Photobiol. Chem. 2012, 235, 21–28. [Google Scholar] [CrossRef]
- Correia, R.F.; Viseu, M.I.; Andrade, S.M. Aggregation/disaggregation of chlorophyll a in model phospholipid–detergent vesicles and micelles. Photochem. Photobiol. Sci. 2014, 13, 907–916. [Google Scholar] [CrossRef]
- Lucky, S.; Soo, K.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Huang, H.; Song, W.; Rieffel, J.; Lovell, J.F. Emerging applications of porphyrins in photomedicine. Front. Phys. 2015, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Srivatsan, A.; Missert, J.R.; Upadhyay, S.K.; Pandey, R.K. Porphyrin-based photosensitizers and the corresponding multifunctional nanoplatforms for cancer-imaging and phototherapy. J. Porphyrins Phthalocyanines 2015, 19, 109–134. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, X.; Dai, Z. Porphyrin-Loaded Nanoparticles for Cancer Theranostics. Nanoscale 2016, 8, 12394–12405. [Google Scholar] [CrossRef]
- Rajora, M.A.; Lou, J.W.H.; Zheng, G. Advancing Porphyrin’s Biomedical Utility via Supramolecular Chemistry. Chem. Soc. Rev. 2017, 46, 6433–6469. [Google Scholar] [CrossRef]
- Zhang, Y.; Lovell, J.F. Recent Applications of Phthalocyanines and Naphthalocyanines for Imaging and Therapy. Wiley Interdiscip. Rev. 2017, 9, e1420. [Google Scholar] [CrossRef] [PubMed]
- Bryden, F.; Boyle, R.W. Metalloporphyrins for Medical Imaging Applications. In Advances in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; Volume 68, pp. 141–221. [Google Scholar]
- Park, S.Y.; Baik, H.J.; Oh, Y.T.; Oh, K.T.; Youn, Y.S.; Lee, E.S. A Smart Polysaccharide/Drug Conjugate for Photodynamic Therapy. Angew. Chem. Int. Ed. 2011, 50, 1644–1647. [Google Scholar] [CrossRef] [PubMed]
- Napp, J.; Behnke, T.; Fischer, L.; Wurth, C.; Wottawa, M.; Katschinski, D.M.; Alves, F.; Resch-Genger, U.; Schaferling, M. Targeted Luminescent Near-Infrared Polymer-Nanoprobes for In Vivo Imaging of Tumor Hypoxia. Anal. Chem. 2011, 83, 9039–9046. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, W.; Na, K. Doxorubicin loaded singlet-oxygen producible polymeric micelle based on chlorine e6 conjugated pluronic F127 for overcoming drug resistance in cancer. Biomaterials 2014, 35, 7963–7969. [Google Scholar] [CrossRef]
- Li, Y.; Lin, T.Y.; Luo, Y.; Liu, Q.; Xiao, W.; Guo, W.; Lac, D.; Zhang, H.; Feng, C.; Wachs-mann-Hogiu, S.; et al. A smart and versatile theranostic nanomedicine platform based on nanoporphyrin. Nat. Commun. 2014, 5, 4712. [Google Scholar] [CrossRef]
- Jiang, D.; Gao, X.; Kang, T.; Feng, X.; Yao, J.; Yang, M.; Jing, Y.; Zhu, Q.; Feng, J.; Chen, J. Actively targeting D-alpha-tocopheryl polyethylene glycol 1000 succinate-poly(lactic acid) nanoparticles as vesicles for chemo-photodynamic combination therapy of doxorubicin-resistant breast cancer. Nanoscale 2016, 8, 3100–3118. [Google Scholar] [CrossRef]
- Paulo, P.M.R.; Lopes, J.N.C.; Costa, S.M.B. Molecular Dynamics Simulations of Charged Dendrimers: Low-to-Intermediate Half-Generation PAMAMs. J. Phys. Chem. 2007, 111, 10651–10664. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.D.; Nishiyama, N.; Zhang, G.D.; Harada, A.; Jiang, D.L.; Kawauchi, S.; Morimoto, Y.; Kikuchi, M.; Koyama, H.; Aida, T.; et al. Supramolecular Nanocarrier of Anionic Dendrimer Porphyrins with Cationic Block Copolymers Modified with Polyethylene Glycol to Enhance Intracellular Photodynamic Efficacy. Angew. Chem. Int. Ed. 2005, 44, 419–423. [Google Scholar] [CrossRef]
- Battah, S.; Balaratnam, S.; Casas, A.; O’Neill, S.; Edwards, C.; Batlle, A.; Dobbin, P.; MacRobert, A.J. Macromolecular delivery of 5-aminolaevulinic acid for photodynamic therapy using dendrimer conjugates. Mol. Cancer Ther. 2007, 6, 876. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Toi, Y.; Harada, A.; Kono, K. Preparation of Poly(Ethylene Glycol)-Attached Dendrimers Encapsulating Photosensitizers for Application to Photodynamic Therapy. Bioconjugate Chem. 2007, 18, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.D.; Nishiyama, N.; Kataoka, K. Supramolecular Assembly of Photofunctional Dendrimers for Biomedical Nano-Devices. Supramol. Chem. 2007, 19, 309–314. [Google Scholar] [CrossRef]
- Nishiyama, N.; Nakagishi, Y.; Morimoto, Y.; Lai, P.S.; Miyazaki, K.; Urano, K.; Horie, S.; Kumagai, M.; Fukushima, S.; Cheng, Y.; et al. Enhanced photodynamic cancer treatment by supramolecular nanocarriers charged with dendrimer phthalocyanine. J. Control. Release 2009, 133, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Schumann, C.; Naleway, M.A.; Pang, A.J.; Chon, K.J.; Taratula, O. A multifunctional theranostic platform based on phthalocyanine-loaded dendrimer for image-guided drug delivery and photodynamic therapy. Mol. Pharm. 2013, 10, 3946–3958. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Schumann, C.; Duong, T.; Taylor, K.L.; Taratula, O. Dendrimer-Encapsulated Naphthalocyanine as a Single Agent-Based Theranostic Nanoplatform for Near-Infrared Fluorescence Imaging and Combinatorial Anticancer Phototherapy. Nanoscale 2015, 7, 3888–3902. [Google Scholar] [CrossRef]
- Pereira, P.M.R.; Silva, S.; Bispo, M.; Zuzarte, M.; Gomes, C.; Girão, H.; Cavaleiro, J.A.S.; Ribeiro, C.A.F.; Tomé, J.P.C.; Fernandes, R. Mitochondria-Targeted Photodynamic Therapy with a Galactodendritic Chlorin to Enhance Cell Death in Resistant Bladder Cancer Cells. Bioconj. Chem. 2016, 27, 2762–2769. [Google Scholar] [CrossRef]
- Pereira, P.M.R.; Silva, S.; Ramalho, J.S.; Gomes, C.M.; Girão, H.; Cavaleiro, J.A.S.; Ribeiro, C.A.F.; Tomé, J.P.C.; Fernandes, R. The role of galectin-1 in in vitro and in vivo photodynamic therapy with a galactodendritic porphyrin. Eur. J. Cancer 2016, 68, 60–69. [Google Scholar] [CrossRef]
- Togashi, D.M.; Costa, S.M.B.; Sobral, A.J.F.N. Lipophilic porphyrin microparticles induced by AOT reverse micelles A fluorescence lifetime imaging study. Biophys. Chem. 2006, 119, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Cai, X.L.; Xu, S.D.; Fateminia, S.M.A.; Liu, J.; Liang, J.; Feng, G.X.; Wu, W.B.; Liu, B. Decoration of porphyrin with tetraphenylethene: Converting a fluorophore with aggregation-caused quenching to aggregation-induced emission enhancement. J. Mater. Chem. 2016, 4, 4690–4695. [Google Scholar] [CrossRef]
- Shakiba, M.; Ng, K.K.; Huynh, E.; Chan, H.; Charron, D.M.; Chen, J.; Muhanna, N.; Foster, F.S.; Wilson, B.C.; Zheng, G. Stable J-aggregation enabled dual photoacoustic and fluorescence nanoparticles for intraoperative cancer imaging. Nanoscale 2016, 8, 12618–12625. [Google Scholar] [CrossRef]
- Wang, D.; Niu, L.J.; Qiao, Z.Y.; Cheng, D.B.; Wang, J.F.; Zhong, Y.; Bai, F.; Wang, H.; Fan, H.Y. Synthesis of Self-Assembled Porphyrin Nanoparticle Photosensitizers. ACS Nano 2018, 12, 3796–3803. [Google Scholar] [CrossRef]
- Ng, K.K.; Zheng, G. Molecular Interactions in Organic Nanoparticles for Phototheranostic Applications. Chem. Rev. 2015, 115, 11012–11042. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Hizume, Y.; Tashiro, K.; Charvet, R.; Yamamoto, Y.; Saeki, A.; Seki, S.; Aida, T. Chiroselective Assembly of a Chiral Porphyrin–Fullerene Dyad: Photoconductive Nanofiber with a Top-Class Ambipolar Charge-Carrier Mobility. J. Am. Chem. Soc. 2010, 132, 6628–6629. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Nihashi, W.; Umeyama, T.; Matano, Y.; Seki, S.; Shimizu, Y.; Imahori, H. Segregated Donor–Acceptor Columns in Liquid Crystals That Exhibit Highly Efficient Ambipolar Charge Transport. J. Am. Chem. Soc. 2011, 133, 10736–10739. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Ebeling, D.; Patwardhan, S.; Zhang, X.; von Berlepsch, H.; Bottcher, C.; Stepanenko, V.; Uemura, S.; Hentschel, C.; Fuchs, H.; et al. Biosupramolecular nanowires from chlorophyll dyes with exceptional charge-transport properties. Angew. Chem. Int. Ed. 2012, 51, 6378–6382. [Google Scholar] [CrossRef]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.; Liu, Z. Photothermally Enhanced Photodynamic Therapy Delivered by Nano-Graphene Oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef]
- Huang, P.; Xu, C.; Lin, J.; Wang, C.; Wang, X.; Zhang, C.; Zhou, X.; Guo, S.; Cui, D. Folic Acid-conjugated Graphene Oxide loaded with Photosensitizers for Targeting Photodynamic Therapy. Theranostics 2011, 1, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Patel, M.; Schumann, C.; Naleway, M.; Pang, A.; He, H.; Taratula, O. Phthalocyanine-loaded graphene nanoplatform for imaging-guided combinatorial phototherapy. Int. J. Nanomed. 2015, 10, 2347–2362. [Google Scholar] [CrossRef] [PubMed]
- Derycke, A.S.L.; De Witte, P.A. Liposomes for photodynamic therapy. Adv. Drug Deliv. Rev. 2004, 56, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Pogue, B.W.; Hasan, T. Liposomal delivery of photosensitising agents. Expert Opin. Drug Deliv. 2005, 2, 477–487. [Google Scholar] [CrossRef]
- Buschmann, M.D.; Carrasco, M.J.; Alishetty, S.; Paige, M.; Alameh, M.G.; Weissman, D. Nanomaterial Delivery Systems for mRNA Vaccines. Vaccines 2021, 9, 65. [Google Scholar] [CrossRef]
- Allison, R.R.; Sibata, C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagn. Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Hasan, T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 195–214. [Google Scholar] [CrossRef]
- De Leeuw, J.; Van Der Beek, N.; Bjerring, P.; Martino Neumann, H. Photodynamic therapy of acne vulgaris using 5-aminolevulinic acid 0.5% liposomal spray and intense pulsed light in combination with topical keratolytic agents. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.M.; Taylor, V.M.; Cedeño, D.L.; Padhee, S.; Robledo, S.M.; Jones, M.A.; Lash, T.D.; Vélez, I.D. Association of Acenaphthoporphyrins with Liposomes for the Photodynamic Treatment of Leishmaniasis. Photochem. Photobiol. 2010, 86, 645–652. [Google Scholar] [CrossRef]
- Huynh, E.; Zheng, G. Porphysome Nanotechnology: A Paradigm Shift in Lipid-Based Supramolecular Structures. Nano Today 2014, 9, 212–222. [Google Scholar] [CrossRef]
- Valic, M.S.; Zheng, G. Rethinking translational nanomedicine: Insights from the ‘bottom-up’ design of the Porphysome for guiding the clinical development of imageable nanomaterials. Curr. Opin. Chem. Biol. 2016, 33, 126–134. [Google Scholar] [CrossRef]
- Lovell, J.F.; Jin, C.S.; Huynh, E.; Jin, H.; Kim, C.; Rubinstein, J.L.; Chan, W.C.; Cao, W.; Wang, L.V.; Zheng, G. Porphysome Nanovesicles Generated by Porphyrin Bilayers for Use as Multimodal Biophotonic Contrast Agents. Nat. Mater. 2011, 10, 324–332. [Google Scholar] [CrossRef]
- Jin, C.S.; Lovell, J.F.; Chen, J.; Zheng, G. Hypoxic Tumors with Dose-Equivalent Photothermal, but Not Photodynamic, Therapy Using a Nanostructured Porphyrin Assembly. ACS Nano 2013, 7, 2541–2550. [Google Scholar] [CrossRef]
- Luo, D.; Li, N.; Carter, K.A.; Lin, C.; Geng, J.; Shao, S.; Huang, W.C.; Qin, Y.; Atilla-Gokcumen, G.E.; Lovell, J.F. Rapid Light-Triggered Drug Release in Liposomes Containing Small Amounts of Unsaturated and Porphyrin–Phospholipids. Small 2016, 12, 3039–3047. [Google Scholar] [CrossRef]
- He, C.; Liu, D.; Lin, W. Self-assembled core-shell nanoparticles for combined chemotherapy and photodynamic therapy of resistant head and neck cancers. ACS Nano 2015, 9, 991–1003. [Google Scholar] [CrossRef]
- Rieffel, J.; Chen, F.; Kim, J.; Chen, G.; Shao, W.; Shao, S.; Chitgupi, U.; Hernandez, R.; Graves, S.A.; Nickles, R.J.; et al. Hexamodal Imaging with Porphyrin-Phospholipid-Coated Upconversion Nanoparticles. Adv. Mater. 2015, 27, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tao, H.; Cheng, L.; Liu, Z. Near-Infrared Light Induced in Vivo Photodynamic Therapy of Cancer Based on Upconversion Nanoparticles. Biomaterials 2011, 32, 6145–6154. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, K.; Yang, G.; Cheng, L.; He, L.; Liu, Y.; Li, Y.; Guo, L.; Liu, Z. Near-infrared light triggered photodynamic therapy in combination with gene therapy using upconversion nanoparticles for effective cancer cell killing. Nanoscale 2014, 6, 9198–9205. [Google Scholar] [CrossRef]
- Xu, J.; Xu, L.; Wang, C.; Yang, R.; Zhuang, Q.; Han, X.; Dong, Z.; Zhu, W.; Peng, R.; Liu, Z. Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano 2017, 11, 4463–4474. [Google Scholar] [CrossRef]
- Khadria, A.; Fleischhauer, J.; Boczarow, I.; Wilkinson, J.D.; Kohl, M.M.; Anderson, H.L. Porphyrin Dyes for Nonlinear Optical Imaging of Live Cells. Iscience 2018, 4, 153–163. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Kaskel, S. Porphyrin-Based Metal–Organic Frameworks for Biomedical Applications. Angew. Chem. Int. Ed. 2021, 60, 5010. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.M.; Li, Y.H.; Cai, S.J.; Yin, X.B.; He, X.W.; Zhang, Y.K. Fluorescent Imaging-Guided Chemotherapy-and-Photodynamic Dual Therapy with Nanoscale Porphyrin Metal–Organic Framework. Small 2017, 13, 1603459. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakamura, M.; Ishimura, K. Silica-porphyrin hybrid nanotubes for in vivo cell tracking by near infrared fluorescence imaging. Chem. Commun. 2012, 48, 3830–3832. [Google Scholar] [CrossRef]
- Zijlstra, P.; Orrit, M. Single metal nanoparticles: Optical detection, spectroscopy and applications. Rep. Prog. Phys. 2011, 74, 106401. [Google Scholar] [CrossRef]
- Khatua, S.; Paulo, P.M.R.; Yuan, H.; Gupta, A.; Zijlstra, P.; Orrit, M. Resonant Plasmonic Enhancement of Single-Molecule Fluorescence by Individual Gold Nanorods. ACS Nano 2014, 8, 4440–4449. [Google Scholar] [CrossRef] [PubMed]
- Paulo, P.M.R.; Botequim, D.; Jośkowiak, A.; Martins, S.; Prazeres, D.M.F.; Zijlstra, P.; Costa, S.M.B. Enhanced Fluorescence of a Dye on DNA-Assembled Gold Nanodimers Discriminated by Lifetime Correlation Spectroscopy. J. Phys. Chem. 2018, 122, 10971–10980. [Google Scholar] [CrossRef]
- Giannini, V.; Fernańdez-Domínguez, A.I.; Heck, S.C.; Maier, S.A. Plasmonic Nanoantennas: Fundamentals and Their Use in Controlling the Radiative Properties of Nanoemitters. Chem. Rev. 2011, 111, 3888–3912. [Google Scholar] [CrossRef]
- Botequim, D.; Silva, I.I.R.; Serra, S.G.; Melo, E.P.; Prazeres, D.M.F.; Costa, S.M.B.; Paulo, P.M.R. Fluorescent Dye Nano-Assemblies by Thiol Attachment Directed to the Tips of Gold Nanorods for Effective Emission Enhancement. Nanoscale 2020, 12, 6334–6345. [Google Scholar] [CrossRef]
- Oliveira-Silva, R.; Sousa-Jerónimo, M.; Botequim, D.; Silva, N.J.O.; Prazeres, D.M.F.; Paulo, P.M.R. Density Gradient Selection of Colloidal Silver Nanotriangles for Assembling Dye-Particle Plasmophores. Nanomaterials 2019, 9, 893. [Google Scholar] [CrossRef]
- Teixeira, R.; Paulo, P.M.R.; Viana, A.S.; Costa, S.M.B. Plasmon-Enhanced Emission of a Phthalocyanine in Polyelectrolyte Films Induced by Gold Nanoparticles. J. Phys. Chem. C 2011, 115, 24674–24680. [Google Scholar] [CrossRef]
- Teixeira, R.; Paulo, P.M.R.; Costa, S.M.B. Gold Nanoparticles in Core−Polyelectrolyte−Shell Assemblies Promote Large Enhancements of Phthalocyanine Fluorescence. J. Phys. Chem. 2015, 119, 21612–21619. [Google Scholar] [CrossRef]
- Francisco, A.P.; Botequim, D.; Prazeres, D.M.F.; Serra, V.V.; Costa, S.M.B.; Laia, C.A.T.; Paulo, P.M.R. Extreme Enhancement of Single-Molecule Fluorescence from Porphyrins Induced by Gold Nanodimer Antennas. J. Phys. Chem. Lett. 2019, 10, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cheng, X.; Chen, M.; Sheng, J.; Ren, J.; Jiang, Z.; Cai, J.; Hu, Y. Fluorescence guided photothermal/photodynamic ablation of tumours using pH-responsive chlorin e6-conjugated gold nanorods. Colloids Surf. 2017, 160, 345–354. [Google Scholar] [CrossRef]
- Huang, X.; Tian, X.J.; Yang, W.L.; Ehrenberg, B.; Chen, J.Y. The conjugates of gold nanorods and chlorin E6 for enhancing the fluorescence detection and photodynamic therapy of cancers. Phys. Chem. Chem. Phys. 2013, 15, 15727–15733. [Google Scholar] [CrossRef]
- Zeng, J.Y.; Zhang, M.K.; Peng, M.Y.; Gong, D.; Zhang, X.Z. Porphyrinic Metal–Organic Frameworks Coated Gold Nanorods as a Versatile Nanoplatform for Combined Photodynamic/Photothermal/Chemotherapy of Tumor. Adv. Funct. Mater. 2018, 28, 1705451. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, J.; Di, Z.; Liu, B.; Li, Z.; Wu, X.; Li, L. Core–shell gold nanorod@mesoporous-MOF heterostructures for combinational phototherapy. Nanoscale 2021, 13, 131–137. [Google Scholar] [CrossRef]
- Wang, S.; Huang, P.; Nie, L.; Xing, R.; Liu, D.; Wang, Z.; Lin, J.; Chen, S.; Niu, G.; Lu, G.; et al. Single Continuous Wave Laser Induced Photodynamic/Plasmonic Photothermal Therapy Using Photosensitizer-Functionalized Gold Nanostars. Adv. Mater. 2013, 25, 3055–3061. [Google Scholar] [CrossRef]
- Wang, L.; Song, Q.; Liu, Q.; He, D.; Ouyang, J. Plasmon-Enhanced Fluorescence-Based Core–Shell Gold Nanorods as a Near-IR Fluorescent Turn-On Sensor for the Highly Sensitive Detection of Pyrophosphate in Aqueous Solution. Adv. Funct. Mater. 2015, 25, 7017–7027. [Google Scholar] [CrossRef]
- Jang, B.; Park, J.; Tung, C.; Kim, I.; Choi, Y. Gold nanorod-photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS Nano 2011, 5, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Yu, K.; Li, L.; Zhang, T.; Guan, Z.; Gao, N.; Yuan, P.; Li, S.; Yao, S.Q.; Xu, Q.H.; et al. Gold nanorod enhanced two-photon excitation fluorescence of photosensitizers for two-photon imaging and photodynamic therapy. ACS Appl. Mater. Interfaces 2014, 6, 2700–2708. [Google Scholar] [CrossRef] [PubMed]
- Weidtkamp-Peters, S.; Felekyan, S.; Bleckmann, A.; Simon, R.; Becker, W.; Kühnemuth, R.; Seidel, C.A.M. Multiparameter fluorescence image spectroscopy to study molecular interactions. Photochem. Photobiol. Sci. 2009, 8, 470–480. [Google Scholar] [CrossRef] [PubMed]
- König, K. Multiphoton Microscopy and Fluorescence Lifetime Imaging; De Gruyter: Berlin, Germany; Boston, MA, USA, 2018. [Google Scholar]
- Lian, X.; Wei, M.Y.; Ma, Q. Nanomedicines for Near-Infrared Fluorescent Lifetime-Based Bioimaging. Front. Bioeng. Biotechnol. 2019, 7, 386. [Google Scholar] [CrossRef]
- Togashi, D.M.; Romão, R.I.S.; da Silva, A.M.G.; Sobral, A.J.F.N.; Costa, S.M.B. Self-organization of a sulfonamido-porphyrin in Langmuir monolayers and Langmuir–Blodgett films. Phys. Chem. Chem. Phys. 2005, 7, 3874–3883. [Google Scholar] [CrossRef]
- Serra, V.V.; Teixeira, R.; Andrade, S.M.; Costa, S.M.B. Design of polyelectrolyte core-shells with DNA to control TMPyP binding. Colloids Surf. 2016, 146, 127–135. [Google Scholar] [CrossRef]
- Zander, C.; Enderlein, J.; Keller, R.A. Single Molecule Detection in Solution; Wiley-VCH Verlag Berlin GmbH: Berlin, Germany, 2002; Chapter 2. [Google Scholar]
- Mitsuishi, M.; Tanaka, H.; Obata, M.; Miyashita, T. Plasmon-Enhanced Luminescence from Ultrathin Hybrid Polymer Nanoassemblies for Microscopic Oxygen Sensor Application. Langmuir 2010, 26, 15117–15120. [Google Scholar] [CrossRef]
- López-Ruiz, N.; Martínez-Olmos, A.; Pérez de Vargas-Sansalvador, I.M.; Fernández-Ramos, M.D.; Carvajal, M.A.; Capitan-Vallvey, L.F.; Palma, A.J. Determination of O2 using colour sensing from image processing with mobile devices. Sens. Actuators Chem. 2012, 171–172, 938–945. [Google Scholar] [CrossRef]
- Huang, H.; Song, W.; Chen, G.; Reynard, J.M.; Ohulchanskyy, T.Y.; Prasad, P.N.; Bright, F.V.; Lovell, J.F. Pd-Porphyrin-Cross-Linked Implantable Hydrogels with Oxygen-Responsive Phosphorescence. Adv. Healthc. Mater. 2014, 3, 891–896. [Google Scholar] [CrossRef]
- Kuimova, M.; Botchway, S.; Parker, A.; Balaz, M.; Collins, H.A.; Anderson, H.L.; Suhling, K.; Ogilby, P.R. Imaging intracellular viscosity of a single cell during photoinduced cell death. Nat. Chem. 2009, 1, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, A.; Kim, G.H.; Rhim, W.K.; Hartman, K.L.; Nam, J.M. Myoglobin and Polydopamine-Engineered Raman Nanoprobes for Detecting, Imaging, and Monitoring Reactive Oxygen Species in Biological Samples and Living Cells. Small 2017, 13, 1701584. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, R.; Serra, V.V.; Botequim, D.; Paulo, P.M.R.; Andrade, S.M.; Costa, S.M.B. Fluorescence Spectroscopy of Porphyrins and Phthalocyanines: Some Insights into Supramolecular Self-Assembly, Microencapsulation, and Imaging Microscopy. Molecules 2021, 26, 4264. https://doi.org/10.3390/molecules26144264
Teixeira R, Serra VV, Botequim D, Paulo PMR, Andrade SM, Costa SMB. Fluorescence Spectroscopy of Porphyrins and Phthalocyanines: Some Insights into Supramolecular Self-Assembly, Microencapsulation, and Imaging Microscopy. Molecules. 2021; 26(14):4264. https://doi.org/10.3390/molecules26144264
Chicago/Turabian StyleTeixeira, Raquel, Vanda Vaz Serra, David Botequim, Pedro M. R. Paulo, Suzana M. Andrade, and Sílvia M. B. Costa. 2021. "Fluorescence Spectroscopy of Porphyrins and Phthalocyanines: Some Insights into Supramolecular Self-Assembly, Microencapsulation, and Imaging Microscopy" Molecules 26, no. 14: 4264. https://doi.org/10.3390/molecules26144264
APA StyleTeixeira, R., Serra, V. V., Botequim, D., Paulo, P. M. R., Andrade, S. M., & Costa, S. M. B. (2021). Fluorescence Spectroscopy of Porphyrins and Phthalocyanines: Some Insights into Supramolecular Self-Assembly, Microencapsulation, and Imaging Microscopy. Molecules, 26(14), 4264. https://doi.org/10.3390/molecules26144264






