Abstract
The catalyst-free conjugate addition of pyrroles to β-Fluoro-β-nitrostyrenes was investigated. The reaction was found to proceed under solvent-free conditions to form 2-(2-Fluoro-2-nitro-1-arylethyl)-1H-pyrroles. The effectiveness of this approach was demonstrated through the preparation of a series of the target products in a quantitative yield. The kinetics of a conjugate addition of pyrrole was studied in detail to reveal the substituent effect and activation parameters of the reaction. The subsequent base-induced elimination of nitrous acid afforded a series of novel 2-(2-Fluoro-1-arylvinyl)-1H-pyrroles prepared in up to an 85% isolated yield. The two-step sequence herein proposed is an indispensable alternative to a direct reaction with elusive and unstable 1-Fluoroacetylenes.
1. Introduction
The pyrrole ring is a constituent subunit of many naturally produced molecules and pharmaceutically active compounds [1]. Pyrrole and its derivatives are widely used as intermediates in the synthesis of pharmaceuticals, agrochemicals, dyes and other valuable organic compounds [2,3,4,5]. Many drugs containing a pyrrole moiety are available in the market or are currently undergoing clinical trials [6]. They exhibit a wide range of biological activities, such as antibacterial, anticoccidial, anti-inflammatory, antipsychotic, anticonvulsant, antifungal, antiviral, anticancer, etc. [7,8]. The conjugate addition of 2-unsubstituted pyrroles to Michael acceptors is a powerful tool used for their further functionalization [9,10,11,12]. Nitroalkenes are highly reactive and attractive Michael acceptors, giving useful compounds for the subsequent transformation of the nitro group [13]. Indeed, the introduction of a 2-Nitroalkyl moiety into the pyrrole nucleus has attracted much research interest. 2-(2-Nitroalkyl)pyrroles were demonstrated to be appealing intermediates for the synthesis of bioactive compounds [14].
On the other hand, the incorporation of a fluorine into a molecule can positively modulate a number of important pharmacokinetic and physicochemical properties of the drug, such as lipophilicity, electrophilicity, conformation, pKa, metabolic and chemical stability, membrane permeability and the binding affinity to a target protein [15,16,17,18]. Indeed, about one-fourth of the currently manufactured agrochemical and pharmaceutical products contain at least one fluorine atom, and their number tends to persistently rise [19]. In this respect, the development of new routes to fluorinated pyrrole derivatives is of great research interest and importance [20,21].
We recently reported an effective and stereoselective method for the preparation of β-Fluoro-β-nitrostyrenes based on the radical nitration of 2-Bromo-2-fluorostyrenes. This process takes place with the simultaneous elimination of bromine and gives the target structures solely as Z isomers in high yields (up to 92%) [22]. These molecules are very attractive building blocks for the construction of numerous monofluorinated molecules [23,24,25,26,27,28,29,30]. This paper is devoted to the development of routes to new classes of monofluorinated derivatives of pyrrole. We report a catalyst-free conjugate addition of pyrroles to β-Fluoro-β-nitrostyrenes, yielding a novel 2-(2-Fluoro-2-nitro-1-arylethyl)-1H-pyrroles 3. In turn, the latter prove to be effective precursors for the synthesis of novel 2-(2-Fluoro-1-arylvinyl)-1H-pyrroles 4 by the base-induced elimination of nitrous acid. This two-step sequence opens up a straightforward way to monofluorinated compounds not previously available. The approach reported herein is an indispensable alternative to the direct addition of pyrroles to elusive and unstable 1-Fluoroacetylenes (Scheme 1).
Scheme 1.
Overview of this work.
2. Results and Discussion
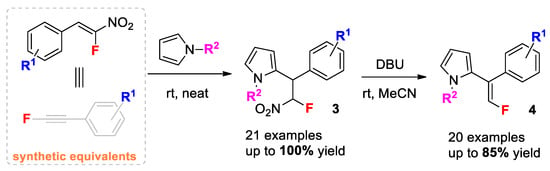
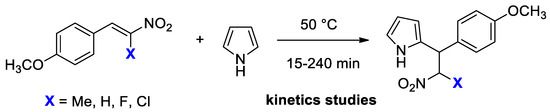
Initially, we studied the conjugated addition of pyrrole 2a to β-Fluoro-β-nitrostyrenes 1 to prepare a series of novel 2-(2-Fluoro-2-nitro-1-arylethyl)-1H-pyrroles 3. The transformations were carried out in excess of pyrrole, which acted as both reagent and solvent (Scheme 2).
Scheme 2.
Scope of β-Fluoro-β-nitrostyrenes 1 in reaction with pyrrole under solvent-free conditions. Reaction conditions: nitrostyrene 1 (0.5 mmol) was reacted with pyrrole (0.5 mL) at rt for 25–30 h; dr was determine by 19F NMR spectroscopy; isolated yields in parentheses were obtained by column chromatography on silica gel; *—a gram-scale reaction resulted in a 99% yield.
We found that the reaction proceeded very efficiently without any catalyst at room temperature. The reaction took place only at the α-position of pyrrole, affording the corresponding monofluorinated adducts 3 in a quantitative yield. The reaction demonstrated a broad scope in terms of nitrostyrenes 1 (Scheme 2). It can easily be scaled up to a gram scale without a loss of effectiveness. The NMR spectroscopic analysis showed the formation of a diastereoisomeric mixture of products 3 in a ratio of ca. 40:60. The formation of two diastereomers was probably caused by the high acidity of the proton on the carbon bearing the fluorine and nitro groups (the estimated pKa of CH2FNO2 was around 9.5) [31]. We also studied the reaction with 1,3-bis((Z)-2-Fluoro-2-nitrovinyl)benzene 1o. The formation of bis-pyrrole adducts 3o proceeded in a quantitative yield, giving a mixture of four isomers. Due to the partial overlapping of the resonance signals of F, we did not estimate the molar ratio of all the isomers with 19F NMR-spectroscopy. All the structures obtained were elucidated by a combination of NMR spectroscopy and HRMS (See Supplementary Materials).
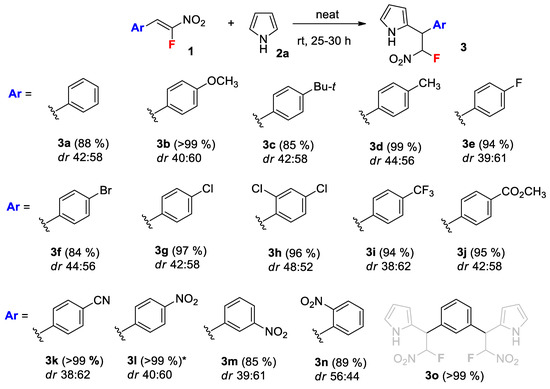
To gain deeper insights into the reaction, we carried out some kinetic studies to estimate the activation parameters and substituent effect. All the kinetic runs were performed under pseudo-first order conditions using a 216-molar excess of 1H-pyrrole. Conversions (F) of 1 were measured by 19F NMR spectroscopy. The total effective pseudo-first order rate constants k* were obtained by plotting the experimental values of ln (C0/C) versus time with good correlations (Table 1 and Table 2). The overall second-order rate total constants ktotal were calculated from the effective k* and initial concentration of pyrrole (Table 1 and Table 2). The individual constants for the minor and major isomers (kmin and kmaj) were evaluated by the multiplication of ktotal with the molar fractions of the isomers (Table 1 and Table 2). The reactions were found to proceed under the kinetic control, since the isomer ratio remained constant throughout the reactions course (Table 1 and Table 2). First, the activation parameters were estimated for the reaction of nitrostyrene 1g with pyrrole. The kinetic studies were conducted in the temperature range of 30–90 °C (Figure 1). The calculated rate constants are presented in Table 1.

Table 1.
Kinetic parameters for the reaction of 1h with pyrrole.

Table 2.
Kinetic data of the reactions of nitrostyrenes 1 with pyrrole.
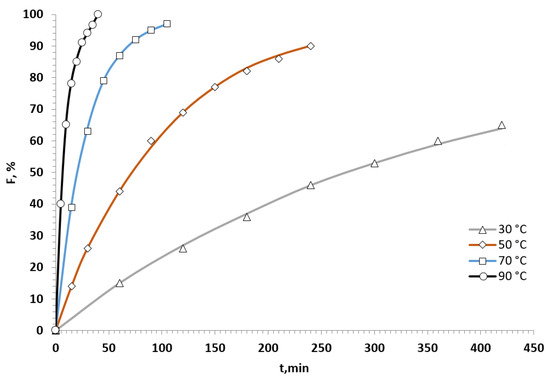
Figure 1.
Kinetic curves of the reaction of 1h with pyrrole at 30–90 °C.
The activation parameters were estimated for both the reaction pathways (the formation of two diastereomers) by the Eyring equation [32] (1) from the plots of ln(k/T) versus 1/T (2–3). The activation enthalpies () and entropies () were found to be 51.72 kJ/mol and −183.45 J/mol K for the major isomer and 55.03 kJ/mol and −178.57 J/mol K for the minor isomer.
ln(k/T) = ln(kb/ħ) + ΔS≠/R − ΔH≠/RT
ln(kmaj/T) = 1.675 − 6222.5/T (R > 0.999)
ln(kmin/T) = 2.271 − 6622.7/T (R > 0.999)
Next, we studied the substituent effect. The kinetic curves were obtained for the reactions of pyrrole with differently para-position substituted nitrostyrenes 1 at 50 °C. The rate constants were calculated and summarized in Table 2. It was found that the reaction rate depended on the nature of a substituent on the benzene ring of the nitrostyrenes 1. The substituent effect was estimated first by plotting the log k against the Hammett constants σp for the para-substituents (Figure 2).
Figure 2.
Plot of log k versus the Hammett constants (σp).
The following linear relationships were obtained:
log ktotal = 0.33σp − 5.00 (R = 0.889)
log kmin = 0.25σp − 5.45 (R = 0.760)
log kmaj = 0.38σp − 5.45 (R = 0.928)
The positive value of the reaction constant ρ indicates that the reaction is favored by the withdrawal of electron pairs from the reaction site. However, the low value of ρ (0.3 ÷ 0.4) demonstrates a low sensitivity to the substituent influences. As a consequence, this leads to relatively low correlations of the linear relationships obtained (0.76 ÷ 0.93). Indeed, the ratio of the maximum (p-NO2-substituted 1l) and minimum (p-OCH3-substituted 1b) values of k is around 3, whereas, for the Diels–Alder reaction of nitrostyrenes 1 with 2,3-dimethylbutadiene (ρ = 1.00), the same ratio is around 14 [24].
Another parameter frequently used for the estimation of reactivity is a global electrophilicity index (ω). This parameter is in good correlation with σp. The ground-state geometries of nitrostyrenes 1 were optimized using the DFT B3LYP/6-31G* level of theory [24]. The values of ω were evaluated from the HOMO and LUMO energies of nitrostyrenes 1:
ω = μ2/2η = 1/8 (EHOMO + ELUMO)2/(ELUMO − EHOMO)
Next, the linearization of the log k versus ω was made for the formation of both diastereomers (Figure 3). However, the correlations of the linear relationships obtained were worse (8–10).
log ktotal = 0.31ω − 5.90 (R = 0.843)
log kmin = 0.22ω − 6.10 (R = 0.700)
log kmaj = 0.35ω − 6.22 (R = 0.887)
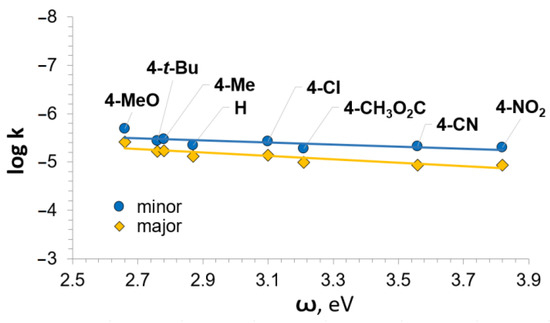
Figure 3.
Plot of log k versus electrophilicity indexes (ω).
Next, we selected a series of nitrostyrenes differing only in a substituent at the double bond to evaluate the substituent effect at β-carbon (Scheme 3). The kinetics studies of reactions of pyrrole with nitrostyrenes bearing H, F, Cl and Me at the terminal carbon were conducted at 50 °C (Figure 4). The data obtained are summarized in Table 3. In this case, a high sensitivity to the substituent influences was observed because of the near position of the substituent to the reaction site. The least reactivity was demonstrated by β-methylated nitrostyrene 1b′ (Table 3, Entry 1). Its conversion was only 17% after 4 h of the reaction. Fluorinated nitrostyrene 1b showed a 6.5 times faster total reaction rate than that of the methylated one 1b′.
Scheme 3.
Kinetics studies of the substituent effect at the double bond.
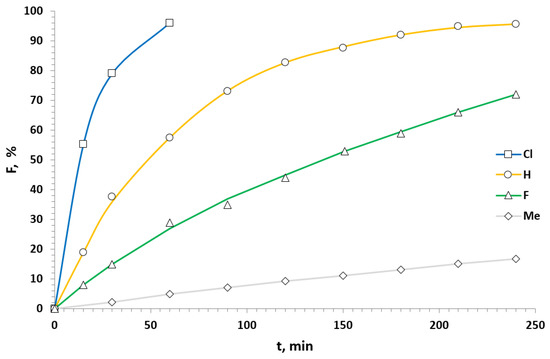
Figure 4.
Kinetic curves of the reactions of differently β-substituted nitrostyrenes with pyrrole at 50 °C.

Table 3.
Kinetic data of the reactions of β-substituted nitrostyrenes with pyrrole.
However, β-unsubstituted nitrostyrene 1b″ having the minimum sterical demand was about 18 times more reactive than 1b′. To our surprise, β-chlorinated nitrostyrene 1b‴ (Table 3, Entry 4) proved the most reactive one. Almost a full conversion was achieved only after 1 h. It was nearly 69 times more reactive than 1b′. Such an effect of the substituent at β-carbon can be explained by the combination of electronic and sterical effects.
The novel 2-(2-Fluoro-2-nitro-1-arylethyl)-1H-pyrroles 3 obtained are interesting compounds due to their potential synthetic utility for the subsequent transformations. We proposed that they could be appropriate and effective precursors for novel 2-(2-Fluoro-1-arylvinyl)-1H-pyrrole 4. C-Vinylpyrroles have attracted much research interest due to their synthetic utility as building blocks for pyrrole derivatives [33]. However, by now, 2-(2-Fluoro-vinyl)pyrroles have not been obtained yet. Apparently, this is related to the unavailability of suitable precursors. Indeed, 1-Fluoroacetylenes that could be potential precursors for the synthesis of such structures are elusive, unstable compounds. However, the use of β-fluoro-β-nitrostyrenes 1 as synthetic equivalents of 1-fluoroacetylenes is a promising way to overcome this synthetic problem. We applied this strategy based on the formation of a double bond in the final step by the base-induced elimination of nitrous acid from the adducts 3 (Scheme 4).
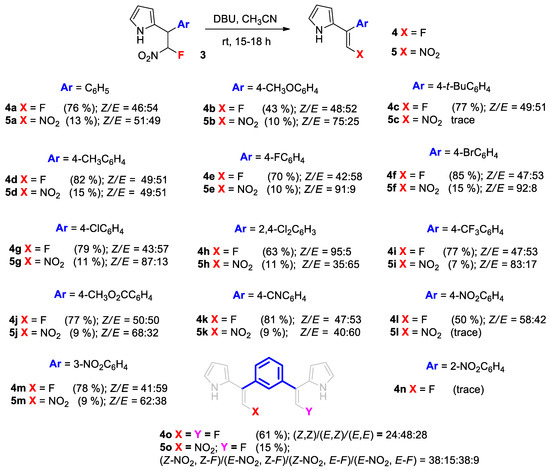
Scheme 4.
Scope of the pyrrole adducts 3 in the preparation of 2-(2-Fluoro-1-arylvinyl)-1H-pyrroles 4. Reaction conditions: adduct 3 (0.4–0.5 mmol) was treated with DBU (0.8–3 mmol) in MeCN (3 mL) at rt for 15–18 h; Z/E was determined from the isolated yields of isomers or by 1H and 19F NMR spectroscopy; isolated yields in parentheses were obtained by column chromatography on silica gel.
The reactions were conducted in acetonitrile using DBU as a base at an ambient temperature (Scheme 4). In principle, the competitive elimination of either HNO2 or HF can be expected, leading to the formation of either fluorine-substituted 4 or nitro-substituted 2-Vinylpyrrole 5. However, to our delight, the reaction demonstrated a high selectivity towards the desired 2-(2-Fluoro-1-arylvinyl)-1H-pyrroles 4. The reaction demonstrated a high effectiveness to furnish the desired products 4 in up to 85% yields, whereas the side products 5 did not exceed 15%. Somewhat lower yields (43–50%) were achieved in the case of the adducts 3b and 3l, having a strong electron-donating (EDG) methoxy- or a strong electron-withdrawing (EWG) nitro group at the para-position of the benzene ring, correspondingly. The presence of a nitro group at the ortho-position of the benzene substituent of 3n resulted in the decomposition of the reaction mixture under these reaction conditions. Only trace amounts of adduct 4n were detected. However, when the nitro-group was posed at the meta-position, a high yield of product 4m (78%) was obtained. All the other adducts 3 were also successfully transformed into the corresponding 2-vinylpyrroles 4.
Both the fluorinated product 4 and nitro product 5 were formed as a mixture of Z- and E-isomers. However, both the fluorinated isomers 4, if necessary, can be separated by column chromatography on silica gel, whereas, in the case of the side product 5, both isomers have the same retention time and, therefore, are isolated together. We demonstrated the possibility of separation (Z)- and (E)-2-(2-Fluoro-1-phenylvinyl)-1H-pyrrole for a large number of examples and fully characterized them. As expected, the elimination of the bis-pyrrole adduct 3o resulted in the formation of the desired difluorinated product 4o as a mixture of three isomers, with the Z,Z-, E,Z- and E,E- configurations in a 24:48:28 ratio, correspondingly. The side products were monofluorinated nitro-derivatives 5o formed as a mixture of four isomers. We isolated two mixtures of isomers in the Z-NO2 and Z-F, along with E-NO2 and Z-F configurations and the Z-NO2 and E-F, along with E-NO2 and E-F configurations.
The stereochemistry of the products 4 was unambiguously assigned with NMR spectroscopy. The 1H NMR spectra displayed a significant downfield shift (ca. 1 ppm) of pyrrolic H5 in a Z-configuration in reference to that in the E-configuration (Figure 5). Additionally, the splitting of the resonance signal of fluorine into a double doublet in the Z-configuration was observed with 19F NMR spectroscopy (Figure 5), whereas, in the E-configuration, the fluorine resonance signal was a doublet. Probably, this indicated the formation of an intermolecular hydrogen bond with fluorine. Indeed, a similar pattern was observed for the side nitro products 5. In this case, the downfield shift of the pyrrolic proton in the Z-configuration was much greater ca. 3.7 ppm. The difference in the frequencies of the NH vibrations regarding the Z- and E-positions of fluorine observed with IR spectroscopy was about 20 cm−1 (Figure 6).
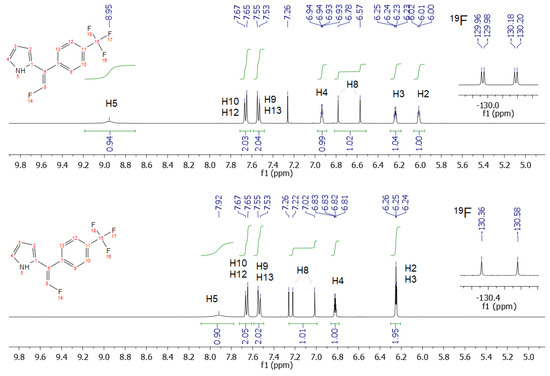
Figure 5.
Comparison of the 1H and 19F NMR spectra for the Z- and E-isomers of 4i.
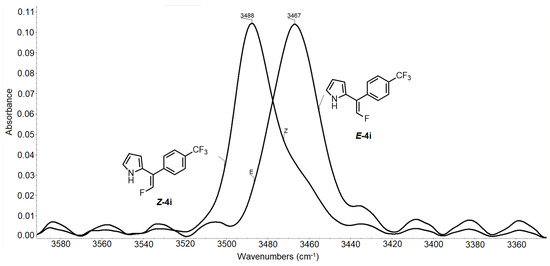
Figure 6.
Fragment of IR spectra of the isomers of 4i with NH vibrations.
As expected, the resulting ratio of the diastereomers 4 remained similar to that of the precursors 3. However, the elimination of the 2,4-dichlorophenyl-substituted adduct 3b with dr = 48:52 led to the predominant formation of a Z-isomer 4 in high diastereomeric excess (90% de).
Next, we demonstrated the versatility of our methodology for N-substituted pyrroles. Due to the reduced nucleophilicity of N-aryl pyrroles and high steric demand of substituents, the conjugate addition required harsher conditions. Moreover, most of N-aryl pyrroles used are solid at room temperature (except for pyrrole 2g) and need heating to be melted. We applied catalyst-free conditions for the reactions of nitrostyrene 1g with N-substituted pyrroles (Scheme 5). The reactions were carried out under solvent-free conditions and microwave-activated at elevated temperatures (150–200 °C) for 16–20 h. This, in turn, intensified the undesirable side reactions and, as a consequence, in some cases, decreased the selectivity. Indeed, some amount of β-substituted adducts was observed along with the target products. The reaction with 1-methyl-1H-pyrrole 2b was completed at 150 °C with a good overall yield of 3p (76%). However, the formation of 13% of the β-substituted regioisomers was detected by 1H and 19F-NMR spectroscopy. The reactions of nitrostyrene 1g with 1-Phenyl-1H-pyrrole 2c and 1-(4-Ethylphenyl)-1H-pyrrole 2d demonstrated poor reactivity. Low yields of adducts 3q and 3r (28–35%) were obtained after the full conversion of 1g. However, the reaction with 1-(p-Tolyl)-1H-pyrrole 2e displayed a better reactivity to give adduct 3s in a 51% yield. It was also found that the reactivity of N-aryl-substituted pyrroles was increased in the presence of an electron-donating methoxy group in the benzene ring. For instance, the reaction with 1-(3-Methoxyphenyl)-1H-pyrrole 2g resulted in a good yield of adduct 3u (74%), whereas, in the case of the simultaneous presence of EDG (-OMe) and weak EWG (-Cl), the yield of the adduct 3t (46%) diminished compared to 3u. On the other hand, we failed to obtain the adducts based on pyrroles having only electron-withdrawing groups onthe aryl-substituent under these conditions.
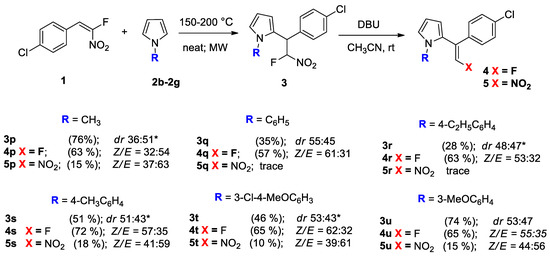
Scheme 5.
Scope of N-substituted pyrroles 2 in a conjugate addition–elimination sequence. Reaction conditions: 1 step: nitrostyrene 1 (0.5 mmol) was reacted with N-substituted pyrrole (0.4–0.5 g) under microwave irradiation at 150–200 °C for 15–24 h; 2 step: adduct 3 (1 mol. equiv.) was treated with DBU (6 mol. equiv.) in MeCN at rt for 15–18h; Z/E was determined by 1H and 19F NMR spectroscopy; isolated yields in parentheses were obtained by column chromatography on silica gel; * the formation of 3-substituted products was also observed (4–13%).
After, the subsequent elimination of nitrous acid from the adducts 3p–3u was conducted (Scheme 5). Similar to their N-unsubstituted analogs, these compounds also reacted smoothly. The corresponding 2-vinyl pyrroles 4p–4s were obtained in good yields (57–72%). The side nitro product 5 was isolated in the range of 10–18%. It was found that N-aryl-substituted 2-vinylpyrroles formed with a predominance of the Z-isomer. It can be clearly explained by the steric demand of the aryl substituent with nitrogen compared to the hydrogen or methyl groups. Indeed, in the course of conjugate addition (Scheme 3), the orientation of the small fluorine near a bulky aryl is sterically more favorable.
The pyrrole derivatives are also of great importance for the modification at the free α-position. Therefore, we studied the reaction of adduct 3l with 4-Chlorobenzaldehyde to prepare the corresponding dipyrromethane 6 (Scheme 6). The reaction proceeded in the presence of trifluoroacetic acid (TFA) as the catalyst and gave 6 as a mixture of the diastereomers in a 48% yield. This transformation can open up the straightforward way to novel valuable classes of fluorinated pyrrole derivatives, such as dipyrromethanes and their boron difluoride complexes (BODIPYs).
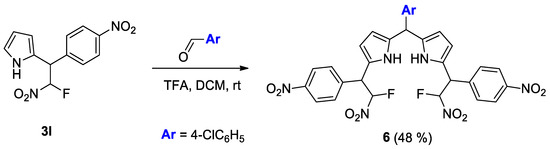
Scheme 6.
Synthesis of dipyrromethane 6.
3. Materials and Methods
All reagents were purchased from commercial sources and used without any further purification. All solvents were dried before use by the standard procedure [34]. Melting points (m.p.) were measured with a Büchi B-545 melting point apparatus (Büchi, Flawil, Switzerland). Microwave activated reactions were conducted in Monowave 200 (Anton Paar, Graz, Austria). NMR (1H, 13C and 19F) spectra were obtained with the Bruker AVANCE 400 (Bruker Corp., Karlsruhe, Germany) and Agilent 400-MR spectrometers (Agilent Technologies, Santa Clara, CA, USA) using deuterated chloroform (CDCl3). Chemical shifts for the 1H NMR spectroscopic data were referenced to the internal tetramethylsilane (δ = 0.0 ppm) and the residual solvent resonance (δ = 7.26 ppm); chemical shifts for the 13C NMR spectroscopic data were referenced to the residual solvent resonance (δ = 77.16 ppm); chemical shifts for the 19F NMR spectroscopic data were referenced to PhCF3 (δ = −63.72 ppm) or C6F6 (δ = −164.90 ppm). Data were reported as follows: chemical shift; integration multiplicity (s = singlet, d = doublet, t = triplet, q = quadruplet, qui = quintet, sext = sextet, sept = septet, br = board, m = multiplet, dd = doublet of doublets and ddd = doublet of doublet of doublets) and coupling constants (Hz). Starting β-fluoro-β-nitrostyrenes were prepared according to the described procedures [22,35]. All nitrostyrenes are known compounds [22,23,24,25,26,27,28,29,30].
General procedure for the conjugate addition of 1H-pyrrole to β-fluoro-β-nitrostyrenes.
In a typical experiment, β-fluoro-β-nitrostyrene (0.5 mmol) and pyrrole (0.5 mL) were successively loaded into a vial. The reaction mixture was stirred at room temperature for 25–30 h. After completion of the reaction (TLC monitoring), the excess of pyrrole was evaporated under vacuum. The desired product was isolated by column chromatography on silica gel as a mixture of diastereomers.
2-(2-Fluoro-2-nitro-1-phenylethyl)-1H-pyrrole (3a). Eluent: Hex/DCM 1:1, DCM; 107 mg (88 %); dr = 42:58; colorless oil. 1H NMR (400 MHz, CDCl3): (major isomer) δ = 7.99 (br s, 1H), 7.46–7.23 (m, 5H), 6.78–6.71 (m, 1H), 6.33–6.28 (m, 1H), 6.28 (dd, 2JHF = 50.0, 3.5 Hz, 1H), 6.20 (t, J = 2.3 Hz, 1H), 4.97 (dd, 3JHF = 28.5, 3.4 Hz, 1H) ppm; (minor isomer) δ = 8.11 (br s, 1H), 7.46–7.23 (m, 5H), 6.78–6.71 (m, 1H), 6.26–6.23 (m, 1H), 6.20 (t, J = 2.3 Hz, 1H), 6.19 (dd, 2JHF = 50.0, 3.4 Hz, 1H), 4.99 (dd, 3JHF = 28.0, 4.0 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): (major isomer) δ = 133.2, 129.2, 129.1, 128.4, 125.7, 118.9, 111.5 (d, 1JCF = 245.4 Hz), 109.0, 107.6, 47.9 (d, 2JCF = 18.7 Hz) ppm; (minor isomer) δ = 135.1 (d, 3JCF = 1.6 Hz), 129.4, 128.8, 128.6, 123.8, 119.2, 112.4 (d, 1JCF = 243.7 Hz), 109.2 (d, 4JCF = 0.8 Hz), 108.9, 48.0 (d, 2JCF = 18.6 Hz) ppm; 19F NMR (376 MHz, CDCl3): (major isomer) δ = −153.26 (dd, J = 50.0, 28.5 Hz) ppm; (minor isomer) δ = −151.19 (dd, J = 50.0, 28.0 Hz) ppm. HRMS (ESI-TOF) m/z: [M − H]− calcd. for C12H10FN2O2 233.0732; found 233.0735.
2-(2-Fluoro-1-(4-methoxyphenyl)-2-nitroethyl)-1H-pyrrole (3b). Eluent: Hex/DCM 1:1, DCM; 67 mg (>99%); dr = 40:60; colorless oil. 1H NMR (400 MHz, CDCl3): (major isomer) δ = 7.99 (br s, 1H), 7.16 (d, J = 8.5 Hz, 2H), 6.86 (d, J = 8.7 Hz, 2H), 6.77–6.69 (m, 1H), 6.27 (dd, 2JHF = 50.2, 3.2 Hz, 1H), 6.26 (s, 1H), 6.24–6.14 (m, 1H), 4.92 (dd, 3JHF = 29.0, 3.5 Hz, 1H), 3.78 (s, 3H) ppm; (minor isomer) δ = 8.11 (br s, 1H), 7.23 (d, J = 8.6 Hz, 2H), 6.91 (d, J = 8.7 Hz, 2H), 6.77–6.69 (m, 1H), 6.14 (dd, 2JHF = 50.1, 3.8 Hz, 1H), 6.24–6.13 (m, 2H), 4.91 (dd, 3JHF = 27.0, 4.3 Hz, 1H), 3.81 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): (major isomer) δ = 159.9, 130.4, 126.1, 125.0, 118.7, 114.5, 111.5 (d, 1JCF = 244.9 Hz), 109.0, 107.3, 55.4, 47.2 (d, 2JCF = 18.9 Hz) ppm; (minor isomer) δ = 159.7, 129.7, 126.8 (d, 3JCF = 2.3 Hz), 124.3, 119.0, 114.7, 112.7 (d, 1JCF = 243.5 Hz), 108.9 (d, 4JCF = 0.8 Hz), 108.9, 55.4, 47.3 (d, 2JCF = 18.7 Hz) ppm. 19F NMR (376 MHz, CDCl3): (major isomer) δ = −153.92 (dd, J = 50.2, 29.0 Hz) ppm; (minor isomer) δ = −150.63 (dd, J = 50.1, 27.0 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C13H14FN2O3 265.0983; found 265.0986.
2-(1-(4-(Tert-butyl)phenyl)-2-fluoro-2-nitroethyl)-1H-pyrrole (3c). Eluent: Hex/DCM 1:1, DCM; 131 mg (85 %); dr = 42:58; yellowish oil. 1H NMR (400 MHz, CDCl3): (major isomer) δ = 7.96 (br s, 1H), 7.38 (d, J = 8.4 Hz, 2H), 7.20 (d, J = 8.2 Hz, 2H), 6.75–6.71 (m, 1H), 6.29 (br s, 1H), 6.24 (dd, J = 5.8, 3.2 Hz, 1H), 6.27 (dd, 2JHF = 50.1, 3.6 Hz, 1H), 4.96 (dd, 3JHF = 28.3, 3.5 Hz, 1H), 1.33 (s, 9H) ppm; (minor isomer) δ = 8.09 (br s, 1H), 7.44 (d, J = 8.4 Hz, 2H), 7.27 (d, J = 9.0 Hz, 2H), 6.75–6.71 (m, 1H), 6.17–6.21 (m, 2H), 6.18 (dd, 2JHF = 50.1, 3.7 Hz, 1H), 4.99 (dd, 3JHF = 28.4, 3.8 Hz, 1H), 1.36 (s, 9H) ppm. 13C NMR (100 MHz, CDCl3): (major isomer) δ = 151.7, 130.1, 128.9, 126.1, 125.9 (d, 4JCF = 0.9 Hz), 118.7, 111.6 (d, 1JCF = 245.1 Hz), 109.0, 107.4, 47.5 (d, 2JCF = 18.9 Hz), 34.6, 31.3 ppm; (minor isomer) δ = 151.8, 132.1 (d, 3JCF = 1.3 Hz), 128.1, 126.3, 123.9, 119.0, 112.4 (d, 1JCF = 243.9 Hz), 109.1, 108.9, 47.5 (d, 2JCF = 18.4 Hz), 34.7, 31.3 ppm.19F NMR (376 MHz, CDCl3): (major isomer) δ = −152.75 (dd, J = 50.1, 28.3 Hz) ppm; (minor isomer) δ = −151.15 (ddd, J = 50.1, 28.4, 5.0 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C16H20FN2O2 291.1509; found 291.1503.
2-(2-Fluoro-2-nitro-1-(p-tolyl)ethyl)-1H-pyrrole (3d). Eluent: Hex/DCM 1:1; 122 mg (99 %); dr = 44:56; yellowish oil. 1H NMR (400 MHz, CDCl3): (major isomer) δ = 8.09 (br s, 1H), 7.20–7.12 (m, 4H), 6.77–6.70 (m, 1H), 6.31–6.27 (m, 1H), 6.27 (dd, 2JHF = 50.1, 3.5 Hz, 1H), 6.25 (dd, J = 5.7, 2.5 Hz, 1H), 4.94 (dd, 3JHF = 28.4, 3.4 Hz, 1H), 2.36 (s, 3H) ppm; (minor isomer) δ = 7.97 (br s, 1H), 7.22 (s, 4H), 6.77–6.70 (m, 1H), 6.21–6.17 (m, 2H), 6.17 (dd, 2JHF = 50.1, 4.2 Hz, 1H), 4.95 (dd, 3JHF = 27.6, 4.3 Hz, 1H), 2.39 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): (major isomer) δ = 138.7, 130.1, 129.8, 129.1, 125.9, 118.7, 111.5 (d, 1JCF = 245.3 Hz), 109.0, 107.4 (d, 4JCF = 1.0 Hz), 47.6 (d, 2JCF = 18.9 Hz), 21.2 ppm; (minor isomer) δ = 138.6 ppm, 131.9 (d, 3JCF = 2.1 Hz), 130.0, 128.3, 124.1, 119.0, 112.6 (d, 1JCF = 243.5 Hz), 109.0, 108.8, 47.6 (d, 2JCF = 18.9 Hz), 21.2 ppm. 19F NMR (376 MHz, CDCl3): (major isomer) δ = −153.31 (dd, J = 50.1, 28.4 Hz) ppm; (minor isomer) δ = −150.74 (dd, J = 50.1, 27.6 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C13H14FN2O2 249.1039; found 249.1054.
2-(2-Fluoro-1-(4-fluorophenyl)-2-nitroethyl)-1H-pyrrole (3e). Eluent: Hex/DCM 1:1, DCM; 118 mg (94%); dr = 44:56; yellowish oil. 1H NMR (400 MHz, CDCl3): (major isomer) δ = 8.01 (br s, 1H), 7.22 (dd, J = 8.5, 5.2 Hz, 2H), 7.06 –6.98 (m, 2H), 6.80–6.72 (m, 1H), 6.29 (br s, 1H), 6.28 (dd, 2JHF = 50.0, 3.2 Hz, 1H), 6.26–6.15 (m, 1H), 4.97 (dd, 3JHF = 28.8, 3.1 Hz, 1H) ppm; (minor isomer) δ = 8.14 (br s, 1H), 7.30 (dd, J = 8.5, 5.2 Hz, 2H), 7.05–7.13 (m, 2H), 6.72–6.80 (m, 1H), 6.15–6.26 (m, 2H), 6.16 (dd, 2JHF = 50.0, 3.5 Hz, 1H), 4.97 (dd, 3JHF = 27.6, 3.1 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): (major isomer) δ = 162.9 (d, 1JCF = 248.2 Hz), 131.0 (d, 3JCF = 8.2 Hz), 129.0 (d, 3JCF = 3.1 Hz), 125.5, 119.0, 116.1 (d, 2JCF = 21.4 Hz), 111.2 (d, 1JCF = 245.4 Hz), 109.1, 107.7, 47.2 (d, 2JCF = 18.8 Hz) ppm; (minor isomer) δ = 162.7 (d, 1JCF = 248.2 Hz), 130.9 (t, 3JCF = 2.6 Hz), 130.2 (d, 3JCF = 8.2 Hz), 123.6, 119.3, 116.3 (d, 2JCF = 21.3 Hz), 47.3 (d, 2JCF = 18.6 Hz), 109.0, 109.3, 112.3 (d, 1JCF = 243.7 Hz) ppm. 19F NMR (376 MHz, CDCl3): (major isomer) δ = −113.92 (tt, J = 8.5, 5.2 Hz), −154.01 (dd, J = 50.0, 28.8 Hz) ppm; (minor isomer) δ = −114.10 (tt, J = 8.5, 5.2 Hz), −151.24 (dd, J = 50.0, 27.6 Hz) ppm. HRMS (ESI-TOF) m/z: [M − H]− calcd. for C12H9F2N2O2 251.0638; found 251.0644.
2-(1-(4-Bromophenyl)-2-fluoro-2-nitroethyl)-1H-pyrrole (3f). Eluent: Hex/DCM 1:1; 133 mg (84 %); dr = 44:56; yellowish oil; 1H NMR (400 MHz, CDCl3): (major isomer) δ = 8.01 (br s, 1H), 7.49–7.44 (m, 2H), 7.11 (d, J = 8.3 Hz, 2H), 6.76 (ddd, J = 4.1, 2.8, 1.5 Hz, 1H), 6.31–6.27 (m, 1H), 6.27 (dd, 2JHF = 49.9, 3.3 Hz, 1H), 6.24 (dd, J = 6.1, 2.8 Hz, 1H), 4.94 (dd, 3JHF = 28.6, 3.5 Hz, 1H) ppm; (minor isomer) δ = 8.13 (br s, 1H), 7.55–7.50 (m, 2H), 7.19 (d, J = 8.5 Hz, 2H), 6.76 (ddd, J = 4.1, 2.8, 1.5 Hz, 1H), 6.16 (dd, 2JHF = 50.0, 4.0 Hz, 1H), 6.14–6.20 (m, 2H), 4.95 (dd, 3JHF = 28.2, 5.2 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 134.1 (d, 3JCF = 1.8 Hz), 132.5, 132.3, 132.2, 130.1, 125.1, 123.1, 122.8, 119.4, 119.1, 111.9 (d, 1JCF = 244.0 Hz), 111.0 (d, 1JCF = 245.7 Hz), 109.5, 109.1, 109.0, 107.9 (d, 4JCF = 1.2 Hz), 47.4 (d, 2JCF = 18.7 Hz), 47.3 (d, 2JCF = 18.7 Hz) ppm.19F NMR (376 MHz, CDCl3): (major isomer) δ = −153.62 (dd, J = 49.9, 28.6 Hz) ppm; (minor isomer) δ = −151.36 (dd, J = 50.0, 28.2 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C12H1180BrFN2O2 312.9982; found 312.9951.
2-(1-(4-Chlorophenyl)-2-fluoro-2-nitroethyl)-1H-pyrrole (3g). Eluent: Hex/DCM 1:1, DCM; 131 mg, (97 %); dr = 42:58; yellowish oil. 1H NMR (400 MHz, CDCl3): (major isomer) δ = 8.02 (br s, 1H), 7.32 (d, J = 8.4 Hz, 2H), 7.18 (d, J = 8.4 Hz, 2H), 6.76 (s, 1H), 6.29 (br s, 1H), 6.27 (dd, J = 50.0, 3.3 Hz, 1H), 6.25 (dd, J = 5.8, 2.8 Hz, 1H), 4.95 (dd, 3JHF = 28.6, 3.2 Hz, 1H) ppm; (minor isomer) δ = 8.14 (br s, 1H), 7.37 (d, J = 8.4 Hz, 2H), 7.26 (d, J = 8.4 Hz, 1H), 6.76 (s, 1H), 6.16 (dd, 2JHF = 50.0, 4.0 Hz, 2H), 6.21–6.15 (m, 2H), 4.97 (dd, 3JHF = 27.9, 5.0 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3): (major isomer) δ = 134.9, 131.7, 130.5, 129.3, 125.1, 119.1, 111.1 (d, 1JCF = 245.6 Hz), 109.1, 107.8 (d, 4JCF = 1.1 Hz), 47.2 (d, 2JCF = 18.7 Hz) ppm; (minor isomer) δ = 134.6, 133.6 (d, 3JCF = 1.5 Hz), 129.8, 129.5, 123.2, 119.4, 112.0 (d, 1JCF = 244.0 Hz), 109.4, 109.0, 47.3 (d, 2JCF = 18.8 Hz) ppm; 19F NMR (376 MHz, CDCl3): (major isomer) δ = −153.65 (dd, J = 50.0, 28.6 Hz) ppm; (minor isomer) δ = −151.35 (dd, J = 50.0, 27.9 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C12H1135ClFN2O2 269.0493; found 269.0485.
2-(1-(2,4-Dichlorophenyl)-2-fluoro-2-nitroethyl)-1H-pyrrole (3h). Eluent: Hex/DCM 1:1; 145 mg, (96 %); dr = 48:52; yellowish oil; 1H NMR (400 MHz, CDCl3): δ = 8.21 (br s, 1H) ppm, 8.11 (br s, 1H), 7.51–7.47 (m, 1H), 7.46–7.41 (m, 2H), 7.29–7.22 (m, 3H), 6.78–6.73 (m, 2H), 6.31 (dd, 2JHF = 50.0, 3.8 Hz, 1H), 6.29–6.24 (m, 2H), 6.21 (td, J = 6.3, 2.8 Hz, 2H), 6.19 (dd, 2JHF = 49.9, 3.3 Hz, 1H), 5.59 (dd, 3JHF = 26.5, 4.0 Hz, 1H), 5.57 (dd, 3JHF = 30.8, 3.2 Hz, 1H) ppm.13C NMR (100 MHz, CDCl3): δ = 135.3, 135.2, 134.9, 134.0, 131.8, 131.5 (d, JCF = 1.0 Hz), 131.1 (d, JCF = 4.1 Hz), 130.5, 130.0, 129.9, 128.1, 128.0, 124.3 (d, JCF = 1.8 Hz), 121.8, 119.3, 119.2, 110.8 (d, 1JCF = 246.1 Hz), 110.1 (d, 1JCF = 244.1 Hz), 110.1 (d, 4JCF = 2.0 Hz), 109.3, 109.2, 108.3, 44.2 (d, 2JCF = 18.4 Hz), 42.9 (d, 2JCF = 18.7 Hz) ppm. 19F NMR (376 MHz, CDCl3): (major isomer) δ = −155.07 (dd, J = 49.8, 30.7 Hz) ppm; (minor isomer) δ = −151.19 (dd, J = 49.8, 26.3 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C12H1035Cl2FN2O2 303.0098; found 303.0095.
2-(2-Fluoro-2-nitro-1-(4-(trifluoromethyl)phenyl)ethyl)-1H-pyrrole (3i). Eluent: Hex/DCM 1:1, DCM; 142 mg (94%); dr = 38:62; yellow solid; M.p. = 68–72 ºC. 1H NMR (400 MHz, CDCl3): (major isomer) δ = 8.04 (br s, 1H), 7.61 (d, J = 8.1 Hz, 2H), 7.39 (d, J = 8.1 Hz, 2H), 6.81–6.75 (m, 1H), 6.31 (dd, 2JHF = 49.8, 3.4 Hz, 1H), 6.35–6.30 (m, 1H), 6.21 (t, J = 2.4 Hz, 1H), 5.05 (dd, 3JHF = 28.3, 3.3 Hz, 1H) ppm; (minor isomer) δ = 8.17 (br s, 1H), 7.67 (d, J = 8.2 Hz, 2H), 7.46 (d, J = 8.1 Hz, 2H), 6.75–6.81 (m, 1H), 6.22–6.30 (m, 1H), 6.22 (dd, 2JHF = 49.9, 3.7 Hz, 1H), 6.21 (t, J = 2.4 Hz, 1H), 5.08 (dd, 3JHF = 28.6, 3.7 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): (major isomer) δ = 137.4, 131.0 (q, 2JCF = 32.8 Hz), 129.7, 126.1 (q, 3JCF = 3.7 Hz), 124.7, 123.9 (q, 1JCF = 272.2 Hz), 119.4, 111.1 (d, 1JCF = 245.9 Hz), 109.3, 108.2 (d, 4JCF = 1.1 Hz), 47.6 (d, 2JCF = 18.8 Hz) ppm; (minor isomer) δ = 139.2, 130.9 (q, 2JCF = 32.6 Hz), 128.9, 126.3 (q, 3JCF = 3.6 Hz), 123.9 (q, 1JCF = 272.2 Hz), 122.7, 119.7, 111.8 (d, 1JCF = 244.2 Hz), 109.8, 109.1, 47.7 (d, 2JCF = 18.6 Hz) ppm; 19F NMR (376 MHz, CDCl3): (major isomer) δ = −65.89 (s, 1F), −155.34 (dd, J = 49.8, 28.3 Hz, 3F) ppm; (minor isomer) δ = −65.86 (s, 1F), -153.71 (dd, J = 49.9, 28.6 Hz, 3F) ppm. HRMS (ESI-TOF) m/z: [M − H]− calcd. for C13H10F4N2O2 301.0606; found 301.0612.
Methyl 4-(2-fluoro-2-nitro-1-(1H-pyrrol-2-yl)ethyl)benzoate (3j). Eluent: Hex/DCM 1:1, DCM; 138 mg (95%), dr = 42:58); yellowish oil; 1H NMR (400 MHz, CDCl3): (major isomer) δ = 8.46 (br s, 1H), 7.93 (d, J = 8.3 Hz, 2H), 7.30 (d, J = 8.2 Hz, 2H), 6.78–6.74 (m, 1H), 6.31 (dd, 2JHF = 49.8, 3.4 Hz, 1H), 6.27–6.31 (m, 1H), 6.23 (dd, J = 5.9, 2.8 Hz, 1H), 5.03 (dd, 3JHF = 28.2, 3.4 Hz, 1H), 3.88 (s, 3H) ppm; (minor isomer) δ = 8.46 (br s, 1H), 8.00 (d, J = 8.3 Hz, 2H), 7.37 (d, J = 8.2 Hz, 2H), 6.78–6.74 (m, 1H), 6.22 (dd, 2JHF = 49.9, 3.9 Hz, 1H), 6.20–6.16 (m, 2H), 5.05 (dd, 3JHF = 28.3, 3.8 Hz, 1H), 3.91 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): (major isomer) δ = 166.8, 138.51, 130.32, 130.17, 129.27, 124.84, 119.22, 111.14 (d, 1JCF = 245.8 Hz), 109.04, 108.02 (d, 4JCF = 1.1 Hz), 52.38, 47.69 (d, 2JCF = 18.7 Hz) ppm; (minor isomer) δ = 166.7, 140.3 (d, 3JCF = 0.9 Hz), 130.4, 130.3, 128.5, 122.9, 119.5, 111.8 (d, 1JCF = 244.1 Hz), 109.5, 108.9, 52.4, 47.8 (d, 2JCF = 18.8 Hz) ppm. 19F NMR (376 MHz, CDCl3): (major isomer) δ = −153.04 (dd, J = 49.8, 28.2 Hz) ppm; (minor isomer) δ = −151.51 (dd, J = 49.9, 28.3 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C14H14FN2O4 293.0932; found 293.0936.
4-(2-Fluoro-2-nitro-1-(1H-pyrrol-2-yl)ethyl)benzonitrile (3k). Eluent: Hex/DCM 1:1, DCM; 130 mg (85 %); dr = 38:62; yellowish oil; 1H NMR (400 MHz, CDCl3): (major isomer) δ = 8.36 (br s, 1H), 7.61–7.55 (m, 2H), 7.37 (d, J = 8.2 Hz, 2H), 6.81–6.76 (m, 1H), 6.31 (dd, 2JHF = 49.7, 3.3 Hz, 1H), 6.32–6.28 (m, 1H), 6.23 (dd, J = 6.0, 2.8 Hz, 1H), 5.06 (dd, 3JHF = 28.4, 3.2 Hz, 1H) ppm; (minor isomer) δ = 8.36 (br s, 1H), 7.67–7.62 (m, 2H), 7.45 (d, J = 8.3 Hz, 2H), 6.81–6.76 (m, 1H), 6.22 (dd, 2JHF = 49.8, 3.7 Hz, 1H), 6.22–6.17 (m, 2H), 5.09 (dd, 3JHF = 28.6, 3.6 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): (major isomer) δ = 138.9, 132.7, 130.0, 124.2, 119.5, 118.4, 112.4, 110.8 (d, 1JCF = 246.2 Hz), 109.2, 108.4 (d, 4JCF = 1.3 Hz), 47.6 (d, 2JCF = 18.5 Hz) ppm; (minor isomer) δ = 140.7, 133.0, 129.3, 122.2, 119.7, 118.3, 112.2, 111.3 (d, 1JCF = 244.5 Hz), 109.8, 109.1, 47.8 (d, 2JCF = 17.1 Hz) ppm. 19F NMR (376 MHz, CDCl3): (major isomer) δ = −153.37 (dd, J = 49.7, 28.4 Hz) ppm; (minor isomer) δ = −151.89 (dd, J = 49.8, 28.6 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C13H11FN3O2 260.0830; found 260.0829.
2-(2-Fluoro-2-nitro-1-(4-nitrophenyl)ethyl)-1H-pyrrole (3l). Eluent: Hex/DCM 1:1, DCM; 428 mg (>99% yield), scaled-up 1303 mg (99%); dr = 40:60; brown waxy solid. 1H NMR (400 MHz, CDCl3): (major isomer) δ = 8.24 (br s, 1H), 8.14 (d, J = 8.6 Hz, 2H), 7.43 (d, J = 8.6 Hz, 1H), 6.84–6.79 (m, 1H), 6.33 (dd, 2JHF = 49.7, 3.2 Hz, 2H), 6.35–6.29 (m, 1H), 6.24 (dd, J = 5.7, 2.8 Hz, 1H), 5.12 (dd, 3JHF = 28.2, 3.1 Hz, 1H) ppm; (minor isomer) δ = 8.31 (br s, 1H), 8.21 (d, J = 8.7 Hz, 1H), 6.84–6.79 (m, 1H), 6.25 (dd, 2JHF = 49.9, 3.7 Hz, 3H), 6.22–6.17 (m, 2H), 5.13 (dd, 3JHF = 28.4, 3.7 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): (major isomer) δ = 148.0, 140.7, 130.3, 124.1, 124.1, 119.7, 110.9 (d, 1JCF = 246.1 Hz), 109.4, 108.6 (d, 4JCF = 1.3 Hz), 47.5 (d, 2JCF = 18.9 Hz) ppm; (minor isomer) δ = 147.9, 142.5, 129.5, 124.4, 122.1, 119.9, 111.3 (d, 1JCF = 244.4 Hz), 110.1, 109.3, 47.7 (d, 2JCF = 19.2 Hz) ppm. 19F NMR (376 MHz, CDCl3): (major isomer) δ = −153.27 (dd, J = 49.7, 28.2 Hz) ppm; (minor isomer) δ = −151.61 (dd, J = 49.9, 28.4 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C12H11FN3O4 280.0728; found 280.0728.
2-(2-Fluoro-2-nitro-1-(3-nitrophenyl)ethyl)-1H-pyrrole (3m). Eluent: Hex/DCM 1:1, DCM; 117 mg (85%); dr = 39:61; yellow oil. 1H NMR (400 MHz, CDCl3): (major isomer) δ = 8.37 (br s, 1H), 8.24–8.09 (m, 2H), 7.61 (d, J = 7.7 Hz, 1H), 7.51 (t, J = 7.9 Hz, 1H), 6.83–6.78 (m, 1H), 6.34 (s, 1H), 6.34 (dd, 2JHF = 49.6, 3.3 Hz, 1H), 6.26–6.21 (m, 1H), 5.13 (dd, 3JHF = 28.4, 3.2 Hz, 1H) ppm; (minor isomer) δ = 8.37 (br s, 1H), 8.24–8.09 (m, 2H), 7.68 (d, J = 7.8 Hz, 1H), 7.57 (t, J = 8.0 Hz, 1H), 6.83–6.78 (m, 1H), 6.26 (dd, 2JHF = 49.8, 3.6 Hz, 1H), 6.26–6.16 (m, 2H), 5.15 (dd, 3JHF = 28.8, 3.6 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): (major isomer) δ = 148.3, 135.4, 134.7, 130.2, 124.2, 124.1, 123.8, 119.6, 110.9 (d, 1JCF = 245.9 Hz), 109.3, 108.5 (d, 4JCF = 1.6 Hz), 47.3 (d, 2JCF = 18.6 Hz) ppm; (minor isomer) δ = 148.5, 137.5, 135.7, 130.4, 123.6, 123.3, 122.2, 119.9, 111.4 (d, 1JCF = 244.3 Hz), 109.9, 109.2, 47.5 (d, 2JCF = 18.9 Hz) ppm. 19F NMR (376 MHz, CDCl3): (major isomer) δ = −153.52 (dd, J = 49.6, 28.4 Hz) ppm; (minor isomer) δ = −152.26 (dd, J = 49.8, 28.8 Hz) ppm. HRMS (ESI-TOF) m/z: [M − H]− calcd. for C12H9FN3O4 278.0583; found 278.0587.
2-(2-Fluoro-2-nitro-1-(2-nitrophenyl)ethyl)-1H-pyrrole (3n). Eluent: Hex/DCM 1:1, DCM; 126 mg (89%); dr = 56:44; pale brown oil. 1H NMR (400 MHz, CDCl3): (major isomer) δ = 8.58 (br s, 1H), 7.85 (d, J = 8.1 Hz, 1H), 7.69 (d, J = 7.9 Hz, 1H), 7.42–7.64 (m, 2H), 6.79 (s, 1H), 6.34 (br s, 1H), 6.34 (dd, 2JHF = 49.5, 4.3 Hz, 1H), 6.21 (dd, J = 5.4, 2.6 Hz, 1H), 5.83 (dd, 3JHF = 26.6, 3.8 Hz, 1H) ppm; (minor isomer) δ = 8.38 (br s, 1H), 8.01 (d, J = 8.1 Hz, 1H), 7.42–7.64 (m, 3H), 6.74 (s, 1H), 6.34 (br s, 1H), 6.42 (dd, J = 50.0, 3.0 Hz, 1H), 6.17 (dd, J = 5.4, 2.6 Hz, 1H), 5.83 (dd, J = 30.2, 3.8 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): (major isomer) δ = 149.5, 133.5, 131.0 (d, 4JCF = 4.2 Hz), 129.5, 128.6, 125.1, 124.2 (d, 3JCF = 1.1 Hz), 119.3, 111.0 (d, 1JCF = 242.4 Hz), 109.1, 108.0 (d, 4JCF = 1.7 Hz), 41.1 (d, 2JCF = 18.2 Hz) ppm; (minor isomer) δ = 148.6, 134.0, 132.0, 130.4, 129.5, 125.3, 122.4, 119.3, 110.7 (d, 1JCF = 244.1 Hz), 109.8, 109.1, 42.9 (d, 2JCF = 18.1 Hz) ppm. 19F NMR (376 MHz, CDCl3): (major isomer) δ = −151.14 (dd, J = 49.5, 26.6 Hz) ppm; (minor isomer) δ = −155.79 (dd, J = 50.0, 30.2 Hz) ppm. HRMS (ESI-TOF) m/z: [M − H]− calcd. for C12H9FN3O4 278.0583; found 278.0587.
1,3-Bis(2-fluoro-2-nitro-1-(1H-pyrrol-2-yl)ethyl)benzene (3o). Eluent: Hex/DCM 1:1, DCM; 191 mg (>99%); mixture of four isomers dr = 41:59 (two pair of isomers); greenish oil. 1H NMR (400 MHz, CDCl3): δ = 8.15 (s, 1H), 8.11 (s, 1H), 8.05 (s, 3H), 8.02 (br s, 1H), 7.96 (br s, 2H), 7.12–7.44 (m, 15H), 7.07 (s, 1H), 6.65–6.76 (m, 8H), 6.23 (dd, 2JHF = 49.5, 3.6 Hz, 1H), 6.04–6.34 (m, 21H), 6.08 (dd, 2JHF = 50.1, 4.2 Hz, 1H), 6.06 (dd, 2JHF = 49.8, 4.2 Hz, 1H), 4.95 (dd, J = 28.5, 4.6 Hz, 1H), 4.80–5.03 (m, 5H), 4.91 (dd, 3JHF = 28.1, 4.6 Hz, 1H), 4.90 (dd, 3JHF = 28.5, 3.1 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 136.2, 136.2, 136.1, 136.1, 135.9, 135.9, 135.8, 135.8, 134.4, 134.3, 134.0, 134.0, 130.2, 130.1, 130.0, 130.0, 129.8, 129.8, 129.7, 129.4, 129.3, 128.8, 128.8, 128.7, 128.5, 128.4, 128.3, 125.0, 124.9, 123.3, 123.2, 119.4, 119.4, 119.2, 119.2, 119.1, 119.1, 112.1 (d, 1JCF = 244.2 Hz), 112.0 (d, 1JCF = 244.1 Hz), 111.3 (d, 1JCF = 245.9 Hz), 111.2 (d, 1JCF = 245.8 Hz), 109.3, 109.2, 109.0, 109.0, 108.9, 108.9, 108.8, 107.9, 107.9, 107.8, 47.8 (d, 2JCF = 18.0 Hz), 47.7 (d, 2JCF = 18.0 Hz), 47.7 (d, 2JCF = 18.7 Hz), 47.6 (d, 2JCF = 18.5 Hz), 47.5 (d, 2JCF = 18.5 Hz) ppm (the other signals are overlapped). 19F NMR (376 MHz, CDCl3): δ = −151.12 (dd, J = 49.4, 27.7, 2F Hz), −151.12–−151.41 (m, 2F), −153.23–−153.76 (m, 4F) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C18H17F2N4O4 391.1212; found 391.1206.
General procedure for the conjugate addition of 1-substituted 1H-pyrroles to β-fluoro-β-nitrostyrenes.
In a typical experiment, a mixture of β-fluoro-β-nitrostyrene (0.5 mmol) and pyrrole (0.4–0.5 g) was loaded into a 4-mL vial (vial G4, Anton Paar). The reaction mixture was placed into a microwave reactor and heated at 150–200 °C with stirring until completion (15–24 h). The reaction progress was monitored by TLC or 19F NMR analysis. After completion of the reaction, the resulting mixture was separated by column chromatography on silica gel.
2-(1-(4-Chlorophenyl)-2-fluoro-2-nitroethyl)-1-methyl-1H-pyrrole (3p). Eluent: Hex/DCM 3:1, Hex/DCM 1:1; 114 mg (76%); dr = 36:51; yellow oil. 1H NMR (400 MHz, CDCl3): (major isomer) δ = 7.33–7.27 (m, 2H), 7.10 (d, J = 8.4 Hz, 2H), 6.66–6.61 (m, 1H), 6.37–6.32 (m, 1H), 6.18 (t, J = 3.2 Hz, 1H), 6.11 (dd, 2JHF = 49.8, 4.7 Hz, 1H), 4.93 (dd, 3JHF = 27.1, 2.9 Hz, 1H), 3.33 (s, 3H) ppm; (minor isomer) δ = 7.38–7.32 (m, 2H), 7.22 (d, J = 8.5 Hz, 2H), 6.62–6.58 (m, 1H), 6.40–6.36 (m, 1H), 6.29 (dd, J = 49.4, 3.0 Hz, 1H), 6.14 (t, J = 3.2 Hz, 1H), 4.96 (dd, J = 25.6, 4.7 Hz, 1H), 3.36 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): (major isomer) δ = 134.8, 131.3, 130.7, 129.3, 126.3, 123.6, 111.2 (d, 1JCF = 245.6 Hz), 108.1 (d, 4JCF = 3.4 Hz), 107.4, 46.0 (d, 2JCF = 19.1 Hz), 34.0 ppm; (minor isomer) δ = 134.6, 133.3 (d, 3JCF = 1.9 Hz), 130.1, 129.5, 124.4, 123.5, 112.2 (d, 1JCF = 246.7 Hz), 108.8 (d, 4JCF = 3.3 Hz), 107.4, 46.0 (d, 2JCF = 19.1 Hz), 33.9 ppm. 19F NMR (282 MHz, CDCl3): (major isomer) δ = −153.51 (dd, J = 49.8, 27.1 Hz) ppm; (minor isomer) δ = −152.56 (dd, J = 49.4, 25.6 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C13H13ClFN2O2 283.0644; found 283.0961.
2-(1-(4-Chlorophenyl)-2-fluoro-2-nitroethyl)-1-phenyl-1H-pyrrole (3q). Eluent: Hex/DCM 3:1, Hex/DCM 1:1, DCM; 62 mg (35%); dr = 55:45; yellow oil. 1H NMR (400 MHz, CDCl3): (major isomer) δ = 7.31–7.42 (m, 3H), 7.22–7.17 (m, 2H), 7.02–6.94 (m, 2H), 6.92 (d, J = 8.4 Hz, 2H), 6.81 (dd, J = 2.8, 1.7 Hz, 1H), 6.49–6.53 (m, 1H), 6.34–6.31 (m, 1H), 6.19 (dd, J = 50.0, 3.7 Hz, 1H), 4.76 (dd, J = 27.1, 3.5 Hz, 1H) ppm; (minor isomer) δ = 7.42–7.31 (m, 3H), 7.29–7.22 (m, 2H), 7.05 (d, J = 8.5 Hz, 2H), 7.02–6.94 (m, 2H), 6.77 (dd, J = 2.8, 1.7 Hz, 1H), 6.28–6.31 (m, 1H), 6.58 (dd, J = 4.3, 3.7 Hz, 1H), 5.97 (dd, J = 49.6, 4.4 Hz, 1H), 4.83 (dd, J = 28.7, 3.3 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): (major isomer) δ = 139.0, 134.6, 132.0, 130.6, 129.4, 129.0, 128.5, 127.0, 127.0, 124.0, 111.6 (d, 1JCF = 244.9 Hz), 109.2 (d, 4JCF = 3.2 Hz), 108.8, 46.0 (d, 2JCF = 19.1 Hz) ppm; (minor isomer) δ = 139.0, 134.4, 133.9 (d, 3JCF = 1.4 Hz), 130.1, 129.4, 129.3, 128.4, 127.1, 125.1, 123.8, 112.0 (d, 1JCF = 247.0 Hz), 109.7 (d, 4JCF = 3.6 Hz), 108.9, 45.8 (d, 2JCF = 18.4 Hz) ppm. 19F NMR (282 MHz, CDCl3): (major isomer) δ = −152.19 (dd, J = 50.0, 27.1 Hz), (minor isomer) δ = −153.32 (dd, J = 49.6, 28.7 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C18H15ClFN2O2 345.0801; found 345.0799.
2-(1-(4-Chlorophenyl)-2-fluoro-2-nitroethyl)-1-(4-ethylphenyl)-1H-pyrrole (3r). Eluent: Hex/DCM 5:1, Hex/DCM 3:1; Hex/DCM 1:1; 51 mg (28%); dr = 52:32; yellow oil. 1H NMR (400 MHz, CDCl3): (major isomer) δ = 7.31–7.14 (m, 4H), 6.94 (d, J = 8.4 Hz, 2H), 6.90 (d, J = 6.8 Hz, 2H), 6.79 (dd, J = 2.8, 1.7 Hz, 1H), 6.53–6.45 (m, 1H), 6.33–6.30 (m, 1H), 6.18 (dd, J = 50.0, 3.6 Hz, 1H), 4.76 (dd, J = 27.5, 3.7 Hz, 1H), 2.70 (q, J = 7.6 Hz, 2H), 1.28 (t, J = 7.6 Hz, 3H) ppm; (minor isomer) δ = 7.31–7.14 (m, 4H), 7.07 (d, J = 8.4 Hz, 2H), 6.88 (d, J = 6.9 Hz, 2H), 6.75 (dd, J = 2.8, 1.7 Hz, 1H), 6.60–6.54 (m, 1H), 6.30–6.26 (m, 1H), 5.97 (dd, J = 49.7, 4.4 Hz, 1H), 4.83 (dd, J = 28.8, 4.1 Hz, 1H), 2.70 (q, J = 7.7 Hz, 2H), 1.28 (t, J = 7.6 Hz, 3H) ppm.13C NMR (100 MHz, CDCl3): (major isomer) δ = 144.8, 136.5, 134.5, 132.1, 130.7, 128.9, 128.7, 127.1, 126.8, 124.0, 111.6 (d, 1JCF = 245.1 Hz), 109.0 (d, 4JCF = 3.1 Hz), 108.5, 45.9 (d, 2JCF = 19.0 Hz), 28.6, 15.6 ppm; (minor isomer) δ = 144.6, 136.6, 134.3, 134.0, 130.1, 129.3, 128.7, 126.9, 125.2, 123.8, 112.0 (d, 1JCF = 246.7 Hz), 109.4 (d, 4JCF = 3.6 Hz), 108.6, 45.8 (d, 2JCF = 18.5 Hz), 15.6 ppm. 19F NMR (376 MHz, CDCl3): (major isomer) δ = −152.34 (ddd, J = 50.0, 27.5, 2.3 Hz) ppm; (minor isomer) δ = −153.34 (ddd, J = 49.7, 28.8, 2.5 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C20H19ClFN2O2 373.1114; found 373.1116.
2-(1-(4-Chlorophenyl)-2-fluoro-2-nitroethyl)-1-(p-tolyl)-1H-pyrrole (3s). Eluent: Hex/DCM 5:1, Hex/DCM 4:1, Hex/DCM 1:1; 92 mg (51%); dr =48:47; yellow oil. 1H NMR (400 MHz, CDCl3): (major isomer) δ = 7.25–7.19 (m, 2H), 7.16 (d, J = 7.3 Hz, 2H), 6.96 (d, J = 8.4 Hz, 2H), 6.88 (d, J = 6.4 Hz, 2H), 6.78 (dd, J = 2.6, 1.7 Hz, 1H), 6.53–6.47 (m, 1H), 6.33–6.30 (m, 1H), 6.17 (dd, J = 50.0, 3.6 Hz, 1H), 4.75 (dd, J = 27.5, 3.3 Hz, 1H), 2.40 (s, 3H) ppm; (minor isomer) δ = 7.27 (d, J = 8.6 Hz, 2H), 7.17 (d, J = 7.0 Hz, 2H), 7.08 (d, J = 8.4 Hz, 2H), 6.86 (d, J = 6.5 Hz, 2H), 6.74 (dd, J = 2.6, 1.7 Hz, 1H), 6.60–6.54 (m, 1H), 6.30–6.26 (m, 1H), 5.97 (dd, J = 49.7, 4.3 Hz, 1H), 4.83 (dd, J = 28.3, 3.5 Hz, 1H), 2.40 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): (major isomer) δ = 138.5, 136.3, 134.5, 132.1, 130.7, 129.9, 128.9, 127.0, 126.8, 124.0, 111.6 (d, 1JCF = 245.0 Hz), 109.0 (d, 4JCF = 3.1 Hz), 108.6, 45.8 (d, 2JCF = 18.9 Hz), 21.2 ppm; (minor isomer) δ = 138.38, 136.41, 134.34, 133.94 (d, 3JCF = 1.6 Hz), 130.08, 129.89, 129.26, 126.86, 125.12, 123.76, 111.96 (d, 1JCF = 246.7 Hz), 109.48 (d, J = 3.7 Hz), 108.66, 45.74 (d, 2JCF = 18.4 Hz), 21.21 ppm. 19F NMR (376 MHz, CDCl3): (major isomer) δ = −152.30 (ddd, J = 50.0, 27.5, 2.0 Hz) ppm; (minor isomer) δ = −153.45 (ddd, J = 49.7, 28.3, 2.2 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C19H17ClFN2O2 359.0957; found 359.0949.
1-(3-Chloro-4-methoxyphenyl)-2-(1-(4-chlorophenyl)-2-fluoro-2-nitroethyl)-1H-pyrrole (3t). Eluent: Hex/DCM 5:1, Hex/DCM 4:1, Hex/DCM 1:1; 97 mg (46%); dr = 53:43; yellow oil. 1H NMR (400 MHz, CDCl3): (major isomer) δ = 7.26–7.21 (m, 2H), 6.98–6.92 (m, 3H), 6.87–6.84 (m, 2H), 6.74 (dd, J = 2.8, 1.7 Hz, 1H), 6.51–6.46 (m, 1H), 6.31–6.28 (m, 1H), 6.19 (dd, J = 49.9, 3.6 Hz, 1H), 4.68 (dd, J = 27.2, 3.5 Hz, 1H), 3.93 (s, 3H) ppm; (minor isomer) δ = 7.32–7.27 (m, 2H), 7.09 (d, J = 8.5 Hz, 2H), 6.98 (d, J = 1.7 Hz, 1H), 6.90–6.87 (m, 2H), 6.70 (dd, J = 2.8, 1.7 Hz, 1H), 6.58–6.52 (m, 1H), 6.26 (dd, J = 4.8, 3.6 Hz, 1H), 5.97 (dd, J = 49.6, 4.3 Hz, 1H), 4.77 (dd, J = 28.9, 4.2 Hz, 1H), 3.94 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): (major isomer) δ = 155.1, 134.7, 131.9, 131.9, 130.6, 129.1, 127.3, 126.4, 124.1, 122.7, 111.8, 111.4 (d, 1JCF = 245.0 Hz), 109.1 (d, 4JCF = 3.1 Hz), 108.8, 56.5, 45.9 (d, 2JCF = 19.0 Hz) ppm; (minor isomer) δ = 155.1, 134.5, 133.7 (d, 3JCF = 1.1 Hz), 132.0, 130.1, 129.4, 126.5, 125.4, 123.8, 122.7, 111.9, 111.8 (d, 1JCF = 247.0 Hz), 109.6 (d, 4JCF = 3.6 Hz), 108.9, 56.4, 45.8 (d, 2JCF = 18.4 Hz) ppm. 19F NMR (376 MHz, CDCl3): (major isomer) δ = −152.40 (ddd, J = 49.9, 27.2, 2.4 Hz) ppm; (minor isomer) δ = −153.78 (ddd, J = 49.6, 28.9, 2.5 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C19H16Cl2FN2O3 409.0517; found 409.0522.
2-(1-(4-Chlorophenyl)-2-fluoro-2-nitroethyl)-1-(3-methoxyphenyl)-1H-pyrrole (3u). Eluent: Hex/DCM 3:1, Hex/DCM 1:1; 153 mg (74%); dr = 53:47; yellow oil. 1H NMR (400 MHz, CDCl3): (major isomer) δ = 7.33–7.19 (m, 3H), 6.99 (d, J = 8.4 Hz, 2H), 6.98–6.91 (m, 1H), 6.83 (dd, J = 2.7, 1.7 Hz, 1H), 6.67–6.59 (m, 1H), 6.56–6.50 (m, 1H), 6.49 (t, J = 2.1 Hz, 1H), 6.36–6.32 (m, 1H), 6.21 (dd, J = 50.0, 3.6 Hz, 1H), 4.84 (dd, J = 27.2, 3.6 Hz, 1H), 3.70 (s, 3H) ppm; (minor isomer) δ = 7.33–7.19 (m, 3H), 7.12 (d, J = 8.4 Hz, 2H), 6.98–6.91 (m, 1H), 6.80 (dd, J = 2.7, 1.7 Hz, 1H), 6.67–6.59 (m, 2H), 6.56–6.50 (m, 1H), 6.32–6.29 (m, 1H), 6.00 (dd, J = 49.5, 4.2 Hz, 1H), 4.92 (dd, J = 29.2, 4.2 Hz, 1H), 3.72 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): (major isomer) δ = 160.1, 139.9, 134.4, 132.1, 130.6, 130.0, 128.9, 126.9, 123.8, 119.0, 114.5, 112.3, 111.5 (d, 1JCF = 244.9 Hz), 109.2 (d, 4JCF = 3.1 Hz), 108.7, 55.3, 45.8 (d, 2JCF = 19.1 Hz) ppm; (minor isomer) δ = 160.1, 140.0, 134.3, 133.9, 130.1, 130.0, 129.2, 124.9, 123.6, 119.1, 114.6, 112.3, 111.8 (d, 1JCF = 246.9 Hz), 109.7 (d, 4JCF = 3.7 Hz), 108.8, 55.3, 45.7 (d, 2JCF = 18.4 Hz). 19F NMR (376 MHz, CDCl3) (major isomer) δ = −152.33 (dd, J = 50.0, 27.2 Hz) ppm; (minor isomer) δ = −153.66 (dd, J = 49.5, 29.2 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C19H17ClFN2O3 375.0906; 375.0893.
General procedure for base-induced nitrous acid elimination.
In a typical experiment, the selected adduct 3 (0.4–0.5 mmol, 1 mol equiv.) was treated with a solution of DBU (2.0–6.0 mol equiv.) in acetonitrile (3 mL) with vigorous stirring at room temperature overnight (15–18 h). After completion of the reaction (TLC monitoring), the reaction mixture was concentrated under vacuum. The residue was separated by column chromatography.
Elimination for 3a. Purification by column chromatography on silica gel with Hex/DCM 1:1 gave 59 mg (76%) of the target product 4a as a mixture of diastereomers (Z:E = 46:54) and 11 mg (13 %) of the side product 5a as a mixture of diastereomers (Z:E = 57:43).
(Z)-2-(2-Fluoro-1-phenylvinyl)-1H-pyrrole (Z-4a). Colorless oil. 1H NMR (400 MHz, CDCl3): δ = 8.90 (br s, 1H), 7.50–7.32 (m, 5H), 6.91 (dd, J = 4.1, 2.6 Hz, 1H), 6.69 (d, 2JHF = 84.3 Hz, 1H), 6.31–6.27 (m, 1H), 6.16–6.10 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 143.7 (d, 1JCF = 265.6 Hz), 134.6 (d, 3JCF = 10.0 Hz), 129.8, 129.7 (d, 4JCF = 3.7 Hz), 128.6, 127.0 (d, 3JCF = 3.1 Hz), 119.4 (d, 6JCF = 4.8 Hz), 118.9 (d, 2JCF = 3.6 Hz), 111.1 (d, 4JCF = 4.6 Hz), 108.9 ppm; 19F NMR (376 MHz, CDCl3) δ = −132.04 (dd, J = 84.3, 6.3 Hz) ppm; HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C12H11FN 188.0870; found 188.0867.
(E)-2-(2-Fluoro-1-phenylvinyl)-1H-pyrrole (E-4a). Colorless oil. 1H NMR (400 MHz, CDCl3) δ = 7.90 (br s, 1H), 7.50–7.32 (m, 5H), 7.12 (d, 2JHF = 83.2 Hz, 1H), 6.82–6.77 (m, 1H), 6.26 (dd, J = 5.7, 2.9 Hz, 2H) ppm. 13C NMR (100 MHz, CDCl3): δ = 144.7 (d, 1JCF = 267.3 Hz), 133.8, 129.8, 128.6, 128.3 (d, 4JCF = 3.5 Hz), 127.2 (d, 3JCF = 9.4 Hz), 119.0 (d, 6JCF = 2.4 Hz), 118.9 (d, 2JCF = 10.5 Hz), 109.3, 107.6 (d, 4JCF = 2.6 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ = −133.82 (d, 2JHF = 83.2 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C12H11FN 188.0870; found 188.0867.
(Z)-2-(2-Nitro-1-phenylvinyl)-1H-pyrrole (Z-5a). Yellow oil. 1H NMR (400 MHz, CDCl3): δ = 11.93 (br s, 1H), 7.51–7.36 (m, 4H), 7.34–7.24 (m, 2H), 6.97 (s, 1H), 6.38–6.32 (m, 1H), 6.31–6.26 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 138.4, 138.4, 134.1, 129.6, 129.4, 128.8, 128.3, 126.6, 124.8, 112.0 ppm; HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C12H11N2O2 215.0815; found 215.0819.
(E)-2-(2-Nitro-1-phenylvinyl)-1H-pyrrole (E-5a). Yellow oil. 1H NMR (400 MHz, CDCl3): δ = 11.93 (br s, 1H), 7.58 (s, 1H), 7.51–7.36 (m, 4H), 7.34–7.28 (m, 1H), 7.00 (td, J = 2.7, 1.4 Hz, 1H), 6.67–6.61 (m, 1H), 6.38–6.32 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 142.8, 141.7, 129.6 129.1, 128.5, 128.4, 127.8, 125.0, 114.8, 112.3 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C12H11N2O2 215.0815; found 215.0819.
Elimination for 3b. Purification by column chromatography on silica gel with gradient elution (Hex/DCM 1:1, DCM) gave 23 mg (43%) of the target product 4b as a mixture of diastereomers (Z:E = 48:52) and 6 mg (10%) of the side product 5b as a mixture of diastereomers (Z:E = 75:25).
(Z)-2-(2-Fluoro-1-(4-methoxyphenyl)vinyl)-1H-pyrrole (Z-4b). Colorless oil. 1H NMR (400 MHz, CDCl3): δ = 8.88 (br s, 1H), 7.35 (d, J = 8.5 Hz, 2H), 6.94–6.91 (m, 2H), 6.89 (dd, J = 4.1, 2.7 Hz, 1H), 6.64 (d, 2JHF = 84.7 Hz, 1H), 6.27–6.24 (m, 1H), 6.12–6.07 (m, 1H), 3.84 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ =159.5, 143.4 (d, 1JCF = 265.3 Hz), 131.0 (d, 4JCF = 2.9 Hz), 127.4 (d, 3JCF = 3.1 Hz), 126.8 (d, 3JCF = 10.2 Hz), 119.30 (d, 6JCF = 4.8 Hz), 118.26 (d, 2JCF = 3.4 Hz), 113.98, 110.99 (d, 4JCF = 4.8 Hz), 108.93, 55.44 ppm.19F NMR (376 MHz, CDCl3): δ = −132.54 (dd, J = 84.7, 6.3 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C13H13FNO 218.0976; found 218.0973.
(E)-2-(2-Fluoro-1-(4-methoxyphenyl)vinyl)-1H-pyrrole (E-4b). Colorless oil. 1H NMR (400 MHz, CDCl3): δ = 7.97 (br s, 1H), 7.34–7.30 (m, 2H), 7.05 (d, 2JHF = 83.5 Hz, 1H), 6.94–6.91 (m, 2H), 6.79 (dd, J = 4.2, 2.6 Hz, 1H), 6.23 (dd, J = 5.8, 2.9 Hz, 2H), 3.84 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 159.7, 144.3 (d, 1JCF = 266.1 Hz), 130.9 (d, 4JCF = 4.0 Hz), 127.5 (d, 3JCF = 9.9 Hz), 126.0, 118.9 (d, 6JCF = 2.1 Hz), 118.4 (d, 2JCF = 9.7 Hz), 114.0, 109.2, 107.6 (d, 4JCF = 2.7 Hz), 55.4 ppm. 19F NMR (376 MHz, CDCl3): δ = −134.90 (d, J = 83.5 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C13H13FNO 218.0976; found 218.0973.
(Z)-2-(1-(4-Methoxyphenyl)-2-nitrovinyl)-1H-pyrrole (Z-5b). 1H NMR (400 MHz, CDCl3): δ = 11.87 (br s, 1H), 7.39–7.33 (m, 2H), 7.29–7.22 (m, 1H), 7.03–6.90 (m, 3H), 6.38–6.32 (m, 2H), 3.87 (br s, 3H). 13C NMR (100 MHz, CDCl3) δ = 161.0, 141.6, 131.3, 130.6, 128.0, 126.3, 124.8, 113.8, 111.8, 55.6 ppm. HRMS (ESI-TOF) m/z: [M − H]− calcd. for 243.0770; found 243.0752.
Elimination for 3c. Purification by column chromatography on silica gel with gradient elution (Hex/DCM 3:1; Hex/DCM 2:1; DCM) gave 73 mg (77%) of the target product 4c as a mixture of diastereomers (Z/E = 42:58).
(Z)-2-(1-(4-(Tert-butyl)phenyl)-2-fluorovinyl)-1H-pyrrole (E-4c). Colorless oil. 1H NMR (400 MHz, CDCl3): δ = 8.86 (br s, 1H), 7.49–7.41 (m, 2H), 7.41–7.33 (m, 2H), 6.93–6.87 (m, 1H), 6.69 (d, 2JHF = 84.5 Hz, 1H), 6.32– 6.28 (m, 1H), 6.26–6.16 (m, 1H), 1.39 (s, 9H) ppm. 13C NMR (100 MHz, CDCl3): δ = 151.3, 143.6 (d, 1JCF = 265.5 Hz), 131.6 (d, 4JCF = 10.0 Hz), 129.4 (d, 4JCF = 2.6 Hz), 127.1 (d, 3JCF = 3.2 Hz), 125.5, 119.2 (d, 6JCF = 4.7 Hz), 118.6 (d, 2JCF = 3.7 Hz), 111.1 (d, 4JCF = 5.0 Hz), 109.0, 34.7, 31.4 ppm; 19F NMR (376 MHz, CDCl3): δ = −131.10 (dd, J = 84.5, 6.1 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C16H19FN 244.1496; found 244.1500.
(E)-2-(1-(4-(Tert-butyl)phenyl)-2-fluorovinyl)-1H-pyrrole (E-4c). Colorless oil. 1H NMR (400 MHz, CDCl3): δ = 7.92 (br s, 1H), 7.49–7.41 (m, 2H), 7.41–7.33 (m, 2H), 7.10 (d, 2JHF = 83.4 Hz, 1H), 6.82–6.76 (m, 1H), 6.28–6.22 (m, 2H), 1.40 (s, 9H) ppm. 13C NMR (100 MHz, CDCl3): δ = 151.4, 144.7 (d, 1JCF = 267.0 Hz), 130.8, 129.4 (d, 4JCF = 3.8 Hz), 127.4 (d, 3JCF = 9.9 Hz), 125.5, 118.9 (d, 6JCF = 2.1 Hz), 118.7 (d, 2JCF = 9.8 Hz), 109.2, 107.4 (d, 4JCF = 2.4 Hz), 34.8, 31.5 ppm. 19F NMR (376 MHz, CDCl3): δ = −132.94 (d, 2JHF = 83.4 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C16H19FN 244.1496; found 244.1500.
Elimination for 3d. Purification by column chromatography on silica gel with gradient elution (Hex; Hex/DCM 5:1; Hex/DCM 2:1; Hex/DCM 1:1) gave 29 mg (40%) of the Z-isomer and 31 mg (42%) of the E-isomer of the target product 4d and 13 mg (15%) of the side product 5d as a mixture of diastereomers (Z/E = 49:51).
(Z)-2-(2-Fluoro-1-(p-tolyl)vinyl)-1H-pyrrole (Z-4d). Colorless oil. 1H NMR (400 MHz, CDCl3): δ = 8.87 (br s, 1H), 7.29 (d, J = 8.0 Hz, 2H), 7.19 (d, J = 7.9 Hz, 2H), 6.90–6.86 (m, 1H), 6.64 (d, 2JHF = 84.6 Hz, 1H), 6.24–6.20 (m, 1H), 6.12–6.06 (m, 1H), 2.39 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 2.39 (s, 3H), 143.5 (d, 1JCF = 265.2 Hz), 138.2, 131.6 (d, 3JCF = 10.0 Hz), 129.7 (d, 4JCF = 2.5 Hz), 129.3, 127.2 (d, 3JCF = 3.3 Hz), 119.3 (d, 6JCF = 4.8 Hz), 118.6 (d, 2JCF = 3.3 Hz), 111.0 (d, 4JCF = 4.7 Hz), 108.9, 21.4 ppm. 19F NMR (376 MHz, CDCl3): δ = −132.35 (dd, J = 84.6, 6.3 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C13H13FN 202.1027; found 202.1024.
(E)-2-(2-Fluoro-1-(p-tolyl)vinyl)-1H-pyrrole (E-4d). Colorless oil. 1H NMR (400 MHz, CDCl3): δ = 7.92 (br s, 1H), 7.31 (d, J = 8.0 Hz, 2H), 7.22 (d, J = 7.9 Hz, 2H), 7.08 (d, 2JHF = 83.4 Hz, 1H), 6.79 (dd, J = 3.9, 2.5 Hz, 1H), 6.28–6.24 (m, 1H), 6.23 (dd, J = 5.7, 2.8 Hz, 1H), 2.39 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 144.5 (d, 1JCF = 266.4 Hz), 138.2, 130.8, 129.6 (d, 4JCF = 3.6 Hz), 129.3, 127.4 (d, 3JCF = 9.6 Hz), 118.9 (d, 6JCF = 1.9 Hz), 118.8 (d, 2JCF = 10.0 Hz), 109.2, 107.4 (d, 4JCF = 2.6 Hz), 21.4 ppm. 19F NMR (376 MHz, CDCl3): δ = −134.25 (d, 2JHF = 83.4 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C13H13FN 202.1027; found 202.1024.
2-(2-Nitro-1-(p-tolyl)vinyl)-1H-pyrrole (5d). Pale brown oil. 1H NMR (400 MHz, CDCl3): δ = 11.91 (br s, 1H), 8.21 (br s, 1H), 7.56 (s, 1H), 7.34–7.09 (m, 8H), 7.01–6.96 (m, 1H), 6.97 (s, 1H), 6.80–6.71 (m, 1H), 6.70–6.62 (m, 1H), 6.39–6.29 (m, 3H), 2.43 (s, 3H), 2.42 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 21.4, 21.6, 111.9, 111.9, 112.2, 114.4, 124.6, 125.0, 126.4, 127.9, 128.3, 128.6, 129.0, 129.5, 129.7, 129.9, 135.5, 139.7, 139.9, 141.9 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for 229.0972; found 229.0972.
Elimination for 3e. Purification by column chromatography on silica gel with Hex/DCM 1:1 gave 63 mg (70%) of the target product 4e as a mixture of diastereomers (Z/E = 42:58) and 10 mg (10 %) of the side product 5e as a mixture of diastereomers (Z/E = 91:9).
(Z)-2-(2-Fluoro-1-(4-fluorophenyl)vinyl)-1H-pyrrole (Z-4e). Colorless oil. 1H NMR (400 MHz, CDCl3): (Z-isomer) δ = 8.93 (br s, 1H), 7.44–7.35 (m, 2H), 7.14–7.06 (m, 2H), 6.94–6.89 (m, 1H), 6.65 (d, J = 84.1 Hz, 1H), 6.25–6.22 (m, 1H), 6.06–6.02 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): (Z-isomer) δ = 162.5 (d, 1JCF = 248.1 Hz), 143.5 (d, 1JCF = 265.4 Hz), 131.5 (dd, 3JCF = 6.0, 4JCF = 3.2 Hz), 130.5 (dd, 3JCF = 10.4, 4JCF = 3.3 Hz), 127.1 (d, 3JCF = 3.1 Hz), 119.7 (d, 6JCF = 5.0 Hz), 118.0 (d, 2JCF = 4.1 Hz), 115.5 (d, 2JCF = 21.5 Hz), 111.1 (d, 4JCF = 4.3 Hz), 109.0 ppm. 19F NMR (376 MHz, CDCl3): δ = −114.24 (tt, J = 8.6, 5.4 Hz, 1F), −131.98 (dd, J = 84.1, 6.5 Hz, 1F) ppm. HRMS (ESI-TOF) m/z: [M − H]− calcd. for C12H8F2N 204.0630; found 204.0638.
(E)-2-(2-Fluoro-1-(4-fluorophenyl)vinyl)-1H-pyrrole (E-4e). Colorless oil. 1H NMR (400 MHz, CDCl3): δ = 7.92 (s, 1H), 7.44–7.35 (m, 2H), 7.14–7.06 (m, 2H), 7.08 (d, 2JHF = 83.1 Hz, 1H), 6.81 (dd, J = 4.6, 2.3 Hz, 1H), 6.25–6.22 (m, 2H) ppm. 13C NMR (100 MHz, CDCl3): δ = 162.53 (d, 1JCF = 248.1 Hz), 144.67 (d, 1JCF = 267.6 Hz), 131.4 (dd, 3JCF = 8.3, 4JCF = 3.9 Hz), 129.73 (d, 3JCF = 3.3 Hz), 127.02 (d, 3JCF = 12.7 Hz), 119.14 (d, 6JCF = 2.0 Hz), 118.05 (d, 2JCF = 9.7 Hz), 115.56 (d, 2JCF = 21.5 Hz), 109.39, 108.06 (d, 4JCF = 2.8 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ = −114.75–−114.85 (m), −133.82 (d, 2JHF = 83.1 Hz) ppm. HRMS (ESI-TOF) m/z: [M − H]− calcd. for C12H8F2N 204.0630; found 204.0638.
(Z)-2-(1-(4-Fluorophenyl)-2-nitrovinyl)-1H-pyrrole (Z-5e). Pale brown oil. 1H NMR (400 MHz, CDCl3): δ = 11.89 (br s, 1H), 7.45–7.37 (m, 2H), 7.30–7.24 (m, 1H), 7.17–7.08 (m, 2H), 6.93 (s, 1H), 6.38–6.32 (m, 1H), 6.28–6.24 (m, 1H). 13C NMR (100 MHz, CDCl3): δ = 163.5 (d, 1JCF = 249.8 Hz), 140.6, 134.4 (d, 4JCF = 3.5 Hz), 131.6 (d, 3JCF = 8.3 Hz), 128.4, 127.7, 126.7, 124.9, 115.5 (d, 2JCF = 21.8 Hz), 112.1 ppm. 19F NMR (376 MHz, CDCl3) δ = −112.21 (tt, J = 8.5, 5.2 Hz) ppm. HRMS (ESI-TOF) m/z: [M − H]− calcd. for C12H8FN2O2 231.0575; found 231.0572.
Elimination for 3f. Purification by column chromatography on silica gel with gradient elution (Hex; Hex/DCM 6:1; Hex/DCM 4:1; Hex/DCM 1:1) gave 43 mg (40%) of the Z-isomer and 49 mg (45%) of the E-isomer of the target product 4f and 18 mg (15%) of the side product 5f as a mixture of diastereomers (Z/E = 92:8).
(Z)-2-(1-(4-Bromophenyl)-2-fluorovinyl)-1H-pyrrole (Z-4f). Colorless oil. 1H NMR (400 MHz, CDCl3): δ = 8.92 (br s, 1H), 7.56–7.49 (m, 2H), 7.32–7.26 (m, 2H), 6.94–6.89 (m, 1H), 6.64 (d, 2JHF = 83.9 Hz, 1H), 6.26–6.20 (m, 1H), 6.06–6.00 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 143.5 (d, 1JCF = 266.0 Hz), 133.6 (d, 3JCF = 10.3 Hz), 131.8, 131.4 (d, 4JCF = 2.6 Hz), 126.6 (d, 3JCF = 3.1 Hz), 122.5, 119.8 (d, 6JCF = 5.0 Hz), 118.1 (d, 2JCF = 4.2 Hz), 111.2 (d, 4JCF = 4.2 Hz), 109.0 ppm. 19F NMR (376 MHz, CDCl3): δ = −131.66 (dd, J = 83.9, 6.3 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C12H1079BrFN 265.9975; found 265.9971.
(E)-2-(1-(4-Bromophenyl)-2-fluorovinyl)-1H-pyrrole (E-4f). Bluish oil. 1H NMR (400 MHz, CDCl3): δ = 7.91 (br s, 1H), 7.57–7.54 (m, 2H), 7.29 (d, J = 8.4 Hz, 2H), 7.07 (d, 2JHF = 82.8 Hz, 1H), 6.81 (dd, J = 4.5, 2.3 Hz, 1H), 6.24 (t, J = 2.3 Hz, 2H) ppm. 13C NMR (100 MHz, CDCl3): δ = 145.0 (d, 1JCF = 268.9 Hz), 132.7, 131.8, 131.3 (d, 4JCF = 4.0 Hz), 126.6 (d, 3JCF = 9.3 Hz), 122.4, 119.3 (d, 6JCF = 2.0 Hz), 118.1 (d, 2JCF = 9.4 Hz), 108.3 (d, 4JCF = 2.8 Hz), 109.4 ppm. 19F NMR (376 MHz, CDCl3): δ = −132.42 (d, J = 82.8 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C12H1081BrFN 267.9955; found 267.9953.
(Z)-2-(1-(4-Bromophenyl)-2-nitrovinyl)-1H-pyrrole (Z-5f). Yellow oil. 1H NMR (400 MHz, CDCl3): (Z-isomer) δ = 11.90 (br s, 1H), 7.60–7.53 (m, 2H), 7.32–7.24 (m, 3H), 6.92 (s, 1H), 6.38–6.32 (m, 1H), 6.29–6.24 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): (Z-isomer) δ = 140.4, 137.3, 131.6, 131.2, 128.2, 127.4, 126.9, 124.9, 124.0, 112.1 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C12H1079BrN2O2 292.9920; found 292.9914.
Elimination for 3g. Purification by column chromatography on silica gel with gradient elution (Hex; Hex/DCM 6:1; Hex/DCM 4:1; Hex/DCM 1:1) gave 36 mg (34%) of the Z-isomer and 47 mg (45 %) of the E-isomer of the target product 4g and 13 mg (11%) of the side product 5g as a mixture of diastereomers (Z/E = 87:13).
(Z)-2-(1-(4-Chlorophenyl)-2-fluorovinyl)-1H-pyrrole (Z-4g). Colorless oil. 1H NMR (400 MHz, CDCl3): δ = 8.93 (br s, 1H), 7.42–7.30 (m, 4H), 6.94–6.90 (m, 1H), 6.65 (d, 2JHF = 83.9 Hz, 1H), 6.26–6.21 (m, 1H), 6.06–6.02 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 143.5 (d, 1JCF = 265.8 Hz), 134.3, 133.1 (d, 3JCF = 10.3 Hz), 131.1 (d, 4JCF = 2.7 Hz), 128.8, 126.7 (d, 3JCF = 3.2 Hz), 119.8 (d, 6JCF = 5.0 Hz), 118.1 (d, 2JCF = 4.2 Hz), 111.2 (d, 4JCF = 4.3 Hz), 109.0 ppm. 19F NMR (376 MHz, CDCl3) δ = −131.75 (dd, J = 83.9, 6.3 Hz) ppm. HRMS (ESI-TOF) m/z: [M − H]− calcd. for C12H835ClFN 220.0335; found 220.0343.
(E)-2-(1-(4-chlorophenyl)-2-fluorovinyl)-1H-pyrrole (E-4g). Colorless oil. 1H NMR (400 MHz, CDCl3): δ = 7.92 (br s, 1H), 7.41–7.33 (m, 4H), 7.07 (d, 2JHF = 82.8 Hz, 1H), 6.81 (dd, J = 4.6, 2.3 Hz, 1H), 6.24 (t, J = 2.4 Hz, 2H) ppm. 13C NMR (100 MHz, CDCl3): δ = 145.0 (d, 1JCF = 268.8 Hz), 134.1, 132.3, 131.0 (d, 4JCF = 4.0 Hz), 128.8, 126.7 (d, 3JCF = 9.4 Hz), 119.2 (d, 6JCF = 1.9 Hz), 118.0 (d, 2JCF = 9.4 Hz), 109.4, 108.2 (d, 4JCF = 2.8 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ = −132.62 (d, J = 82.9 Hz) ppm. HRMS (ESI-TOF) m/z: [M − H]− calcd. for C12H835ClFN 220.0335; found 220.0343.
(Z)-2-(1-(4-Chlorophenyl)-2-nitrovinyl)-1H-pyrrole (Z-5g). Orange solid. M.p. = 122–124 °C (Hex). 1H NMR (400 MHz, CDCl3): δ = 11.90 (br s, 1H), 7.44–7.38 (m, 2H), 7.38–7.33 (m, 2H), 7.30–7.24 (m, 1H), 6.92 (s, 1H), 6.38–6.32 (m, 1H), 6.30–6.22 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 140.4, 136.8, 135.9, 131.0, 130.0, 128.7, 128.3, 126.9, 124.9, 112.1 ppm. HRMS (ESI-TOF) m/z: [M − H]− calcd. for C12H1035ClN2O2 249.0425; found 249.0421.
Elimination for 3h. Purification by column chromatography on silica gel with gradient elution (Hex; Hex/DCM 4:1; Hex/DCM 1:1; DCM) gave 70 mg (60%) of the Z-isomer and 4 mg (3%) of the E-isomer of the target product 4h and 14 mg (11%) of the side product 5h as a mixture of diastereomers (Z/E = 35:65).
(Z)-2-(1-(2,4-Dichlorophenyl)-2-fluorovinyl)-1H-pyrrole (Z-4h). Yellowish oil. 1H NMR (400 MHz, CDCl3): δ = 8.91 (br s, 1H), 7.50 (s, 1H), 7.33–7.28 (m, 2H), 6.94–6.89 (m, 1H), 6.60 (d, 2JHF = 83.2 Hz, 1H), 6.23–6.18 (m, 1H), 5.83–5.79 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 144.4 (d, 1JCF = 267.6 Hz), 136.3 (d, 4JCF = 3.1 Hz), 135.2, 133.6 (d, 4JCF = 2.5 Hz), 131.6 (d, 3JCF = 11.8 Hz), 129.9, 127.2, 126.1 (d, 3JCF = 3.4 Hz), 119.9 (d, 6JCF = 5.3 Hz), 115.4 (d, 2JCF = 6.2 Hz), 110.8 (d, 4JCF = 4.2 Hz), 109.1 ppm.19F NMR (376 MHz, CDCl3): δ = −130.76 (dd, J = 83.2, 6.2 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C12H935Cl2FN 256.0091; found 256.0101.
(E)-2-(1-(2,4-Dichlorophenyl)-2-fluorovinyl)-1H-pyrrole (E-4h). Yellowish oil. 1H NMR (400 MHz, CDCl3): δ = 7.88 (br s, 1H), 7.51 (d, J = 2.1 Hz, 1H), 7.31 (dd, J = 8.3, 2.0 Hz, 1H), 7.27 (s, 1H), 7.15 (d, 2JHF = 82.0 Hz, 1H), 6.79–6.75 (m, 1H), 6.21 (dd, J = 6.0, 2.8 Hz, 1H), 6.15–6.10 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 144.8 (d, 1JCF = 264.1 Hz), 135.1, 135.1 (d, 4JCF = 1.4 Hz), 132.7 (d, 4JCF = 1.6 Hz), 131.1, 130.0, 127.5, 126.02 (d, 3JCF = 7.5 Hz), 119.1 (d, 6JCF = 2.3 Hz), 116.2 (d, 2JCF = 13.2 Hz), 109.8, 107.2 (d, 4JCF = 3.8 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ = −130.75 (d, J = 82.0 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C12H935Cl37ClFN 258.0061; found 258.0069.
2-(1-(2,4-Dichlorophenyl)-2-nitrovinyl)-1H-pyrrole (5h). Red-brown solid; M.p. = 52–54 °C (DCM) with decomposition. 1H NMR (400 MHz, CDCl3): (Z-isomer) δ = 11.87 (br s, 1H), 7.50 (d, J = 2.0 Hz, 1H), 7.41–7.22 (m, 3H), 6.82 (s, 1H), 6.40–6.30 (m, 1H), 6.14–6.07 (m, 1H) ppm; (E-isomer) δ = 8.49 (br s, 1H), 7.62 (s, 1H), 7.54 (d, J = 2.0 Hz, 1H), 7.39–7.32 (m, 1H), 7.18 (d, J = 8.2 Hz, 1H), 7.07–7.02 (m, 1H), 6.52–6.46 (m, 1H), 6.38–6.31 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 138.4, 137.0, 135.9, 135.7, 135.2, 134.5, 133.6, 132.1, 131.8, 130.3, 130.0, 129.9, 128.6, 127.5, 127.3, 127.1, 126.9, 126.5, 125.6, 124.2, 116.6, 112.7, 112.5 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C12H935Cl2N2O2 283.0036; found: 283.0038.
Elimination for 3i. Purification by column chromatography on silica gel with gradient elution (Hex; Hex/DCM 3:1; Hex/DCM 1:1; DCM) gave 42 mg (36%) of the Z-isomer and 47 mg (41%) of the E-isomer of the target product 4i and 12 mg (7%) of the side product 5i as a mixture of diastereomers (Z/E = 85:15).
(Z)-2-(2-Fluoro-1-(4-(trifluoromethyl)phenyl)vinyl)-1H-pyrrole (Z-4i). Colorless oil. 1H NMR (400 MHz, CDCl3): δ = 8.95 (br s, 1H), 7.66 (d, J = 8.2 Hz, 2H), 7.54 (d, J = 8.0 Hz, 2H), 6.93 (dd, J = 4.1, 2.7 Hz, 1H), 6.68 (d, 2JHF = 83.5 Hz, 1H), 6.26–6.21 (m, 1H), 6.04–5.99 (m, 1H). 13C NMR (100 MHz, CDCl3): δ = 143.9 (d, 1JCF = 266.5 Hz), 138.6 (d, 3JCF = 10.2 Hz), 130.6 (q, 2JCF = 32.5 Hz), 130.2 (d, 4JCF = 2.6 Hz), 126.4 (d, 3JCF = 2.7 Hz), 125.5 (q, 3JCF = 3.8 Hz), 124.2 (q, 1JCF = 272.9 Hz), 120.0 (d, 6JCF = 5.1 Hz), 118.3 (d, 2JCF = 4.4 Hz), 111.3 (d, 4JCF = 4.1 Hz), 109.1 ppm. 19F NMR (376 MHz, CDCl3) δ = −65.78 (s, 3F), −133.27 (dd, J = 83.5, 6.5 Hz, 1F). HRMS (ESI-TOF) m/z: [M − H]− calcd. for C13H8F4N 254.0598; found 254.0601.
(E)-2-(2-Fluoro-1-(4-(trifluoromethyl)phenyl)vinyl)-1H-pyrrole (E-4i). Colorless oil. 1H NMR (400 MHz, CDCl3): δ = 7.92 (br s, 1H), 7.66 (d, J = 8.2 Hz, 2H), 7.54 (d, J = 8.4 Hz, 2H), 7.12 (d, 2JHF = 82.5 Hz, 1H), 6.82 (dd, J = 4.6, 2.3 Hz, 1H), 6.25 (t, J = 2.4 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ = 145.6 (d, 1JCF = 270.4 Hz), 137.6, 130.3 (q, 2JCF = 32.5 Hz), 130.0 (d, 4JCF = 4.0 Hz), 126.3 (d, 3JCF = 9.3 Hz), 125.5 (q, 3JCF = 3.7 Hz), 124.1 (d, 1JCF = 270.6 Hz), 119.5 (d, 6JCF = 1.9 Hz), 118.1 (d, 2JCF = 9.3 Hz), 109.6, 108.6 (d, J = 2.9 Hz) ppm. 19F NMR (376 MHz, CDCl3) δ = δ − 65.87 (s, 3F), −133.67 (d, J = 82.5 Hz, 1F) ppm. HRMS (ESI-TOF) m/z: [M − H]− calcd. for C13H8F4N 254.0598; found 254.0601.
(Z)-(2-Nitro-1-(4-(trifluoromethyl)phenyl)vinyl)-1H-pyrrole (5i). Orange waxy solid. 1H NMR (400 MHz, CDCl3): δ = 11.92 (br s, 1H), 7.71 (d, J = 8.1 Hz, 2H), 7.55 (d, J = 8.0 Hz, 2H), 7.31–7.27 (m, 1H), 6.91 (s, 1H), 6.38–6.33 (m, 1H), 6.23–6.18 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 141.9, 139.9, 131.7 (q, 2JCF = 33.0 Hz), 130.0, 128.3, 127.1, 125.4 (q, 3JCF = 3.7 Hz), 124.9, 123.9 (q, 1JCF = 272.4 Hz), 112.3, 112.3 ppm.19F NMR (376 MHz, CDCl3) δ = −65.96 ppm. HRMS (ESI-TOF) m/z: [M-H]− calcd. for C13H8F3N2O2 281.0543; found 281.0548.
Elimination for 3j. Purification by column chromatography on silica gel with gradient elution (Hex; Hex/DCM 3:1; Hex/DCM 2:1; Hex/DCM 1:2; DCM) gave 43 mg (38%) of the Z-isomer and 45 mg (39%) of the E-isomer of the target product 4j and 12 mg (9%) of the side product 5j as a mixture of diastereomers (Z/E = 68:32).
(Z)-Methyl 4-(2-fluoro-1-(1H-pyrrol-2-yl)vinyl)benzoate (Z-4j). Colorless oil. 1H NMR (400 MHz, CDCl3): δ = 8.95 (br s, 1H), 8.09–8.02 (m, 2H), 7.52–7.45 (m, 2H), 6.95–6.89 (m, 1H), 6.69 (d, 2JHF = 83.6 Hz, 1H), 6.27–6.19 (m, 1H), 6.07–6.00 (m, 1H), 3.94 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 166.9, 143.9 (d, 1JCF = 266.6 Hz), 139.6 (d, 3JCF = 10.3 Hz), 130.1, 129.8, 129.8 (d, 4JCF = 2.6 Hz), 126.3 (d, 3JCF = 2.9 Hz), 119.8 (d, 6JCF = 5.0 Hz), 118.6 (d, 2JCF = 4.3 Hz), 111.2 (d, 3JCF = 4.2 Hz), 109.0, 52.4 ppm.19F NMR (376 MHz, CDCl3): δ = −131.15 (dd, J = 83.6, 6.4 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C14H13FNO2 246.0925; found 246.0921.
(E)-Methyl 4-(2-fluoro-1-(1H-pyrrol-2-yl)vinyl)benzoate (E-4j). Yellowish oil. 1H NMR (400 MHz, CDCl3): δ = 8.11 (br s, 1H), 7.97–8.05 (m, 2H), 7.48 (d, J = 8.2 Hz, 2H), 7.11 (d, 2JHF = 82.6 Hz, 1H), 6.82 (dd, J = 4.4, 2.4 Hz, 1H), 6.21–6.27 (m, 2H), 3.92 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 166.9, 145.6 (d, 1JCF = 270.7 Hz), 138.7, 129.8, 129.7, 129.6 (d, 4JCF = 4.0 Hz), 126.4 (d, 3JCF = 9.4 Hz), 119.4 (d, 6JCF = 2.0 Hz), 118.4 (d, 2JCF = 9.0 Hz), 109.4, 108.5 (d, 3JCF = 2.7 Hz), 52.3 ppm. 19F NMR (376 MHz, CDCl3): δ = −130.88 (d, 2JHF = 82.6 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C14H13FNO2 246.0925; found 246.0921.
Methyl 4-(2-nitro-1-(1H-pyrrol-2-yl)vinyl)benzoate (5j). Red-orange solid. M.p. = 153–155 °C with decomposition. 1H NMR (400 MHz, CDCl3): (Z-isomer) δ = 11.93 (br s, 1H), 8.12–8.06 (m, 2H), 7.53–7.45 (m, 2H), 7.30–7.26 (m, 1H), 6.93 (s, 1H), 6.37–6.32 (m, 1H), 6.23–6.19 (m, 1H), 3.96 (s, 3H) ppm; (E-isomer) δ = 8.41 (s, 1H), 8.17–8.12 (m, 2H), 7.57 (s, 1H), 7.41–7.36 (m, 2H), 7.05–7.01 (m, 1H), 6.56–6.52 (m, 1H), 6.37–6.32 (m, 1H), 3.96 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 52.5, 52.6, 112.2, 112.5, 116.1, 124.9, 125.3, 127.0, 127.3, 127.5, 128.2, 128.5, 129.1, 129.6, 129.7, 130.0, 130.9, 131.2, 139.1, 140.5, 141.8, 142.8, 166.5, 166.7 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C14H13N2O4 273.0870; found 273.0873.
Elimination for 3k. Purification by column chromatography on silica gel with gradient elution (Hex/DCM 1:1; DCM) gave 88 mg (81%) of the target product 4k as a mixture of diastereomers (Z/E = 47:53) and 11 mg (9%) of the side product 5k as a mixture of diastereomers (Z/E = 40:60).
(Z)-4-(2-Fluoro-1-(1H-pyrrol-2-yl)vinyl)benzonitrile (Z-4k). Greenish solid. M.p. = 97–99 °C (Hex:DCM 1:1). 1H NMR (400 MHz, CDCl3): δ = 9.00 (br s, 1H), 7.71–7.63 (m, 2H), 7.57–7.48 (m, 2H), 6.96–6.91 (m, 1H), 6.68 (d, 2JHF = 83.0 Hz, 1H), 6.26–6.18 (m, 1H), 6.00–5.95 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 143.9 (d, 1JCF = 267.5 Hz), 139.8 (d, 3JCF = 10.4 Hz), 132.3, 130.4 (d, 4JCF = 2.6 Hz), 125.7 (d, 3JCF = 2.9 Hz), 120.2 (d, 6JCF = 4.9 Hz), 118.6, 118.2 (d, 2JCF = 5.0 Hz), 112.1, 111.2 (d, 4JCF = 3.9 Hz), 109.0 ppm. 19F NMR (376 MHz, CDCl3): (Z-isomer) δ = −129.86 (dd, 2JHF = 82.2, 6.0 Hz) ppm. HRMS (ESI-TOF) m/z: [M − H]− calcd. for C13H8FN2 211.0677; found 211.0674.
(E)-4-(2-Fluoro-1-(1H-pyrrol-2-yl)vinyl)benzonitrile (E-4k). Greenish solid. M.p. = 97–99 °C (Hex:DCM 1:1). 1H NMR (400 MHz, CDCl3): δ = 8.11 (br s, 1H), 7.71–7.63 (m, 2H), 7.56–7.49 (m, 2H), 7.11 (d, 2JHF = 82.2 Hz, 1H), 6.86–6.82 (m, 1H), 6.28–6.17 (m, 2H) ppm. 13C NMR (100 MHz, CDCl3): δ = 145.9 (d, 1JCF = 272.2 Hz), 138.9, 132.2, 130.31 (d, 4JCF = 4.3 Hz), 125.6 (d, 3JCF = 9.3 Hz), 119.7 (d, 6JCF = 1.3 Hz), 118.7, 117.9 (d, 2JCF = 8.5 Hz), 111.6, 109.4, 109.1 (d, 4JCF = 3.0 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ = −130.34 (d, 2JHF = 83.0 Hz) ppm.
4-(2-Nitro-1-(1H-pyrrol-2-yl)vinyl)benzonitrile (5k). Yellow oil. 1H NMR (400 MHz, CDCl3): (Z-isomer) δ = 11.90 (br s, 1H), 7.80–7.72 (m, 2H), 7.58–7.51 (m, 2H), 7.33–7.28 (m, 1H), 6.88 (s, 1H), 6.39–6.33 (m, 1H), 6.18–6.14 (m, 1H) ppm; (E-isomer) δ = 8.52 (br s, 1H), 7.80–7.72 (m, 2H), 7.54 (s, 1H), 7.47–7.40 (m, 2H), 7.09–7.05 (m, 1H), 6.48–6.41 (m, 1H), 6.39–6.33 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 142.9, 140.9, 139.4, 139.4, 132.4, 132.2, 130.4, 129.3, 129.0, 128.2, 127.4, 127.0, 126.8, 125.8, 124.8, 118.4, 118.2, 117.4, 113.6, 112.7, 112.4, 112.4, ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C13H10N3O2 240.0768; found 240.0771.
Elimination for 3l. Purification by column chromatography on silica gel with gradient elution (Hex; Hex/DCM 5:1; Hex/DCM 2:1; Hex/DCM 1:1) gave 25 mg (29%) of the Z-isomer and 18 mg (21%) of the E-isomer of the target product 4l.
(Z)-2-(2-Fluoro-1-(4-nitrophenyl)vinyl)-1H-pyrrole (Z-4l). Yellow solid. M.p. = 114–115 °C (Hex/DCM 5:1) with decomposition. 1H NMR (400 MHz, CDCl3): δ = 8.99 (br s, 1H), 8.25 (d, J = 8.5 Hz, 2H), 7.59 (d, J = 8.4 Hz, 2H), 6.97–6.92 (m, 1H), 6.71 (d, 2JHF = 82.8 Hz, 1H), 6.26–6.21 (m, 1H), 6.00–5.95 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 148.0, 144.0 (d, 1JCF = 267.7 Hz), 141.8 (d, 3JCF = 10.5 Hz), 130.6 (d, 4JCF = 2.4 Hz), 125.8 (d, 3JCF = 3.0 Hz), 123.8, 120.3 (d, 6JCF = 5.0 Hz), 118.1 (d, 2JCF = 5.3 Hz), 111.4 (d, 4JCF = 3.7 Hz), 109.2 ppm. 19F NMR (376 MHz, CDCl3): δ = −130.01 (dd, J = 82.8, 6.5 Hz) ppm. HRMS (ESI-TOF) m/z: [M − H]− calcd. for C12H8FN2O2 231.0575; found 231.0584.
(E)-2-(2-fluoro-1-(4-nitrophenyl)vinyl)-1H-pyrrole (E-4l). Pale brown oil. 1H NMR (400 MHz, CDCl3): δ = 8.23 (d, J = 8.7 Hz, 2H), 8.00 (br s, 1H), 7.59 (d, J = 8.6 Hz, 2H), 7.13 (d, 2JHF = 82.0 Hz, 1H), 6.80–6.89 (m, 1H), 6.20–6.28 (m, 2H) ppm. 13C NMR (100 MHz, CDCl3): δ = 147.5, 146.3 (d, 1JCF = 273.8 Hz), 140.8, 130.5 (d, 4JCF = 4.5 Hz), 125.6 (d, 3JCF = 8.8 Hz), 123.8, 119.8 (d, 6JCF = 1.4 Hz), 117.7 (d, 2JCF = 8.5 Hz), 109.7, 109.4 (d, 4JCF = 3.1 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ = −128.64 (d, 2JHF = 82.0 Hz) ppm. HRMS (ESI-TOF) m/z: [M − H]− calcd. for C12H8FN2O2 231.0575; found 231.0584.
Elimination for 3m. Purification by column chromatography on silica gel with gradient elution (Hex; Hex/DCM 3:1; Hex/DCM 1:1; DCM) gave 31 mg (32%) of the Z-isomer and 44 mg (46%) of the E-isomer of the target product 4m and 11.6 mg (11%) of the side product 5m as a mixture of diastereomers (Z/E = 62:38).
(Z)-2-(2-Fluoro-1-(3-nitrophenyl)vinyl)-1H-pyrrole (Z-4m). Yellow oil. 1H NMR (400 MHz, CDCl3) δ = 9.02 (br s, 1H), 8.33–8.22 (m, 2H), 7.78–7.73 (m, 1H), 7.58 (t, J = 7.9 Hz, 1H), 6.96 (dd, J = 4.0, 2.6 Hz, 1H), 6.71 (d, 2JHF = 82.9 Hz, 1H), 6.29–6.17 (m, 1H), 5.94 (s, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 148.4, 144.0 (d, 1JCF = 266.9 Hz), 136.6 (d, 3JCF = 10.8 Hz), 135.9 (d, 4JCF = 2.6 Hz), 129.6, 126.10 (d, 3JCF = 3.0 Hz), 124.7 (d, 4JCF = 2.7 Hz), 123.4, 120.4 (d, 6JCF = 5.2 Hz), 117.8 (d, 2JCF = 5.4 Hz), 111.4 (d, 4JCF = 3.6 Hz), 109.2 ppm. 19F NMR (376 MHz, CDCl3) δ = −130.64 (dd, J = 82.9, 6.6 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C12H10FN2O2 233.0721; found 233.0730.
(E)-2-(2-Fluoro-1-(3-nitrophenyl)vinyl)-1H-pyrrole (E-4m). Yellow oil. 1H NMR (400 MHz, CDCl3): δ = 8.29 (br s, 1H), 8.18 (dd, J = 8.2, 1.3 Hz, 1H), 8.07 (s, 1H), 7.74 (d, J = 7.7 Hz, 1H), 7.56 (t, J = 8.0 Hz, 1H), 7.13 (d, 2JHF = 82.1 Hz, 1H), 6.85 (dd, J = 4.0, 2.6 Hz, 1H), 6.25 (dd, J = 5.8, 2.9 Hz, 1H), 6.22 (s, 1H). 13C NMR (100 MHz, CDCl3): δ = 148.4, 145.8 (d, 1JCF = 271.4 Hz), 135.8, 135.7 (d, 4JCF = 4.0 Hz), 129.5, 125.7 (d, 3JCF = 8.9 Hz), 124.5 (d, 4JCF = 4.4 Hz), 123.1, 119.8 (d, 6JCF = 1.8 Hz), 117.4 (d, 2JCF = 8.8 Hz), 109.7, 109.3 (d, 4JCF = 3.4 Hz) ppm. 19F NMR (376 MHz, CDCl3) δ = −130.84 (d, 2JHF = 82.1 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C12H10FN2O2 233.0721; found 233.0730.
2-(2-Nitro-1-(3-nitrophenyl)vinyl)-1H-pyrrole (5m). Yellow oil. 1H NMR (400 MHz, CDCl3) (Z-isomer) δ = 11.91 (br s, 1H), 8.41–8.32 (m, 1H), 8.33–8.27 (m, 1H), 7.71–7.61 (m, 1H), 7.34–7.30 (m, 1H), 6.92 (s, 1H), 6.40–6.34 (m, 1H), 6.21–6.10 (m, 1H) ppm; (E-isomer) δ = 8.52 (br s, 1H), 8.41–8.32 (m, 1H), 8.23–8.16 (m, 1H), 7.80–7.73 (m, 2H), 7.56 (s, 1H), 7.12–7.07 (m, 1H), 6.46–6.40 (m, 1H), 6.40–6.34 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 148.3, 148.1, 140.2, 139.9, 138.7, 136.1, 135.4, 134.6, 129.7, 129.6, 129.2, 128.4, 127.5, 127.2, 127.0, 126.0, 124.9, 124.6, 124.5, 124.2, 123.7, 117.8, 112.9, 112.5 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C12H10N3O4 260.0666; found 260.0662.
Elimination for 3o. Purification by column chromatography on silica gel with gradient elution (Hex; Hex/DCM 3:1; Hex/DCM 1:1; Hex/DCM 1:2; DCM) gave 21 mg (15%) of the Z,Z-isomer, 42 mg (29%) of the E,Z-isomer and 24 mg (17 %) of the E,E-isomer of the target product 4o and 13 mg (8 %) of the mixture of (Z-NO2-Z-F)/(E-NO2-Z-F) = 73:27 and 11 mg (7%) of the mixture of (Z-NO2-E-F)/(E-NO2-E-F) = 81:19 of the side product 5o.
1,3-Bis((Z)-2-fluoro-1-(1H-pyrrol-2-yl)vinyl)benzene (Z,Z-4o). Colorless oil. 1H NMR (400 MHz, CDCl3) δ 8.93 (br s, 2H), 7.47–7.37 (m, 4H), 6.91 (dd, J = 4.0, 2.6 Hz, 2H), 6.68 (d, 2JHF = 84.1 Hz, 2H), 6.23 (dd, J = 5.3, 2.6 Hz, 2H), 6.10–6.05 (m, 2H) ppm. 13C NMR (100 MHz, CDCl3): δ = 143.8 (d, 1JCF = 265.7 Hz), 135.0 (d, 3JCF = 10.2 Hz), 131.0 (d, 4JCF = 2.7 Hz), 129.7 (d, 4JCF = 2.6 Hz), 128.7, 126.9 (d, 3JCF = 3.1 Hz), 119.6 (d, 6JCF = 5.0 Hz), 118.6 (d, 2JCF = 3.6 Hz), 111.2 (d, 4JCF = 4.3 Hz), 109.0. 19F NMR (376 MHz, CDCl3): δ = −131.92 (dd, J = 84.1, 6.5 Hz). HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C18H15F2N2 297.1198; found 297.1197.
1-((E)-2-Fluoro-1-(1H-pyrrol-2-yl)vinyl)-3-((Z)-2-fluoro-1-(1H-pyrrol-2-yl)vinyl)benzene (E,Z-4o). Colorless oil. 1H NMR (400 MHz, CDCl3) δ = 8.92 (br s, 1H), 7.95 (br s, 1H), 7.48 (s, 1H), 7.46–7.35 (m, 3H), 7.10 (d, 2JHF = 83.0 Hz, 1H), 6.91 (dd, J = 4.0, 2.6 Hz, 1H), 6.80 (dd, J = 4.0, 2.5 Hz, 1H), 6.68 (d, 2JHF = 84.1 Hz, 1H), 6.29–6.20 (m, 3H), 6.15–6.09 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 144.9 (d, 1JCF = 267.9 Hz), 143.8 (d, 1JCF = 265.7 Hz), 134.9 (d, 3JCF = 10.2 Hz), 134.1, 130.8 (t, 4JCF = 3.2 Hz), 129.7 (d, 4JCF = 2.5 Hz), 129.5 (d, 4JCF = 3.5 Hz), 128.7, 126.9 (d, 3JCF = 9.6 Hz), 126.9 (d, 3JCF = 3.3 Hz), 119.6 (d, 6JCF = 5.0 Hz), 119.2 (d, 6JCF = 2.0 Hz), 118.7 (d, 2JCF = 9.2 Hz), 118.6 (d, 2JCF = 3.5 Hz), 111.1 (d, 4JCF = 4.5 Hz), 109.4, 109.0, 108.0 (d, 4JCF = 2.7 Hz). 19F NMR (376 MHz, CDCl3) δ −131.77 (dd, J = 84.1, 6.5 Hz), −133.23 (d, 2JHF = 83.0 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C18H15F2N2 297.1198; found 297.1197.
1,3-Bis((E)-2-fluoro-1-(1H-pyrrol-2-yl)vinyl)benzene (E,E-4o). Colorless solid. M.p. = 140-141 ºC with decomposition. 1H NMR (400 MHz, CDCl3): δ = 7.96 (br s, 2H), 7.52–7.33 (m, 4H), 7.10 (d, 2JHF = 83.1 Hz, 2H), 6.79 (dd, J = 4.1, 2.6 Hz, 2H), 6.27–6.23 (m, 2H), 6.22 (dd, J = 5.9, 2.8 Hz, 2H) ppm. 13C NMR (100 MHz, CDCl3): δ = 144.82 (d, 1JCF = 267.3 Hz), 133.99, 130.81 (t, 4JCF = 3.8 Hz), 129.54 (d, 4JCF = 3.4 Hz), 128.75, 126.92 (d, 3JCF = 9.4 Hz), 119.14 (d, 6JCF = 2.1 Hz), 118.74 (d, 2JCF = 9.8 Hz), 109.38, 107.71 (d, 4JCF = 2.6 Hz) ppm. 19F NMR (376 MHz, CDCl3) δ = −133.77 (d, 2JHF = 83.1 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C18H15F2N2 297.1198; found 297.1197.
2-((Z)-1-(3-((Z)-2-fluoro-1-(1H-pyrrol-2-yl)vinyl)phenyl)-2-nitrovinyl)-1H-pyrrole (Z-NO2-Z-F-5o) and 2-((E)-1-(3-((Z)-2-fluoro-1-(1H-pyrrol-2-yl)vinyl)phenyl)-2-nitrovinyl)-1H-pyrrole (E-NO2-Z-F-5o). Yellow oil. 1H NMR (400 MHz, CDCl3): (major) δ = 11.93 (br s, 1H), 8.98 (br s, 1H), 7.55–7.48 (m, 2H), 7.48–7.42 (m, 2H), 7.29–7.24 (m, 1H), 6.98 (s, 1H), 6.93 (dd, J = 4.1, 2.7 Hz, 1H), 6.69 (d, 2JHF = 83.8 Hz, 1H), 6.39–6.33 (m, 1H), 6.34–6.28 (m, 1H), 6.26–6.19 (m, 1H), 6.06–5.98 (m, 1H) ppm; (minor) δ = 8.87 (br s, 1H), 8.34 (br s, 1H), 7.56 (s, 1H), 7.48–7.42 (m, 2H), 7.39–7.37 (s, 1H), 7.34–7.29 (m, 1H), 7.03 (dd, J = 3.9, 2.7 Hz, 1H), 6.90 (dd, J = 4.1, 2.7 Hz, 1H), 6.71 (d, 2JHF = 83.6 Hz, 1H), 6.64–6.59 (m, 1H), 6.39–6.33 (m, 1H), 6.26–6.19 (m, 1H), 6.18–6.14 (m, 1H). 13C NMR (100 MHz, CDCl3) δ = 145.2, 143.8 (d, 1JCF = 266.1 Hz), 142.6, 142.4, 141.1, 139.9 (d, 1JCF = 245.0 Hz), 135.1 (d, 3JCF = 10.4 Hz), 134.9 (d, 3JCF = 10.4 Hz), 134.3, 130.9, 130.8 (d, 4JCF = 2.7 Hz), 130.6 (d, 4JCF = 2.1 Hz), 130.0 (d, 4JCF = 2.9 Hz), 129.5, 129.1, 129.0, 128.5, 128.4, 128.2, 128.9, 127.7, 126.7, 126.7, 126.5 (d, 3JCF = 2.9 Hz), 125.1, 124.9, 119.9 (d, 6JCF = 5.1 Hz), 119.7 (d, 2JCF = 4.7 Hz), 118.4 (d, 2JCF = 3.9 Hz), 115.7, 112.5, 112.1, 111.3 (d, 4JCF = 5.1 Hz), 111.2 (d, 4JCF = 4.0 Hz), 109.2, 109.0 ppm. 19F NMR (376 MHz, CDCl3): (major) δ = −131.57 (dd, J = 83.8, 6.7 Hz) ppm; (minor) δ = −131.01 (dd, J = 83.6, 5.9 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C18H15FN3O2 324.1143; found 324.1140.
2-((Z)-1-(3-((E)-2-fluoro-1-(1H-pyrrol-2-yl)vinyl)phenyl)-2-nitrovinyl)-1H-pyrrole (Z-NO2-E-F-5o) and 2-((E)-1-(3-((E)-2-fluoro-1-(1H-pyrrol-2-yl)vinyl)phenyl)-2-nitrovinyl)-1H-pyrrole (E-NO2-E-F-5o). Yellow oil. 1H NMR (400 MHz, CDCl3): (major) δ = 11.92 (br s, 1H), 8.01 (br s, 1H), 7.54–7.47 (m, 2H), 7.45 (t, J = 7.6 Hz, 1H), 7.39 (dt, J = 7.6, 1.5 Hz, 1H), 7.30–7.25 (m, 1H), 7.10 (d, 2JHF = 82.7 Hz, 1H), 6.97 (s, 1H), 6.84–6.78 (m, 1H), 6.39–6.31 (m, 2H), 6.23 (t, J = 2.3 Hz, 1H), 6.20 (dd, J = 6.0, 2.7 Hz, 1H) ppm; (minor) δ = 8.38 (br s, 1H), 8.08 (br s, 1H), 7.59 (d, J = 7.8 Hz, 1H), 7.56 (s, 1H), 7.54–7.47 (m, 1H), 7.35–7.30 (m, 1H), 7.30–7.25 (m, 1H), 7.14 (d, 2JHF = 83.2 Hz, 1H), 7.05–7.00 (m, 1H), 6.84–6.78 (m, 1H), 6.67 (ddd, J = 3.9, 2.7, 1.3 Hz, 1H), 6.39–6.31 (m, 1H), 6.31–6.26 (m, 1H), 6.23 (t, J = 2.3 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 146.3, 145.2 (d, 1JCF = 269.1 Hz), 143.7, 139.9 (d, 1JCF = 261.7 Hz), 134.2 (d, 3JCF = 5.1 Hz), 134.0, 131.0, 130.9, 130.7 (d, 4JCF = 4.1 Hz), 130.7 (d, 4JCF = 3.5 Hz), 129.6, 129.6, 129.4, 129.1, 129.0, 128.4, 128.4, 128.1, 127.8, 127.7, 126.7, 126.6 (d, 3JCF = 9.0 Hz), 125.3, 125.0, 119.6 (d, 6JCF = 2.5 Hz), 119.4 (d, 6JCF = 2.0 Hz), 118.3 (d, 2JCF = 9.1 Hz), 115.3, 112.5, 112.1, 109.5, 109.2, 108.6 (d, 4JCF = 3.0 Hz), 107.5 (d, 4JCF = 2.0 Hz). 19F NMR (376 MHz, CDCl3) (major) δ = −132.42 (d, 2JHF = 82.7 Hz) ppm; (minor) δ = −133.65 (d, 2JHF = 83.2 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C18H15FN3O2 324.1143; found 324.1140.
Elimination for 3p. Purification by column chromatography on silica gel with gradient elution (Hex/DCM 2:1; DCM) gave 58 mg (63%) of the target product 4p as a mixture of diastereomers (Z/E = 32:54) and 15 mg (15%) of the side product 5p as a mixture of diastereomers (Z/E = 37:63).
2-(1-(4-Chlorophenyl)-2-fluorovinyl)-1-methyl-1H-pyrrole(4p). Colorless oil. 1H NMR (400 MHz, CDCl3): (E-isomer) δ = 7.34–7.28 (m, 2H), 7.20–7.15 (m, 2H), 7.04 (d, 2JHF = 82.9 Hz, 1H), 6.75–6.71 (m, 1H), 6.24–6.21 (m, 1H), 6.20 (dd, J = 3.6, 1.8 Hz, 1H), 3.36 (d, J = 0.8 Hz, 3H) ppm; (Z-isomer) δ = 7.38–7.26 (m, 4H), 6.92 (d, 2JHF = 83.6 Hz, 1H), 6.72–6.69 (m, 1H), 6.25–6.21 (m, 1H), 6.18–6.15 (m, 1H), 3.22 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 147.5 (d, 1JCF = 278.6 Hz), 146.6 (d, 1JCF = 267.6 Hz), 135.2 (d, 3JCF = 6.7 Hz), 133.9, 133.6 (d, 4JCF = 1.9 Hz), 133.2 (d, 4JCF = 3.6 Hz), 130.1 (d, 4JCF = 5.9 Hz), 129.0, 128.8, 128.6 (d, J = 3.4 Hz), 127.4 (d, 3JCF = 12.6 Hz), 125.5, 124.3 (d, 6JCF = 1.0 Hz), 123.7, 118.1 (d, 2JCF = 8.6 Hz), 116.6 (d, 2JCF = 6.2 Hz), 111.5 (d, 4JCF = 2.5 Hz), 111.3 (d, 4JCF = 1.3 Hz), 108.0, 107.4, 34.9 (d, 5JCF = 2.7 Hz), 34.6 ppm. 19F NMR (282 MHz, CDCl3): (E-isomer) δ = −124.05 (d, 2JHF = 82.9 Hz) ppm; (Z-isomer) δ = −122.67 (d, 2JHF = 83.6 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C13H12ClFN 236.0637; found 236.0640.
2-(1-(4-Chlorophenyl)-2-nitrovinyl)-1-methyl-1H-pyrrole (5p). Yellow oil. 1H NMR (400 MHz, CDCl3) (E-isomer) δ = 7.42–7.37 (m, 2H), 7.35 (s, 1H), 7.31–7.27 (m, 2H), 6.91–6.88 (m, 1H), 6.26 (dd, J = 3.8, 2.6 Hz, 1H), 6.22 (dd, J = 3.8, 1.7 Hz, 1H), 3.38 (s, 3H) ppm; (Z-isomer) δ = 7.42–7.37 (m, 2H), 7.32 (s, 1H), 7.25–7.19 (m, 2H), 6.88–6.84 (m, 1H), 6.28 (dd, J = 3.9, 1.7 Hz, 1H), 6.19 (dd, J = 3.9, 2.7 Hz, 1H), 3.41 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 141.8, 140.7, 137.8, 135.9, 133.9, 133.1, 131.3, 131.1, 130.8, 130.7, 130.7, 129.3, 128.9, 128.5, 127.7, 127.1, 119.1, 116.2, 109.7, 109.4, 36.7, 35.0 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C13H12ClN2O2 263.0582; found 263.0583.
Elimination for 3q. Purification by column chromatography on silica gel with gradient elution (Hex/DCM 3:1; Hex/DCM 1:1) gave 19 mg (57%) of the target product 4q as a mixture of diastereomers (Z/E = 61:31).
2-(1-(4-Chlorophenyl)-2-fluorovinyl)-1-phenyl-1H-pyrrole (4q). Colorless oil. 1H NMR (400 MHz, CDCl3) (Z-isomer) δ = 7.26–7.06 (m, 7H), 7.04–6.97 (m, 2H), 6.97–6.93 (m, 1H), 6.83 (d, 2JHF = 82.4 Hz, 1H), 6.41 (dd, J = 3.6, 1.8 Hz, 1H), 6.38 (dd, J = 3.5, 2.9 Hz, 1H) ppm; (E-isomer) δ = 7.26–7.06 (m, 7H), 7.04–6.97 (m, 2H), 6.97–6.93 (m, 1H), 6.94 (d, 2JHF = 83.4 Hz, 1H), 6.36 (dd, J = 3.5, 1.8 Hz, 1H), 6.33–6.30 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 147.3 (d, 1JCF = 275.6 Hz), 146.65 (d, 1JCF = 268.1 Hz), 140.3, 140.0, 134.8 (d, 3JCF = 6.6 Hz), 133.5, 133.1, 133.0 (d, 3JCF = 2.9 Hz), 130.2 (d, 4JCF = 5.1 Hz), 128.9, 128.9, 128.6 (d, 4JCF = 3.3 Hz), 128.5, 128.1, 127.7 (d, 3JCF = 11.8 Hz), 126.9, 126.8, 125.4, 125.3, 124.9, 124.5 (d, 6JCF = 1.0 Hz), 123.9, 118.5 (d, 2JCF = 8.6 Hz), 117.6 (d, 2JCF = 7.4 Hz), 113.4 (d, 4JCF = 2.6 Hz), 113.04 (d, 4JCF = 1.5 Hz), 109.3, 108.7. 19F NMR (282 MHz, CDCl3) (Z-isomer) δ = −124.11 (d, 2JHF = 82.4 Hz) ppm; (E-isomer) δ = −124.83 (d, 2JHF = 83.3 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C18H14ClFN 298.0793; found 298.0786.
Elimination for 3r. Purification by column chromatography on silica gel with gradient elution (Hex/DCM 5:1; Hex/DCM 1:1) gave 27 mg (63%) of the target product 4q as a mixture of diastereomers (Z/E = 53:32).
2-(1-(4-Chlorophenyl)-2-fluorovinyl)-1-(4-ethylphenyl)-1H-pyrrole (4r). Yellowish oil. 1H NMR (400 MHz, CDCl3) (Z-isomer) δ = 7.14–6.95 (m, 8H), 6.94–6.90 (m, 1H), 6.82 (d, 2JHF = 82.4 Hz, 1H), 6.40 (dd, J = 3.6, 1.7 Hz, 1H), 6.39–6.36 (m, 1H), 2.59 (q, J = 7.7 Hz, 2H), 1.19 (t, J = 7.5 Hz, 3H) ppm; (E-isomer) δ = 7.14–6.95 (m, 8H), 6.94–6.90 (m, 1H), 6.92 (d, 2JHF = 83.5 Hz, 1H), 6.34 (dd, J = 3.4, 1.8 Hz, 1H), 6.32–6.28 (m, 1H), 2.57 (q, J = 7.7 Hz, 2H), 1.17 (t, 3H) ppm. 13C NMR (100 MHz, CDCl3) δ = 147.2 (d, 1JCF = 275.0 Hz), 146.6 (d, 1JCF = 268.1 Hz), 143.2, 143.0, 138.0, 137.7, 134.9 (d, 3JCF = 6.7 Hz), 133.4, 133.1 (d, 3JCF = 2.8 Hz), 133.06 (d, 3JCF = 1.3 Hz), 130.3 (d, 4JCF = 5.0 Hz), 129.1 (d, 4JCF = 2.2 Hz), 128.7 (d, 4JCF = 3.3 Hz), 128.4, 128.2, 128.2, 128.0, 125.5, 125.4, 125.0, 124.5 (d, 6JCF = 1.1 Hz), 123.8, 118.5 (d, 2JCF = 8.4 Hz), 117.6 (d, 2JCF = 7.5 Hz), 113.0 (d, 4JCF = 2.6 Hz), 112.6 (d, 4JCF = 1.8 Hz), 109.1, 108.5, 28.5, 28.5, 15.7, 15.7 ppm. 19F NMR (376 MHz, CDCl3) (Z-isomer) δ = −124.17 (d, 2JHF = 82.5 Hz) ppm; (E-isomer) δ = −125.20 (d, 2JHF = 83.4 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C20H18ClFN 326.1106; found 326.1097.
Elimination for 3s. Purification by column chromatography on silica gel with gradient elution (Hex/DCM 4:1; Hex/DCM 1:1) gave 47 mg (72%) of the target product 4s as a mixture of diastereomers (Z/E = 57:35) and 13 mg (18%) of the side product 5s as a mixture of diastereomers (Z/E = 41:59).
2-(1-(4-Chlorophenyl)-2-fluorovinyl)-1-(p-tolyl)-1H-pyrrole (4s). Yellowish oil. 1H NMR (400 MHz, CDCl3) (Z-isomer) δ = 7.18–6.98 (m, 8H), 6.96–6.90 (m, 1H), 6.83 (d, 2JHF = 82.3 Hz, 1H), 6.41–6.36 (m, 2H), 2.31 (s, 3H) ppm; (E-isomer) δ = 7.18–6.98 (m, 8H), 6.96–6.90 (m, 1H), 6.91 (d, 2JHF = 82.1 Hz, 1H), 6.34 (dd, J = 3.3, 1.8 Hz, 1H), 6.31 (t, J = 3.1 Hz, 1H), 2.28 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 147.3 (d, 1JCF = 275.6 Hz), 146.6 (d, 1JCF = 267.9 Hz), 137.9, 137.5, 136.7, 136.6, 134.9 (d, 3JCF = 6.7 Hz), 133.5, 133.1, 133.1, 130.2 (d, 4JCF = 5.1 Hz), 129.5, 129.4, 128.6 (d, 4JCF = 3.4 Hz), 128.5, 128.1, 127.7 (d, 3JCF = 12.4 Hz), 125.4, 125.1, 124.7, 124.5 (d, 6JCF = 1.0 Hz), 123.9, 118.5 (d, 2JCF = 8.7 Hz), 117.5 (d, 2JCF = 7.3 Hz), 113.0 (d, 4JCF = 2.3 Hz), 112.9 (d, 4JCF = 1.8 Hz), 109.1, 108.5, 21.1, 21.1 ppm. 19F NMR (376 MHz, CDCl3) (Z-isomer) δ = −123.99 (d, 2JHF = 82.4 Hz) ppm; (E-isomer) δ = −124.76 (d, 2JHF = 83.4 Hz) ppm. HRMS (ESI-TOF) m/z: [M + O + H]+ calcd. for C19H15ClFNO (adduct with oxygen) 328.0899; found 328.0890.
2-(1-(4-Chlorophenyl)-2-nitrovinyl)-1-(p-tolyl)-1H-pyrrole (5s). Yellow oil. 1H NMR (400 MHz, CDCl3) (E-isomer) δ = 7.33–7.16 (m, 4H), 7.14–6.96 (m, 5H), 6.75 (s, 1H), 6.32 (dd, J = 3.9, 2.7 Hz, 1H), 6.28 (dd, J = 3.9, 1.7 Hz, 1H), 2.39 (s, 3H) ppm; (Z-isomer) δ = 7.33–7.16 (m, 4H), 7.07 (s, 1H), 7.14–6.96 (m, 5H), 6.42 (d, J = 2.2 Hz, 2H), 2.30 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3) δ = 140.9, 140.7, 138.6, 137.4, 137.3, 137.1, 136.8, 135.6, 135.3, 134.0, 133.5, 132.2, 131.3, 130.6, 130.5, 130.4, 129.9, 129.2, 129.1, 128.4, 127.4, 126.2, 125.9, 124.6, 121.1, 117.3, 110.6, 110.4, 21.2, 21.1 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C19H16ClN2O2 339.0895; found 339.0895.
Elimination for 3t. Purification by column chromatography on silica gel with gradient elution (Hex/DCM 5:1; Hex/DCM 4:1) gave 46 mg (65%) of the target product 4t as a mixture of diastereomers (Z/E = 62:32) and 10 mg (10%) of the side product 5t as a mixture of diastereomers (Z/E = 39:61).
1-(3-Chloro-4-methoxyphenyl)-2-(1-(4-chlorophenyl)-2-fluorovinyl)-1H-pyrrole (4t). Colorless oil. 1H NMR (400 MHz, CDCl3): (Z-isomer) δ = 7.19–7.09 (m, 3H), 7.07–6.91 (m, 3H), 6.89–6.85 (m, 1H), 6.84 (d, 3JHF = 82.4 Hz, 1H), 6.75 (d, J = 6.8 Hz, 1H), 6.40 (dd, J = 3.5, 1.7 Hz, 1H), 6.38–6.33 (m, 1H), 3.86 (s, 3H) ppm; (E-isomer) δ = 7.19–7.09 (m, 3H), 7.07–6.91 (m, 3H), 6.95 (d, 3JHF = 83.2 Hz, 1H), 6.89–6.85 (m, 1H), 6.71 (d, J = 8.8 Hz, 1H), 6.38–6.33 (m, 1H), 6.29 (t, J = 3.2 Hz, 1H), 3.84 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 153.9, 153.8, 147.3 (d, 1JCF = 276.0 Hz), 146.8 (d, 1JCF = 268.6 Hz), 134.7 (d, 3JCF = 6.6 Hz), 133.7, 133.6, 133.3, 133.3, 132.9 (d, 3JCF = 3.0 Hz), 130.2 (d, 4JCF = 5.0 Hz), 128.6, 128.6, 128.2, 127.4, 127.2, 125.6, 124.6, 124.5, 124.3, 123.8, 122.3, 122.3, 118.2 (d, 2JCF = 8.4 Hz), 117.4 (d, 2JCF = 7.7 Hz), 113.3 (d, 4JCF = 2.7 Hz), 112.8 (d, 4JCF = 1.3 Hz), 111.7, 109.4, 108.8, 56.5, 56.5. 19F NMR (376 MHz, CDCl3): (Z-isomer) δ = −123.66 (d, 3JHF = 82.4 Hz), (E-isomer) δ = −124.46 (d, 3JHF = 83.2 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C19H1535Cl2FNO 362.0509; found 362.0504.
1-(3-Chloro-4-methoxyphenyl)-2-(1-(4-chlorophenyl)-2-nitrovinyl)-1H-pyrrole (5t). Yellow oil. 1H NMR (400 MHz, CDCl3) (E-isomer) δ = 7.33–7.27 (m, 2H), 7.20–7.16 (m, 2H), 7.13 (d, J = 2.6 Hz, 1H), 7.11 (s, 1H), 7.06–6.95 (m, 2H), 6.79 (d, J = 8.8 Hz, 1H), 6.45 (dd, J = 3.8, 1.7 Hz, 1H), 6.41 (dd, J = 3.8, 2.8 Hz, 1H), 3.87 (s, 3H) ppm; (Z-isomer) δ = 7.26–7.22 (m, 2H), 7.16 (d, J = 2.6 Hz, 1H), 7.06–6.95 (m, 4H), 6.94 (s, 1H), 6.84 (d, J = 8.7 Hz, 1H), 6.39 (dd, J = 3.9, 1.7 Hz, 1H), 6.32 (dd, J = 3.9, 2.8 Hz, 1H), 3.92 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3) δ = 155.0, 154.3, 141.0, 140.4, 137.5, 135.4, 135.3, 133.9, 133.6, 132.6, 132.3, 131.0, 130.5, 130.4, 129.7, 129.2, 128.4, 128.1, 127.3, 126.9, 126.4, 125.5, 124.3, 123.2, 122.7, 120.0, 117.2, 112.2, 112.0, 110.7, 110.6, 56.6, 56.5 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C19H1535Cl2N2O3 389.0454; found 389.0451.
Elimination for 3u. Purification by column chromatography on silica gel with gradient elution (Hex/DCM 2:1; Hex/DCM 1:1; DCM) gave 81 mg (65%) of the target product 4u as a mixture of diastereomers (Z/E = 55:35) and 20 mg (15%) of the side product 5u as a mixture of diastereomers (Z/E = 44:56).
2-(1-(4-Chlorophenyl)-2-fluorovinyl)-1-(3-methoxyphenyl)-1H-pyrrole (4u). Yellowish oil. 1H NMR (400 MHz, CDCl3) (Z-isomer) δ = 7.20–7.05 (m, 4H), 7.02 (d, J = 8.5 Hz, 1H), 7.00–6.94 (m, 1H), 6.87 (d, 2JHF = 82.4 Hz, 1H), 6.80–6.65 (m, 3H), 6.44 (dd, J = 3.4, 1.6 Hz, 1H), 6.42–6.36 (m, 1H), 3.73 (s, 3H) ppm; (E-isomer) δ = 7.20–7.05 (m, 4H), 7.00–6.94 (m, 2H), 6.97 (d, 2JHF = 83.4 Hz, 1H), 6.80–6.65 (m, 3H), 6.42–6.36 (m, 1H), 6.36–6.31 (m, 1H), 3.71 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3) δ = 159.8, 147.2 (d, 1JCF = 275.7 Hz), 146.7 (d, 1JCF = 268.2 Hz), 141.4, 141.0, 134.7 (d, 3JCF = 6.6 Hz), 133.4, 133.1, 133.0 (d, 3JCF = 2.9 Hz), 130.2 (d, 4JCF = 5.2 Hz), 129.6, 129.6, 128.8 (d, 3JCF = 3.5 Hz), 128.6 (d, 4JCF = 3.2 Hz), 128.5, 128.1, 127.7 (d, 3JCF = 4.1 Hz), 125.3, 124.4, 123.8, 118.5 (d, 2JCF = 8.4 Hz), 117.6, 117.6 (d, 2JCF = 7.2 Hz), 117.3, 113.4 (d, 4JCF = 2.5 Hz), 113.1 (d, 4JCF = 1.3 Hz), 112.5, 112.4, 111.1, 110.9, 109.3, 108.7, 55.4, 55.4 ppm. 19F NMR (376 MHz, CDCl3) (Z-isomer) δ = −124.12 (d, J = 82.4 Hz) ppm; (E-isomer) δ = −124.80 (d, 2JHF = 83.3 Hz) ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C19H1635ClFNO 328.0899; found 328.0893 ppm.
2-(1-(4-chlorophenyl)-2-nitrovinyl)-1-(3-methoxyphenyl)-1H-pyrrole (5u). Yellow oil. 1H NMR (400 MHz, CDCl3): δ = 7.32–7.22 (m, 3H), 7.21–7.06 (m, 4H), 7.11 (s, 1H), 6.79–6.65 (m, 2H), 6.47 (dd, J = 3.8, 1.7 Hz, 1H), 6.45–6.40 (m, 1H), 3.73 (s, 3H) ppm; δ = 7.32–7.22 (m, 1H), 7.21–7.06 (m, 3H), 7.04 (dd, J = 2.5, 2.0 Hz, 1H), 6.91–6.85 (m, 1H), 6.86 (s, 1H), 6.79–6.65 (m, 3H), 6.37–6.31 (m, 2H), 3.80 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3) δ = 160.5, 160.1, 140.9, 140.6, 140.6, 140.4, 137.3, 135.4, 135.3, 133.8, 133.7, 132.2, 130.9, 130.6, 130.5, 130.4, 130.0, 129.3, 129.0, 128.3, 127.1, 126.2, 120.6, 118.4, 117.4, 117.1, 113.7, 112.8, 112.2, 110.9, 110.6, 110.4, 55.6, 55.5 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd. for C19H16ClN2O3 355.0844; found 355.0831.
5,5′-((4-Chlorophenyl)methylene)bis(2-(2-fluoro-2-nitro-1-(4-nitrophenyl)ethyl)-1H-pyrrole) (6). A solution of adduct 3l (90 mg, 2.3 mol. equiv.) and 4-chlorobenzaldehyde (23 mg, 1 mol. equiv.) in DCM (1 mL) was loaded into a vial. Next, TFA (1 drop) was added to the solution. The reaction mixture was stirred overnight at room temperature. After completion of the reaction (TLC monitoring), the reaction mixture was concentrated under vacuum. The product was isolated as a mixture of isomers by column chromatography on silica using DCM as the eluent. Pale brown oil. 1H NMR (400 MHz, CDCl3) δ = 8.21–8.06 (m, 4H), 8.03–7.85 (m, 2H), 7.52–7.34 (m, 4H), 7.27–7.20 (m, 2H), 7.07–7.00 (m, 2H), 6.41–6.03 (m, 4H), 5.87–5.69 (m, 2H), 5.37–5.30 (m, 1H), 5.13–4.89 (m, 2H) ppm. 13C NMR (100 MHz, CDCl3) δ = 148.0, 147.8, 142.3, 142.2, 142.2, 140.7, 140.7, 139.5, 139.5, 139.4, 139.4, 133.8, 133.8, 133.7, 133.6, 133.6, 133.3, 133.3, 133.3, 130.2, 130.2, 130.1, 129.7, 129.7, 129.7, 129.4, 129.4, 129.0, 129.0, 129.9, 124.4, 124.2, 124.1, 124.1, 122.2, 122.1, 122.0, 112.3, 111.1 (d, 1JCF = 246.1 Hz), 111.1 (d, 1JCF = 244.8 Hz), 110.9 (d, 1JCF = 246.7 Hz), 110.8, 110.8 (d, 1JCF = 246.4 Hz), 110.8, 110.7, 110.7, 110.6, 110.6, 109.1, 109.1, 109.0, 108.8, 108.8, 108.7, 47.7 (d, 2JCF = 17.5 Hz), 47.6 (d, 2JCF = 17.0 Hz), 47.6 (d, 2JCF = 18.3 Hz), 47.5 (d, 2JCF = 19.2 Hz), 43.3, 43.3 (the other signals are overlapped) ppm. 19F NMR (376 MHz, CDCl3) δ = −150.39–−150.95 (m), −151.00–−151.72 (m) ppm.
4. Conclusions
In summary, new synthetic approaches to novel classes of monofluorinated 2-substituted pyrroles were developed. The conjugate addition of pyrroles to β-Fluoro-β-nitrostyrenes was investigated to yield 2-(2-Fluoro-2-nitro-1-arylethyl)-1H-pyrroles. The reaction proceeded under catalyst-free neat conditions. It was demonstrated that structurally diverse β-Fluoro-β-nitrostyrenes and pyrroles efficiently participated in this transformation. As a result, a family of novel adducts was synthesized in a quantitative yield. The kinetics of this reaction were studied to evaluate the activation parameters and substituent effects. Furthermore, it was found that 2-(2-Fluoro-2-nitro-1-arylethyl)-1H-pyrroles are prone to efficiently undergo the subsequent base-induced elimination of nitrous acid to furnish 2-(2-Fluoro-1-arylvinyl)-1H-pyrroles. This approach demonstrated a broad scope in terms of differently substituted nitrostyrenes and pyrroles. The chemo- and stereoselectivity of this process were studied. A family of novel monofluorinated 2-vinylpyrroles was prepared in up to an 85% isolated yield. The compounds herein obtained can be potential building blocks to diverse fluorine-substituted pyrrole derivatives. The preparation of the corresponding dipyrromethane was demonstrated. Thus, the proposed synthetic sequence involving a conjugate addition and HNO2 elimination represents a remarkable example of an effective alternative route in which β-Fluoro-β-nitrostyrenes serve as a formal analog of 1-Fluoroacetylenes.
Supplementary Materials
NMR spectra of the compounds obtained and kinetics data are available online.
Author Contributions
Conceptualization, A.S.A. and V.G.N.; methodology, A.S.A. and V.G.N.; validation, A.A.T. and S.L.I.; investigation, A.S.A.; resources, V.G.N.; data curation, V.G.N.; writing—original draft preparation, A.S.A.; writing—review and editing, A.A.T. and V.G.N.; visualization, A.S.A. and project administration, V.G.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by RFBR, project number 20-33-70132.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Acknowledgments
The authors acknowledge partial support from M. V. Lomonosov Moscow State University Program of Development.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 3–5 are available from the authorsupon request.
References
- Gholap, S.S. Pyrrole: An emerging scaffold for construction of valuable therapeutic agents. Eur. J. Med. Chem. 2016, 110, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Estévez, V.; Villacampa, M.; Menéndez, J.C. Multicomponent reactions for the synthesis of pyrroles. Chem. Soc. Rev. 2010, 39, 4402–4421. [Google Scholar] [CrossRef]
- Estévez, V.; Villacampa, M.; Menéndez, J.C. Recent advances in the synthesis of pyrroles by multicomponent reactions. Chem. Soc. Rev. 2014, 43, 4633–4657. [Google Scholar] [CrossRef] [PubMed]
- Balakrishna, A.; Aguiar, A.; Sobral, P.J.M.; Wani, M.Y.; Almeida e Silva, J.; Sobral, A.J.F.N. Paal–Knorr synthesis of pyroles: From conventional to green synthesis. Catal. Rev. Sci. Eng. 2019, 61, 84–110. [Google Scholar] [CrossRef]
- Albano, G.; Aronica, L.A. Acyl Sonogashira Cross-Coupling: State of the Art and Application to the Synthesis of Heterocyclic Compounds. Catalysts 2020, 10, 25. [Google Scholar] [CrossRef]
- Kaur, R.; Rani, V.; Abbot, V.; Kapoor, Y.; Konar, D.; Kumar, K. Recent synthetic and medicinal perspectives of pyrroles: An overview. Pharm. Chem. Chem. Sci. 2017, 1, 17–32. Available online: https://www.alliedacademies.org/articles/recent-synthetic-and-medicinal-perspectives-of-pyrroles-an-overview-9135.html (accessed on 8 June 2021).
- Ahmad, S.; Alam, O.; Naim, M.J.; Shaquiquzzaman, M.; Alam, M.M.; Iqbal, M. Pyrrole: An insight into recent pharmacological advances with structure activity relationship. Eur. J. Med. Chem. 2018, 157, 527–561. [Google Scholar] [CrossRef]
- Petri, G.L.; Spanò, V.; Spatola, R.; Holl, R.; Raimondi, M.V.; Barraja, P.; Montalbano, A. Bioactive pyrrole-based compounds with target selectivity. Eur. J. Med. Chem. 2020, 208, 112783. [Google Scholar] [CrossRef]
- Das, B.; Chowdhury, N.; Damodar, K. Iodine-catalyzed efficient conjugate addition of pyrroles to α,β-unsaturated ketones. Tetrahedron Lett. 2007, 48, 2867–2870. [Google Scholar] [CrossRef]
- Kusurkar, R.S.; Nayak, S.K.; Chavan, N.L. Conjugate addition of pyrroles to a,b-unsaturated ketones using copper bromide as a catalyst. Tetrahedron Lett. 2006, 7, 7323–7326. [Google Scholar] [CrossRef]
- Yadav, J.S.; Abraham, S.; Reddy, B.V.S.; Sabitha, G. Addition of pyrroles to electron deficient olefins employing InCl3. Tetrahedron Lett. 2001, 42, 8063–8065. [Google Scholar] [CrossRef]
- Ünaleroglu, C.; Temelli, B.; Demir, A.S. Metal Triflates-Catalyzed Conjugate Addition of Homochiral Pyrroles to a,b-Unsaturated Esters. Synthesis 2004, 15, 2574–2578. [Google Scholar] [CrossRef]
- Vlasov, V.M. Effects of substituents on activation parameter changes in the Michael-type reactions of nucleophilic addition to activated alkenes and alkynes in solution. Monatsh. Chem. 2016, 147, 319–328. [Google Scholar] [CrossRef]
- Palmieri, A.; Petrini, M. Synthesis and practical applications of 2-(2-nitroalkyl)pyrroles. Org. Biomol. Chem. 2020, 18, 4533–4546. [Google Scholar] [CrossRef] [PubMed]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzym. Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef]
- Wang, B.C.; Wang, L.J.; Jiang, B.; Wang, S.Y.; Wu, N.; Li, X.Q.; Shi, D.Y. Application of Fluorine in Drug Design during 2010-2015 Years: A Mini-Review. Mini Rev. Med. Chem. 2017, 17, 683–692. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef]
- Politanskaya, L.V.; Selivanova, G.A.; Panteleeva, E.V.; Tretyakov, E.V.; Platonov, V.E.; Nikul’shin, P.V.; Vinogradov, A.S.; Zonov, Y.V.; Karpov, V.M.; Mezhenkova, T.V.; et al. Organofluorine chemistry: Promising growth areas and challenges. Russ. Chem. Rev. 2019, 88, 425–569. [Google Scholar] [CrossRef]
- Serdyuk, O.V.; Muzalevskiy, V.M.; Nenajdenko, V.G. Synthesis and Properties of Fluoropyrroles and Their Analogues. Synthesis 2012, 44, 2115–2137. [Google Scholar] [CrossRef]
- Nenajdenko, V.G.; Muzalevskiy, V.M.; Serdyuk, O.V. Chemistry of Fluorinated Pyrroles. In Fluorine in Heterocyclic Chemistry Volume 1: 5-Membered Heterocycles and Macrocycles; Nenajdenko, V.G., Ed.; Springer: Cham, Switzerland, 2014; Volume 1, pp. 55–115. [Google Scholar] [CrossRef]
- Motornov, V.A.; Muzalevskiy, V.M.; Tabolin, A.A.; Novikov, R.A.; Nelyubina, Y.V.; Nenajdenko, V.G.; Ioffe, S.L. Radical Nitration-Debromination of α-Bromo-α-fluoroalkenes as a Stereoselective Route to Aromatic α-Fluoronitroalkenes—Functionalized Fluorinated Building Blocks for Organic Synthesis. J. Org. Chem. 2017, 82, 5274–5284. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, S.A.; Larkovich, R.V.; Aldoshin, A.S.; Tabolin, A.A.; Ioffe, S.L.; Groß, J.; Opatz, T.; Nenajdenko, V.G. Diels–Alder reaction of β-fluoro-β-nitrostyrenes with cyclic dienes. Beilstein J. Org. Chem. 2021, 17, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Larkovich, R.V.; Ponomarev, S.A.; Aldoshin, A.S.; Tabolin, A.A.; Ioffe, S.L.; Nenajdenko, V.G. Diels-Alder Reaction of β-Fluoro-β-nitrostyrenes. Synthesis of Mono-fluorinated Six-Membered Derivatives. Eur. J. Org. Chem. 2020, 2020, 2479–2492. [Google Scholar] [CrossRef]
- Motornov, V.A.; Tabolin, A.A.; Nelyubina, Y.V.; Nenajdenko, V.G.; Ioffe, S.L. Copper-mediated oxidative [3 + 2]-annulation of nitroalkenes and pyridinium imines: Efficient synthesis of 3-fluoro- and 3-nitro-pyrazolo[1,5-a]pyridines. Org. Biomol. Chem. 2020, 18, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Motornov, V.A.; Tabolin, A.A.; Novikov, R.A.; Nelyubina, Y.V.; Nenajdenko, V.G.; Ioffe, S.L. Fluoronitroalkenes in tandem [4 + 1]/[3 + 2]-cycloaddition: One-pot three-component assembly of fluorinated bicyclic nitroso acetals. Org. Chem. Front. 2018, 5, 2588–2594. [Google Scholar] [CrossRef]
- Motornov, V.A.; Tabolin, A.A.; Nelyubina, Y.V.; Nenajdenko, V.G.; Ioffe, S.L. Copper-mediated oxidative [3 + 2]-annulation of nitroalkenes and pyridinium ylides: General access to functionalized indolizines and efficient synthesis of 1-fluoroindolizines. Org. Biomol. Chem. 2019, 17, 1442–1454. [Google Scholar] [CrossRef]
- Aldoshin, A.S.; Tabolin, A.A.; Ioffe, S.L.; Nenajdenko, V.G. Green, Catalyst-Free Reaction of Indoles with β-Fluoro-β-nitrostyrenes in Water. Eur. J. Org. Chem. 2018, 2018, 3816–3825. [Google Scholar] [CrossRef]
- Motornov, V.A.; Tabolin, A.A.; Novikov, R.A.; Nelyubina, Y.V.; Ioffe, S.L.; Smolyar, I.V.; Nenajdenko, V.G. Synthesis and Regioselective N-2 Functionalization of 4-Fluoro-5-aryl-1,2,3-NH-triazoles. Eur. J. Org. Chem. 2017, 6851–6860. [Google Scholar] [CrossRef]
- Aldoshin, A.S.; Tabolin, A.A.; Ioffe, S.L.; Nenajdenko, V.G. One-Pot Synthesis of 3-(2-Fluoroalkenyl)indoles. Eur. J. Org. Chem. 2019, 2019, 4384–4396. [Google Scholar] [CrossRef]
- Lorand, J.P.; Urban, J.; Overs, J.; Ahmed, Q.A. Fluoronitromethane: Synthesis and estimation of acid strength. J. Org. Chem. 1969, 34, 4176–4178. [Google Scholar] [CrossRef]
- Chang, R. Physical Chemistry for the Biosciences; University Science Books: Sausalito, CA, USA, 2005; pp. 338–342. [Google Scholar]
- Trofimov, B.A.; Sobenina, L.N.; Demenev, A.P.; Mikhaleva, A.I. C-Vinylpyrroles as Pyrrole Building Blocks. Chem. Rev. 2004, 104, 2481–2506. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.B.G.; Lawton, M. Drying of Organic Solvents: Quantitative Evaluation of the Efficiency of Several Desiccants. J. Org. Chem. 2010, 75, 8351–8354. [Google Scholar] [CrossRef] [PubMed]
- Shastin, A.V.; Muzalevsky, V.M.; Balenkova, E.S.; Nenajdenko, V.G. Stereoselective synthesis of 1-bromo-1-fluorostyrenes. Mendeleev Commun. 2006, 16, 178–180. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).