Importance of the C12 Carbon Chain in the Biological Activity of Rhamnolipids Conferring Protection in Wheat against Zymoseptoria tritici
Abstract
1. Introduction
2. Results
2.1. Chemical Description of the Synthesized RLs
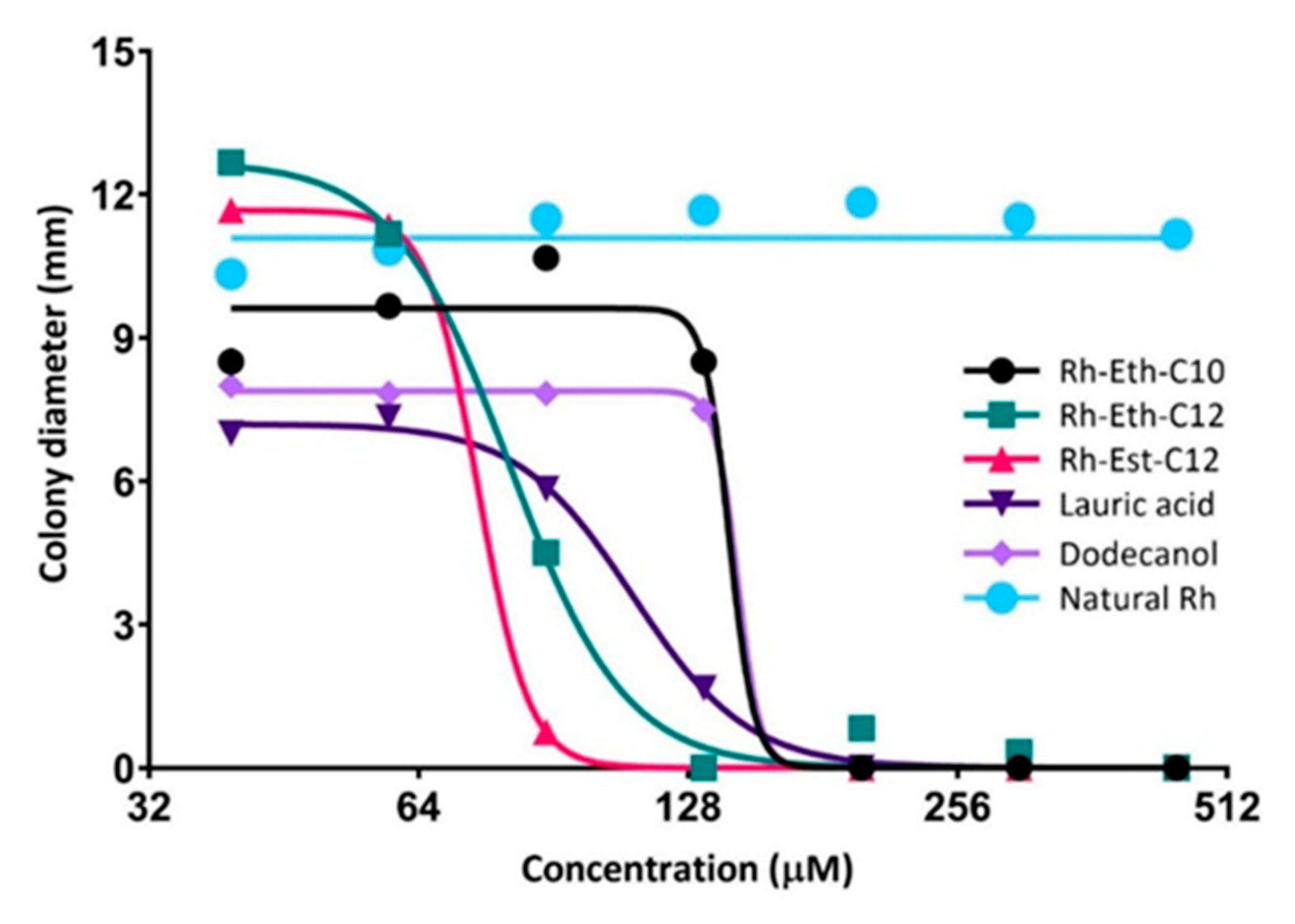
2.2. Biological Activities of RLs Differ According to the Length of Their Carbon Chain
2.3. Correlations between In Vitro Antifungal Activity, in Planta Defence Elicitation and Protection Efficacy
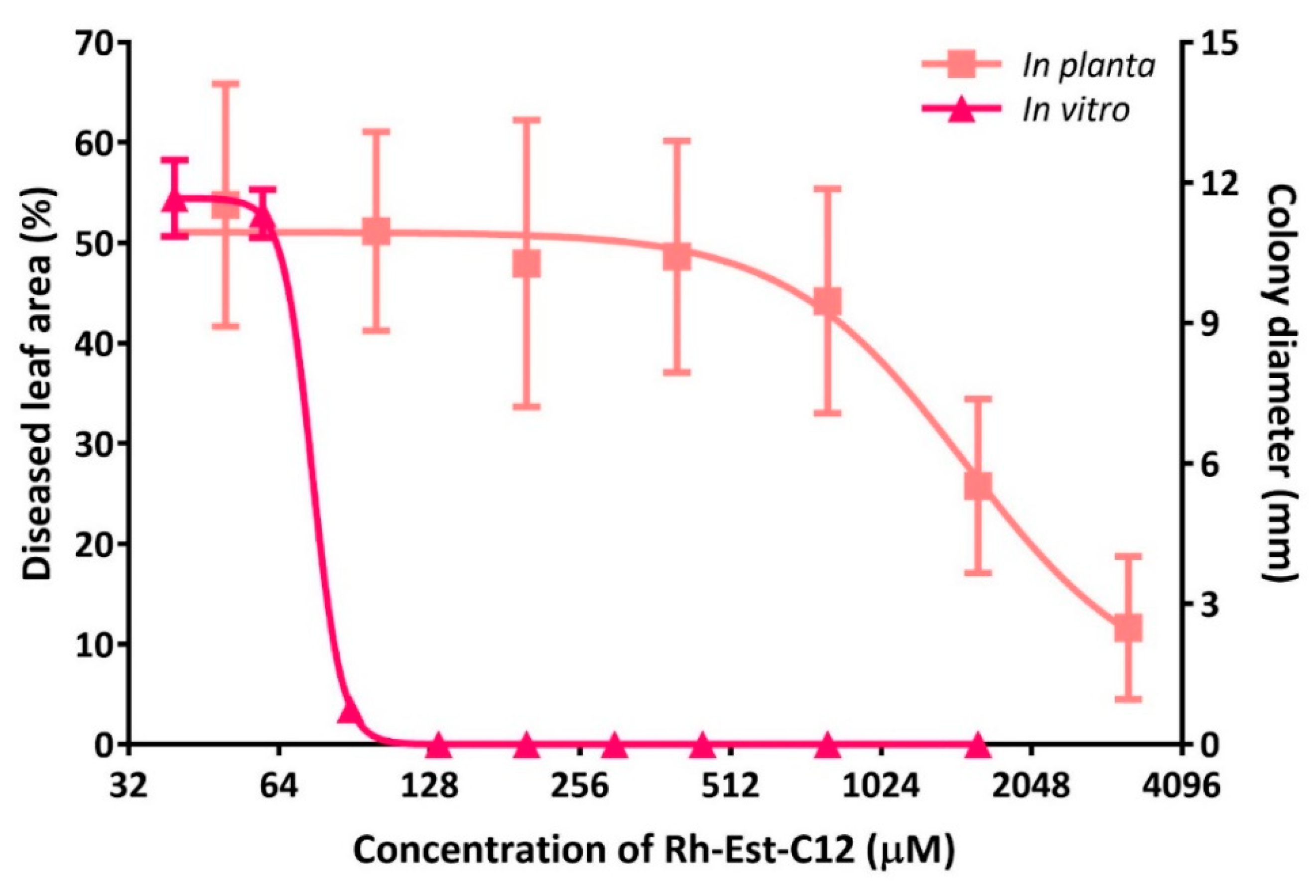
2.4. Comparison of In Vitro Versus in Planta Antifungal Effects of Rh-Est-C12
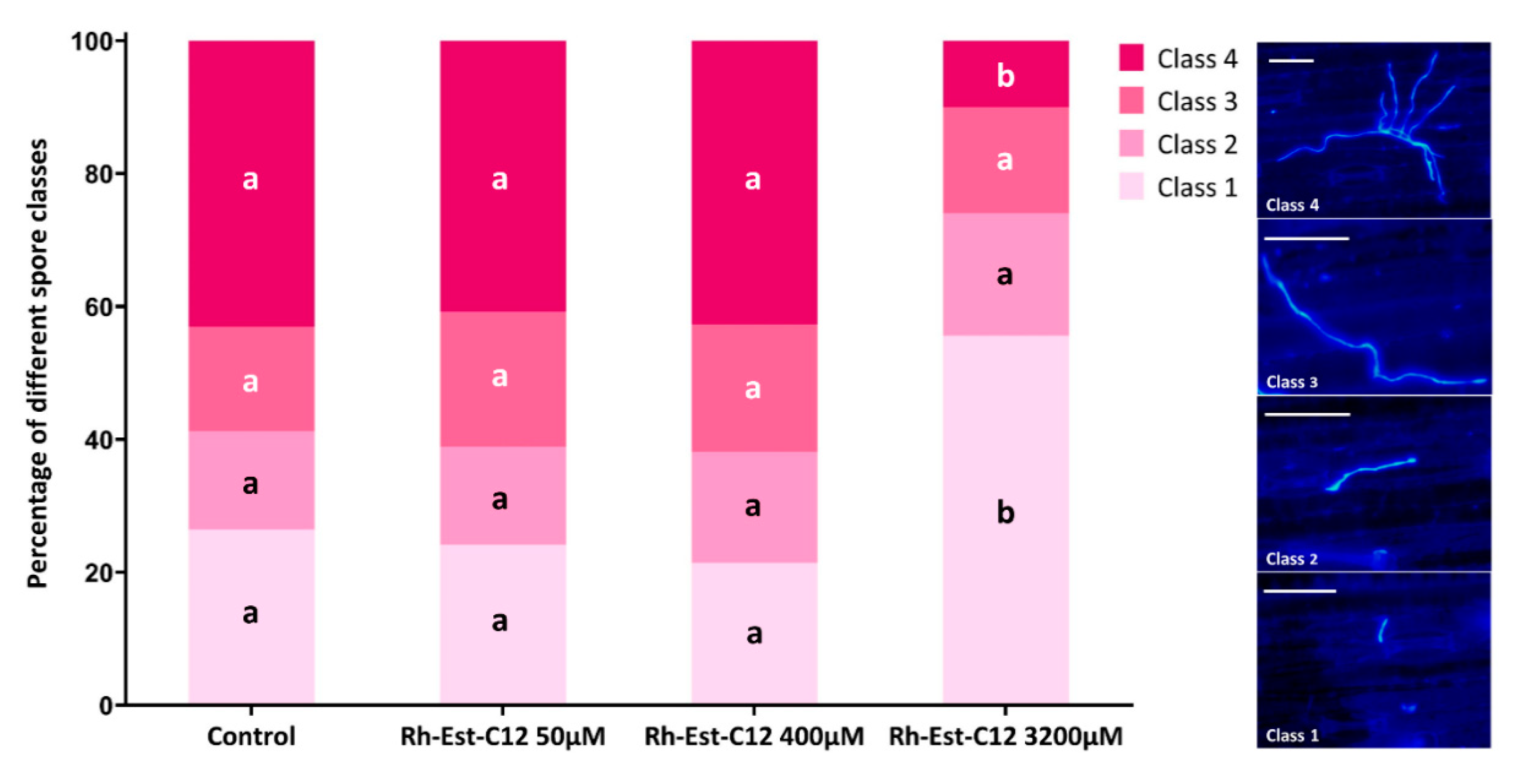
2.5. Direct In Vitro Antifungal Activity of Rh-Est-C12 Does not Vary Depending on Z. tritici Strains
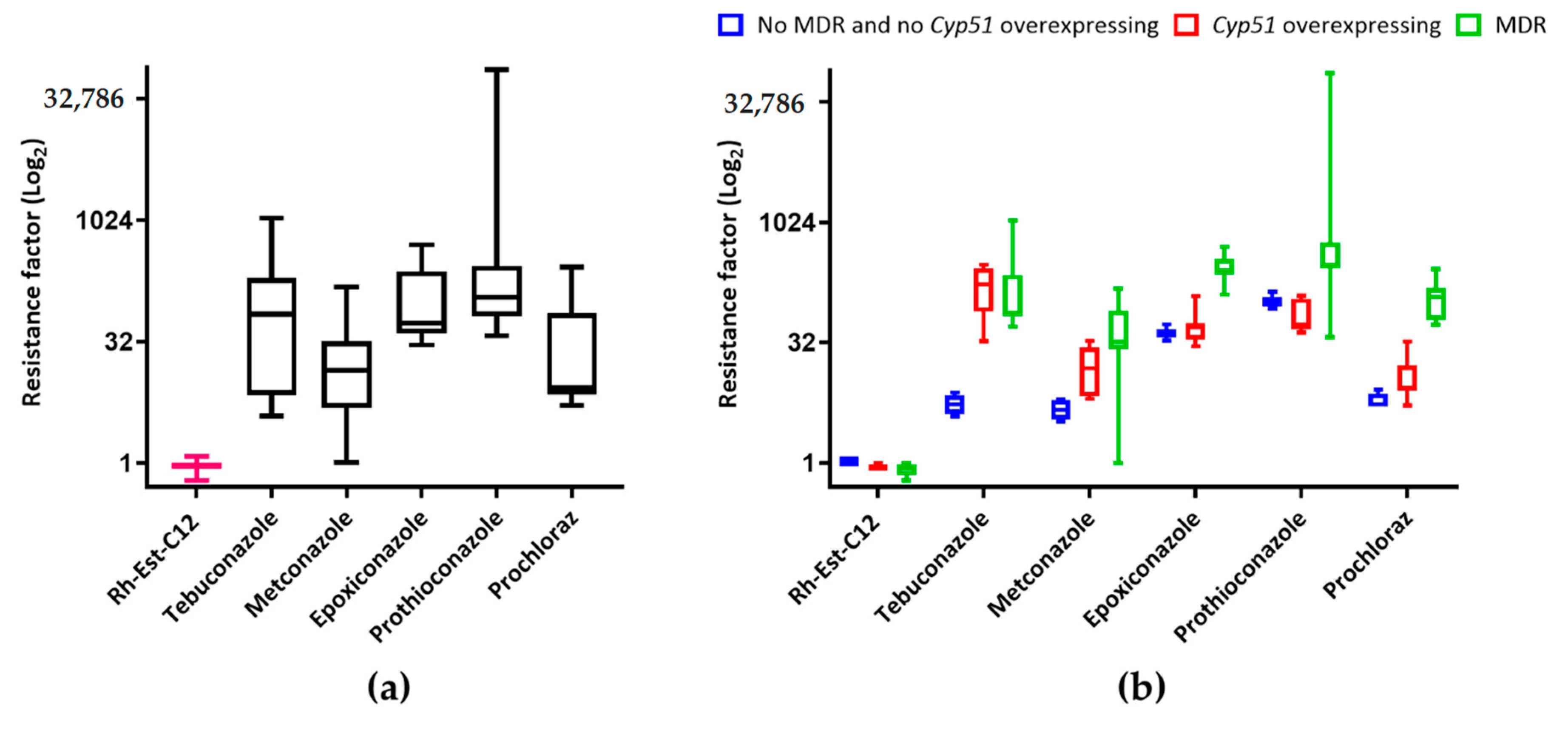
3. Discussion
4. Materials and Methods
4.1. Chemical Compounds Used
4.2. General Procedures for RL Synthesis
4.3. RL Chemical Analysis
4.3.1. Octadecyl α/β-L-Rhamnopyranoside (Rh-Eth-C18)
4.3.2. Octanoyl α/β-L-Rhamnopyranoside (Rh-Est-C8)
4.3.3. Decanoyl α/β-L-Rhamnopyranoside (Rh-Est-C10)
4.3.4. Octenylsuccinate α/β-L-Rhamnopyranoside (Rh-Suc-C8)
4.3.5. Iso-Dodecenylsuccinate α/β-L-Rhamnopyranoside (Rh-Suc-C12b)
4.3.6. 3-Octenyloctylsuccinate α/β-L-Rhamnopyranoside (Rh-Suc-C8-C8)
4.3.7. 3-Octenyldodecylsuccinate α/β-L-Rhamnopyranoside (Rh-Suc-C8-C12)
4.3.8. 3-Dodecenyloctylsuccinate α/β-L-Rhamnopyranoside (Rh-Suc-C12-C8)
4.3.9. 3-Dodecenyldodecylsuccinate α/β-L-Rhamnopyranoside (Rh-Suc-C12-C12)
4.4. Plant Treatment and Inoculation
4.5. Plant Defence-Related Enzyme Assays
4.6. In Planta Antifungal Effect of Rh-Est-C12
4.7. In Vitro Direct Antifungal Effect of Rhamnolipids
4.8. Fungal Strain Effect of Rh-Est-C12 Antifungal Activity
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- FAO Cereal Supply and Demand Brief | World Food Situation | Food and Agriculture Organization of the United Nations [WWW Document], n.d. Available online: http://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 18 January 2019).
- Fones, H.; Gurr, S. The impact of Septoria tritici Blotch disease on wheat: An EU perspective. Fungal Genet. Biol. 2015, 79, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Torriani, S.F.F.; Melichar, J.P.E.; Mills, C.; Pain, N.; Sierotzki, H.; Courbot, M. Zymoseptoria tritici: A major threat to wheat production, integrated approaches to control. Fungal Genet. Biol. 2015, 79, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Cools, H.J.; Hawkins, N.J.; Fraaije, B.A. Constraints on the evolution of azole resistance in plant pathogenic fungi. Plant Pathol. 2013, 62, 36–42. [Google Scholar] [CrossRef]
- McDonald, M.C.; Renkin, M.; Spackman, M.; Orchard, B.; Croll, D.; Solomon, P.S.; Milgate, A. Rapid Parallel Evolution of Azole Fungicide Resistance in Australian Populations of the Wheat Pathogen Zymoseptoria tritici. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, D.; Salat, M.; Frey, R.; Mosbach, A.; Luksch, T.; Balmer, D.; Hansen, R.; Widdison, S.; Logan, G.; Dietrich, R.A.; et al. A dispensable paralog of succinate dehydrogenase subunit C mediates standing resistance towards a subclass of SDHI fungicides in Zymoseptoria tritici. PLoS Pathog. 2019, 15. [Google Scholar] [CrossRef] [PubMed]
- D’aes, J.; De Maeyer, K.; Pauwelyn, E.; Höfte, M. Biosurfactants in plant-Pseudomonas interactions and their importance to biocontrol. Environ. Microbiol. Rep. 2010, 2, 359–372. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Sachdev, D.P.; Cameotra, S.S. Biosurfactants in agriculture. Appl. Microbiol. Biotechnol. 2013, 97, 1005–1016. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Natural surfactants used in cosmetics: Glycolipids. Int. J. Cosmet. Sci. 2009, 31, 255–261. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Q.; Hua, Y.; Chen, J.; Zhang, H.; Wang, H. Potential applications of biosurfactant rhamnolipids in agriculture and biomedicine. Appl. Microbiol. Biotechnol. 2017, 101, 8309–8319. [Google Scholar] [CrossRef]
- Shao, B.; Liu, Z.; Zhong, H.; Zeng, G.; Liu, G.; Yu, M.; Liu, Y.; Yang, X.; Li, Z.; Fang, Z.; et al. Effects of rhamnolipids on microorganism characteristics and applications in composting: A review. Microbiol. Res. 2017, 200, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Vatsa, P.; Sanchez, L.; Clement, C.; Baillieul, F.; Dorey, S. Rhamnolipid Biosurfactants as New Players in Animal and Plant Defense against Microbes. Int. J. Mol. Sci. 2010, 11, 5095–5108. [Google Scholar] [CrossRef] [PubMed]
- Stanghellini, M.E.; Miller, R.M. BIOSURFACTANTS: Their Identity and Potential Efficacy in the Biological Control of Zoosporic Plant Pathogens. Plant Dis. 1997, 81, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Haba, E.; Pinazo, A.; Jauregui, O.; Espuny, M.J.; Infante, M.R.; Manresa, A. Physicochemical characterization and antimicrobial properties of rhamnolipids produced by Pseudomonas aeruginosa 47T2 NCBIM 40044. Biotechnol. Bioeng. 2003, 81, 316–322. [Google Scholar] [CrossRef]
- Yoo, D.S.; Lee, B.S.; Kim, E.K. Characteristics of Microbial Biosurfactant as an Antifungal Agent Against Plant Pathogenic Fungus. J. Microbiol. Biotechnol. 2005, 15, 1164–1169. [Google Scholar]
- Varnier, A.-L.; Sanchez, L.; Vatsa, P.; Boudesocque, L.; Garcia-Brugger, A.; Rabenoelina, F.; Sorokin, A.; Renault, J.-H.; Kauffmann, S.; Pugin, A.; et al. Bacterial rhamnolipids are novel MAMPs conferring resistance to Botrytis cinerea in grapevine. Plant Cell Environ. 2009, 32, 178–193. [Google Scholar] [CrossRef]
- Monnier, N.; Furlan, A.; Botcazon, C.; Dahi, A.; Mongelard, G.; Cordelier, S.; Clément, C.; Dorey, S.; Sarazin, C.; Rippa, S. Rhamnolipids From Pseudomonas aeruginosa Are Elicitors Triggering Brassica napus Protection against Botrytis cinerea without Physiological Disorders. Front Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Sanchez, L.; Courteaux, B.; Hubert, J.; Kauffmann, S.; Renault, J.-H.; Clément, C.; Baillieul, F.; Dorey, S. Rhamnolipids elicit defense responses and induce disease resistance against biotrophic, hemibiotrophic, and necrotrophic pathogens that require different signaling pathways in Arabidopsis and highlight a central role for salicylic acid. Plant Physiol. 2012, 160, 1630–1641. [Google Scholar] [CrossRef]
- Johann, S.; Seiler, T.-B.; Tiso, T.; Bluhm, K.; Blank, L.M.; Hollert, H. Mechanism-specific and whole-organism ecotoxicity of mono-rhamnolipids. Sci. Total Environ. 2016, 548–549, 155–163. [Google Scholar] [CrossRef]
- Hogan, D.E.; Tian, F.; Malm, S.W.; Olivares, C.; Palos Pacheco, R.; Simonich, M.T.; Hunjan, A.S.; Tanguay, R.L.; Klimecki, W.T.; Polt, R.; et al. Biodegradability and toxicity of monorhamnolipid biosurfactant diastereomers. J. Hazard. Mater. 2019, 364, 600–607. [Google Scholar] [CrossRef]
- Funston, S.J.; Tsaousi, K.; Rudden, M.; Smyth, T.J.; Stevenson, P.S.; Marchant, R.; Banat, I.M. Characterising rhamnolipid production in Burkholderia thailandensis E264, a non-pathogenic producer. Appl. Microbiol. Biotechnol. 2016, 100, 7945–7956. [Google Scholar] [CrossRef] [PubMed]
- Bahia, F.M.; de Almeida, G.C.; de Andrade, L.P.; Campos, C.G.; Queiroz, L.R.; da Silva, R.L.V.; Abdelnur, P.V.; Corrêa, J.R.; Bettiga, M.; Parachin, N.S. Rhamnolipids production from sucrose by engineered Saccharomyces cerevisiae. Sci. Rep. 2018, 8, 2905. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga-Loaiza, W.P.; Schellenberger, R.; De Gaetano, Y.; Obounou Akong, F.; Villaume, S.; Crouzet, J.; Haudrechy, A.; Baillieul, F.; Clément, C.; Lins, L.; et al. Synthetic Rhamnolipid Bolaforms trigger an innate immune response in Arabidopsis thaliana. Sci. Rep. 2018, 8, 8534. [Google Scholar] [CrossRef] [PubMed]
- Robineau, M.; Le Guenic, S.; Sanchez, L.; Chaveriat, L.; Lequart, V.; Joly, N.; Calonne, M.; Jacquard, C.; Declerck, S.; Martin, P.; et al. Synthetic Mono-Rhamnolipids Display Direct Antifungal Effects and Trigger an Innate Immune Response in Tomato against Botrytis Cinerea. Molecules 2020, 25, 3108. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Sun, Y.; Li, P.; Deng, M.; Shen, D.; Dai, G.; Yao, N.; Lu, Y. The Ralstonia solanacearum effector RipAK suppresses plant hypersensitive response by inhibiting the activity of host catalases. Cell. Microbiol. 2017, 19, e12736. [Google Scholar] [CrossRef]
- Shetty, N.P.; Mehrabi, R.; Lütken, H.; Haldrup, A.; Kema, G.H.J.; Collinge, D.B.; Jørgensen, H.J.L. Role of hydrogen peroxide during the interaction between the hemibiotrophic fungal pathogen Septoria tritici and wheat. New Phytol. 2007, 174, 637–647. [Google Scholar] [CrossRef]
- Shetty, N.P.; Kristensen, B.K.; Newman, M.-A.; Møller, K.; Gregersen, P.L.; Jørgensen, H.J.L. Association of hydrogen peroxide with restriction of Septoria tritici in resistant wheat. Physiol. Mol. Plant Pathol. 2003, 62, 333–346. [Google Scholar] [CrossRef]
- Lagrimini, L.M. Wound-induced deposition of polyphenols in transgenic plants overexpressing peroxidase. Plant Physiol. 1991, 96, 577–583. [Google Scholar] [CrossRef]
- Monnier, N.; Furlan, A.L.; Buchoux, S.; Deleu, M.; Dauchez, M.; Rippa, S.; Sarazin, C. Exploring the Dual Interaction of Natural Rhamnolipids with Plant and Fungal Biomimetic Plasma Membranes through Biophysical Studies. Int. J. Mol. Sci. 2019, 20, 1009. [Google Scholar] [CrossRef]
- Henry, G.; Deleu, M.; Jourdan, E.; Thonart, P.; Ongena, M. The bacterial lipopeptide surfactin targets the lipid fraction of the plant plasma membrane to trigger immune-related defence responses. Cell. Microbiol. 2011, 13, 1824–1837. [Google Scholar] [CrossRef] [PubMed]
- Siah, A.; Bomble, M.; Tisserant, B.; Cadalen, T.; Holvoet, M.; Hilbert, J.-L.; Halama, P.; Reignault, P. Genetic Structure of Zymoseptoria tritici in Northern France at Region, Field, Plant, and Leaf Layer Scales. Phytopathology 2018, 108, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Huf, A.; Rehfus, A.; Lorenz, K.H.; Bryson, R.; Voegele, R.T.; Stammler, G. Proposal for a new nomenclature for CYP51 haplotypes in Zymoseptoria tritici and analysis of their distribution in Europe. Plant Pathol. 2018, 67, 1706–1712. [Google Scholar] [CrossRef]
- Price, C.L.; Parker, J.E.; Warrilow, A.G.; Kelly, D.E.; Kelly, S.L. Azole fungicides—Understanding resistance mechanisms in agricultural fungal pathogens. Pest Manag. Sci. 2015, 71, 1054–1058. [Google Scholar] [CrossRef]
- Mejri, S.; Siah, A.; Coutte, F.; Magnin-Robert, M.; Randoux, B.; Tisserant, B.; Krier, F.; Jacques, P.; Reignault, P.; Halama, P. Biocontrol of the wheat pathogen Zymoseptoria tritici using cyclic lipopeptides from Bacillus subtilis. Environ. Sci. Pollut Res. Int. 2018, 25, 29822–29833. [Google Scholar] [CrossRef]
- Siah, A.; Deweer, C.; Duyme, F.; Sanssené, J.; Durand, R.; Halama, P.; Reignault, P. Correlation of in planta endo-beta-1,4-xylanase activity with the necrotrophic phase of the hemibiotrophic fungus Mycosphaerella graminicola. Plant Pathol. 2010, 59, 661–670. [Google Scholar] [CrossRef]
- Mitchell, H.J.; Hall, J.L.; Barber, M.S. Elicitor-Induced Cinnamyl Alcohol Dehydrogenase Activity in Lignifying Wheat (Triticum aestivum L.) Leaves. Plant Physiol. 1994, 104, 551–556. [Google Scholar] [CrossRef]
- Shindler, J.S.; Childs, R.E.; Bardsley, W.G. Peroxidase from human cervical mucus. The isolation and characterisation. Eur. J. Biochem. 1976, 65, 325–331. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar]
- García-Limones, C.; Hervás, A.; Navas-Cortés, J.A.; Jiménez-Díaz, R.M.; Tena, M. Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp.ciceris. Physiol. Mol. Plant Pathol. 2002, 61, 325–337. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kema, G.H.; Verstappen, E.C.; Waalwijk, C. Avirulence in the wheat septoria tritici leaf blotch fungus Mycosphaerella graminicola is controlled by a single locus. Mol. Plant Microbe Interact. 2000, 13, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
| Scheme | Compound | Name | Molecular Weight (g moL−1) |
|---|---|---|---|
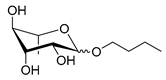 | Rh-Eth-C4 | Butyl α/β-L-rhamnopyranoside | 220 |
 | Rh-Eth-C6 | Hexyl α/β-L-rhamnopyranoside | 248 |
 | Rh-Eth-C8 | Octyl α/β-L-rhamnopyranoside | 276 |
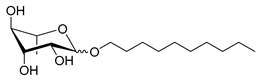 | Rh-Eth-C10 | Decyl α/β-L-rhamnopyranoside | 304 |
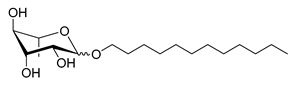 | Rh-Eth-C12 | Dodecyl α/β-L-rhamnopyranoside | 332 |
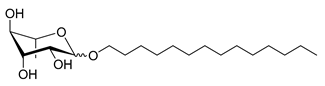 | Rh-Eth-C14 | Tetradecyl α/β-L-rhamnopyranoside | 360 |
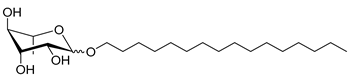 | Rh-Eth-C16 | Hexadecyl α/β-L-rhamnopyranoside | 388 |
 | Rh-Eth-C18 * | Octadecyl α/β-L-rhamnopyranoside | 416 |
 | Rh-Est-C8 * | Octanoyl α/β-L-rhamnopyranoside | 290 |
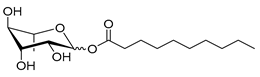 | Rh-Est-C10 * | Decanoyl α/β-L-rhamnopyranoside | 318 |
 | Rh-Est-C12 | Dodecanoyl α/β-L-rhamnopyranoside | 346 |
 | Rh-Suc-C8 * | Octenylsuccinate α/β-L-rhamnopyranoside | 374 |
 | Rh-Suc-C12 | Dodecenylsuccinate α/β-L-rhamnopyranoside | 430 |
 | Rh-Suc-C12b * | Iso-Dodecenylsuccinate α/β-L-rhamnopyranoside | 430 |
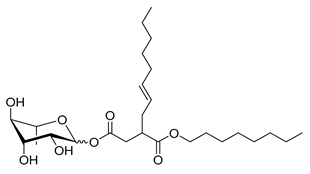 | Rh-Suc-C8-C8 * | 3-Octenyloctylsuccinate α/β-L-rhamnopyranoside | 486 |
 | Rh-Suc-C8-C12 * | 3-Octenyldodecylsuccinate α/β-L-rhamnopyranoside | 542 |
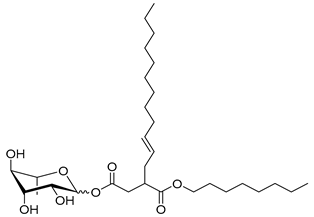 | Rh-Suc-C12-C8 * | 3-Dodecenyloctylsuccinate α/β-L-rhamnopyranoside | 542 |
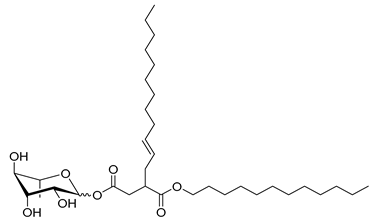 | Rh-Suc-C12-C12 * | 3-Dodecenyldodecylsuccinate α/β-L-rhamnopyranoside | 598 |
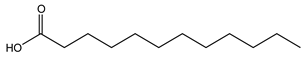 | Lauric acid | Lauric acid | 200 |
 | Dodecanol | Dodecanol | 186 |
| Mixture of rhamnolipids (data from Agae Technologies LLC) | Nat-Rh | Bacterial rhamnolipids (see Section 4) | 578 |
| Molecule | In Vitro Antifungal Activity | In Planta Defense Elicitation Activity | In Planta Protection Efficacy | ||
|---|---|---|---|---|---|
| IC50 (μM) | MIC (μM) | Catalase Activity (U min−1 g−1 Proteins) | Peroxidase Activity (U min−1 g−1 Proteins) | Leaf Diseased Area (%) • | |
| Control | - | - | 83.4 ab | 51.7 bc | 55.7 a (0%) |
| Natural-Rh | >1500 | >1500 | 71.9 a | 53.4 c | 46.3 b (−17.0%) |
| Rh-Eth-C4 | >1500 | >1500 | 110.4 abcd | 64.8 bc | 51.4 ab (−7.8%) |
| Rh-Eth-C6 | >1500 | >1500 | 111.7 abcd | 55.9 bc | 54.4 ab (−2.3%) |
| Rh-Eth-C8 | >1500 | >1500 | 121.1 abcd | 74.2 abc | 54.3 ab (−2.6%) |
| Rh-Eth-C10 | 158 | 200 | 131.6 bcd | 54.2 bc | 46.0 b (−17.4%) |
| Rh-Eth-C12 | 81 | 450 | 155.2 d | 71.0 abc | 46.2 b (−15.5%) |
| Rh-Eth-C14 | >1500 | >1500 | 99.2 abcd | 71.2 abc | 49.4 ab (−11.3%) |
| Rh-Eth-C16 | >1500 | >1500 | 130.9 bcd | 67.7 bc | 50.3 ab (−9.7%) |
| Rh-Eth-C18 | >1500 | >1500 | Nd * | Nd * | Nd * |
| Rh-Est-C8 | >1500 | >1500 | 112.9 abcd | 44.9 c | 50.6 ab (−9.1%) |
| Rh-Est-C10 | >1500 | >1500 | 98.4 abcd | 58.2 bc | 50.6 ab (−9.3%) |
| Rh-Est-C12 | 75 | 133 | 142.5 cd | 117.3 a | 35.9 c (−35.6%) |
| Rh-Suc-C8 | 1399 | 1500 | 123.3 abcd | 60.3bc | 54.1 ab (−3.0%) |
| Rh-Suc-C12 | >1500 | >1500 | 124.9 abcd | 76.6 abc | 50.3 ab (−9.7%) |
| Rh-Suc-C12b | >1500 | >1500 | 96.8 abc | 116.9 a | 46.2 b (−16.2%) |
| Rh-Suc-C8-C8 | >1500 | >1500 | 102.4 abcd | 62.1 bc | 52.4 ab (−6.0%) |
| Rh-Suc-C8-C12 | >1500 | >1500 | 113.7 abcd | 96.9 ab | 51.2 ab (−8.1%) |
| Rh-Suc-C12-C8 | >1500 | >1500 | 120.1 abcd | 79.9 abc | 55.8 a (+0.1%) |
| Rh-Suc-C12-C12 | >1500 | >1500 | Nd * | Nd * | Nd * |
| Lauric acid | 109 | 200 | 94.0 abc | 76.8 abc | 50.9 ab (−8.7%) |
| Dodecanol | 164 | 200 | 107.4 abcd | 51.1 bc | 54.4 ab (−2.4%) |
| IC50 | MIC | Catalase Activity | Peroxidase Activity | |
|---|---|---|---|---|
| IC50 | - | - | - | - |
| MIC | 0.99 | - | - | - |
| Catalase activity | −0.40 | −0.35 | - | - |
| Peroxidase activity | −0.10 | −0.10 | 0.19 | - |
| Leaf diseased area | 0.46 | 0.46 | −0.25 | −0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Platel, R.; Chaveriat, L.; Le Guenic, S.; Pipeleers, R.; Magnin-Robert, M.; Randoux, B.; Trapet, P.; Lequart, V.; Joly, N.; Halama, P.; et al. Importance of the C12 Carbon Chain in the Biological Activity of Rhamnolipids Conferring Protection in Wheat against Zymoseptoria tritici. Molecules 2021, 26, 40. https://doi.org/10.3390/molecules26010040
Platel R, Chaveriat L, Le Guenic S, Pipeleers R, Magnin-Robert M, Randoux B, Trapet P, Lequart V, Joly N, Halama P, et al. Importance of the C12 Carbon Chain in the Biological Activity of Rhamnolipids Conferring Protection in Wheat against Zymoseptoria tritici. Molecules. 2021; 26(1):40. https://doi.org/10.3390/molecules26010040
Chicago/Turabian StylePlatel, Rémi, Ludovic Chaveriat, Sarah Le Guenic, Rutger Pipeleers, Maryline Magnin-Robert, Béatrice Randoux, Pauline Trapet, Vincent Lequart, Nicolas Joly, Patrice Halama, and et al. 2021. "Importance of the C12 Carbon Chain in the Biological Activity of Rhamnolipids Conferring Protection in Wheat against Zymoseptoria tritici" Molecules 26, no. 1: 40. https://doi.org/10.3390/molecules26010040
APA StylePlatel, R., Chaveriat, L., Le Guenic, S., Pipeleers, R., Magnin-Robert, M., Randoux, B., Trapet, P., Lequart, V., Joly, N., Halama, P., Martin, P., Höfte, M., Reignault, P., & Siah, A. (2021). Importance of the C12 Carbon Chain in the Biological Activity of Rhamnolipids Conferring Protection in Wheat against Zymoseptoria tritici. Molecules, 26(1), 40. https://doi.org/10.3390/molecules26010040











