Advances in Enzyme and Ionic Liquid Immobilization for Enhanced in MOFs for Biodiesel Production
Abstract
1. Introduction
2. Biodiesel Production
2.1. Conventional Catalysts
2.2. Enzymatic Biodiesel Production
2.2.1. Factors Affecting Enzymatic Biodiesel Production
Lipid Source
Alcohols
2.2.2. Immobilized Enzymes in Biodiesel Production
3. MOFs
3.1. MOF Structure and Properties
3.2. MOF Preparation
3.2.1. Conventional Methods
3.2.2. Microwave Synthesis
3.2.3. Sonochemical Synthesis
3.2.4. Electrochemical Synthesis
3.3. Properties of MOF-Immobilized Enzymes
3.3.1. Enzyme Stability
3.3.2. Enzyme Recovery and Reusability
3.3.3. Allosteric Effect
3.4. Lipase Immobilization on MOFs
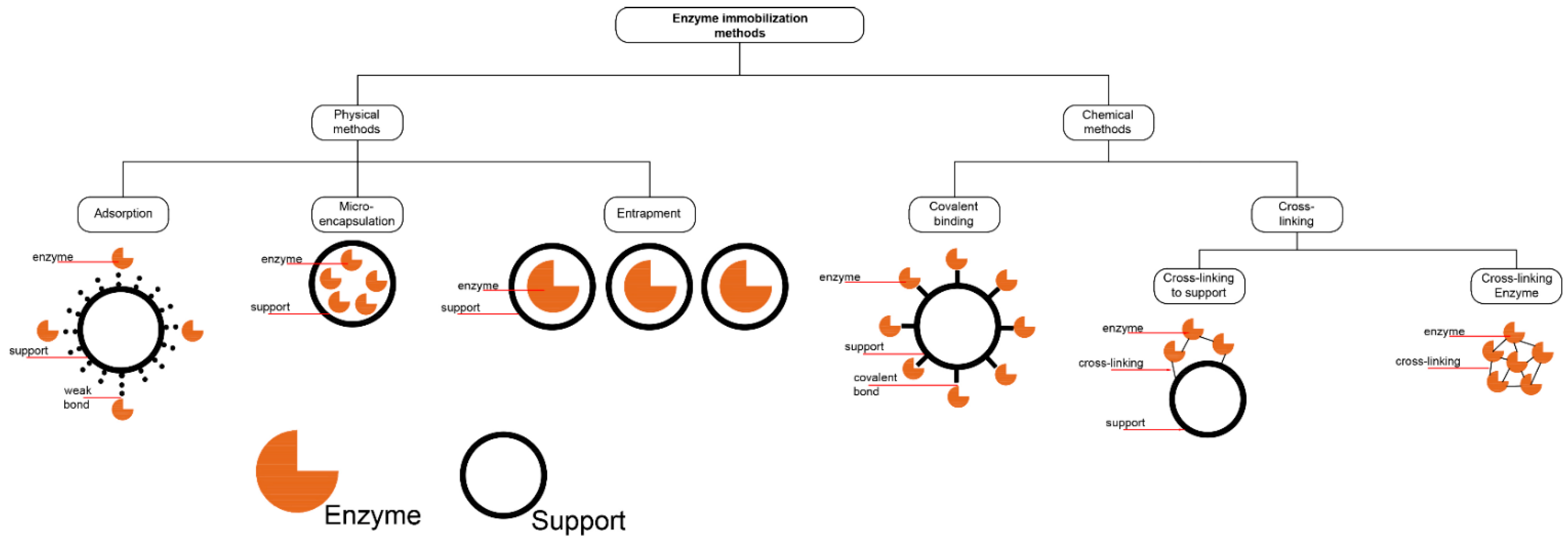
3.4.1. Physical Adsorption
3.4.2. Covalent Binding
3.4.3. Cross-Linking
3.4.4. Entrapment/Encapsulation
4. ILs in Biodiesel Production
4.1. Acidic ILs
4.1.1. Acidic ILs as Catalyst Supports
4.1.2. Acidic ILs as Sole Catalysts
4.1.3. Polymeric Acidic ILs
4.1.4. Immobilized Acidic ILs
4.2. Basic ILs
4.3. Limitations of ILs
5. Use of Immobilized Enzyme in Biodiesel Production
6. IL–MOF Systems for Biodiesel Production
7. Cost Analysis and Viability of Immobilized Enzymes
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Basha, S.A.; Gopal, K.R.; Jebaraj, S. A review on biodiesel production, combustion, emissions and performance. Renew. Sustain. Energy Rev. 2009, 13, 1628–1634. [Google Scholar] [CrossRef]
- Casiello, M.; Catucci, L.; Fracassi, F.; Fusco, C.; Laurenza, A.G.; Di Bitonto, L.; Pastore, C.; D’Accolti, L.; Nacci, A. ZnO/Ionic Liquid Catalyzed Biodiesel Production from Renewable and Waste Lipids as Feedstocks. Catalysts 2019, 9, 71. [Google Scholar] [CrossRef]
- Pradhan, P.; Mahajani, S.M.; Arora, A. Production and utilization of fuel pellets from biomass: A review. Fuel Process. Technol. 2018, 181, 215–232. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Mishra, V.K.; Goswami, R. A review of production, properties and advantages of biodiesel. Biofuels 2018, 9, 273–289. [Google Scholar] [CrossRef]
- Masri, A.N.; Mutalib, M.A.; Aminuddin, N.F.; Lévêque, J. Novel SO3H-functionalized dicationic ionic liquids—A comparative study for esterification reaction by ultrasound cavitation and mechanical stirring for biodiesel production. Sep. Purif. Technol. 2018, 196, 106–114. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Gupta, V.K.; Manikanta, A.; Mishra, K.; Singh, S.; Singh, S.; Ramteke, P.W.; Mishra, P.K. Recent development on sustainable biodiesel production using sewage sludge. 3 Biotech. 2018, 8, 245. [Google Scholar] [CrossRef]
- Lam, H.L.; Varbanov, P.S.; Klemeš, J.J. Regional renewable energy and resource planning. Appl. Energy 2011, 88, 545–550. [Google Scholar] [CrossRef]
- Yun, H.; Wang, M.; Feng, W.; Tan, T. Process simulation and energy optimization of the enzyme-catalyzed biodiesel production. Energy 2013, 54, 84–96. [Google Scholar] [CrossRef]
- Alhassan, F.H.; Rashid, U.; Taufiq-Yap, Y. Synthesis of waste cooking oil-based biodiesel via effectual recyclable bi-functional Fe2O3MnOSO42−/ZrO2 nanoparticle solid catalyst. Fuel 2015, 142, 38–45. [Google Scholar] [CrossRef]
- Yusuf, N.; Kamarudin, S.; Yaakub, Z. Overview on the current trends in biodiesel production. Energy Convers. Manag. 2011, 52, 2741–2751. [Google Scholar] [CrossRef]
- Kim, D.-S.; Hanifzadeh, M.; Kumar, A. Trend of biodiesel feedstock and its impact on biodiesel emission characteristics. Environ. Prog. Sustain. Energy 2018, 37, 7–19. [Google Scholar] [CrossRef]
- Jeevahan, J.; Mageshwaran, G.; Joseph, G.B.; Raj, R.D.; Kannan, R.T. Various strategies for reducing No x emissions of biodiesel fuel used in conventional diesel engines: A review. Chem. Eng. Commun. 2017, 204, 1202–1223. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Yang, T.; Yue, C.; Pu, Q.; Zhang, Y. Facile synthesis of polyoxometalates tethered to post Fe-BTC frameworks for esterification of free fatty acids to biodiesel. RSC Adv. 2019, 9, 8113–8120. [Google Scholar] [CrossRef]
- Adnan, M.; Li, K.; Xu, L.; Yan, Y. X-shaped zif-8 for immobilization rhizomucor miehei lipase via encapsulation and its application toward biodiesel production. Catalysts 2018, 8, 96. [Google Scholar] [CrossRef]
- Šánek, L.; Pecha, J.; Kolomazník, K.; Bařinová, M. Pilot-scale production of biodiesel from waste fats and oils using tetramethylammonium hydroxide. Waste Manag. 2016, 48, 630–637. [Google Scholar] [CrossRef]
- Meng, X.; Yang, J.; Xu, X.; Zhang, L.; Nie, Q.; Xian, M. Biodiesel production from oleaginous microorganisms. Renew. Energy 2009, 34, 1–5. [Google Scholar] [CrossRef]
- Han, M.; Li, Y.; Gu, Z.; Shi, H.; Chen, C.; Wang, Q.; Wan, H.; Guan, G. Immobilization of thiol-functionalized ionic liquids onto the surface of MIL-101 (Cr) frameworks by SCr coordination bond for biodiesel production. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 593–600. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Dalai, A.K. Waste Cooking OilAn Economical Source for Biodiesel: A Review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z. Structure Evolution of Synthetic Amino Acids-Derived Basic Ionic Liquids for Catalytic Production of Biodiesel. ACS Sustain. Chem. Eng. 2016, 5, 1237–1247. [Google Scholar] [CrossRef]
- Anwar, M.; Rasul, M.G.; Ashwath, N. Production optimization and quality assessment of papaya (Carica papaya) biodiesel with response surface methodology. Energy Convers. Manag. 2018, 156, 103–112. [Google Scholar] [CrossRef]
- Cho, H.U.; Park, J.M. Biodiesel production by various oleaginous microorganisms from organic wastes. Bioresour. Technol. 2018, 256, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, S.; Cheng, J.; Lou, W. Acidic ionic liquid-catalyzed esterification of oleic acid for biodiesel synthesis. Chin. J. Catal. 2014, 35, 396–406. [Google Scholar] [CrossRef]
- Deshmane, C.A.; Wright, M.W.; Lachgar, A.; Rohlfing, M.; Liu, Z.; Le, J.; Hanson, B.E. A comparative study of solid carbon acid catalysts for the esterification of free fatty acids for biodiesel production. Evidence for the leaching of colloidal carbon. Bioresour. Technol. 2013, 147, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.F.; Wilson, K. Recent developments in heterogeneous catalysis for the sustainable production of biodiesel. Catal. Today 2015, 242, 3–18. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Mazaheri, H. A review on novel processes of biodiesel production from waste cooking oil. Appl. Energy 2013, 104, 683–710. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Neumann, K.; Werth, K.; Martín, A.; Górak, A. Biodiesel production from waste cooking oils through esterification: Catalyst screening, chemical equilibrium and reaction kinetics. Chem. Eng. Res. Des. 2016, 107, 52–62. [Google Scholar] [CrossRef]
- Wu, S.; Song, L.; Sommerfeld, M.; Hu, Q.; Chen, W. Optimization of an effective method for the conversion of crude algal lipids into biodiesel. Fuel 2017, 197, 467–473. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, P.; Ansari, F.A.; Singh, B.; Bux, F. Biodiesel synthesis from microalgal lipids using tungstated zirconia as a heterogeneous acid catalyst and its comparison with homogeneous acid and enzyme catalysts. Fuel 2017, 187, 180–188. [Google Scholar] [CrossRef]
- Ramos, L.P.; da Silva, F.R.; Mangrich, A.S.; Cordeiro, C.S. Tecnologias de produção de biodiesel. Rev. Virtual Química 2011, 3, 385–405. [Google Scholar]
- Demirbas, A. Importance of biodiesel as transportation fuel. Energy Policy 2007, 35, 4661–4670. [Google Scholar] [CrossRef]
- Sani, Y.M.; Aziz, A.R.A.; Daud, W.M.A.W. Biodiesel Feedstock and Production Technologies: Successes, Challenges and Prospects; INTECH Open Access Publisher: London, UK, 2012. [Google Scholar]
- Tangy, A.; Pulidindi, I.N.; Gedanken, A. SiO2 Beads Decorated with SrO Nanoparticles for Biodiesel Production from Waste Cooking Oil Using Microwave Irradiation. Energy Fuels 2016, 30, 3151–3160. [Google Scholar] [CrossRef]
- Gamba, M.; Lapis, A.A.M.; Dupont, J. Supported Ionic Liquid Enzymatic Catalysis for the Production of Biodiesel. Adv. Synth. Catal. 2008, 350, 160–164. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, F.; Ma, P.; Zheng, W.; Zhao, Y.; Chen, H. Biodiesel Production by Catalytic Esterification of Oleic Acid over Copper (II)–Alginate Complexes. J. Oleo Sci. 2017, 66, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Veljković, V.B.; Stamenković, O.S.; Tasić, M.B. The wastewater treatment in the biodiesel production with alkali-catalyzed transesterification. Renew. Sustain. Energy Rev. 2014, 32, 40–60. [Google Scholar] [CrossRef]
- Xu, Y.; Hanna, M. Synthesis and characterization of hazelnut oil-based biodiesel. Ind. Crops Prod. 2009, 29, 473–479. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, D.-K.; Lee, J.-S. Esterification of free fatty acids using water-tolerable Amberlyst as a heterogeneous catalyst. Bioresour. Technol. 2010, 101, S62–S65. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2019, 3, 2–23. [Google Scholar] [CrossRef]
- Math, M.C.; Chandrashekhara, K.N. Optimization of Alkali Catalyzed Transesterification of Safflower Oil for Production of Biodiesel. J. Eng. 2016, 2016, 1–7. [Google Scholar] [CrossRef][Green Version]
- Hossain, A.S. Alkaline and Acid Catalyzed Transesterification Bioprocess in Biodiesel Preparation from Fresh Water Algae. Asian J. Biochem. 2015, 10, 205–213. [Google Scholar] [CrossRef][Green Version]
- Zheng, Y.; Zhiming, P.; Xinchen, W. Advances in photocatalysis in China. Chin. J. Catal. 2013, 34, 524–535. [Google Scholar] [CrossRef]
- Wan, H.; Chen, C.; Wu, Z.; Que, Y.; Feng, Y.; Wang, W.; Wang, L.; Guan, G.; Liu, X. Encapsulation of heteropolyanion-based ionic liquid within the metal–organic framework MIL-100 (Fe) for biodiesel production. ChemCatChem 2015, 7, 441–449. [Google Scholar] [CrossRef]
- Ishak, Z.I.; Sairi, N.A.; Alias, Y.; Aroua, M.K.T.; Yusoff, R. A review of ionic liquids as catalysts for transesterification reactions of biodiesel and glycerol carbonate production. Catal. Rev. 2017, 59, 44–93. [Google Scholar] [CrossRef]
- Fan, P.; Xing, S.; Wang, J.; Fu, J.; Yang, L.; Yang, G.; Miao, C.; Lv, P. Sulfonated imidazolium ionic liquid-catalyzed transesterification for biodiesel synthesis. Fuel 2017, 188, 483–488. [Google Scholar] [CrossRef]
- Rafiee, E.; Mirnezami, F. Temperature regulated Brønsted acidic ionic liquid-catalyze esterification of oleic acid for biodiesel application. J. Mol. Struct. 2017, 1130, 296–302. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Z.; Wang, R. Functionalized Metal–organic Framework Catalysts for Sustainable Biomass Valorization. Adv. Polym. Technol. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, B.; Mutanda, T.; Permaul, K.; Bux, F. Advances in synthesis of biodiesel via enzyme catalysis: Novel and sustainable approaches. Renew. Sustain. Energy Rev. 2015, 41, 1447–1464. [Google Scholar] [CrossRef]
- Poppe, J.K.; Garcia-Galan, C.; Matte, C.R.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Optimization of synthesis of fatty acid methyl esters catalyzed by lipase B from Candida antarctica immobilized on hydrophobic supports. J. Mol. Catal. B Enzym. 2013, 94, 51–56. [Google Scholar] [CrossRef]
- Rana, Q.u.a.; Rehman, M.L.U.; Irfan, M.; Ahmed, S.; Hasan, F.; Shah, A.A.; Khan, S.; Badshah, M. Lipolytic bacterial strains mediated transesterification of non-edible plant oils for generation of high quality biodiesel. J. Biosci. Bioeng. 2019, 127, 609–617. [Google Scholar] [CrossRef]
- Guil-Laynez, J.L.; Guil-Guerrero, J.L.; Guil-Laynez, Á. Bioprospecting for seed oils in tropical areas for biodiesel production. Ind. Crops Prod. 2019, 128, 504–511. [Google Scholar] [CrossRef]
- Sun, S.; Li, K. Biodiesel production from phoenix tree seed oil catalyzed by liquid lipozyme TL100L. Renew. Energy 2020, 151, 152–160. [Google Scholar] [CrossRef]
- Doğan, T.H. The testing of the effects of cooking conditions on the quality of biodiesel produced from waste cooking oils. Renew. Energy 2016, 94, 466–473. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.; Li, D.; Yang, B.; Wang, Y. One-step synthesis of high-yield biodiesel from waste cooking oils by a novel and highly methanol-tolerant immobilized lipase. Bioresour. Technol. 2017, 235, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Mannu, A.; Ferro, M.; Dugoni, G.C.; Panzeri, W.; Petretto, G.L.; Urgeghe, P.; Mele, A. Improving the recycling technology of waste cooking oils: Chemical fingerprint as tool for non-biodiesel application. Waste Manag. 2019, 96, 1–8. [Google Scholar] [CrossRef]
- Pirouzmand, M.; Anakhatoon, M.M.; Ghasemi, Z. One-step biodiesel production from waste cooking oils over metal incorporated MCM-41; positive effect of template. Fuel 2018, 216, 296–300. [Google Scholar] [CrossRef]
- Gude, V.G.; Grant, G.E. Biodiesel from waste cooking oils via direct sonication. Appl. Energy 2013, 109, 135–144. [Google Scholar] [CrossRef]
- Eze, V.C.; Phan, A.N.; Harvey, A.P. Intensified one-step biodiesel production from high water and free fatty acid waste cooking oils. Fuel 2018, 220, 567–574. [Google Scholar] [CrossRef]
- Hariprasath, P.; Vijayakumar, V.; Selvamani, S.T.; Vigneshwar, M.; Palanikumar, K. Some Studies on Waste Animal Tallow Biodiesel Produced by Modified Transesterification Method Using Heterogeneous Catalyst. Mater. Today Proc. 2019, 16, 1271–1278. [Google Scholar] [CrossRef]
- Öner, C.; Altun, Ş. Biodiesel production from inedible animal tallow and an experimental investigation of its use as alternative fuel in a direct injection diesel engine. Appl. Energy 2009, 86, 2114–2120. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Oh, J.-H.; Herring, J.L. Fast biodiesel production from beef tallow with radio frequency heating. Renew. Energy 2011, 36, 1003–1007. [Google Scholar] [CrossRef]
- da Cunha, M.E.; Krause, L.C.; Moraes, M.S.A.; Faccini, C.S.; Jacques, R.A.; Almeida, S.R.; Rodrigues, M.R.A.; Caramão, E.B. Beef tallow biodiesel produced in a pilot scale. Fuel Process. Technol. 2009, 90, 570–575. [Google Scholar] [CrossRef]
- Adewale, P.; Dumont, M.-J.; Ngadi, M. Enzyme-catalyzed synthesis and kinetics of ultrasonic-assisted biodiesel production from waste tallow. Ultrason. Sonochem. 2015, 27, 1–9. [Google Scholar] [CrossRef]
- Chung, K.-H.; Kim, J.; Lee, K.-Y. Biodiesel production by transesterification of duck tallow with methanol on alkali catalysts. Biomass Bioenergy 2009, 33, 155–158. [Google Scholar] [CrossRef]
- Bhatti, H.; Hanif, M.; Qasim, M. Ataurrehman Biodiesel production from waste tallow. Fuel 2008, 87, 2961–2966. [Google Scholar] [CrossRef]
- Vicente, G.; Martinez, M.; Aracil, J. A Comparative Study of Vegetable Oils for Biodiesel Production in Spain. Energy Fuels 2006, 20, 394–398. [Google Scholar] [CrossRef]
- Watanabe, Y.; Shimada, Y.; Sugihara, A.; Tominaga, Y. Conversion of degummed soybean oil to biodiesel fuel with immobilized Candida antarctica lipase. J. Mol. Catal. B Enzym. 2002, 17, 151–155. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Abdul Aziz, A.R.; Sulaiman, N.M.N. The effects of water on biodiesel production and refining technologies: A review. Renew. Sustain. Energy Rev. 2012, 16, 3456–3470. [Google Scholar] [CrossRef]
- Khan, F.I.; Lan, D.; Durrani, R.; Huan, W.; Zhao, Z.; Wang, Y. The Lid Domain in Lipases: Structural and Functional Determinant of Enzymatic Properties. Front. Bioeng. Biotechnol. 2017, 5, 16. [Google Scholar] [CrossRef]
- Lotti, M.; Pleiss, J.; Valero, F.; Ferrer, P. Effects of methanol on lipases: Molecular, kinetic and process issues in the production of biodiesel. Biotechnol. J. 2015, 10, 22–30. [Google Scholar] [CrossRef]
- Shimada, Y.; Watanabe, Y.; Sugihara, A.; Tominaga, Y. Enzymatic alcoholysis for biodiesel fuel production and application of the reaction to oil processing. J. Mol. Catal. B Enzym. 2002, 17, 133–142. [Google Scholar] [CrossRef]
- Soumanou, M.M.; Bornscheuer, U.T. Improvement in lipase-catalyzed synthesis of fatty acid methyl esters from sunflower oil. Enzym. Microb. Technol. 2003, 33, 97–103. [Google Scholar] [CrossRef]
- Chen, G.; Ying, M.; Li, W. Enzymatic conversion of waste cooking oils into alternative fuel—Biodiesel. Appl. Biochem. Biotechnol. 2006, 132, 911–921. [Google Scholar] [CrossRef]
- Lu, J.; Nie, K.; Xie, F.; Wang, F.; Tan, T. Enzymatic synthesis of fatty acid methyl esters from lard with immobilized Candida sp. 99-125. Process. Biochem. 2007, 42, 1367–1370. [Google Scholar] [CrossRef]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef]
- Batista, A.; Silva, T.; Vieira, A.; de Oliveira, M. Biotechnological Applications of Lipases in Biodiesel Production. Fungal Enzymes. 2013. [CrossRef]
- Mat Radzi, S.; Basri, M.; Bakar Salleh, A.; Ariff, A.; Mohammad, R.; Abdul Rahman, M.B.; Abdul Rahman, R.N.Z.R. High performance enzymatic synthesis of oleyl oleate using immobilised lipase from Candida antartica. Electron. J. Biotechnol. 2005, 8, 291–298. [Google Scholar] [CrossRef]
- Klibanov, A.M. Improving enzymes by using them in organic solvents. Nat. Cell Biol. 2001, 409, 241–246. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P. Comparative analysis of effect of methanol and ethanol on Karanja biodiesel production and its optimisation. Fuel 2016, 180, 164–174. [Google Scholar] [CrossRef]
- Almeida, L.; Barbosa, A.S.; Fricks, A.T.; Freitas, L.; Lima, Á.S.; Soares, C.M. Use of conventional or non-conventional treatments of biochar for lipase immobilization. Process. Biochem. 2017, 61, 124–129. [Google Scholar] [CrossRef]
- Ycel, S.; Terziolu, P.; zime, D. Lipase Applications in Biodiesel Production. In Biodiesel–Feedstocks, Production and Applications; INTECHOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Rashid, U. Supported solid and heteropoly acid catalysts for production of biodiesel. Catal. Rev. 2017, 59, 165–188. [Google Scholar] [CrossRef]
- Corma, A.; García, H.; Llabrés i Xamena, F.X. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.J.; Peters, A.W.; Vermeulen, N.A.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Best Practices for the Synthesis, Activation, and Characterization of Metal–Organic Frameworks. Chem. Mater. 2017, 29, 26–39. [Google Scholar] [CrossRef]
- Bobbitt, N.S.; Mendonca, M.L.; Howarth, A.J.; Islamoglu, T.; Hupp, J.T.; Farha, O.K.; Snurr, R.Q. Metal–organic frameworks for the removal of toxic industrial chemicals and chemical warfare agents. Chem. Soc. Rev. 2017, 46, 3357–3385. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Vilela, S.M.F.; Tomé, J.P.C.; Paz, F.A.A. Multifunctional metal–organic frameworks: From academia to industrial applications. Chem. Soc. Rev. 2015, 44, 6774–6803. [Google Scholar] [CrossRef]
- Van de Voorde, B.; Bueken, B.; Denayer, J.; De Vos, D. Adsorptive separation on metal–organic frameworks in the liquid phase. Chem. Soc. Rev. 2014, 43, 5766–5788. [Google Scholar] [CrossRef]
- Giménez-Marqués, M.; Hidalgo, T.; Serre, C.; Horcajada, P. Nanostructured metal–organic frameworks and their bio-related applications. Co-ord. Chem. Rev. 2016, 307, 342–360. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.-Q.; Jiang, H.-L.; Sun, L.-B. Metal–Organic Frameworks for Heterogeneous Basic Catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef]
- Adil, K.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurin, G.; Eddaoudi, M. Gas/vapour separation using ultra-microporous metal–organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. [Google Scholar] [CrossRef]
- Yamada, T.; Sadakiyo, M.; Shigematsu, A.; Kitagawa, H. Proton-conductive metal–organic frameworks. Bull. Chem. Soc. Jpn. 2015, 89, 1–10. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, H.; Vardhan, H.; Aguila, B.; Zhong, C.; Perman, J.A.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Facile Approach to Graft Ionic Liquid into MOF for Improving the Efficiency of CO2 Chemical Fixation. ACS Appl. Mater. Interfaces 2018, 10, 27124–27130. [Google Scholar] [CrossRef]
- Cohen, S.M. Postsynthetic Methods for the Functionalization of Metal–Organic Frameworks. Chem. Rev. 2011, 112, 970–1000. [Google Scholar] [CrossRef]
- Abd El Rahman, S.K.; Hassan, H.M.; El-Shall, M.S. Metal–organic frameworks with high tungstophosphoric acid loading as heterogeneous acid catalysts. Appl. Catal. A Gen. 2014, 487, 110–118. [Google Scholar]
- Zhang, Y.-B.; Furukawa, H.; Ko, N.; Nie, W.; Park, H.J.; Okajima, S.; Cordova, K.E.; Deng, H.; Kim, J.; Yaghi, O. Introduction of Functionality, Selection of Topology, and Enhancement of Gas Adsorption in Multivariate Metal–Organic Framework-177. J. Am. Chem. Soc. 2015, 137, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar]
- Nozari, V.; Keskin, S.; Uzun, A. Toward Rational Design of Ionic Liquid/Metal–Organic Framework Composites: Effects of Interionic Interaction Energy. ACS Omega 2017, 2, 6613–6618. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Li, N.; Zhong, X.; Jiang, Y. Metal–organic Frameworks: A Potential Platform for Enzyme Immobilization and Related Applications. Front. Bioeng. Biotechnol. 2020, 8, 695. [Google Scholar] [CrossRef]
- Pangestu, T.; Kurniawan, Y.; Soetaredjo, F.E.; Santoso, S.P.; Irawaty, W.; Yuliana, M.; Hartono, S.B.; Ismadji, S. The synthesis of biodiesel using copper based metal–organic framework as a catalyst. J. Environ. Chem. Eng. 2019, 7, 103277. [Google Scholar] [CrossRef]
- Khan, N.A.; Jhung, S.H. Synthesis of metal–organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Co-ord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, D.; Luo, Q.; Wang, J.; Deng, T.; Zhang, Y.; Ma, P. Efficient biodiesel production from oleic acid using metal–organic framework encapsulated Zr-doped polyoxometalate nano-hybrids. RSC Adv. 2020, 10, 8766–8772. [Google Scholar] [CrossRef]
- AbdelSalam, H.; El-Maghrabi, H.; Zahran, F.; Zaki, T. Microwave-assisted production of biodiesel using metal–organic framework Mg3(bdc)3(H2O)2. Korean J. Chem. Eng. 2020, 37, 670–676. [Google Scholar] [CrossRef]
- Nikseresht, A.; Daniyali, A.; Ali-Mohammadi, M.; Afzalinia, A.; Mirzaie, A. Ultrasound-assisted biodiesel production by a novel composite of Fe(III)-based MOF and phosphotangestic acid as efficient and reusable catalyst. Ultrason. Sonochem. 2017, 37, 203–207. [Google Scholar] [CrossRef]
- Lestari, W.W.; Nugraha, R.E.; Winarni, I.D.; Adreane, M.; Rahmawati, F. Optimization on electrochemical synthesis of HKUST-1 as candidate catalytic material for Green diesel production. AIP Conf. Proc. 2016, 1725, 20038. [Google Scholar] [CrossRef]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.-C. Enzyme–MOF (metal–organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef] [PubMed]
- Vaitsis, C.; Sourkouni, G.; Argirusis, C. Metal Organic Frameworks (MOFs) and ultrasound: A review. Ultrason. Sonochem. 2019, 52, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Bajpai, P.K.; Arora, C. A review on metal–organic framework: Synthesis, properties and application. Charact. Appl. Nanomater. 2018, 2, 551. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Encapsulation of lipase within metal–organic framework (MOF) with enhanced activity intensified under ultrasound. Enzym. Microb. Technol. 2018, 108, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, Q.; Li, B.-G.; Zhu, S. Engineering Elastic ZIF-8-Sponges for Oil-Water Separation. Adv. Mater. Interfaces 2017, 4, 1700560. [Google Scholar] [CrossRef]
- Nadar, S.; Rathod, V.K. One pot synthesis of α-amylase metal organic framework (MOF)-sponge via dip-coating technique. Int. J. Biol. Macromol. 2019, 138, 1035–1043. [Google Scholar] [CrossRef]
- Feng, Y.; Yao, J. Design of Melamine Sponge-Based Three-Dimensional Porous Materials toward Applications. Ind. Eng. Chem. Res. 2018, 57, 7322–7330. [Google Scholar] [CrossRef]
- Valizadeh, B.; Nguyen, T.N.; Stylianou, K.C. Shape engineering of metal–organic frameworks. Polyhedron 2018, 145, 1–15. [Google Scholar] [CrossRef]
- Sijbesma, R.P.; Nolte, R.J.M. A molecular clip with allosteric binding properties. J. Am. Chem. Soc. 1991, 113, 6695–6696. [Google Scholar] [CrossRef]
- Wang, L.-B.; Wang, Y.-C.; He, R.; Zhuang, A.; Wang, X.; Zeng, J.; Hou, J.G. A New Nanobiocatalytic System Based on Allosteric Effect with Dramatically Enhanced Enzymatic Performance. J. Am. Chem. Soc. 2013, 135, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Lu, J.; Nie, K.; Deng, L.; Wang, F. Biodiesel production with immobilized lipase: A review. Biotechnol. Adv. 2010, 28, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.-G.; Xu, T.; Li, Z.-Q.; Wang, W.; Wu, Y.; Jiang, X.; Tian, X.-Y.; Zhang, L.-D. Hierarchically Micro- and Mesoporous Metal–organic Frameworks with Tunable Porosity. Angew. Chem. Int. Ed. 2008, 47, 9487–9491. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Modica, J.A.; Howarth, A.J.; Vargas, L.E.; Moghadam, P.Z.; Snurr, R.Q.; Mrksich, M.; Hupp, J.T.; Farha, O.K. Toward Design Rules for Enzyme Immobilization in Hierarchical Mesoporous Metal–organic Frameworks. Chem 2016, 1, 154–169. [Google Scholar] [CrossRef]
- Jegannathan, K.R.; Abang, S.; Poncelet, D.; Chan, E.S.; Ravindra, P. Production of Biodiesel Using Immobilized Lipase—A Critical Review. Crit. Rev. Biotechnol. 2008, 28, 253–264. [Google Scholar] [CrossRef]
- Jung, S.; Kim, Y.; Kim, S.-J.; Kwon, T.-H.; Huh, S.; Park, S. Bio-functionalization of metal–organic frameworks by covalent protein conjugation. Chem. Commun. 2011, 47, 2904–2906. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Kim, M. An Overview of Techniques in Enzyme Immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163. [Google Scholar] [CrossRef]
- Nobakht, N.; Faramarzi, M.A.; Shafiee, A.; Khoobi, M.; Rafiee, E. Polyoxometalate-metal organic framework-lipase: An efficient green catalyst for synthesis of benzyl cinnamate by enzymatic esterification of cinnamic acid. Int. J. Biol. Macromol. 2018, 113, 8–19. [Google Scholar] [CrossRef]
- Andreani, L.; Rocha, J.D. Use of ionic liquids in biodiesel production: A review. Braz. J. Chem. Eng. 2012, 29, 1–13. [Google Scholar] [CrossRef]
- Ratti, R. Ionic Liquids: Synthesis and Applications in Catalysis. Adv. Chem. 2014, 2014, 729842. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Lo, W.-S.; Huang, Y.-D.; Si, X.; Liao, F.-S.; Lin, S.-W.; Williams, B.P.; Sun, T.-Q.; Lin, H.-W.; An, Y.; et al. Probing Interactions between Metal–Organic Frameworks and Freestanding Enzymes in a Hollow Structure. Nano Lett. 2020, 20, 6630–6635. [Google Scholar] [CrossRef]
- Shieh, F.-K.; Wang, S.-C.; Yen, C.-I.; Wu, C.-C.; Dutta, S.; Chou, L.-Y.; Morabito, J.V.; Hu, P.; Hsu, M.H.; Wu, K.C.-W.; et al. Imparting Functionality to Biocatalysts via Embedding Enzymes into Nanoporous Materials by ade NovoApproach: Size-Selective Sheltering of Catalase in Metal–Organic Framework Microcrystals. J. Am. Chem. Soc. 2015, 137, 4276–4279. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.-S.; Lo, W.-S.; Hsu, Y.-S.; Wu, C.-C.; Wang, S.-C.; Shieh, F.-K.; Morabito, J.V.; Chou, L.-Y.; Wu, K.C.-W.; Tsung, C.-K. Shielding against Unfolding by Embedding Enzymes in Metal–Organic Frameworks via a de Novo Approach. J. Am. Chem. Soc. 2017, 139, 6530–6533. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.-H.; Wu, S.-H.; Huang, Y.-D.; Lo, W.-S.; Williams, B.P.; Chen, S.-Y.; Yang, H.-C.; Hsu, Y.-S.; Lin, Z.-Y.; Chen, X.-H.; et al. Rapid mechanochemical encapsulation of biocatalysts into robust metal–organic frameworks. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Z.; Wang, F.; Stiles, A.R.; Guo, C. Ionic liquids for biofuel production: Opportunities and challenges. Appl. Energy 2012, 92, 406–414. [Google Scholar] [CrossRef]
- Guo, F.; Fang, Z.; Tian, X.; Long, Y.-D.; Jiang, L.-Q. One-step production of biodiesel from Jatropha oil with high-acid value in ionic liquids. Bioresour. Technol. 2011, 102, 6469–6472. [Google Scholar] [CrossRef]
- Zhao, H.; Baker, G.A. Ionic liquids and deep eutectic solvents for biodiesel synthesis: A review. J. Chem. Technol. Biotechnol. 2013, 88, 3–12. [Google Scholar] [CrossRef]
- Troter, D.Z.; Todorović, Z.B.; Đokić-Stojanović, D.R.; Stamenković, O.S.; Veljković, V.B. Application of ionic liquids and deep eutectic solvents in biodiesel production: A review. Renew. Sustain. Energy Rev. 2016, 61, 473–500. [Google Scholar] [CrossRef]
- Welton, T. Ionic liquids in catalysis. Coord. Chem. Rev. 2004, 248, 2459–2477. [Google Scholar] [CrossRef]
- Ghiaci, M.; Aghabarari, B.; Habibollahi, S.; Gil, A. Highly efficient Brønsted acidic ionic liquid-based catalysts for biodiesel synthesis from vegetable oils. Bioresour. Technol. 2011, 102, 1200–1204. [Google Scholar] [CrossRef]
- Hallett, J.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Yan, P.; Xu, Z.; Zhang, C.; Liu, X.; Xu, W.; Zhang, Z.-C. Fractionation of lignin from eucalyptus bark using amine-sulfonate functionalized ionic liquids. Green Chem. 2015, 17, 4913–4920. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Alves, M.B.; Lapis, A.A.; Nachtigall, F.M.; Eberlin, M.N.; Dupont, J.; Suarez, P.A.Z. 1-n-Butyl-3-methylimidazolium tetrachloro-indate (BMI⋅ InCl4) as a media for the synthesis of biodiesel from vegetable oils. J. Catal. 2007, 249, 154–161. [Google Scholar]
- Alegría, A.; de Arriba, Á.L.F.; Morán, J.R.; Cuellar, J. Biodiesel production using 4-dodecylbenzenesulfonic acid as catalyst. Appl. Catal. B Environ. 2014, 160, 743–756. [Google Scholar] [CrossRef]
- Alegría, A.; Cuellar, J. Esterification of oleic acid for biodiesel production catalyzed by 4-dodecylbenzenesulfonic acid. Appl. Catal. B Environ. 2015, 179, 530–541. [Google Scholar] [CrossRef]
- Olkiewicz, M.; Plechkova, N.V.; Earle, M.J.; Fabregat, A.; Stuber, F.; Fortuny, A.; Font, J.; Bengoa, C. Biodiesel production from sewage sludge lipids catalysed by Brønsted acidic ionic liquids. Appl. Catal. B Environ. 2016, 181, 738–746. [Google Scholar] [CrossRef]
- Ullah, H.; Wilfred, C.D.; Shaharun, M. Ionic liquid-based extraction and separation trends of bioactive compounds from plant biomass. Sep. Sci. Technol. 2019, 54, 559–579. [Google Scholar] [CrossRef]
- Laus, G.; Bentivoglio, G.; Schottenberger, H.; Kahlenberg, V.; Kopacka, H.; Röder, T.; Sixta, H. Ionic liquids: Current developments, potential and drawbacks for industrial applications. Lenzing. Ber. 2005, 84, 71–85. [Google Scholar]
- Sharikh, A.M.; Sulaiman, S.; Azmi, A. A Review on Multiple Functions of Ionic Liquid in Biodiesel Production. J. Adv. Res. Fluid Mech. Therm. Sci. 2017, 39, 26–35. [Google Scholar]
- Sahoo, S.; Kumar, P.; Lefebvre, F.; Halligudi, S. Oxidative kinetic resolution of alcohols using chiral Mn–salen complex immobilized onto ionic liquid modified silica. Appl. Catal. A Gen. 2009, 354, 17–25. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Chen, J. Synthesis of N-Methylimidazolium Functionalized Strongly Basic Anion Exchange Resins for Adsorption of Cr(VI). Ind. Eng. Chem. Res. 2009, 48, 3261–3267. [Google Scholar] [CrossRef]
- Wang, H.; Meng, X.; Zhao, G.; Zhang, S. Isobutane/butene alkylation catalyzed by ionic liquids: A more sustainable process for clean oil production. Green Chem. 2017, 19, 1462–1489. [Google Scholar] [CrossRef]
- Dang, D.T.; Ha, S.H.; Lee, S.-M.; Chang, W.-J.; Koo, Y.-M. Enhanced activity and stability of ionic liquid-pretreated lipase. J. Mol. Catal. B Enzym. 2007, 45, 118–121. [Google Scholar] [CrossRef]
- Lozano, P.; De Diego, T.; Carrie, D.; Vaultier, M.; Iborra, J.L. Over-stabilization of Candida antarctica lipase B by ionic liquids in ester synthesis. Biotechnol. Lett. 2001, 23, 1529–1533. [Google Scholar] [CrossRef]
- Ha, S.H.; Lan, M.N.; Lee, S.H.; Hwang, S.M.; Koo, Y.-M. Lipase-catalyzed biodiesel production from soybean oil in ionic liquids. Enzym. Microb. Technol. 2007, 41, 480–483. [Google Scholar] [CrossRef]
- Lai, J.-Q.; Hu, Z.-L.; Wang, P.-W.; Yang, Z. Enzymatic production of microalgal biodiesel in ionic liquid [BMIm][PF6]. Fuel 2012, 95, 329–333. [Google Scholar] [CrossRef]
- Abdi, Y.; Shomal, R.; Taher, H.; Al-Zuhair, S. Improving the reusability of an immobilized lipase-ionic liquid system for biodiesel production. Biofuels 2019, 10, 635–641. [Google Scholar] [CrossRef]
- Carvalho, N.B.; Vidal, B.T.; Barbosa, A.S.; Pereira, M.M.; Mattedi, S.; Freitas, L.; Lima, Á.S.; Soares, C.M.F. Lipase Immobilization on Silica Xerogel Treated with Protic Ionic Liquid and its Application in Biodiesel Production from Different Oils. Int. J. Mol. Sci. 2018, 19, 1829. [Google Scholar] [CrossRef]
- Roman, F.F.; Ribeiro, A.E.; Queiroz, A.; Lenzi, G.G.; Chaves, E.S.; Brito, P. Optimization and kinetic study of biodiesel production through esterification of oleic acid applying ionic liquids as catalysts. Fuel 2019, 239, 1231–1239. [Google Scholar] [CrossRef]
- Ullah, Z.; Bustam, M.A.; Man, Z.; Khan, A.S.; Sarwono, A.; Muhammad, N.; Farooq, M.; Shah, S.N.; Ahmad, P.; Haider, S. Phosphonium-based hydrophobic ionic liquids with fluorous anions for biodiesel production from waste cooking oil. Int. J. Environ. Sci. Technol. 2019, 16, 1269–1276. [Google Scholar] [CrossRef]
- Ding, H.; Ye, W.; Wang, Y.; Wang, X.; Li, L.; Liu, D.; Gui, J.; Song, C.; Ji, N. Process intensification of transesterification for biodiesel production from palm oil: Microwave irradiation on transesterification reaction catalyzed by acidic imidazolium ionic liquids. Energy 2018, 144, 957–967. [Google Scholar] [CrossRef]
- Gholami, A.; Pourfayaz, F.; Maleki, A. Recent Advances of Biodiesel Production Using Ionic Liquids Supported on Nanoporous Materials as Catalysts: A Review. Front. Energy Res. 2020, 8. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Pan, H.; Wang, A.; Souzanchi, S.; Xu, C.; Yang, S. Magnetically recyclable acidic polymeric ionic liquids decorated with hydrophobic regulators as highly efficient and stable catalysts for biodiesel production. Appl. Energy 2018, 223, 416–429. [Google Scholar] [CrossRef]
- Zhen, B.; Li, H.; Jiao, Q.; Li, Y.; Wu, Q.; Zhang, Y. SiW12O40-Based Ionic Liquid Catalysts: Catalytic Esterification of Oleic Acid for Biodiesel Production. Ind. Eng. Chem. Res. 2012, 51, 10374–10380. [Google Scholar] [CrossRef]
- Jianxiang Wu, J.; Gao, Y.; Zhang, W.; Tan, Y.; Tang, A.; Men, Y.; Tang, B. Esterification of cooking oil for biodiesel production using composites Cs2.5H0.5PW12O40/ionic liquids catalysts. Appl. Petrochem. Res. 2014, 4, 305–312. [Google Scholar] [CrossRef]
- Huang, Y. Synthesis of Biodiesel by Phase Transfer Catalysis. Appl. Mech. Mater. 2013, 291–294, 355–358. [Google Scholar] [CrossRef]
- Zimmerman, W.B.; Kokoo, R. Esterification for biodiesel production with a phantom catalyst: Bubble mediated reactive distillation. Appl. Energy 2018, 221, 28–40. [Google Scholar] [CrossRef]
- Fang, D.; Yang, J.; Jiao, C. Dicationic Ionic Liquids as Environmentally Benign Catalysts for Biodiesel Synthesis. ACS Catal. 2010, 1, 42–47. [Google Scholar] [CrossRef]
- Song, H.; Jin, F.; Kang, M.; Chen, J. Novel polymeric acidic ionic liquids as green catalysts for the preparation of polyoxymethylene dimethyl ethers from the acetalation of methylal with trioxane. RSC Adv. 2019, 9, 40662–40669. [Google Scholar] [CrossRef]
- Pan, H.; Li, H.; Zhang, H.; Wang, A.; Yang, S. Acidic ionic liquid-functionalized mesoporous melamine-formaldehyde polymer as heterogeneous catalyst for biodiesel production. Fuel 2019, 239, 886–895. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhen, B.; Li, H.; Feng, Y. Basic ionic liquid as catalyst and surfactant: Green synthesis of quinazolinone in aqueous media. RSC Adv. 2018, 8, 36769–36774. [Google Scholar] [CrossRef]
- Xie, W.; Hu, L.; Yang, X. Basic Ionic Liquid Supported on Mesoporous SBA-15 Silica as an Efficient Heterogeneous Catalyst for Biodiesel Production. Ind. Eng. Chem. Res. 2015, 54, 1505–1512. [Google Scholar] [CrossRef]
- Hosseini, S.; Moradi, G.; Bahrami, K. Synthesis of a novel stabilized basic ionic liquid through immobilization on boehmite nanoparticles: A robust nanocatalyst for biodiesel production from soybean oil. Renew. Energy 2019, 138, 70–78. [Google Scholar] [CrossRef]
- Ren, Q.; Zuo, T.; Pan, J.; Chen, C.; Li, W. Preparation of Biodiesel from Soybean Catalyzed by Basic Ionic Liquids [Hnmm]OH. Mater. 2014, 7, 8012–8023. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Feng, Y.; Wang, G.; Wang, Z.; Bilal, M.; Lv, H.; Jia, S.; Cui, J. Production and use of immobilized lipases in/on nanomaterials: A review from the waste to biodiesel production. Int. J. Biol. Macromol. 2020, 152, 207–222. [Google Scholar] [CrossRef]
- Yu, D.; Tian, L.; Wu, H.; Wang, S.; Wang, Y.; Ma, D.; Fang, X. Ultrasonic irradiation with vibration for biodiesel production from soybean oil by Novozym 435. Process. Biochem. 2010, 45, 519–525. [Google Scholar] [CrossRef]
- Maceiras, R.; Vega, M.; Costa, C.; Ramos, P.; Márquez, M. Effect of methanol content on enzymatic production of biodiesel from waste frying oil. Fuel 2009, 88, 2130–2134. [Google Scholar] [CrossRef]
- De Diego, T.; Manjón, A.; Lozano, P.; Vaultier, M.; Iborra, J.L. An efficient activity ionic liquid-enzyme system for biodiesel production. Green Chem. 2011, 13, 444. [Google Scholar] [CrossRef]
- Rafiei, S.; Tangestaninejad, S.; Horcajada, P.; Moghadam, M.; Mirkhani, V.; Mohammadpoor-Baltork, I.; Kardanpour, R.; Zadehahmadi, F. Efficient biodiesel production using a lipase@ZIF-67 nanobioreactor. Chem. Eng. J. 2018, 334, 1233–1241. [Google Scholar] [CrossRef]
- Adnan, M.; Li, K.; Wang, J.; Xu, L.; Yan, Y. Hierarchical ZIF-8 toward Immobilizing Burkholderia cepacia Lipase for Application in Biodiesel Preparation. Int. J. Mol. Sci. 2018, 19, 1424. [Google Scholar] [CrossRef]
- Corrêa, I.N.D.S.; De Souza, S.L.; Catran, M.; Bernardes, O.L.; Portilho, M.F.; Langone, M.A.P. Enzymatic Biodiesel Synthesis Using a Byproduct Obtained from Palm Oil Refining. Enzym. Res. 2011, 2011, 814507. [Google Scholar] [CrossRef]
- Ruzich, N.I.; Bassi, A.S. Investigation of enzymatic biodiesel production using ionic liquid as a co-solvent. Can. J. Chem. Eng. 2010, 88, 277–282. [Google Scholar] [CrossRef]
- Xie, W.; Huang, M. Enzymatic Production of Biodiesel Using Immobilized Lipase on Core-Shell Structured Fe3O4@MIL-100(Fe) Composites. Catalysts 2019, 9, 850. [Google Scholar] [CrossRef]
- Liu, X.; Liu, P.; An, N.; Liu, C. Metal-organic Frameworks as a Robust Biocatalysis Platform for Enzymatic Production of Biodiesel. E3S Web Conf. 2021, 245, 01023. [Google Scholar] [CrossRef]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal–organic frameworks: Versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Wan, F. Immobilization of polyoxometalate-based sulfonated ionic liquids on UiO-66-2COOH metal-organic frameworks for biodiesel production via one-pot transesterification-esterification of acidic vegetable oils. Chem. Eng. J. 2019, 365, 40–50. [Google Scholar] [CrossRef]
- Ye, C.; Qi, Z.; Cai, D.; Qiu, T. Design and Synthesis of Ionic Liquid Supported Hierarchically Porous Zr Metal–Organic Framework as a Novel Brønsted–Lewis Acidic Catalyst in Biodiesel Synthesis. Ind. Eng. Chem. Res. 2018, 58, 1123–1132. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, C.; Wan, H.; Wang, L.; Li, Z.; Li, B.; Guo, Q.; Guan, G. Fabrication of Magnetic NH2-MIL-88B (Fe) Confined Brønsted Ionic Liquid as an Efficient Catalyst in Biodiesel Synthesis. Energy Fuels 2016, 30, 10739–10746. [Google Scholar] [CrossRef]
- Xie, W.; Wan, F. Basic ionic liquid functionalized magnetically responsive Fe3O4@HKUST-1 composites used for biodiesel production. Fuel 2018, 220, 248–256. [Google Scholar] [CrossRef]
- Leng, K.; Sun, Y.; Li, X.; Sun, S.; Xu, W. Rapid synthesis of metal–organic frameworks MIL-101 (Cr) without the addition of solvent and hydrofluoric acid. Cryst. Growth Des. 2016, 16, 1168–1171. [Google Scholar] [CrossRef]
- Xie, W.; Wan, F. Biodiesel Production from Acidic Oils Using Polyoxometalate-Based Sulfonated Ionic Liquids Functionalized Metal–Organic Frameworks. Catal. Lett. 2019, 149, 2916–2929. [Google Scholar] [CrossRef]
- Sebastian, J.; Muraleedharan, C.; Santhiagu, A. A comparative study between chemical and enzymatic transesterification of high free fatty acid contained rubber seed oil for biodiesel production. Cogent Eng. 2016, 3, 225–240. [Google Scholar] [CrossRef]
- Lv, L.; Dai, L.; Du, W.; Liu, D. Progress in Enzymatic Biodiesel Production and Commercialization. Processes 2021, 9, 355. [Google Scholar] [CrossRef]
- Stoytcheva, M.; Montero, G.; Toscano, L.; Gochev, V.; Valdez, B. The Immobilized Lipases in Biodiesel Production. Biodiesel Feedstocks Process. Technol. 2011. [Google Scholar] [CrossRef]
- Jegannathan, K.R.; Eng-Seng, C.; Ravindra, P. Economic assessment of biodiesel production: Comparison of alkali and biocatalyst processes. Renew. Sustain. Energy Rev. 2011, 15, 745–751. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.; Pillai, S.C.; Ehimen, E.; Bartlett, J. Production of Biodiesel Using Ionic Liquids. In Nanotechnology-Based Industrial Applications of Ionic Liquids; Springer International Publishing: Cham, Switzerland, 2020; pp. 245–269. [Google Scholar]
- Liu, J.; Thallapally, P.K.; McGrail, B.P.; Brown, D.R.; Liu, J. Progress in adsorption-based CO2 capture by metal–organic frameworks. Chem Soc. Rev. 2012, 41, 2308–2322. [Google Scholar] [CrossRef]
| MOF | Oil Source/Alcohol | Synthesis | Pore Size | Time | Temperature/Energy | Yield | Ref. |
|---|---|---|---|---|---|---|---|
| CuBTC-MOF | Palm oil/methanol (1:5) | Solvothermal method | 1.68 cm3/g | 4 h | 60 °C | 91% | [100] |
| ZrSiW/Fe-BTC | Oleic acid/methanol (20:1) | Hydrothermal method |
0.135 m3/g 191.5 m2/g | 4 h | 160 °C | 85% | [102] |
| ZrSiW/UiO-66 | Oleic acid/methanol (20:1) | Hydrothermal method |
0.243 m3/g 249.4 m2/g | 4 h | 150 °C | 98% | [102] |
| Mg3(bdc)3(H2O)2 | Oleic acid/methanol (15:1) | Microwave irradiation | - | 8 min | 150 Watt | 97% | [103] |
| MIL-53 (Fe) | Oleic acid/ethanol (1:16) | Ultrasonic irradiation | 239 cm3/g 1050 m2/g | 15 min | 150 Watt | 96% | [104] |
| MIL-53 (Fe) | Oleic acid/n-butanol (1:16) | Ultrasonic irradiation | 239 cm3/g 1050 m2/g | 15 min | 150 Watt | 98% | [104] |
| HKUST-1 | Palm oil/ethanol (1:1) | Electrolysis | 324.33 m²/g 0.19 cc/g | 2 h | room temperature, 15 V | 54% | [105] |
| Catalyst | Substrate | Ionic Liquid | Loading (w/w%) | Temperature (°C) | Alcohol Ratio | Time (h) | Yield (%) | Reusability | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Catalytic assist Acidic IL—Transesterification | |||||||||
| KOH | Jatropha oil | [Bmim][CH3SO3]–FeCl3 | 13.8 | 120 | 6:1 | 5 | 99.7 | Not reported | [143] |
| No catalyst | - | 12 | Not reported | [143] | |||||
| Acidic IL—Esterification | |||||||||
| No catalyst | Oleic acid | [HMIM][HSO4] | 15 | 110 | 15:1 | 8 | 95.9 | Not reported | [153] |
| Acidic IL—Transesterification | |||||||||
| No catalyst | Waste cooking oil | [TBP][NTf2] | 4.5 | 70 | 12:1 | 1 | 29.7 | 4 cycles with 4% decrease in activity | [154] |
| 60 | 18:1 | 10 | 81.0 | Not reported | [154] | ||||
| Palm oil | [HSO3-Bmim][HSO4] | 9.2 | 108 | 11:1 | 8 | 92.9 | Not studied the conventional experiment | [155] | |
| Microwave (168 W) | Palm oil | [HSO3-Bmim][HSO4] | 9.2 | 108 | 11:1 | 6.4 | 98.9 | 6 cycles with 14.1% decrease in activity | [155] |
| Microwave (120 W) | [MIM][HSO4] | 10 | Not mentioned | 12:1 | 6 | 4.0 | Not reported | [155] | |
| Polymeric IL—Esterification | |||||||||
| No catalyst | Oleic acid | [VSIM][HSO4] | 8.5 | 80 | 12:1 | 4.5 | 92.6 | 6 cycles without leaching acidic sites | [156] |
| FnmS-PIL (1a, C8) | 5 | 75 | 17:1 | 3 | 95.3 | Not reported | [157] | ||
| Polymeric IL—Transesterification | |||||||||
| No catalyst | Caper spurge oil | FnmS-PIL (1a, C8) | 5 | 75 | 17:1 | 3 | 97.1 | 5 cycles with 4% decrease in activity | [157] |
| Immobilized IL—Esterification | |||||||||
| No catalyst | Oleic acid | SiW12O40 (SWIL/SiO2) | 4 | 100 | 30:1 | 4 | 98.5 | Significant reduction after 7 cycles | [158] |
| Catalyst | Substrate | Ionic Liquid | Loading (w/w%) | Temp (°C) | Alcohol Ratio | Time (h) | Yield (%) | Reusability (Cycles) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| No catalyst | Cottonseed oil | Bis-(3-methyl-1-imidazolium)-ethylene dihydroxide | 0.4 | 55 | 12:1 | 4 | 98.5 | 7 | [123] |
| Support | |||||||||
| SBA-15 silica | Soybean oil | 4-Butyl-1,2,4-triazolium hydroxide on SBA-15 | 7.0 | Not reported | 20:1 | 4 | 95.4 | 4 | [166] |
| Boehmite nanoparticles (BNPs) | Chlorocholine hydroxide (CCH) on BNPs | 4.1 | 60 | 11:1 | 4.4 | 95.2 | 5 | [167] | |
| No catalyst | 1-Butyl-3-methyl morpholine hydroxide ([Hnmm]OH) | 4.0 | 70 | 8:1 | 1.5 | 97.0 | 5 | [168] | |
| Enzyme | Support | Immobilization Support | Temperature (°C) | Time (h) | Biodiesel Yield% | Ref. |
|---|---|---|---|---|---|---|
| Novozym@435 | - | Cross linking | 60 | 2.5 | 93 | [175] |
| Novozym@435 | [BMIM][PF6] | Cross linking | 55 | 6 | 80 | [176] |
| Candida rugosa | Fe3O4@MIL-100(Fe) | Covalent attached | 40 | 60 | 92 | [177] |
| Candida antarctica | CALB@MOF Bio-based | Encapsulated | 46 | 12 | 99 | [178] |
| Novozym@435 | [Emim][TfO] | Cross linking | 50 | 12 | 80 | [172] |
| Novozyme@435 | [C16MIM] [NTf2] | Cross linking | 60 | 6 | 98 | [149] |
| Novozyme@435 | [BMIm][PF6] | Cross linking | 40 | 48 | 86 | [150] |
| Candida sp | Zif-67 | Encapsulation | 45 | 60 | 78 | [173] |
| Rhizomucor miehei | X-shaped ZIF-8 | Encapsulation | 45 | 24 | 92 | [15] |
| Burkholderia cepacia | Mesoporous ZIF-8 | Adsorption | 40 | 12 | 93 | [174] |
| MOF | Substrate | Ionic Liquid | Loading (w/w%) | Temp (°C) | Alcohol Ratio | Time (h) | Yield (%) | Reusability | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Encapsulation—Esterification | |||||||||
| MIL-100 | Oleic acid | [SO3H-(CH2)3-HIM]3PW12O40 | 15 | 111 | 11:1 | 5 | 94.6 | 6 cycles | [180] |
| MIL-100(Fe) | (DAILs, 1,4-bis[3-(propyl-3-sulfonate) imidazolium] butane hydrogen sulfate) | 15 | 67 | 8:1 | 5 | 93.5 | 5 cycles | [18] | |
| MIL-101(Cr) | 2 -Mercaptobenzimidazole with electron rich-SH groups (MBIAILs) | 11 | 67 | 10:1 | 4 | 91.0 | 6 cycles | [18] | |
| H-UiO-66 | [(CH2COOH)2IM]HSO4 | 6.28 | 80 | 10:1 | 5 | 93.8 | 5 cycles | [181] | |
| (Fe3O4@NH2-MIL-88B(Fe)) | 1,4-Butanediyl-3,3′-bis-(3-sulfopropyl) imidazolium dihydrogensulfate | 8.5 | 90 | 10.5:1 | 4 | 93.2 | 6 cycles Insignificant reduction | [182] | |
| Encapsulation—Transesterification | |||||||||
| Fe3O4@HKUST-1 | Soybean oil | Fe3O4@HKUST-1 | 1.2 | 65 | 30:1 | 3 | 92.3 | 5 cycles Insignificant reduction | [183] |
| Functionalized with POM—Transesterification | |||||||||
| MIL-100(Fe) | Soybean oil | Phosphomolybdenum-based sulfonated | 9 | 120 | 30:1 | 8 | 92.3 | 5 cycles with 89.5% biodiesel yield | [44] |
| UiO-66-2COOH | Oil-based with high FFA (9%) and water (3%) content | polyoxometalate-based sulfonated | 10 | 110 | 35:1 | 6 | 95.8 | 5 cycles Insignificant reduction | [180] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shomal, R.; Ogubadejo, B.; Shittu, T.; Mahmoud, E.; Du, W.; Al-Zuhair, S. Advances in Enzyme and Ionic Liquid Immobilization for Enhanced in MOFs for Biodiesel Production. Molecules 2021, 26, 3512. https://doi.org/10.3390/molecules26123512
Shomal R, Ogubadejo B, Shittu T, Mahmoud E, Du W, Al-Zuhair S. Advances in Enzyme and Ionic Liquid Immobilization for Enhanced in MOFs for Biodiesel Production. Molecules. 2021; 26(12):3512. https://doi.org/10.3390/molecules26123512
Chicago/Turabian StyleShomal, Reem, Babatunde Ogubadejo, Toyin Shittu, Eyas Mahmoud, Wei Du, and Sulaiman Al-Zuhair. 2021. "Advances in Enzyme and Ionic Liquid Immobilization for Enhanced in MOFs for Biodiesel Production" Molecules 26, no. 12: 3512. https://doi.org/10.3390/molecules26123512
APA StyleShomal, R., Ogubadejo, B., Shittu, T., Mahmoud, E., Du, W., & Al-Zuhair, S. (2021). Advances in Enzyme and Ionic Liquid Immobilization for Enhanced in MOFs for Biodiesel Production. Molecules, 26(12), 3512. https://doi.org/10.3390/molecules26123512








