Obtaining Bioactive Compounds from the Coffee Husk (Coffea arabica L.) Using Different Extraction Methods
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects and Interactions of Extraction Variables on the Antioxidant Activity of Coffee Husk Extracts (Coffea arabica L.)
2.2. Determination of Bioactive Compounds
2.3. Profile of Phenolic Compounds
2.4. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Experimental Design
3.2. Husk Dehydration
3.3. Extraction Procedure
3.4. Antioxidant Activity
3.4.1. Sequestering Activity for the 2,2-Diphenyl-1-Picrylhydrazyl Radical (DPPH•)
3.4.2. Sequestering Activity for the 2,2-Azino-bis (3-Ethylbeothiazoline)-6-Sulphonic Acid Radical (ABTS•+)
3.4.3. Ferric Reducing Antioxidant Power (FRAP)
3.4.4. Total Phenolic Content (TPC)
3.4.5. Total Flavonoid Content
3.4.6. Condensed Tannins
3.5. Profile of Phenolic Compounds
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture (USDA). Foreign Agricultural Service. Coffee Summary. 2015. Available online: http://apps.fas.usda.gov/ (accessed on 28 December 2019).
- BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Café. 2016. Available online: http://www.agricultura.gov.br/ (accessed on 28 February 2016).
- ABIC. Associação Brasileira da Industria de Café. 2019. Available online: http://abic.com.br/cafe-com/historia/ (accessed on 29 October 2019).
- Oliveira, L.S.; Franca, A.S. An Overview of the Potential Uses for Coffee Husks; Elsevier Inc.: London, UK, 2015. [Google Scholar] [CrossRef]
- Setter, C.; Silva, F.T.M.; Assis, M.R.; Ataíde, C.H.; Trugilho, P.F.; Oliveira, T.J.P. Slow pyrolysis of coffee husk briquettes: Characterization of the solid and liquid fractions. Fuel 2020, 261, 116–127. [Google Scholar] [CrossRef]
- Andrade, K.S.; Gonalvez, R.T.; Maraschin, M.; Ribeiro-Do-Valle, R.M.; Martínez, J.; Ferreira, S.R.S. Supercritical fluid extraction from spent coffee grounds and coffee husks: Antioxidant activity and effect of operational variables on extract composition. Talanta 2012, 88, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [PubMed]
- Gemechu, F.G. Embracing nutritional qualities, biological, activities and technological properties off coffee byprocts in functional foods formulation. Trends Food Sci. Technol. 2020, 104, 235–261. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Madane, P.; Biswas, S.; Das, A.; Zhang, W.; Lorenzo, J.M. A comprehensive review on antioxidant dietary fibre enriched meat-based functional foods. Trends Food Sci. Technol. 2020, 99, 323–336. [Google Scholar] [CrossRef]
- García, L.R.P.; Del Bianchi, V.L. Antioxidant capacity in coffee industry residues. Braz. J. Food Technol. 2015, 18, 307–313. [Google Scholar] [CrossRef]
- Quatrin, A.; Pauletto, R.; Maurer, L.H.; Minuzzi, N.; Nichelle, S.M.; Carvalho, J.F.C.; Maróstica Júnior, M.R.; Rodrigues, V.C.; Bochi, V.C.; Emanuelli, T. Characterization and quantification of tannins, flavonols, anthocyanins and matrix-bound polyphenols from jaboticaba fruit peel: A comparison between Myrciaria trunciflora and M. jaboticaba. J. Food Compos. Anal. 2019, 78, 59–74. [Google Scholar] [CrossRef]
- Barros, H.D.F.Q.; Baseggio, A.M.; Angolini, C.F.F.; Pastore, G.M.; Cazarin, C.B.B.; Marostica-Junior, M.R. Influence of different types of acids and pH in the recovery of bioactive compounds in Jabuticaba peel (Plinia cauliflora). Food Res. Int. 2019, 124, 16–26. [Google Scholar] [CrossRef]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Vardanega, R.; Santos, D.T.; De Almeida, M.A. Intensification of bioactive compounds extraction from medicinal plants using ultrasonic irradiation. Pharmacogn. Rev. 2014, 8, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Meregalli, M.M.; Puton, B.M.S.; Camera, F.D.M.; Amaral, A.U.; Zeni, J.; Cansin, R.L.; Mignoni, M.L.; Backes, G.T. Conventional and ultrasound-assisted methods for extraction of bioactive compounds from red araçá peel (Psidium cattleianum Sabine). Arab. J. Chem. 2020, 13, 5800–5809. [Google Scholar] [CrossRef]
- Restuccia, D.; Giorgi, G.; Spizzirri, U.G.; Sciubba, F.; Capuani, G.; Rago, V.; Carullo, G.; Aiello, F. Autochthonous white grape pomaces as bioactive source for functional jams. Int. J. Food Sci. Technol. 2018, 54, 1313–1320. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Ritieni, A. In Vitro Bioaccessibility and Antioxidant Activity of Coffee Silverskin Polyphenolic Extract and Characterization of Bioactive Compounds Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 2132. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.; Monente, C.; Juániz, I.; Peña, M.P.; Cid, C. Influence of extraction process on antioxidant capacity of spent coffee. Food Res. Int. 2013, 50, 610–616. [Google Scholar] [CrossRef]
- Thouri, A.; Chahdoura, H.; Arem, A.E.; Hiichri, A.O.; Hassin, R.B.; Achour, L. Effect of solvents extraction onphytochemmical components and biological activies of Tunisian date seeds (var. Korkobbi and Arechti). Complement. Altern. Med. 2017, 17, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Araújo, N.M.P.; Pereira, G.A.; Arruda, H.S.; Prado, L.G.; Ruiz, A.L.T.G.; Eberlin, M.N.; De Castro, R.J.S.; Pastore, G.M. Enzymatic treatment improves the antioxidant and antiproliferative activities of Adenanthera pavonina L. seeds. Biocatal. Agric. Biotechnol. 2019, 18, e101002. [Google Scholar] [CrossRef]
- Pereira, G.A.; Arruda, H.S.; Molina, G.; Pastore, G.M. Extraction optimization and profile analysis of oligosaccharides in banana pulp and peel. J. Food Proc. Preserv. 2017, e13408. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Recovery of Phenolic Antioxidants and Functional Compounds from Coffee Industry By-Products. Food Bioprocess Technol. 2012, 5, 897–903. [Google Scholar] [CrossRef]
- Mokrani, A.; Madani, K. Effect of Solvent, Time and Temperature on the Extraction of Phenolic Compounds and Antioxidant Capacity of Peach (Prunus persica L.) Fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Lou, J.C.; Chang, C.K. Completely treating heavy metal laboratory waste liquid by an improved ferrite process. Sep. Purif. Technol. 2007, 57, 513–518. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzym. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Muñoz, A.E.; Hernández, S.S.; Tolosa, A.R.; Burillo, S.P.; Olalla Herrera, M. Evaluation of differences in the antioxidant capacity and phenolic compounds of green and roasted coffee and their relationship with sensory properties. LWT Food Sci. Technol. 2020, 128, e109457. [Google Scholar] [CrossRef]
- Stępień, A.E.; Gorzelany, J.; Matłok, N.; Lech, K.; Figiel, A. The effect of drying methods on the energy consumption, bioactive potential and colour of dried leaves of Pink Rock Rose (Cistus creticus). J. Food Sci. Technol. 2019, 56, 2386–2394. [Google Scholar] [CrossRef]
- Bagueta, M.R.; Silva, J.T.P.; Moreira, T.F.M.; Canesin, E.A.; Gonçalves, O.H.; Dos Santos, A.R.; Coqueiro, A.; Junior Demczuk, B.; Leimann, F.V. Extração e caracterização de compostos do resíduo vegetal casca de café. Braz. J. Food Res. 2017, 8, 68–89. [Google Scholar] [CrossRef]
- Saada, M.; Kasmi, M.; Ksouri, R. Towards coffee processing by-products valorization: Phenolic compounds and antioxidant activity of spent coffee grounds and coffee silverskin. Int. J. Biotech. Trends Technol. 2019, 9, 11–15. [Google Scholar]
- Castaldo, L.; Graziani, G.; Gasparini, A.; Izzo, L.; Luz, C.; Manes, J.; Rubino, M.; Meca, G.; Ritieni, A. Study of the Chemical Components, Bioactivity and Antifungal Properties of the Coffee Husk. J. Food Res. 2018, 7, 43–54. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L.A.; Sagratini, G.; Vittori, S.; Caprioli, G. Coffee silverskin extracts: Quantification of 30 bioactive compounds by a new HPLC-MS/MS method and evaluation of their antioxidant and antibacterial activities. Food Res. Int. 2020, 133, 109128. [Google Scholar] [CrossRef]
- De Matos, L.P.C. Compostos Fitoquímicos e Atividade Antioxidante de Casca de Café. Trabalho de Conclusão de Curso. Bachelor’s Thesis, Universidade Tecnológica Federal do Paraná, Campo Mourão, Brazil, 2014. [Google Scholar]
- Sousa, J.R.M. Extração de Compostos Fenólicos da Casca do Café por Diferentes Métodos. Trabalho de Conclusão de Curso. Bachelor’s Thesis, Universidade Federal de Uberlândia, Patos de Minas, Brazil, 2018. [Google Scholar]
- Neto, D.C.S. Desenvolvimento e Aplicação de Extrato de Spondias mombim L. Para Manutenção da Qualidade de Hambúrguer de Frango Pronto Para Consumo. Ph.D. Thesis, Universidade Federal da Paraíba, Bananeiras, Brazil, 2019. [Google Scholar]
- Brand-Wiliams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Broadhurst, T.R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
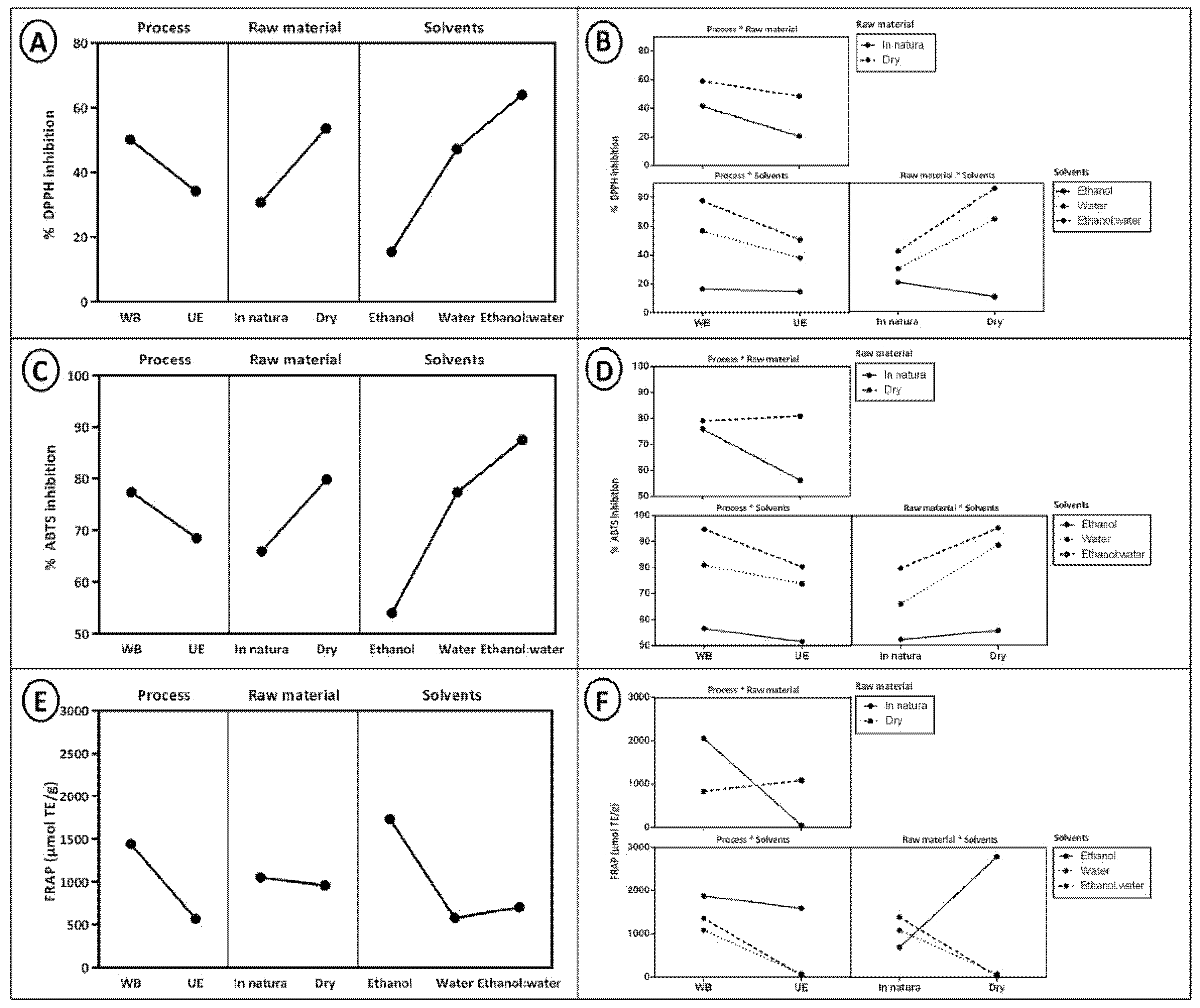
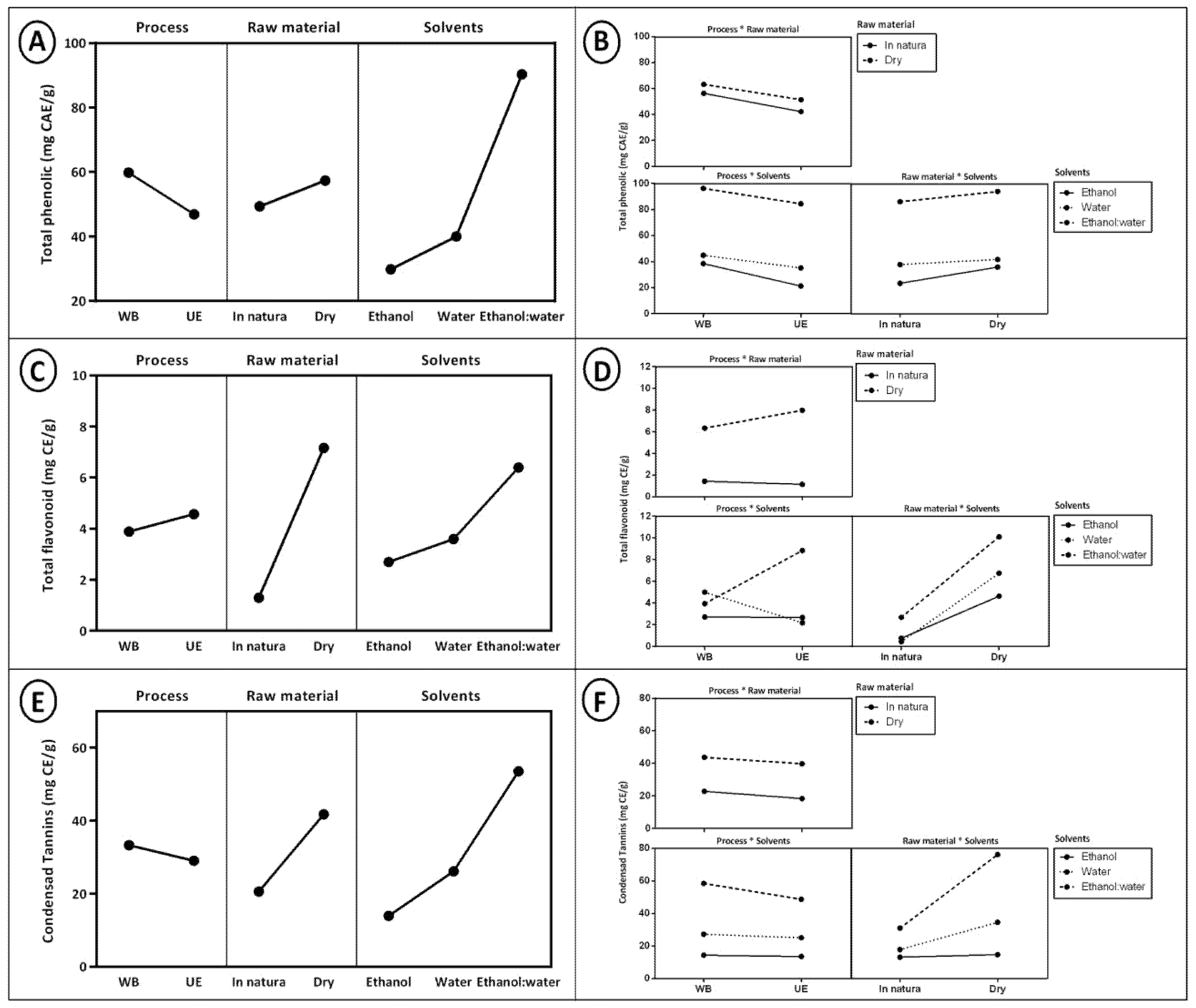
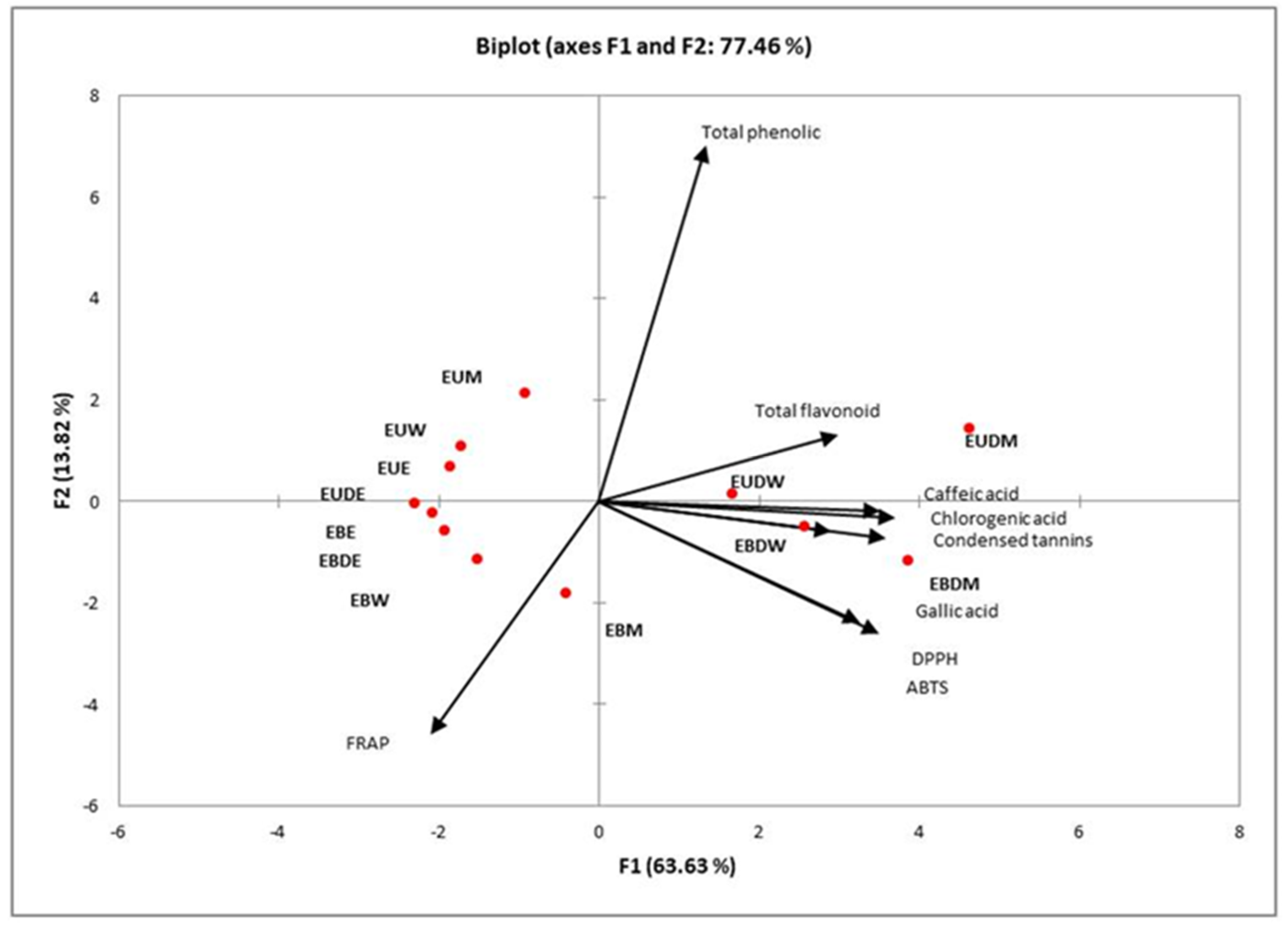
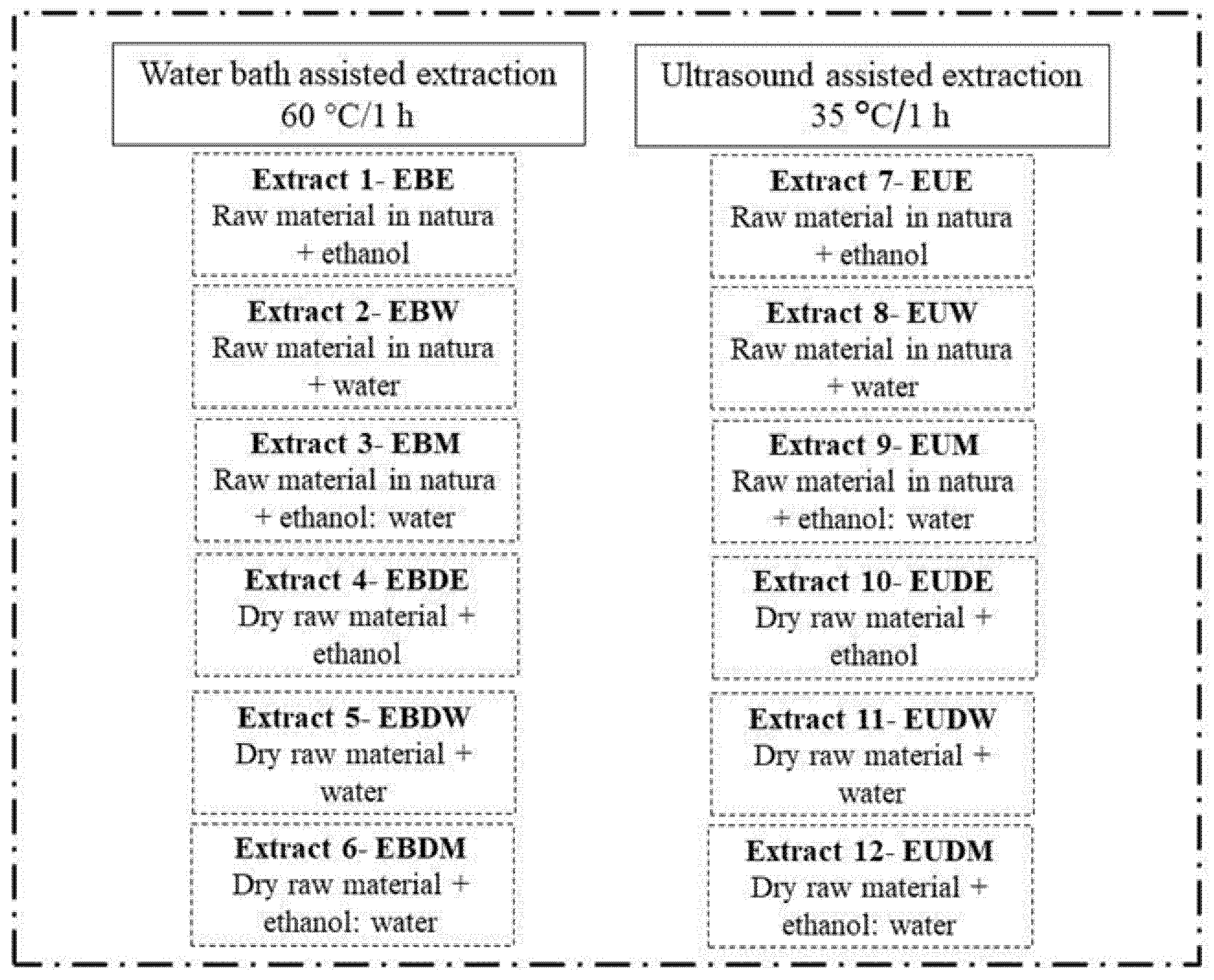
| Extraction Method | Extract | %DPPH Inhibition | %ABTS Inhibition | FRAP (μmol TE/g) |
|---|---|---|---|---|
| Water bath | EBE | 14.17 ± 0.04 | 52.56 ± 0.09 | 1291.85± 0.02 |
| EBW | 37.52 ± 0.1 | 76.05 ± 0.6 | 2103.9 ± 0.8 | |
| EBM | 67.52 ± 0.1 | 91.48 ± 0.1 | 2639.4 ± 0.02 | |
| EBDE | 15.86 ± 0.3 | 56.09 ± 0.8 | 2425.9 ± 0.1 | |
| EBDW | 72.50 ± 0.1 | 81.63 ± 0.01 | 58.73 ± 0.02 | |
| EBDM | 84.95 ± 0.02 | 92.81 ± 0.01 | 23.38 ± 0.1 | |
| Ultrasound | EUE | 23.16 ± 0.01 | 48.75 ± 0.4 | 30.01 ± 0.9 |
| EUW | 19.55 ± 0.01 | 51.97 ± 0.3 | 51.01 ± 0.6 | |
| EUM | 13.24 ± 0.2 | 64.03 ± 0.3 | 64.11 ± 0.02 | |
| EUDE | 2.44 ± 0.1 | 51.24 ± 0.7 | 3136.4 ± 0.1 | |
| EUDW | 53.71 ± 0.02 | 93.24 ± 0.01 | 89.75 ± 0.02 | |
| EUDM | 84.20 ± 0.03 | 97.21 ± 0.01 | 27.62 ± 0.9 |
| Extraction Method | Extract | Total Phenolic (mg CAE/g) | Total Flavonoid (mg CE/g) | Condensed Tannins (mg CE/g) |
|---|---|---|---|---|
| Water bath | EBE | 31.35 ± 1.90 | 0.79 ± 0.12 | 13.50 ± 1.8 |
| EBW | 42.51 ± 0.72 | 0.63 ± 0.07 | 17.43 ± 0.7 | |
| EBM | 95.00 ± 1.39 | 2.81 ± 0.12 | 37.07 ± 1.09 | |
| EBDE | 45.20 ± 1.90 | 4.1 ± 0.5 | 15.12 ± 0.8 | |
| EBDW | 47.57 ± 0.44 | 9.93 ± 0.9 | 35.92 ± 0.4 | |
| EBDM | 97.89 ± 0.72 | 4.53 ± 1 | 79.71 ± 0.8 | |
| Ultrasound | EUE | 16.54 ± 2.18 | 0.75 ± 0.42 | 12.73 ± 0.1 |
| EUW | 34.10 ± 3.78 | 0.21 ± 0.16 | 17.04 ± 4 | |
| EUM | 77.57 ± 0.44 | 2.00 ± 0.19 | 23.75 ± 8 | |
| EUDE | 26.74 ± 2.99 | 5.16 ± 0.3 | 13.21 ± 0.4 | |
| EUDW | 36.16 ± 3.26 | 4.61 ± 0.2 | 32.04 ± 1.30 | |
| EUDM | 90.95 ± 1.73 | 15.69 ± 0.3 | 72.53 ± 1.01 |
| Extraction Method | Extract | Gallic Acid (µg/g) Rt = 4.67 min | Chlorogenic Acid (µg/g) Rt = 6.03 min | Caffeic Acid (µg/g) Rt = 7.28 min |
|---|---|---|---|---|
| Water bath | EBE | 3.18 ± 0.04 | 29.35 ± 2.52 | 1.61 ± 0.35 |
| EBW | 4.45 ± 0.44 | 19.64 ± 3.30 | 1.32 ± 0.26 | |
| EBM | 2.17 ± 0.07 | 25.93 ± 0.84 | 1.40 ± 0.10 | |
| EBDE | Nd | 67.49 ± 1.01 | 1.27 ± 0.01 | |
| EBDW | 60.22 ± 1.95 | 220.89 ± 12.51 | 2.76 ± 0.49 | |
| EBDM | 20.81 ± 4.53 | 337.07 ± 9.88 | 6.15 ± 0.60 | |
| Ultrasound | EUE | 0.72 ± 0.22 | 24.78 ± 2.86 | 1.38 ± 0.11 |
| EUW | 1.91 ± 0.15 | 27.99 ± 0.55 | 1.23 ± 0.15 | |
| EUM | 2.41 ± 0.77 | 27.64 ± 1.68 | 1.95 ± 0.34 | |
| EUDE | Nd | 37.29 ± 1.35 | 1.19 ± 0.04 | |
| EUDW | 33.15 ± 4.15 | 178.68 ± 7.56 | 3.35 ± 0.02 | |
| EUDM | 25.52 ± 4.58 | 304.36 ± 13.01 | 4.53 ± 1.04 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.d.O.; Honfoga, J.N.B.; Medeiros, L.L.d.; Madruga, M.S.; Bezerra, T.K.A. Obtaining Bioactive Compounds from the Coffee Husk (Coffea arabica L.) Using Different Extraction Methods. Molecules 2021, 26, 46. https://doi.org/10.3390/molecules26010046
Silva MdO, Honfoga JNB, Medeiros LLd, Madruga MS, Bezerra TKA. Obtaining Bioactive Compounds from the Coffee Husk (Coffea arabica L.) Using Different Extraction Methods. Molecules. 2021; 26(1):46. https://doi.org/10.3390/molecules26010046
Chicago/Turabian StyleSilva, Mariana de Oliveira, John Nonvignon Bossis Honfoga, Lorena Lucena de Medeiros, Marta Suely Madruga, and Taliana Kênia Alencar Bezerra. 2021. "Obtaining Bioactive Compounds from the Coffee Husk (Coffea arabica L.) Using Different Extraction Methods" Molecules 26, no. 1: 46. https://doi.org/10.3390/molecules26010046
APA StyleSilva, M. d. O., Honfoga, J. N. B., Medeiros, L. L. d., Madruga, M. S., & Bezerra, T. K. A. (2021). Obtaining Bioactive Compounds from the Coffee Husk (Coffea arabica L.) Using Different Extraction Methods. Molecules, 26(1), 46. https://doi.org/10.3390/molecules26010046






