Effect of CO2 Preservation Treatments on the Sensory Quality of Pomegranate Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Supercritical-Carbon Dioxide (CO2)
2.1.2. Supercritical CO2 Combined with Ultrasound (CO2-US)
2.1.3. High Pressure Process (HPP)
2.1.4. Heat Treatment (HT)
2.2. Storage Test at 4 °C
2.3. Sensory Test
2.3.1. Sensory Panel
2.3.2. Procedure
2.4. Analysis of Volatile Compounds
2.5. Chemical-Physical Characterization of Juices
2.6. Data Analysis
2.6.1. Sensory Data
2.6.2. Instrumental Data
3. Results and Discussion
3.1. Changes in Pomegranate Juice Induced by Preservation Treatment
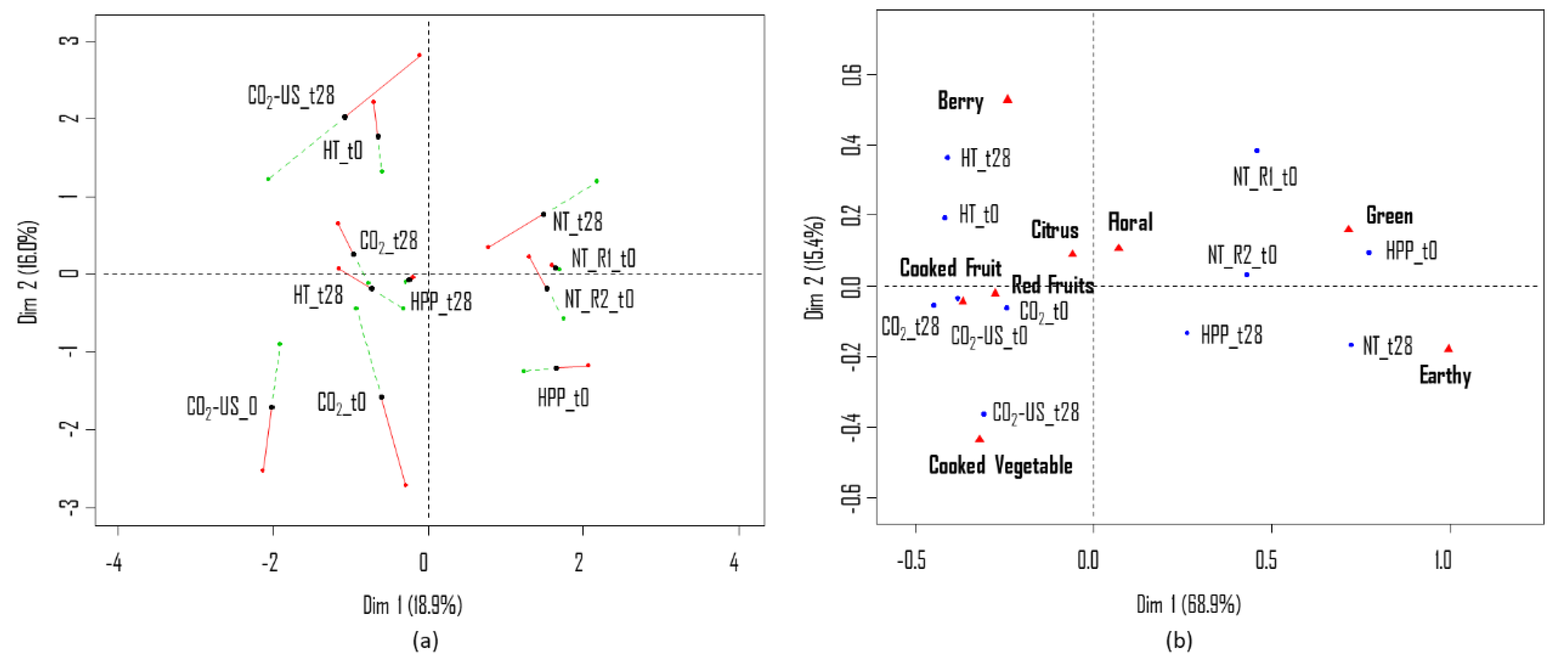
3.1.1. Sensory Quality
3.1.2. Physico-Chemical Parameters
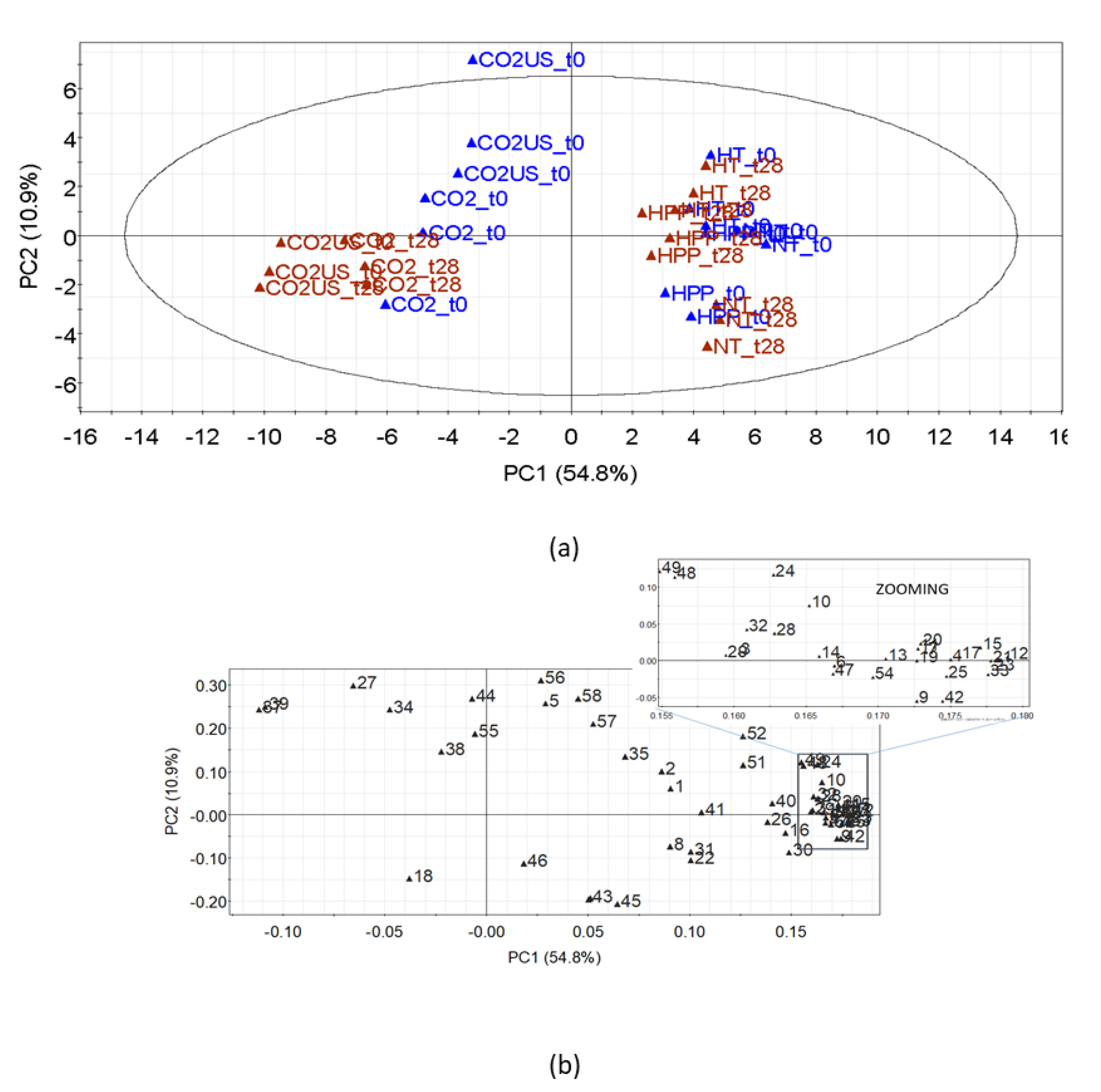
3.1.3. Volatile Compounds
3.2. Changes in Pomegranate Juice Induced by Storage
3.2.1. Sensory Quality
3.2.2. Effect on the Chemical Physical Parameters
3.2.3. Effect on Volatile Compounds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Tzulker, R.; Glazer, I.; Bar-Ilan, I.; Holland, D.; Aviram, M.; Amir, R. Antioxidant activity, polyphenol content, and related compounds in different fruit juices and homogenates prepared from 29 different pomegranate accessions. J. Agric. Food Chem. 2007, 55, 9559–9570. [Google Scholar] [CrossRef] [PubMed]
- Kalaycıoğlu, Z.; Erim, F.B. Total phenolic contents, antioxidant activities, and bioactive ingredients of juices from pomegranate cultivars worldwide. Food Chem. 2017, 221, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, H.R.; Arastoo, M.; Ostad, S.N. A comprehensive review of Punica granatum (Pomegranate) properties in toxicological, pharmacological, cellular and molecular biology researches. Iran. J. Pharm. Res. 2012, 11, 385–400. [Google Scholar]
- Basu, A.; Penugonda, K. Pomegranate juice: A heart-healthy fruit juice. Nutr. Rev. 2009, 67, 49–56. [Google Scholar] [CrossRef]
- Karimi, M.; Sadeghi, R.; Kokini, J. Pomegranate as a promising opportunity in medicine and nanotechnology. Trends Food Sci. Technol. 2017, 69, 59–73. [Google Scholar] [CrossRef]
- Mayuoni-Kirshinbaum, L.; Porat, R. The flavor of pomegranate fruit: A review. J. Sci. Food Agric. 2014, 94, 21–27. [Google Scholar] [CrossRef]
- Koppel, K.; Chambers, E., IV. Development and application of a lexicon to describe the flavor of pomegranate juice. J. Sens. Stud. 2010, 25, 819–837. [Google Scholar] [CrossRef]
- Melgarejo, P.; Domingo, M.S.; Artess, F. Organic acids and sugar composition of harvested pomegranate fruits. Eur. Food Res. Technol. 2000, 211, 185–190. [Google Scholar] [CrossRef]
- Hasnaoui, N.; Mars, M.; Ghaffari, S.; Trifi, M.; Melgarejo, P.; Hernandez, F. Seed and juice characterization of pomegranate fruits grown in Tunisia: Comparison between sour and sweet cultivars revealed interesting properties for prospective industrial applications. Ind. Crops Prod. 2011, 33, 374–381. [Google Scholar] [CrossRef]
- Dafny-Yalin, M.; Glazer, I.; Bar-Ilan, I.; Kerem, Z.; Holland, D.; Amir, R. Color, sugars and organic acids composition in aril juices and peel homogenates prepared from different pomegranate accessions. J. Agric. Food Chem. 2010, 58, 4342–4352. [Google Scholar] [CrossRef] [PubMed]
- Calín-Sánchez, A.; Martínez, J.J.; Vázquez-Araújo, L.; Burló, F.; Melgarejo, P.; Carbonell-Barrachina, A.A. Volatile composition and sensory quality of Spanish pomegranates (Punica granatum L.). J. Sci. Food Agric. 2011, 91, 586–992. [Google Scholar] [CrossRef] [PubMed]
- Melgarejo, P.; Calín-Sánchez, A.; Vázquez-Araújo, L.; Hernández, F.; Martínez, J.J.; Legua, P.; Carbonell-Barrachina, A.A. Volatile composition of pomegranates from 9 Spanish cultivars using headspace solid phase microextraction. J. Food Sci. 2011, 76, 114–120. [Google Scholar] [CrossRef]
- Mayuoni-Kirshinbaum, L.; Tietel, Z.; Porat, R.; Ulrich, D. Identification of aroma-active compounds in ‘Wonderful’ pomegranate fruit using solvent-assisted flavor evaporation and headspace solid-phase micro-extraction methods. Eur. Food Res. Technol. 2012, 235, 277–283. [Google Scholar] [CrossRef]
- Güler, Z.; Gül, E. Volatile organic compounds in the aril juices and seeds from selected five pomegranate (Punica granatum L.) cultivars. Int. J. Food Prop. 2017, 20, 281–293. [Google Scholar] [CrossRef]
- Petruzzi, L.; Campaniello, D.; Speranza, B.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Thermal treatments for fruit and vegetable juices and beverages: A literature overview. Compr. Rev. Food Sci. Food Saf. 2017, 16, 668–691. [Google Scholar] [CrossRef]
- Mena, P.; Martí, N.; Saura, D.; Valero, M.; García-Viguera, C. Combinatory effect of thermal treatment and blending on the quality of pomegranate juices. Food Bioproc. Tech. 2013, 6, 3186–3199. [Google Scholar] [CrossRef]
- Mena, P.; Vegara, S.; Martí, N.; García-Viguera, C.; Saura, D.; Valero, M. Changes on indigenous microbiota, colour, bioactive compounds and antioxidant activity of pasteurised pomegranate juice. Food Chem. 2013, 141, 2122–2129. [Google Scholar] [CrossRef]
- Turfan, O.; Türkyilmaz, M.; Yemi, O.; Özkan, M. Anthocyanin and colour changes during processing of pomegranate (Punica granatum L.; cv. Hicaznar) juice from sacs and whole fruit. Food Chem. 2011, 129, 1644–1651. [Google Scholar] [CrossRef]
- Sánchez-Vega, R.; Elez-Martínez, P.; Martín-Belloso, O. Influence of high-intensity pulsed electric field processing parameters on antioxidant compounds of broccoli juice. Innov. Food Sci. Emerg. Technol. 2015, 29, 70–77. [Google Scholar] [CrossRef]
- Bhat, S.; Singh Saini, C.; Kumar, M.; Sharma, H.K. Effect of thermal and alternate thermal processing on bottle gourd (Lagenaria siceraria) juice. J. Food Process. Preserv. 2017, 41, e12911. [Google Scholar] [CrossRef]
- Yildiz, H.; Bozkurt, H.; Icier, F. Ohmic and conventional heating of pomegranate juice: Effects on rheology, color and total phenolics. Int. J. Food Sci. Technol. 2009, 15, 503–512. [Google Scholar] [CrossRef]
- Ferrari, G.; Maresca, P.; Ciccarone, R. The application of high hydrostatic pressure for the preservation of functional foods: Pomegranate juice. J. Food Eng. 2010, 100, 245–253. [Google Scholar] [CrossRef]
- Chen, D.; Xi, H.; Guo, X.; Qin, Z.; Pang, X.; Hu, X.; Liao, X.; Wu, J. Comparative study of quality of cloudy pomegranate juice treated by high hydrostatic pressure and high temperature short time. Innov. Food Sci. Emerg. Technol. 2013, 19, 85–94. [Google Scholar] [CrossRef]
- Alighourchi, H.R.; Barzegar, M.; Sahari, M.A.; Abbasi, S. Effect of sonication on anthocyanins, total phenolic content, and antioxidant capacity of pomegranate juices. Int. Food Res. J. 2013, 20, 1703–1709. [Google Scholar]
- Pala, Ç.U.; Zorba, N.N.; Özcan, G. Microbial inactivation and physicochemical properties of ultrasound processed pomegranate juice. J. Food Prot. 2015, 78, 531–539. [Google Scholar] [CrossRef]
- Jin, T.Z.; Guo, M.; Yang, R. Combination of pulsed electric field processing and antimicrobial bottle for extending microbiological shelf-life of pomegranate juice. Innov. Food Sci. Emerg. Technol. 2014, 26, 153–158. [Google Scholar] [CrossRef]
- Evrendilek, G.A. Impacts of pulsed electric field and heat treatment on quality and sensory properties and microbial inactivation of pomegranate juice. Food Sci. Technol. Int. 2017, 23, 668–680. [Google Scholar] [CrossRef]
- Pala, Ç.U.; Toklucu, A.K. Effect of UV-C light on anthocyanin content and other quality parameters of pomegranate juice. J. Food Compos. Anal. 2011, 24, 790–795. [Google Scholar] [CrossRef]
- Putnik, P.; Kresoja, Ž.; Bosiljkov, T.; Režek Jambrak, A.; Barba, F.J.; Lorenzo, J.M.; Roohinejad, S.; Granato, D.; Žuntar, I.; Bursać Kovačević, D. Comparing the effects of thermal and non-thermal technologies on pomegranate juice quality: A review. Food Chem. 2019, 279, 150–161. [Google Scholar] [CrossRef]
- Spilimbergo, S.; Bertucco, A. Non-thermal microbial inactivation with dense CO2. Biotechnol. Bioeng. 2003, 84, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Spilimbergo, S.; Ciola, L. Supercritical CO2 and N2O pasteurization of fresh peach and kiwi juice. Int. J. Food Sci. Technol. 2010, 45, 1619–1625. [Google Scholar] [CrossRef]
- Gasperi, F.; Aprea, E.; Biasioli, F.; Carlin, S.; Endrizzi, I.; Pirretti, G.; Spilimbergo, S. Effects of supercritical CO2 and N2O pasteurisation on the quality of fresh apple juice. Food Chem. 2009, 115, 129–136. [Google Scholar] [CrossRef]
- Kincal, D.; Hill, W.S.; Balaban, M.O.; Portier, K.M.; Wei, C.I.; Marshall, M.R. A continuous high-pressure carbon dioxide system for microbial reduction in orange juice. J. Food Sci. 2005, 70, M249–M254. [Google Scholar] [CrossRef]
- Damar, S.; Balaban, M.O.; Sims, C.A. Continuous dense-phase CO2 processing of a coconut water beverage. Int. J. Food Sci. Technol. 2009, 44, 666–673. [Google Scholar] [CrossRef]
- Cappelletti, M.; Ferrentino, G.; Endrizzi, I.; Aprea, E.; Betta, E.; Corollaro, M.L.; Charles, M.; Gasperi, F.; Spilimbergo, S. High pressure carbon dioxide pasteurization of coconut water: A sport drink with high nutritional and sensory quality. J. Food Eng. 2015, 145, 73–81. [Google Scholar] [CrossRef]
- De Marchi, F.; Aprea, E.; Endrizzi, I.; Charles, M.; Betta, E.; Corollaro, M.L.; Cappelletti, M.; Ferrentino, G.; Spilimbergo, S.; Gasperi, F. Effects of pasteurization on volatile compounds and sensory properties of coconut (Cocos nucifera L.) Water: Thermal vs. High-Pressure Carbon Dioxide Pasteurization. Food Bioproc. Tech. 2015, 8, 1393–1404. [Google Scholar] [CrossRef]
- Kincal, D.; Hill, W.S.; Balabam, M.; Portier, K.M.; Sims, C.A.; Wei, C.I.; Marshall, M.R. A continuous high-pressure carbon dioxide system for cloud and quality retention in orange juice. J. Food Sci. 2006, 71, C338–C344. [Google Scholar] [CrossRef]
- Bertolini, F.M.; Morbiato, G.; Facco, P.; Marszałek, K.; Pérez-Esteve, É.; Benedito, J.; Zambon, A.; Spilimbergo, S. Optimization of the supercritical CO2 pasteurization process for the preservation of high nutritional value of pomegranate juice. J. Supercrit. Fluids 2020, 164, 104914. [Google Scholar] [CrossRef]
- Cappelletti, M.; Ferrentino, G.; Spilimbergo, S. Supercritical carbon dioxide combined with high power ultrasound: An effective method for the pasteurization of coconut water. J. Supercrit. Fluids 2014, 92, 257–263. [Google Scholar] [CrossRef]
- Ferrentino, G.; Spilimbergo, S. High pressure carbon dioxide combined with high power ultrasound pasteurization of fresh cut carrot. J. Supercrit. Fluids 2015, 105, 170–178. [Google Scholar] [CrossRef]
- Ferrentino, G.; Spilimbergo, S. A combined high pressure carbon dioxide and high power ultrasound treatment for the microbial preservation of cooked ham. J. Food Eng. 2016, 4, 47–55. [Google Scholar] [CrossRef]
- Michelino, F.; Zambon, A.; Vizzotto, M.T.; Cozzi, S.; Spilimbergo, S. High power ultrasound combined with supercritical carbon dioxide for the drying and microbial inactivation of coriander. J. CO2 Util. 2018, 24, 516–521. [Google Scholar] [CrossRef]
- Paniagua-Martínez, I.; Mulet, A.; García-Alvarado, M.A.; Benedito, J. Orange juice processing using a continuous flow ultrasound-assisted supercritical CO2 system: Microbiota inactivation and product quality. Innov. Food Sci. Emerg. 2018, 47, 362–370. [Google Scholar] [CrossRef]
- Gao, Y.; Nagy, B.; Liu, X.; Simandi, B.; Wang, Q. Supercritical CO2 extraction of lutein esters from marigold (Tagetes erecta L.) enhanced by ultrasound. J. Supercrit. Fluids 2009, 49, 345–350. [Google Scholar] [CrossRef]
- Riera, E.; Blanco, A.; García, J.; Benedito, J.; Mulet, A.; Gallego-Juárez, J.A.; Blasco, M. High-power ultrasonic system for the enhancement of mass transfer in supercritical CO2 extraction processes. Phys. Procedia 2010, 3, 141–146. [Google Scholar] [CrossRef]
- Risvik, E.; McEvan, J.A.; Colwill, J.S.; Rogers, R.; Lyon, D.H. Projective mapping: A tool for sensory analysis and consumer research. Food Qual. Prefer. 1994, 5, 263–269. [Google Scholar] [CrossRef]
- Pagès, J. Collection and analysis of perceived product inter-distances using multiple factor analysis: Application to the study of 10 white wines from the Loire Valley. Food Qual. Prefer. 2005, 16, 642–649. [Google Scholar] [CrossRef]
- Adams, J.; Williams, A.; Lancaster, B.; Foley, M. Advantages and uses of check-all-that-apply response compared to traditional scaling of attributes for salty snacks. In Proceedings of the 7th Pangborn Sensory Science Symposium, Minneapolis, MN, USA, 12–16 August 2007. [Google Scholar]
- Pfeiffer, J.C.; Gilbert, C.C. Napping by modality: A happy medium between analytical and holistic approaches. In Proceedings of the 9th Sensometrics Meeting, St Catharine, ON, Canada, 20–23 July 2008. [Google Scholar]
- Delholm, C.; Brockhoff, P.B.; Meinert, L.; Aaslung, M.D.; Bredie, W.L.P. Rapid descriptive sensory methods—Comparison of Free Multiple Sorting, Partial Napping, Napping, Flash Profiling and conventional profiling. Food Qual. Prefer. 2012, 26, 267–277. [Google Scholar] [CrossRef]
- Louw, L.; Malherbe, S.; Næs, T.; Lambrechts, M.; Van Rensburg, P.; Nieuwoudt, H. Validation of two Napping® techniques as rapid sensory screening tools for high alcohol products. Food Qual. Prefer. 2013, 30, 192–201. [Google Scholar] [CrossRef]
- Liu, J.; Schou Grønbeck, M.; Di Monaco, R.; Giacalone, D.; Bredie, W.L.P. Performance of Flash Profile and Napping with and without training for describing small sensory differences in a model wine. Food Qual. Prefer. 2016, 48, 41–49. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Leang Chheang, S.; Yin, J.; Bava, C.M.; Gimenez, A.; Leticia Vidal, L.; Gastón Ares, G. Check-all-that-apply (CATA) responses elicited by consumers: Within-assessor reproducibility and stability of sensory product characterizations. Food Qual. Prefer. 2013, 30, 56–67. [Google Scholar] [CrossRef]
- Alexi, N.; Nanou, E.; Lazo, O.; Guerrero, L.; Grigorakis, K.; Byrne, D.V. Check-All-That-Apply (CATA) with semi-trained assessors: Sensory profiles closer to descriptive analysis or consumer elicited data? Food Qual. Prefer. 2018, 64, 11–20. [Google Scholar] [CrossRef]
- Gwet, K.L. Computing inter-rater reliability and its variance in the presence of high agreement. Br. J. Math. Stat. Psychol. 2008, 61, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Meyners, M.; Castura, J.C.; Worch, T. Statistical evaluation of panel repeatability in Check-All-That-Apply questions. Food Qual. Prefer. 2016, 49, 197–204. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. SensoMineR: A package for sensory data analysis. J. Sens. Stud. 2008, 23, 14–25. [Google Scholar] [CrossRef]
- Spilimbergo, S.; Cappelletti, M.; Ferrentino, G. High pressure carbon dioxide combined with high power ultrasound processing of dry cured ham spiked with Listeria monocytogenes. Food Res. Int. 2014, 66, 264–273. [Google Scholar] [CrossRef]
- Maskan, M. Production of pomegranate (Punica granatum L.) juice concentrate by various heating methods: Colour degradation and kinetics. J. Food Eng. 2006, 72, 218–224. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, J.I.; Park, J. Effects of a combined process of high-pressure carbon dioxide and high hydrostatic pressure on the quality of carrot juice. Food Eng. Phys. Prop. 2002, 67, 1827–1834. [Google Scholar] [CrossRef]
- Pérez-Vicente, A.; Serrano, P.; Abellán, P.; García-Viguera, C. Influence of packaging material on pomegranate juice colour and bioactive compounds, during storage. J. Sci. Food Agric. 2004, 84, 639–644. [Google Scholar] [CrossRef]
- Vázquez-Araújo, L.; Chambers, E., IV; Adhikari, K.; Carbonell-Barrachina, A.A. Physico-chemical and sensory properties of pomegranate juices with pomegranate albedo and carpellar membranes homogenate. LWT Food Sci. Technol. 2011, 44, 2119–2125. [Google Scholar] [CrossRef]
- Vázquez-Araújo, L.; Koppel, K.; Chambers, E., IV; Adhikari, K.; Carbonell-Barrachina, A.A. Instrumental and sensory aroma profile of pomegranate juices from the USA: Differences between fresh and commercial juice. Flavour Fragr. J. 2011, 26, 129–138. [Google Scholar] [CrossRef]
- Mayuoni-Kirshinbaum, L.; Daus, A.; Porat, R. Changes in sensory quality and aroma volatile composition during prolonged storage of ’Wonderful’ pomegranate fruit. Int. J. Food Sci. Technol. 2013, 48, 1569–1578. [Google Scholar] [CrossRef]
- Pétrier, C.; Combet, E.; Mason, T. Oxygen-induced concurrent ultrasonic degradation of volatile and non-volatile aromatic compounds. Ultrason. Sonochem. 2007, 14, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Fabroni, S.; Amenta, M.; Timpanaro, N.; Rapisarda, P. Supercritical carbon dioxide-treated blood orange juice as a new product in the fresh fruit juice market. Innov. Food Sci. Emerg. Technol. 2010, 11, 477–484. [Google Scholar] [CrossRef]
- Kebede, B.; Lee, P.Y.; Leong, S.Y.; Kethireddy, V.; Ma, Q.; Aganovic, K.; Eyres, G.T.; Hamid, N.; Oey, I. A chemometrics approach comparing volatile changes during the shelf life of apple juice processed by Pulsed Electric Fields, High Pressure and Thermal Pasteurization. Foods 2018, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Vegara, S.; Martí, N.; Mena, P.; Saura, D.; Valero, M. Effect of pasteurization process and storage on color and shelf-life of pomegranate juices. LWT Food Sci. Technol. 2013, 54, 592–596. [Google Scholar] [CrossRef]



| Attribute | Description |
|---|---|
| Odor | Olfactory sensations perceived by smelling (orto-nasally) |
| Citrus | Sensation that recalls the smell of citrus fruits (lemon, orange, grapefruit) |
| Green | Sensation that recalls the smell of freshly cut grass |
| Floral | Sensation that recalls the smell of flowers |
| Unripe fruit | Sensation that recalls the smell of the white film that covers the seed of pomegranate and the smell of unripe fruit |
| Cooked fruit | Sensation that recalls the smell of cooked fruit |
| Berry | Sensation that recalls the smell of wild berries (blueberry, blackberry, mulberry, black currant) |
| Red fruits | Sensation that recalls the smell of red fruits (cherry, raspberry, gooseberry) |
| Earthy | Sensation that recalls the smell of wet earth |
| Fresh vegetable | Sensation that recalls the smell of green vegetables and fresh green vegetable stalks |
| Cooked vegetable | Sensation that recalls the smell of cooked vegetables (green beans, potatoes) |
| Taste | |
| Sweet | Basic taste typical of sucrose (e.g., sugar) |
| Bitter | Basic taste typical of quinine (e.g., coffee) |
| Sour | Basic taste typical of citric acid (e.g., lemon) |
| Flavor | Odors perceived through the mouth (retro-nasally) |
| Citrus | Sensation associated with citrus fruits (lemon, orange, grapefruit) |
| Unripe fruit | Sensation associated with the white film covering the seed of pomegranate and with unripe fruit |
| Cooked fruit, | Sensation associated with cooked fruit |
| Cooked vegetable | Sensation associated with cooked vegetables (beans, potatoes) |
| Mouthfeel/sensation | |
| Astringent | Sensation of dry, puckering, roughing mouthfeel |
| Pungent | Tingling sensation on the tongue not associated with a sensation of heat |
| Throat-itch | Pricking sensation felt only in the throat and not associated with a sensation of heat |
| NT | HPP | HT | CO2 | CO2-US | p | |
|---|---|---|---|---|---|---|
| SSC (brix) | 16.6 (0.1)a | 16.6 (0.2)a | 16.5 (0.2)ab | 16.3 (0.1)ab | 16.2 (0.2)b | 0.005 |
| pH | 3.1 | 3.1 | 3.1 | 3.2 | 3.2 | - |
| L* | 45.4 (1.9) | 42.5 (3.2) | 45.6 (0.3) | 44.9 (2.5) | 44.2 (4.1) | 0.096 (NS) |
| a* | 63.3 (1.4)a | 60.8 (3.4)ab | 59.1 (0.2)b | 61.2 (1.7)ab | 59.3 (3.7)b | 0.007 |
| b* | 14.3 (3.1) | 17.7 (2.4) | 16.1 (0.3) | 15.7 (1.8) | 17.4 (4.0) | 0.059 (NS) |
| Concentration Volatile Compounds (µg/L of 2-Octanol) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | LRI | NT | HPP | HT | CO2 | CO2-US | ||
| 1 | ethyl acetate | 898 | 16 (1)a | 6.9 (0.3)d | 9.33 (0.04)b | 8.4 (0.4)c | 5.2 (0.5)e | <0.0001 |
| 2 | 2-pentanone | 988 | 0.38 (0.01)c | 1.08 (0.03)b | 2.19 (0.09)a | 0.30 (0.03)d | 0.33 (0.02)d | <0.0001 |
| 3 | methyl 2-methylbutanoate | 1019 | 3.1 (0.2)a | 1.25 (0.06)c | 1.91 (0.02)b | 0.29 (0.06)d | 0.33 (0.02)d | <0.0001 |
| 4 | α-pinene | 1024 | 16.3 (1.6)a | 6.5 (0.7)b | 14 (1)a | 1.2 (0.1)d | 2.6 (0.4)c | <0.0001 |
| 5 | toluene | 1048 | 0.28 (0.06)b | 0.3 (0.1)b | 0.21 (0.02)b | 0.10 (0.04)c | 0.7 (0.2)a | 0.0001 |
| 6 | ethyl 2-methylbutanoate | 1060 | 8.5 (0.5)a | 4.5 (0.1)b | 4.6 (0.1)b | 0.9 (0.1)d | 1.4 (0.1)c | <0.0001 |
| 7 | camphene | 1066 | 93.5 (9.8)a | 26 (2)c | 73 (4)b | 5.3 (0.2)e | 14 (2)d | <0.0001 |
| 8 | hexanal | 1093 | 1.9 (0.1)c | 2.7 (0.3)a | 2.2 (0.1)b | 2.02 (0.03)c | 2.4 (0.2)ab | 0.001 |
| 9 | β-pinene | 1111 | 25.8 (1.8)a | 16 (1)b | 10 (1)c | 0.32 (0.07)e | 1.4 (0.2)d | <0.0001 |
| 10 | isoamyl acetate | 1133 | 3.6 (0.3)a | 1.3 (0.2)b | 3.8 (0.4)a | ND | 0.9 (0.1)b | <0.0001 |
| 11 | α-phellandrene | 1171 | 4.2 (0.4)b | 2.8 (0.4)c | 7.1 (0.7)a | 0.42 (0.09)e | 0.71 (0.07)d | <0.0001 |
| 12 | β-myrcene | 1174 | 16.1 (1.2)a | 11.9 (0.8)b | 13 (1)b | 1.1 (0.1)d | 2.8 (0.3)c | < 0.0001 |
| 13 | α-terpinene | 1187 | 2.7 (0.3)b | 2.1 (0.1)c | 3.7 (0.2)a | 0.09 (0.02)e | 0.17 (0.03)d | <0.0001 |
| 14 | methyl hexanoate | 1199 | 2.5 (0.1)a | 0.94 (0.09)c | 1.70 (0.08)b | 0.18 (0.05)e | 0.32 (0.02)d | <0.0001 |
| 15 | limonene | 1206 | 460 (29)a | 444 (11)a | 359 (30)b | 24.5 (0.4)d | 46 (5)c | <0.0001 |
| 16 | 1.8-cineole | 1213 | 4.6 (0.3)a | 3.3 (0.1)c | 3.8 (0.1)b | 3.1 (0.2)c | 2.6 (0.1)d | <0.0001 |
| 17 | β-phellandrene | 1215 | 37 (5)a | 32 (1)a | 30 (4)a | 5.6 (0.3)c | 12 (1)b | <0.0001 |
| 18 | (E)-2-hexenal | 1228 | ND | 0.5 (0.1)a | 0.29 (0.04)b | 0.3 (0.1)b | 0.37 (0.08)ab | <0.0001 |
| 19 | 2-pentyl furan | 1244 | 0.86 (0.04)a | 0.72 (0.02)b | 0.48 (0.06)c | 0.06 (0.02)e | 0.24 (0.02)d | <0.0001 |
| 20 | ethyl hexanoate | 1245 | 4.6 (0.3)a | 2.22 (0.06)c | 2.63 (0.06)b | 0.45 (0.08)e | 0.92 (0.05)d | <0.0001 |
| 21 | γ-terpinene | 1254 | 53 (3)a | 52 (2)a | 45 (4)b | 1.4 (0.1)d | 3.8 (0.3)c | <0.0001 |
| 22 | styrene | 1269 | 0.30 (0.07)a | 0.23 (0.04)a | 0.07 (0.01)b | ND | 0.22 (0.04)a | <0.0001 |
| 23 | p-cymene | 1280 | 17 (1)a | 16.0 (0.4)a | 12.6 (0.7)b | 0.8 (0.1)d | 2.0 (0.1)c | <0.0001 |
| 24 | hexyl acetate | 1284 | 1.50 (0.06)a | 0.87 (0.07)b | 1.00 (0.06)b | 0.21 (0.07)d | 0.6 (0.1)c | <0.0001 |
| 25 | terpinolene | 1292 | 3.9 (0.3)a | 4.4 (0.2)a | 4.4 (0.3)a | 0.15 (0.03)c | 0.47 (0.05)b | <0.0001 |
| 26 | 2-octanone | 1296 | 3.8 (0.1)a | 3.9 (0.3)a | 3.67 (0.03)a | 3.2 (0.1)b | 3.34 (0.08)b | 0.002 |
| 27 | octanal | 1300 | 0.42 (0.07)c | 0.4 (0.2)c | 1.28 (0.02)b | 0.9 (0.2)b | 2.0 (0.2)a | <0.0001 |
| 28 | (Z)-3-hexen-1-ol acetate | 1330 | 1.38 (0.04)a | 0.77 (0.07)b | 0.78 (0.03)b | 0.36 (0.03)d | 0.46 (0.04)c | <0.0001 |
| 29 | 6-methyl 5-hepten-2-one | 1350 | 1.47 (0.05)a | 1.13 (0.07)b | 1.47 (0.05)a | 0.7 (0.1)c | 1.1 (0.1)b | <0.0001 |
| 30 | 1-hexanol | 1365 | 86 (6)bc | 108 (4)a | 91.7 (0.3)b | 78 (2)cd | 72 (6)d | <0.0001 |
| 31 | (E)-3-hexen-1-ol | 1375 | 3.3 (0.3)bc | 4.05 (0.06)a | 4.2 (0.1)a | 3.0 (0.2)c | 3.7 (0.1)b | <0.0001 |
| 32 | (Z)-3-hexen-1-ol | 1396 | 1.53 (0.09)a | 0.73 (0.06)b | 0.74 (0.01)b | 0.28 (0.04)d | 0.39 (0.05)c | <0.0001 |
| 33 | 2-nonanone | 1399 | 11.9 (0.6)a | 11.8 (0.4)a | 8.62 (0.02)b | 2.5 (0.1)d | 4.1 (0.2)c | <0.0001 |
| 34 | nonanal | 1405 | 3.2 (0.6)bc | 2.7 (0.4)c | 4.0 (0.3)b | 3.4 (0.5)bc | 5.7 (0.9)a | 0.001 |
| 35 | furfural | 1478 | 2 (2) | 1.1 (0.4) | 3.9 (0.2) | 0.12 (0.03) | 2 (2) | 0.073 (NS) |
| 36 | tetramethylbenzene 1,2,3,4 | 1498 | ND | ND | ND | 1.15 (0.01)a | 0.82 (0.07)b | <0.0001 |
| 37 | 2-ethyl-1-hexanol | 1502 | 1.16 (0.06)e | 1.6 (0.2)d | 3.0 (0.05)c | 3.78 (0.05)b | 6.1 (0.2)a | <0.0001 |
| 38 | decanal | 1510 | 1.0 (0.1)bc | 0.7 (0.2)c | 1.1 (0.2)bc | 1.2 (0.1)b | 1.6 (0.1)a | 0.001 |
| 39 | benzaldehyde | 1539 | 1.13 (0.06)b | 0.5 (0.1)c | 2.03 (0.09)a | 1.8 (0.4)a | 2.9 (0.7)a | 0.0001 |
| 40 | linalool | 1559 | 0.82 (0.08)b | 0.95 (0.1)b | 1.26 (0.04)a | 0.56 (0.07)c | 0.44 (0.09)c | <0.0001 |
| 41 | 4-terpineol | 1606 | 0.56 (0.07)b | ND | 0.84 (0.02)a | 0.3 (0.1)c | 0.27 (0.04)c | <0.0001 |
| 42 | 2-octen-1-ol acetate | 1639 | 2.38 (0.3)a | 2.4 (0.3)a | 1.4 (0.2)b | 0.18 (0.06)d | 0.40 (0.06)c | <0.0001 |
| 43 | 1-hexadecene | 1651 | 1.0 (0.2)ab | 1.5 (0.8)a | 0.8 (0.5)ab | 1.4 (0.3)ab | 0.20 (0.06)b | 0.040 |
| 44 | acetophenone | 1662 | 0.2 (0.1) | 0.12 (0.04) | 0.3 (0.2) | 0.5 (0.3) | 0.7 (0.7) | 0.4 (NS) |
| 45 | unidentified hydrocarbon | 1674 | 1.8 (0.2) | 5 (5) | 3 (3) | 3 (2) | 0.2 (0.1) | 0.324 (NS) |
| 46 | heptadecane | 1700 | ND | 0.6 (0.6) | 0.3 (0.3) | 0.3 (0.1) | ND | 0.195 (NS) |
| 47 | α-terpineol | 1706 | 1.8 (0.1)ab | 1.67 (0.06)b | 2.05 (0.08)a | 1.02 (0.07)c | 0.83 (0.04)d | <0.0001 |
| 48 | zingiberene | 1730 | 1.1 (0.1)b | 1.1 (0.2)b | 1.43 (0.04)a | 0.71 (0.08)c | 1.0 (0.1)b | 0.0001 |
| 49 | β-bisabolene | 1737 | 0.48 (0.02)b | 0.6 (0.1)ab | 0.7 (0.1)a | 0.29 (0.09)c | 0.44 (0.05)bc | 0.01 |
| 50 | naphthalene | 1751 | 0.14 (0.06)c | 0.3 (0.1)c | 0.3 (0.1)c | 2.12 (0.08)a | 1.44 (0.04)b | <0.0001 |
| 51 | β-sesquiphellandrene | 1779 | 0.43 (0.02) | 0.5 (0.1) | 0.51 (0.04) | 0.45 (0.09) | 0.48 (0.05) | 0.6 (NS) |
| 52 | α-curcumene | 1784 | 0.91 (0.07)c | 1.0 (0.1)bc | 1.26 (0.05)a | 1.0 (0.1)bc | 1.18 (0.03)ab | 0.006 |
| 53 | 3.5-dimethylbenzaldehyde | 1822 | 0.14 (0.04)d | 0.31 (0.08)c | 0.33 (0.09)c | 1.92 (0.09)b | 2.7 (0.2)a | <0.0001 |
| 54 | anethole | 1840 | 0.55 (0.04)a | 0.40 (0.03)b | 0.28 (0.03)c | 0.05 (0.02)e | 0.10 (0.02)d | <0.0001 |
| 55 | hexanoic acid | 1876 | 0.98 (0.05)ab | 1.03 (0.07)ab | 0.88 (0.04)b | 0.9 (0.2)ab | 1.4 (0.4)a | 0.048 |
| 56 | Phenol | 2018 | 0.15 (0.03) | 0.14 (0.02) | 0.16 (0.04) | 0.15 (0.06) | 0.2 (0.1) | 0.653 (NS) |
| 57 | p-cresol | 2094 | 0.09 (0.02) | 0.10 (0.03) | 0.09 (0.03) | 0.09 (0.04) | 0.09 (0.04) | 0.98 (NS) |
| 58 | m-cresol | 2102 | 0.53 (0.08) | 0.54 (0.05) | 0.56 (0.09) | 0.5 (0.1) | 0.6 (0.2) | 0.96 (NS) |
| ΔNT | p | ΔHPP | p | ΔHT | p | ΔCO2 | p | ΔCO2-US | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| SSC (brix) | 0.1 | 0.519 | 0.3 | 0.065 | 0.03 | 0.815 | −0.1 | 0.116 | 0.2 | 0.057 |
| pH | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - |
| L* | −0.5 | 0.608 | 4.4 | 0.006 | 4.1 | <0.0001 | −0.8 | 0.495 | 0.8 | 0.669 |
| a* | −4.6 | 0.0001 | −1.2 | 0.399 | −5.5 | <0.0001 | −5.4 | <0.0001 | −4.1 | 0.033 |
| b* | 2.9 | 0.051 | −2.1 | 0.056 | −1.2 | <0.0001 | 3.8 | 0.001 | 4.4 | 0.031 |
| ΔNT | p | ΔHPP | p | ΔHT | p | ΔCO2 | p | ΔCO2-US | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| ethyl acetate | −11.10 | <0.0001 | 3.61 | 0.001 | 0.03 | 0.871 | −0.44 | 0.436 | −1.21 | 0.015 |
| 2-pentanone | 0.01 | 0.422 | −0.74 | 0.001 | −0.06 | 0.442 | −0.02 | 0.506 | 0.09 | 0.025 |
| methyl 2-methylbutanoate | −1.88 | 0.0001 | 1.68 | 0.0001 | 0.10 | 0.182 | 0.003 | 0.945 | −0.14 | 0.002 |
| α-pinene | 3.22 | 0.029 | 5.61 | 0.001 | −1.54 | 0.122 | −0.80 | 0.0001 | −2.22 | 0.0001 |
| toluene | −0.06 | 0.243 | −0.10 | 0.239 | 0.11 | 0.015 | −0.01 | 0.819 | −0.47 | 0.007 |
| ethyl 2-methylbutanoate | −4.58 | <0.0001 | 3.27 | 0.001 | 0.35 | 0.009 | 0.16 | 0.295 | −0.63 | 0.011 |
| camphene | 22.21 | 0.020 | 35.20 | 0.0001 | −5.71 | 0.134 | −2.50 | <0.0001 | −11.35 | 0.001 |
| hexanal | 1.46 | 0.003 | −1.01 | 0.014 | −0.32 | 0.029 | −0.28 | 0.044 | −1.01 | 0.001 |
| β-pinene | −8.65 | 0.001 | −6.25 | 0.002 | −4.66 | 0.001 | −0.32 | 0.001 | −1.31 | 0.0001 |
| isoamyl acetate | −1.65 | 0.002 | 2.07 | <0.0001 | −0.42 | 0.182 | DL | - | −0.85 | 0.0001 |
| α-phellandrene | 0.80 | 0.028 | 0.27 | 0.326 | −1.38 | 0.024 | −0.21 | 0.013 | −0.71 | <0.0001 |
| β-myrcene | −0.25 | 0.783 | −2.30 | 0.013 | −0.35 | 0.656 | −0.87 | 0.0001 | −2.80 | <0.0001 |
| α-terpinene | −0.46 | 0.036 | −0.17 | 0.074 | 0.15 | 0.384 | −0.05 | 0.020 | −0.17 | 0.001 |
| methyl hexanoate | −1.04 | 0.0001 | 1.39 | <0.0001 | −0.03 | 0.682 | −0.03 | 0.449 | −0.21 | 0.003 |
| limonene | −133.48 | 0.001 | −120.22 | 0.001 | −27.38 | 0.199 | −19.96 | <0.0001 | −43.35 | 0.0001 |
| 1.8-cineole | 0.54 | 0.043 | 0.42 | 0.021 | −0.01 | 0.961 | 0.03 | 0.820 | −1.53 | <0.0001 |
| β-phellandrene | 11.71 | 0.015 | −12.38 | 0.002 | −5.07 | 0.075 | −2.83 | 0.0001 | −10.75 | 0.0001 |
| (E)-2-hexenal | 0.83 | <0.0001 | −0.33 | 0.005 | 0.11 | 0.221 | 0.23 | 0.024 | 0.11 | 0.310 |
| 2-pentyl furan | −0.30 | 0.001 | −0.27 | <0.0001 | 0.05 | 0.317 | −0.06 | 0.003 | −0.24 | <0.0001 |
| ethyl hexanoate | −2.32 | 0.0001 | 1.26 | 0.0001 | −0.09 | 0.252 | −0.19 | 0.019 | −0.75 | <0.0001 |
| γ-terpinene | −16.68 | 0.001 | −15.61 | 0.001 | −4.62 | 0.105 | −1.26 | <0.0001 | −3.71 | <0.0001 |
| styrene | 0.41 | 0.161 | −0.15 | 0.007 | 0.04 | 0.003 | ND | - | −0.22 | 0.001 |
| p-cymene | −4.31 | 0.003 | −3.78 | 0.001 | −1.47 | 0.027 | −0.57 | 0.001 | −1.92 | <0.0001 |
| hexyl acetate | −0.94 | <0.0001 | −0.04 | 0.398 | −0.02 | 0.728 | −0.05 | 0.346 | −0.53 | 0.001 |
| terpinolene | −0.06 | 0.714 | −1.16 | 0.001 | −0.01 | 0.976 | −0.12 | 0.005 | −0.37 | 0.0001 |
| 2-octanone | −0.09 | 0.390 | −0.43 | 0.099 | 0.01 | 0.721 | 0.12 | 0.680 | −0.11 | 0.271 |
| octanal | 0.003 | 0.970 | 0.40 | 0.392 | −0.39 | 0.002 | −0.02 | 0.895 | −1.09 | 0.009 |
| (Z)-3-hexen-1-ol acetate | −0.78 | <0.0001 | 0.02 | 0.625 | −0.03 | 0.317 | −0.07 | 0.056 | −0.28 | 0.0001 |
| 6-methyl 5-hepten-2-one | 0.78 | 0.001 | 0.09 | 0.397 | 0.07 | 0.310 | 0.10 | 0.256 | −0.75 | 0.001 |
| 1-hexanol | 22.11 | 0.004 | −25.08 | 0.001 | 1.43 | 0.127 | −1.58 | 0.429 | −23.28 | 0.002 |
| (E)-3-hexen-1-ol | 2.31 | 0.0001 | −0.83 | 0.001 | 0.13 | 0.480 | 0.32 | 0.091 | −0.52 | 0.050 |
| (Z)-3-hexen-1-ol | −0.95 | <0.0001 | 0.04 | 0.574 | 0.02 | 0.516 | −0.03 | 0.273 | −0.28 | 0.001 |
| 2-nonanone | −1.45 | 0.017 | −2.58 | 0.001 | −0.28 | 0.124 | −0.36 | 0.005 | −2.64 | 0.0001 |
| nonanal | −0.01 | 0.984 | 1.15 | 0.191 | −0.78 | 0.063 | 0.18 | 0.577 | −1.91 | 0.075 |
| furfural | −1.48 | 0.340 | 0.80 | 0.059 | 2.19 | 0.048 | 1.40 | 0.185 | −0.60 | 0.643 |
| tetramethylbenzene 1.2.3.4 | ND | - | ND | - | ND | - | −0.16 | 0.102 | 1.14 | <0.0001 |
| 2-ethyl-1-hexanol | −0.01 | 0.862 | 0.48 | 0.012 | 1.67 | <0.0001 | 0.46 | 0.0001 | −2.54 | 0.0001 |
| decanal | −0.20 | 0.214 | 1.83 | 0.319 | −0.18 | 0.230 | −0.19 | 0.114 | −0.58 | 0.029 |
| benzaldehyde | −0.61 | 0.0001 | 1.36 | 0.0001 | 0.51 | 0.001 | 0.82 | 0.024 | −0.08 | 0.869 |
| linalool | 0.11 | 0.201 | −0.19 | 0.065 | 0.09 | 0.102 | 0.23 | 0.006 | −0.29 | 0.008 |
| hexadecane | 0.05 | 0.001 | ND | - | ND | - | ND | - | ND | - |
| 4-terpineol | 1.03 | 0.001 | 1.18 | 0.0001 | 0.58 | 0.0001 | 0.06 | 0.392 | −0.17 | 0.004 |
| 2-octen-1-ol acetate | −0.45 | 0.073 | −0.96 | 0.005 | 0.02 | 0.870 | −0.01 | 0.845 | −0.40 | 0.0001 |
| 1-hexadecene | −0.11 | 0.469 | −0.74 | 0.208 | −0.47 | 0.148 | −0.56 | 0.288 | 0.04 | 0.468 |
| acetophenone | −0.19 | 0.085 | 0.20 | 0.139 | −0.20 | 0.126 | −0.36 | 0.078 | −0.64 | 0.189 |
| unidentified hydrocarbon | 0.50 | 0.234 | −1.24 | 0.676 | −2.62 | 0.159 | −1.27 | 0.472 | −0.15 | 0.136 |
| heptadecane | ND | - | −0.20 | 0.607 | −0.25 | 0.201 | −0.13 | 0.500 | ND | - |
| α-terpineol | 0.13 | 0. 252 | 0.20 | 0.047 | 0.09 | 0.161 | 0.10 | 0.091 | −0.45 | 0.0001 |
| zingiberene | 0.19 | 0.033 | −0.22 | 0.112 | −0.06 | 0.144 | −0.47 | 0.0001 | −0.78 | 0.0001 |
| β-bisabolene | 0.00 | 1.000 | −0.25 | 0.033 | −0.10 | 0.188 | −0.15 | 0.044 | −0.35 | 0.0001 |
| naphthalene | 0.29 | 0.095 | −0.11 | 0.225 | −0.12 | 0.241 | 0.03 | 0.899 | 1.33 | <0.0001 |
| β-sesquiphellandrene | 0.08 | 0.005 | −0.18 | 0.077 | 0.01 | 0.902 | −0.18 | 0.035 | −0.36 | 0.001 |
| α-curcumene | 0.04 | 0.450 | −0.20 | 0.111 | −0.10 | 0.087 | −0.64 | 0.002 | −0.93 | <0.0001 |
| 3.5-dimethylbenzaldehyde | 0.08 | 0.038 | −0.04 | 0.603 | 0.34 | 0.015 | 2.27 | 0.0001 | 0.88 | 0.010 |
| anethole | −0.24 | 0.001 | −0.05 | 0.066 | 0.01 | 0.842 | −0.003 | 0.820 | −0.10 | 0.0001 |
| hexanoic acid | 0.16 | 0.024 | −0.14 | 0.116 | 0.15 | 0.272 | 0.14 | 0.263 | −0.47 | 0.108 |
| phenol | −0.04 | 0.050 | 0.01 | 0.621 | −0.02 | 0.566 | −0.03 | 0.587 | −0.11 | 0.153 |
| p-cresol | −0.02 | 0.251 | −0.02 | 0.374 | 0.00 | 1.0 | −0.01 | 0.795 | −0.03 | 0.270 |
| m-cresol | −0.09 | 0.172 | −0.03 | 0.647 | −0.02 | 0.799 | −0.05 | 0.541 | −0.17 | 0.144 |
Sample Availability: Samples of the compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosca, A.C.; Menghi, L.; Aprea, E.; Mazzucotelli, M.; Benedito, J.; Zambon, A.; Spilimbergo, S.; Gasperi, F. Effect of CO2 Preservation Treatments on the Sensory Quality of Pomegranate Juice. Molecules 2020, 25, 5598. https://doi.org/10.3390/molecules25235598
Mosca AC, Menghi L, Aprea E, Mazzucotelli M, Benedito J, Zambon A, Spilimbergo S, Gasperi F. Effect of CO2 Preservation Treatments on the Sensory Quality of Pomegranate Juice. Molecules. 2020; 25(23):5598. https://doi.org/10.3390/molecules25235598
Chicago/Turabian StyleMosca, Ana Carolina, Leonardo Menghi, Eugenio Aprea, Maria Mazzucotelli, Jose Benedito, Alessandro Zambon, Sara Spilimbergo, and Flavia Gasperi. 2020. "Effect of CO2 Preservation Treatments on the Sensory Quality of Pomegranate Juice" Molecules 25, no. 23: 5598. https://doi.org/10.3390/molecules25235598
APA StyleMosca, A. C., Menghi, L., Aprea, E., Mazzucotelli, M., Benedito, J., Zambon, A., Spilimbergo, S., & Gasperi, F. (2020). Effect of CO2 Preservation Treatments on the Sensory Quality of Pomegranate Juice. Molecules, 25(23), 5598. https://doi.org/10.3390/molecules25235598









