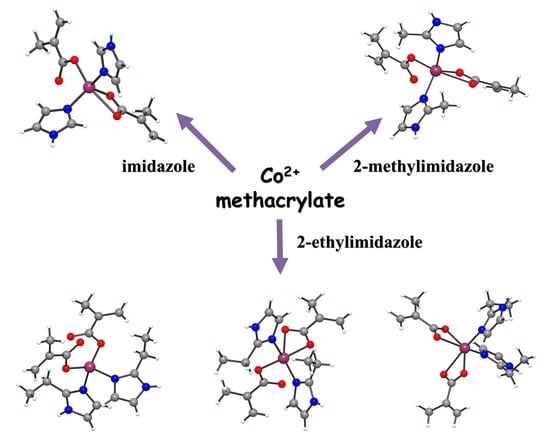
New Cobalt (II) Complexes with Imidazole Derivatives: Antimicrobial Efficiency against Planktonic and Adherent Microbes and In Vitro Cytotoxicity Features
Abstract
:1. Introduction
2. Results and Discussion
2.1. Complexes Synthesis
2.2. Complexes Characterization
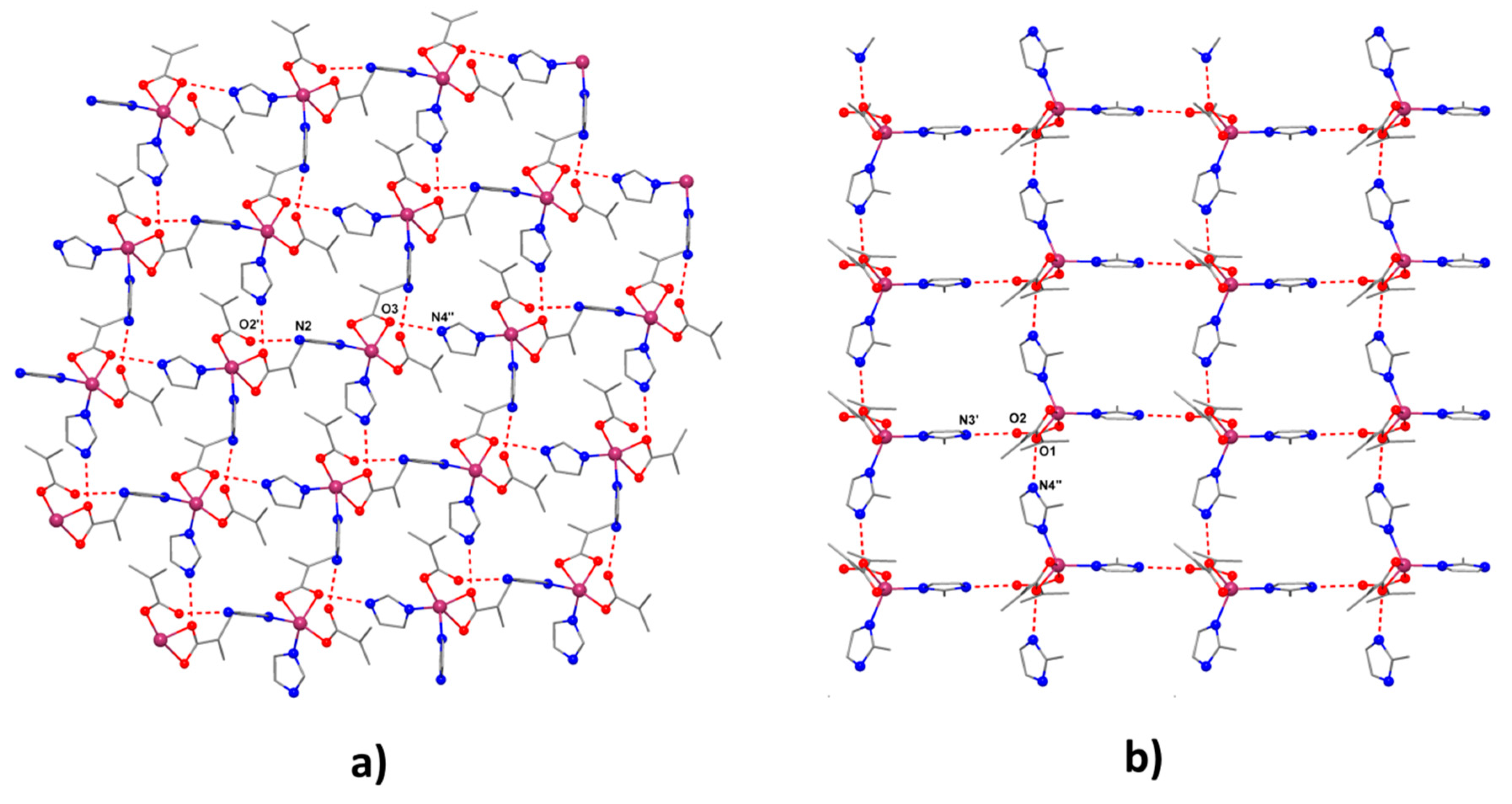
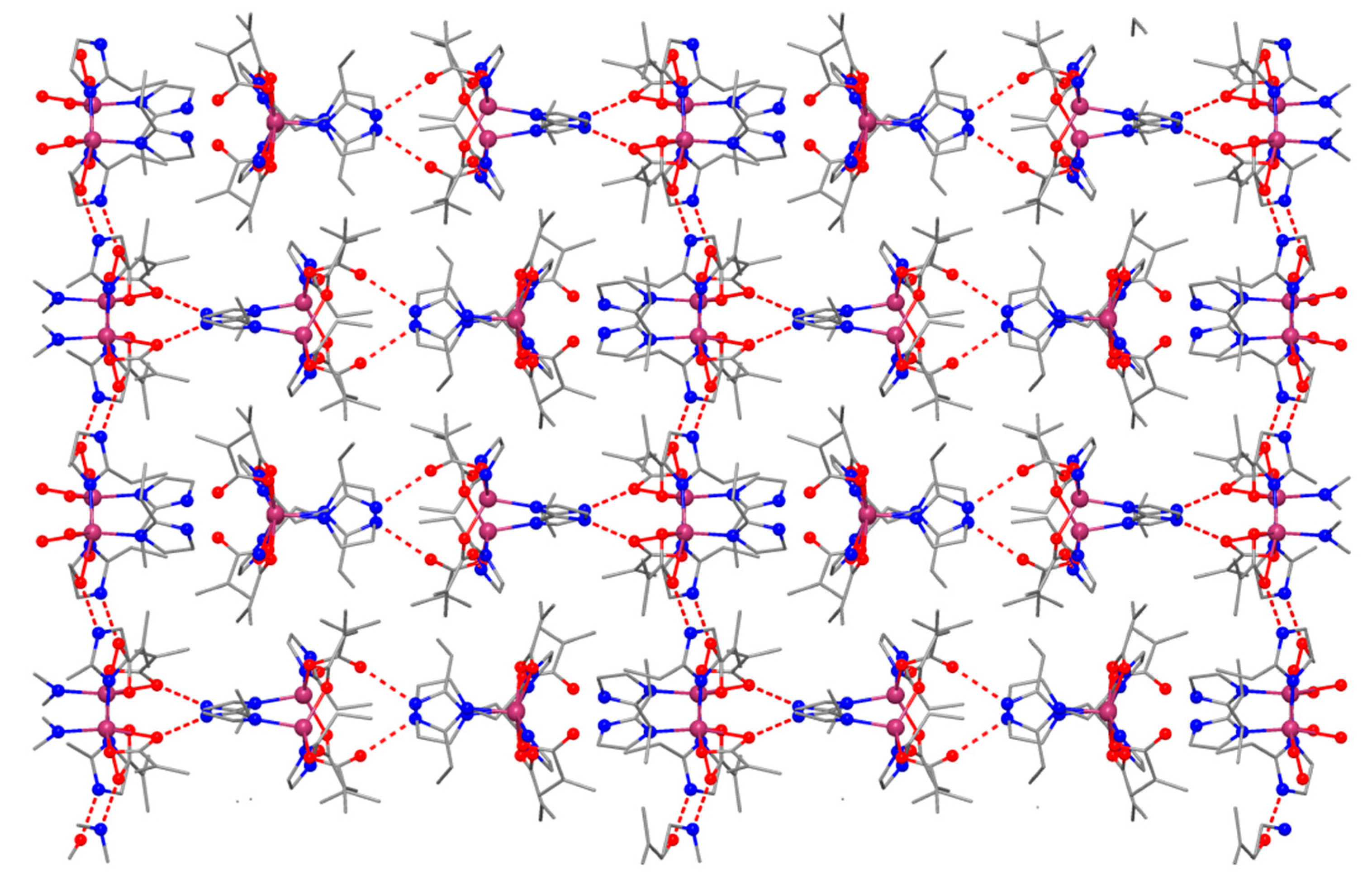
2.2.1. Description of the X-ray Crystal Structures of the Complexes
2.2.2. Infrared Spectra
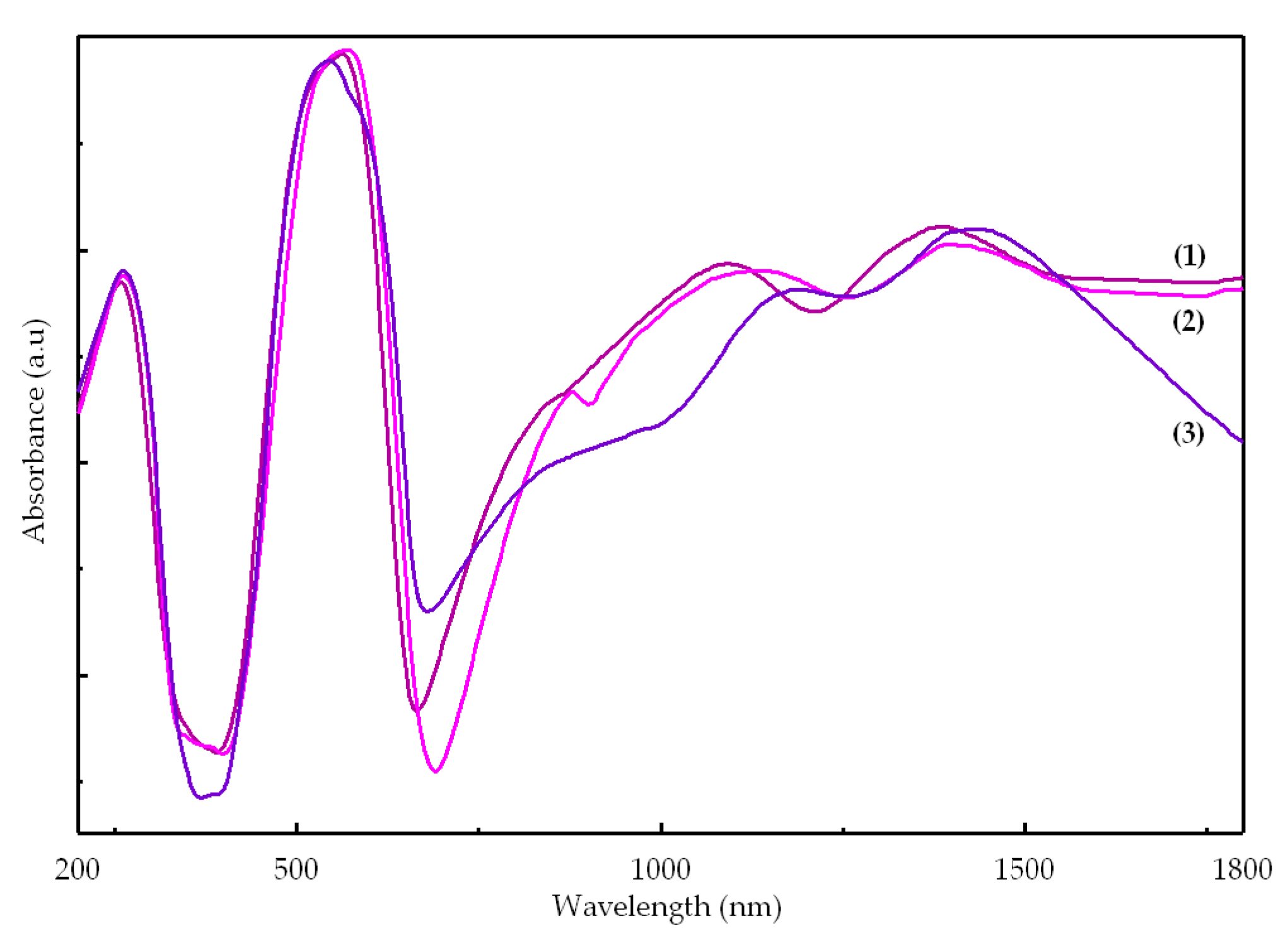
2.2.3. UV-Vis-NIR Spectra
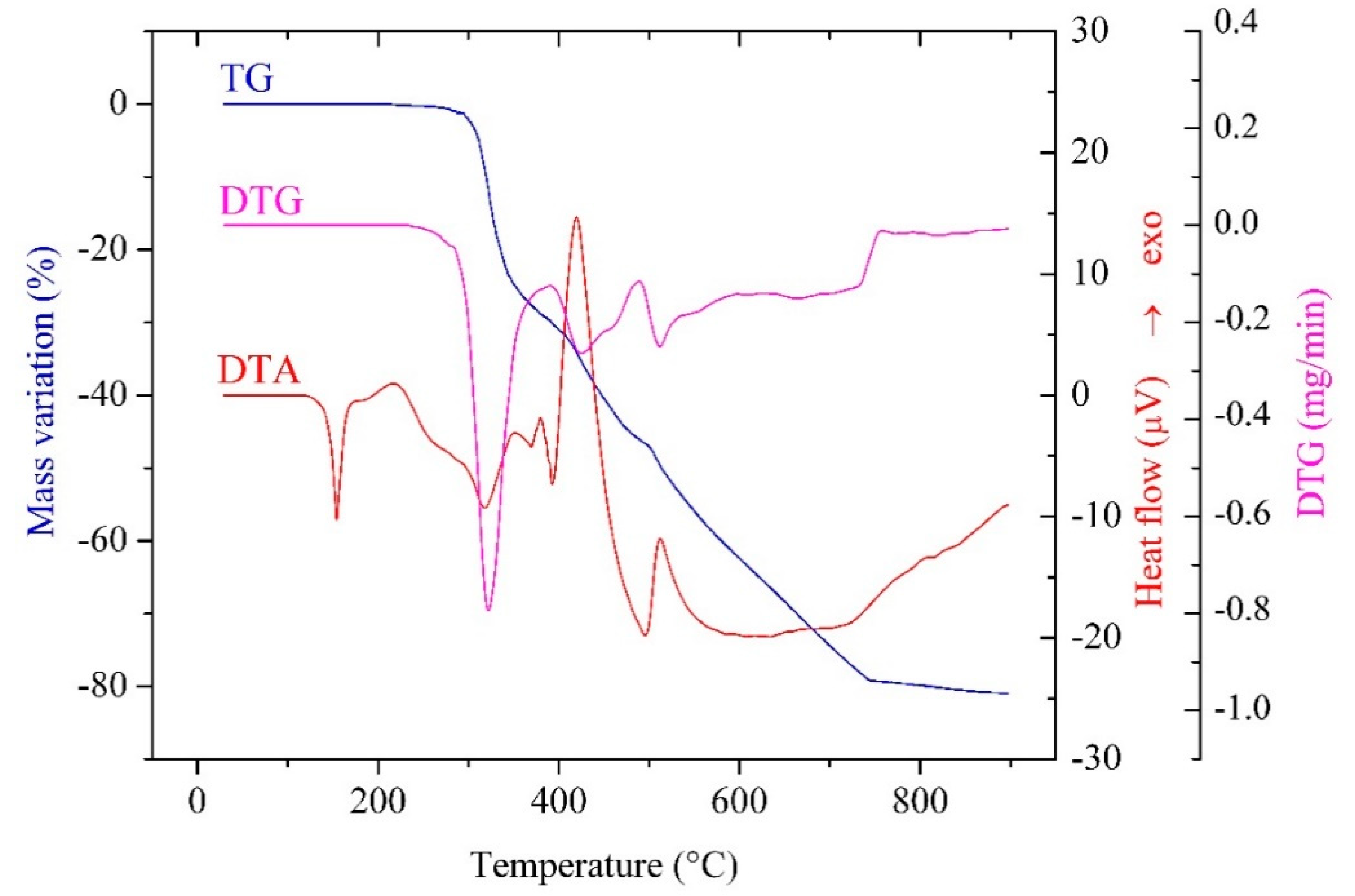
2.2.4. Thermal Behavior
2.2.5. Antimicrobial Assay
2.2.6. Cytotoxicity Tests
3. Materials and Methods
3.1. General Information
3.2. Complexes Synthesis
- (1).
- [Co(Macr)2(HIm)2] (purple crystals); soluble in alcohols (methanol, ethanol), dimethylformamide and dimethyl sulfoxide. Anal. Calc.: Co, 16.13; C, 46.04; H, 4.97; N, 15.34; Found: Co, 16.18; C, 46.11; H, 4.95; N, 15.42.
- (2).
- [Co(Macr)2(2-MeIm)2] (blue violet crystals); soluble in alcohols (methanol, ethanol), dimethylformamide and dimethyl sulfoxide. Anal. Calc.: Co, 14.98; C, 48.86; H, 5.64; N, 14.25; Found: Co, 14.92; C, 48.79; H, 5.61; N, 14.33.
- (3).
- [Co(Macr)2(2-EtIm)2] (dark violet crystals); soluble in alcohols (methanol, ethanol), dimethylformamide and dimethyl sulfoxide. Anal. Calc.: Co, 13.98; C, 51.31; H, 6.22; N, 13.30; Found: Co, 13.93; C, 51.37; H, 6.29; N, 13.37.
3.3. Microbiological Assays
3.3.1. Quantitative Assay of the Antimicrobial Activity against Planktonic Cells
3.3.2. Assessment of the Anti-Biofilm Activity
3.4. Cytotoxicity Tests
3.4.1. Cells
3.4.2. Cytotoxicity Assay
3.4.3. Semi-Quantitative Real-Time RT-PCR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Singh, N.; Pandurangan, A.; Rana, K.; Anand, P.; Ahamad, A.; Tiwari, A.K. Benzimidazole: A short review of their antimicrobial activities. Int. Curr. Pharm. J. 2012, 1, 119–127. [Google Scholar] [CrossRef] [Green Version]
- McCann, M.; Curran, R.; Ben-Shoshan, M.; McKee, V.; Tahir, A.A.; Devereux, M.; Kavanagh, K.; Creaven, B.S.; Kellett, A. Silver (I) complexes of 9-anthracenecarboxylic acid and imidazoles: Synthesis, structure and antimicrobial activity. J. Chem. Soc. Dalton Trans. 2012, 41, 6516–6527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-Y.; Shi, Q.; Shi, Q.-Z.; Gao, Y.-C.; Zhou, Z.-Y. Syntheses, characterization and crystal structure of copper (II) α,β-unsaturated carboxylate complexes with imidazole. Polyhedron 1999, 18, 2009–2015. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhou, L.J.; Shi, Q.; Shi, Q.Z. Novel trinuclear copper (II) complexes with α,β-unsaturated carboxylates and imidazole. Trans. Met. Chem. 2002, 27, 145–148. [Google Scholar] [CrossRef]
- Vlaicu, I.D.; Borodi, G.; Vasile Scaeteanu, G.; Chifiriuc, M.C.; Marutescu, L.; Popa, M.; Stefan, M.; Mercioniu, I.F.; Maurer, M.; Daniliuc, C.; et al. X-ray crystal structure, geometric isomerism, and antimicrobial activity of new copper (II) carboxylate complexes with imidazole derivatives. Molecules 2018, 23, 3253. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.; Luo, Y.T.; Wang, D.; Shi, J.; Li, S.W.; Shen, S.H.; Xu, Y.J. Constructions of five noncovalent-bonded supramolecules from reactions of cadmium (II) and zinc (II) with imidazole/benzimidazole and carboxylate ligands. Z. Anorg. Allg. Chem. 2014, 640, 1717–1726. [Google Scholar] [CrossRef]
- Hernandez, J.; Avila, M.; Jimenez-Vazquez, H.A.; Duque, J.; Reguera, E. Copper dimer with acetate-2-ethylimidazole as ligands. Synth. React. Inorg. M. 2015, 45, 342–345. [Google Scholar] [CrossRef]
- Abdul-Ghani, A.-S.; Abu-Hijleh, A.L.; Qazzaz, M. Effect of bis(acetato)tetrakis(imidazole)copper(II) in delaying the onset and reducing the mortality rate of strycnine- and thiosemicarbazide-induced convulsions. Biol. Trace Elem. Res. 2004, 101, 87–95. [Google Scholar] [CrossRef]
- Abdul-Ghani, A.-S.; Abu-Hijleh, A.-L.; Qazzaz, M.; Muhaisen, A.; Ghani, R.A. Stimulated release of exogenous GABA and glutamate from cerebral cortical synaptosomes and brain slices by bis(acetato)tetrakis(imidazole)copper (II) complex. Biol. Trace Elem. Res. 2005, 108, 205–214. [Google Scholar] [CrossRef]
- Abdul-Ghani, A.-S.; Abu-Hijleh, A.-L.; Nahas, N.; Amin, R. Hypoglicemic effect of copper (II) acetate imidazole complexes. Biol. Trace Elem. Res. 1996, 54, 143–151. [Google Scholar] [CrossRef]
- Puchoňova, M.; Švorek, J.; Švorc, L.; Pavlik, J.; Mazúr, M.; Dlhán, L.; Růžičková, Z.; Moncoľ, J.; Valigura, D. Synthesis, spectral, magnetic, electrochemical evaluation and SOD mimetic activity of four mixed-ligand Cu(II) complexes. Inorg. Chim. Acta 2017, 455, 298–306. [Google Scholar] [CrossRef]
- Tamura, H.; Imai, H.; Kuwahara, J.; Sugiura, Y. A new antitumor complex: Bis(acetato)bis(imidazole)copper(II). J. Am. Chem. Soc. 1987, 109, 6870–6871. [Google Scholar] [CrossRef]
- Abuhijleh, A.L. Catalytic activities of the antitumor complex bis(acetato)bis(imidazole)copper(II) and bis(valproato)bis(imidazole)copper(II) for the oxidation of organic substrates. Polyhedron 1996, 15, 285–293. [Google Scholar] [CrossRef]
- Abuhijleh, L.; Woods, C. Synthesis, spectroscopic and structural characterization of bis(acetato)tetrakis(imidazole)copper(II): A model complex for DNA binding. Inorg. Chim. Acta 1992, 194, 9–14. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Zhao, J.; Yang, P. DNA-binding and cleavage studies of novel binuclear copper (II) complex with 1,1′-dimethyl-2,2′-biimidazole ligand. J. Inorg. Biochem. 2007, 101, 283–290. [Google Scholar] [CrossRef]
- Tabrizi, L.; McArdle, P.; Ektefan, M.; Chiniforoshan, H. Synthesis, crystal structure, spectroscopic and biological properties of mixed ligands of cadmium (II), cobalt (II) and manganese (II) valproate with 1,10-phenanthroline and imidazole. Inorg. Chim. Acta 2016, 439, 138–144. [Google Scholar] [CrossRef]
- McCann, M.; Curran, R.; Ben-Shoshan, M.; McKee, V.; Devereux, M.; Kavanagh, K.; Kellett, A. Synthesis, structure and biological activity of silver (I) complexes of substituted imidazoles. Polyhedron 2013, 56, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Vlaicu, I.D.; Constand, M.; Olar, R.; Marinescu, D.; Grecu, M.N.; Lazar, V.; Chifiriuc, M.C.; Badea, M. Thermal stability of new biologic active copper (II) complexes with 5,6-dimethylbenzimidazole. J. Therm. Anal. Calorim. 2013, 113, 1369–1377. [Google Scholar] [CrossRef]
- Olar, R.; Vlaicu, I.D.; Chifiriuc, M.C.; Bleotu, C.; Stanică, N.; Vasile Scăeţeanu, G.; Silvestro, L.; Dulea, C.; Badea, M. Synthesis, thermal analysis and biological characterisation of some new nickel (II) complexes with unsaturated carboxylates and heterocyclic N-donor ligands. J. Therm. Anal. Calorim. 2017, 127, 731–741. [Google Scholar] [CrossRef]
- Vlaicu, I.D.; Olar, R.; Vasile Scăețeanu, G.; Silvestro, L.; Maurer, M.; Stănică, N.; Badea, M. Thermal, spectral and biological investigation of new nickel complexes with imidazole derivatives. J. Therm. Anal. Calorim. 2018, 134, 503–512. [Google Scholar] [CrossRef]
- Vlaicu, I.D.; Olar, R.; Maxim, C.; Chifiriuc, M.C.; Bleotu, C.; Stanică, N.; Vasile Scăeţeanu, G.; Dulea, C.; Avram, S.; Badea, M. Evaluating the biological potential of some new cobalt (II) complexes with acrylate and benzimidazole derivatives. Appl. Organometal. Chem. 2019, 33, e4976. [Google Scholar] [CrossRef]
- Morzyk-Ociepa, B.; Różycka-Sokołowska, E.; Michalska, D. Revised crystal and molecular structure, FT-IR spectra and DFT studies of chlorotetrakis(imidazole)copper(II) chloride. J. Mol. Struct. 2012, 1028, 49–56. [Google Scholar] [CrossRef]
- Deacon, G.B.; Philips, J.R. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Oldham, C. Carboxylates, squarates and related species. In Comprehensive Coordination Chemistry; Wilkinson, G., Gillard, R.D., McCleverty, J.A., Eds.; Pergamon Press: Oxford, UK, 1987; Volume 2, pp. 435–460. ISBN 0-08-035946-9. [Google Scholar]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands; London, UK; New York, NY, USA, 1984; pp. 481–505. ISBN 0444416994. [Google Scholar]
- Lazǎr, V.; Chifiriuc, M.C. Architecture and physiology of microbial biofilms. Roum. Arch. Microbiol. Immunol. 2010, 69, 95–107. [Google Scholar] [PubMed]
- Cavaliere, R.; Ball, J.L.; Turnbull, L.; Whitchurch, C.B. The biofilm matrix destabilizers, EDTA and DNaseI, enhance the susceptibility of nontypeable Hemophilus influenzae biofilms to treatment with ampicillin and ciprofloxacin. Microbiologyopen 2014, 3, 557–567. [Google Scholar] [CrossRef]
- Shannon, A.M.; Bouchier-Hayes, D.J.; Toomey, D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat. Rev. 2003, 29, 297–307. [Google Scholar] [CrossRef]
- Huo, Y.; Zheng, Z.; Chen, Y.; Wang, Q.; Zhang, Z.; Deng, H. Downregulation of vimentin expression increased drug resistance in ovarian cancer cells. Oncotarget 2016, 7, 45876–45888. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Li, X.F.; Yuan, G.Q.; Hu, H.; Song, X.Y.; Li, J.Y.; Miao, X.K.; Zhou, T.X.; Yang, W.L.; Zhang, X.W.; et al. β-Arrestin 1 has an essential role in neurokinin-1 receptor-mediated glioblastoma cell proliferation and G2/M phase transition. J. Biol. Chem. 2017, 292, 8933–8947. [Google Scholar] [CrossRef] [Green Version]
- Nuţă, I.; Badea, M.; Chifiriuc, M.C.; Bleotu, C.; Popa, M.; Daniliuc, C.-G.; Olar, R. Synthesis, physico-chemical characterization and bioevaluation of Ni(II), Pd(II), and Pt(II) complexes with 1-(o-tolyl)biguanide: Antimicrobial and antitumor studies. Appl. Organometal. Chem. 2020, 34, e5807. [Google Scholar] [CrossRef]
- Rostas, A.M.; Badea, M.; Ruţă, L.L.; Farcaşanu, I.C.; Maxim, C.; Chifiriuc, M.C.; Popa, M.; Luca, M.; Čelan Korošin, N.; Cerc Korošec, R.; et al. Copper(II) complexes with mixed heterocycle ligands as promising antibacterial and antitumor species. Molecules 2020, 25, 3777. [Google Scholar] [CrossRef] [PubMed]
- Matei, L.; Bleotu, C.; Baciu, I.; Diaconu, C.C.; Hanganu, A.; Banu, O.; Ionita, P.; Paun, A.; Tatibouet, A.; Zarafu, I. Synthesis and biological activities of some new isonicotinic acid 2-(2-hydroxy-8-substituted-tricyclo 7.3.1.0(2.7) tridec-13-ylidene)-hydrazides. Bioorg. Med. Chem. 2015, 23, 401–410. [Google Scholar] [CrossRef] [PubMed]


| Compound | (1) | (2) | (3) |
|---|---|---|---|
| Chemical formula | C14H18CoN4O4 | C16H22CoN4O4 | C54H78Co3N12O12 |
| M (g·mol−1) | 365.26 | 393.31 | 1264.09 |
| Temperature (K) | 293 | 293 | 293 |
| Crystal system | monoclinic | monoclinic | monoclinic |
| Space group | P21/a | P21/c | P21/a |
| a (Å) | 12.4662(2) | 13.5660(11) | 16.2171(2) |
| b (Å) | 8.2403(3) | 7.8085(4) | 16.1886(3) |
| c (Å) | 17.4959(4) | 18.0920(15) | 24.4967(4) |
| α (º) | 90 | 90 | 90 |
| β (º) | 104.050(5) | 100.707(6) | 94.440(5) |
| γ (º) | 90 | 90 | 90 |
| V (Å3) | 1743.50(9) | 1883.1(2) | 6411.87(18) |
| Z | 4 | 4 | 4 |
| Dc (g·cm−3) | 1.3914 | 1.3872 | 1.3094 |
| µ (mm−1) | 1.007 | 0.938 | 0.831 |
| F(0 0 0) | 757.7 | 821.8 | 2657.3 |
| Goodness-of-fit (GOF) on F2 | 1.033 | 1.097 | 0.962 |
| Final R1, wR2 [I > 2σ(l)] | R1 = 0.0374, wR2 = 0.0869 | R1 = 0.0529, wR2 = 0.1241 | R1 = 0.0506, wR2 = 0.1135 |
| R1, wR2 (all data) | R1 = 0.0730, wR2 = 0.1114 | R1 = 0.0848, wR2 = 0.1450 | R1 = 0.0974, wR2 = 0.1342 |
| Largest difference in peak and hole (eÅ−3) | 0.37/−0.42 | 0.56/−0.64 | 0.71/−0.94 |
| (1) | (2) | (3) | ||||||
|---|---|---|---|---|---|---|---|---|
| Co(1) | O(1) | 1.982(3) | Co(1) | O(1) | 1.994(3) | Co(1) | N(3) | 2.052(3) |
| Co(1) | O(3) | 2.003(3) | Co(1) | O(5) | 1.982(3) | Co(1) | N(2) | 2.059(3) |
| Co(1) | N(3) | 2.026(3) | Co(1) | N(2) | 2.017(4) | Co(1) | O(1) | 2.047(3) |
| Co(1) | N(1) | 2.008(3) | Co(1) | N(1) | 2.037(4) | Co(1) | O(3) | 2.047(3) |
| Co(1) | O(4) | 2.352(3) | ||||||
| Co(1) | O(2) | 2.383(3) | ||||||
| Co(3) | N(10) | 2.057(4) | ||||||
| Co(3) | N(11) | 2.020(3) | ||||||
| Co(3) | O(12) | 1.980(3) | ||||||
| Co(3) | O(9) | 2.022(3) | ||||||
| Co(2) | N(6) | 2.028(3) | ||||||
| Co(2) | N(7) | 2.026(3) | ||||||
| Co(2) | O(5) | 1.948(3) | ||||||
| Co(2) | O(7) | 1.958(3) | ||||||
| NaMacr | HIm | 2-MeIm | 2-EtIm | (1) | (2) | (3) | Assignments |
|---|---|---|---|---|---|---|---|
| - | 3126 m | 3136 m | 3153 w | 3139 m | 3160 m | 3175 m | ν(CH), ν(NH) |
| 2969 w | - | - | - | 2959 m | 2963 m | 2976 m | νas(CH3) |
| 2928 w | - | - | - | - | 2920 m | 2927 m | νs(CH3) |
| - | 1593 m | 1596 vs. | - | 1581vs. | 1587 vs. | - | δ(NH), ν(CC), ν(CN) |
| 1556 vs. | - | - | - | 1561 vs. 1540 s | 1561 vs. 1548 s | 1568 vs. - | νas(COO) |
| 1367 m | - | - | - | - 1368 m | 1380 m 1368 s | 1386 m 1368 s | νs(COO) |
| - | 1251 w | 1206 w | 1243 w | 1257 m | 1223 m | 1236 m | ν(CN), δ(CH) |
| - | 1142 m | 1155 vs | 1153 m | 1147 m | 1157 m | 1159 m | ν(CC), ν(CN), δ(CH) |
| 920 m | 929 s | 942 s | 956 s | 941 m | 930 m | 933 m | δ(CH), δ(imidazole ring) |
| 855 m | - | 875 w | 875 w | 862 m | 865 m | 856 m | π(CH), δ(imidazole ring) |
| - | 750 s | 756 vs | 751 vs | 756 m | 755 m | 754 m | π(CH) |
| - | 615 m | 627 w | 625 w | 623 m | 627 m | 624 m | π(NH) |
| Compound | Absorption Maxima | Assignments | |
|---|---|---|---|
| λ [nm] | ῦ [cm−1] | ||
| [Co(Macr)2(Im)2] (1) | 260 | 38,460 | π → π* |
| 525 | 19,050 | 4A′2 → 4E′′ (P) | |
| 565 | 17,700 | 4A′2 → 4A′2 (P) | |
| 865 1095 | 11,560 9130 | 4A′2 → 4E′ | |
| 1385 | 7220 | 4A′2 → 4E″ | |
| 1925 | 5194 | 4A′2 → 4A″1, 4A″2 | |
| [Co(Macr)2(2-MeIm)2] (2) | 265 | 37,735 | π → π* |
| 535 | 18,520 | 4A′2 → 4E′′ (P) | |
| 570 | 17,540 | 4A′2 → 4A′2 (P) | |
| 880 1135 | 11,360 8810 | 4A′2 → 4E′ | |
| 1395 | 7170 | 4A′2 → 4E″ | |
| 1840 | 5435 | 4A′2 → 4A″1, 4A″2 | |
| [Co(Macr)2(2-EtIm)2] (3) | 260 | 38,460 | π → π* |
| 545 | 18,350 | 4A2 → 4E (P) | |
| 585 | 17,090 | 4A2 → 4T1(P) Td | |
| 910 | 11,000 | 4A2 → 4B1 | |
| 1190 | 8400 | 4A2 → 4T1(F) Td | |
| 1395 | 7000 | 4A2 → 4E | |
| 1920 | 5200 | 4A2 → 4B2 | |
| Complex | Step | Thermal Effect | Temp./°C | Δmexp/% | Δmcalc/% | Process |
|---|---|---|---|---|---|---|
| [Co(Macr)2(Im)2] (1) | 1. | Endothermic | 135 | - | - | melting |
| 2. | Miscellaneous | 220–480 | 36.10 | 37.27 | imidazole elimination | |
| 3. | Exothermic | 480–900 | 40.58 | 40.77 | methacrylate oxidative degradation | |
| Residue (Co3O4) | 23.32 | 21.96 | ||||
| [Co(Macr)2(2-MeIm)2] (2) | 1. | Endothermic | 119 | - | - | melting |
| 2. | Miscellaneous | 270–490 | 42.33 | 41.75 | 2-methylimidazole elimination | |
| 3. | Exothermic | 490–790 | 36.67 | 37.86 | methacrylate oxidative degradation | |
| Residue (Co3O4) | 21.00 | 20.39 | ||||
| [Co(Macr)2(2-EtIm)2] (3) | 1. | Endothermic | 135 | - | - | melting |
| 2. | Miscellaneous | 220–480 | 45.8 | 45.62 | 2-ethylimidazole elimination | |
| 3. | Exothermic | 480–900 | 35.06 | 35.33 | methacrylate oxidative degradation | |
| Residue (Co3O4) | 19.14 | 19.05 | ||||
| Microbial Strains | Co(Macr)2 | HIm | 2-MeIm | 2-EtIm | (1) | (2) | (3) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBEC | MIC | MBEC | MIC | MBEC | MIC | MBEC | MIC | MBEC | MIC | MBEC | MIC | MBEC | |
| E. coli ATCC 8739 | 62.5 | >500 | 125 | >500 | 125 | >500 | 62.5 | >500 | 31.2 | 31.2 | 31.2 | 31.2 | 125 | 62.5 |
| P. aeruginosa ATCC 1671 | 250 | 500 | 62.5 | 500 | 31.2 | 500 | 31.2 | 500 | 62.5 | 15.6 | 31.2 | 15.6 | 31.2 | 31.2 |
| S. aureus ATCC 6538 | 125 | 500 | >500 | 500 | >500 | 500 | >500 | 500 | 15.6 | 15.6 | 15.6 | 15.6 | 15.6 | 15.6 |
| E. faecalis ATCC 29212 | 250 | 500 | >500 | 500 | >500 | 500 | 500 | 500 | 31.2 | 31.2 | 62.5 | 62.5 | 62.5 | 62.5 |
| C. albicans ATCC 26790 | 250 | >500 | 500 | >500 | 250 | >500 | 500 | >500 | 7.8 | >500 | 7.8 | >500 | 15.6 | >500 |
| (1) | (2) | (3) | Control | ||||
|---|---|---|---|---|---|---|---|
| HeLa | |||||||
| 50 µg/mL | 100 µg/mL | 50 µg/mL | 100 µg/mL | 50 µg/mL | 100 µg/mL | ||
| G1 | 58.29 | 30.98 | 59.48 | 28.43 | 59.65 | 22.74 | 58.84 |
| S | 24.95 | 42.86 | 33.96 | 36.2 | 23 | 40.48 | 25.97 |
| G2/M | 8.36 | 15.95 | 7.73 | 10.59 | 8.14 | 13.64 | 8.51 |
| HCT-8 | |||||||
| G1 | 71.50 | 52.24 | 62.84 | 66.98 | 70.59 | 60.75 | 88.70 |
| S | 8.02 | 26.90 | 10.85 | 21.20 | 7.75 | 23.12 | 8.66 |
| G2/M | 6.86 | 6.82 | 8.24 | 14.92 | 3.33 | 17.18 | 3.81 |
| MG63 | |||||||
| G1 | 34.19 | 26.64 | 57.55 | 15.51 | 34.97 | 21.2 | 81.49 |
| S | 25.29 | 19.38 | 19.86 | 24.93 | 26.68 | 25.67 | 10.75 |
| G2/M | 30.48 | 51.44 | 17.58 | 51.71 | 30.25 | 52.66 | 6.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fudulu, A.; Olar, R.; Maxim, C.; Scăeţeanu, G.V.; Bleotu, C.; Matei, L.; Chifiriuc, M.C.; Badea, M. New Cobalt (II) Complexes with Imidazole Derivatives: Antimicrobial Efficiency against Planktonic and Adherent Microbes and In Vitro Cytotoxicity Features. Molecules 2021, 26, 55. https://doi.org/10.3390/molecules26010055
Fudulu A, Olar R, Maxim C, Scăeţeanu GV, Bleotu C, Matei L, Chifiriuc MC, Badea M. New Cobalt (II) Complexes with Imidazole Derivatives: Antimicrobial Efficiency against Planktonic and Adherent Microbes and In Vitro Cytotoxicity Features. Molecules. 2021; 26(1):55. https://doi.org/10.3390/molecules26010055
Chicago/Turabian StyleFudulu, Alina, Rodica Olar, Cătălin Maxim, Gina Vasile Scăeţeanu, Coralia Bleotu, Lilia Matei, Mariana Carmen Chifiriuc, and Mihaela Badea. 2021. "New Cobalt (II) Complexes with Imidazole Derivatives: Antimicrobial Efficiency against Planktonic and Adherent Microbes and In Vitro Cytotoxicity Features" Molecules 26, no. 1: 55. https://doi.org/10.3390/molecules26010055
APA StyleFudulu, A., Olar, R., Maxim, C., Scăeţeanu, G. V., Bleotu, C., Matei, L., Chifiriuc, M. C., & Badea, M. (2021). New Cobalt (II) Complexes with Imidazole Derivatives: Antimicrobial Efficiency against Planktonic and Adherent Microbes and In Vitro Cytotoxicity Features. Molecules, 26(1), 55. https://doi.org/10.3390/molecules26010055