Comparison of Different HILIC Stationary Phases in the Separation of Hemopexin and Immunoglobulin G Glycopeptides and Their Isomers
Abstract
1. Introduction
2. Results and Discussion
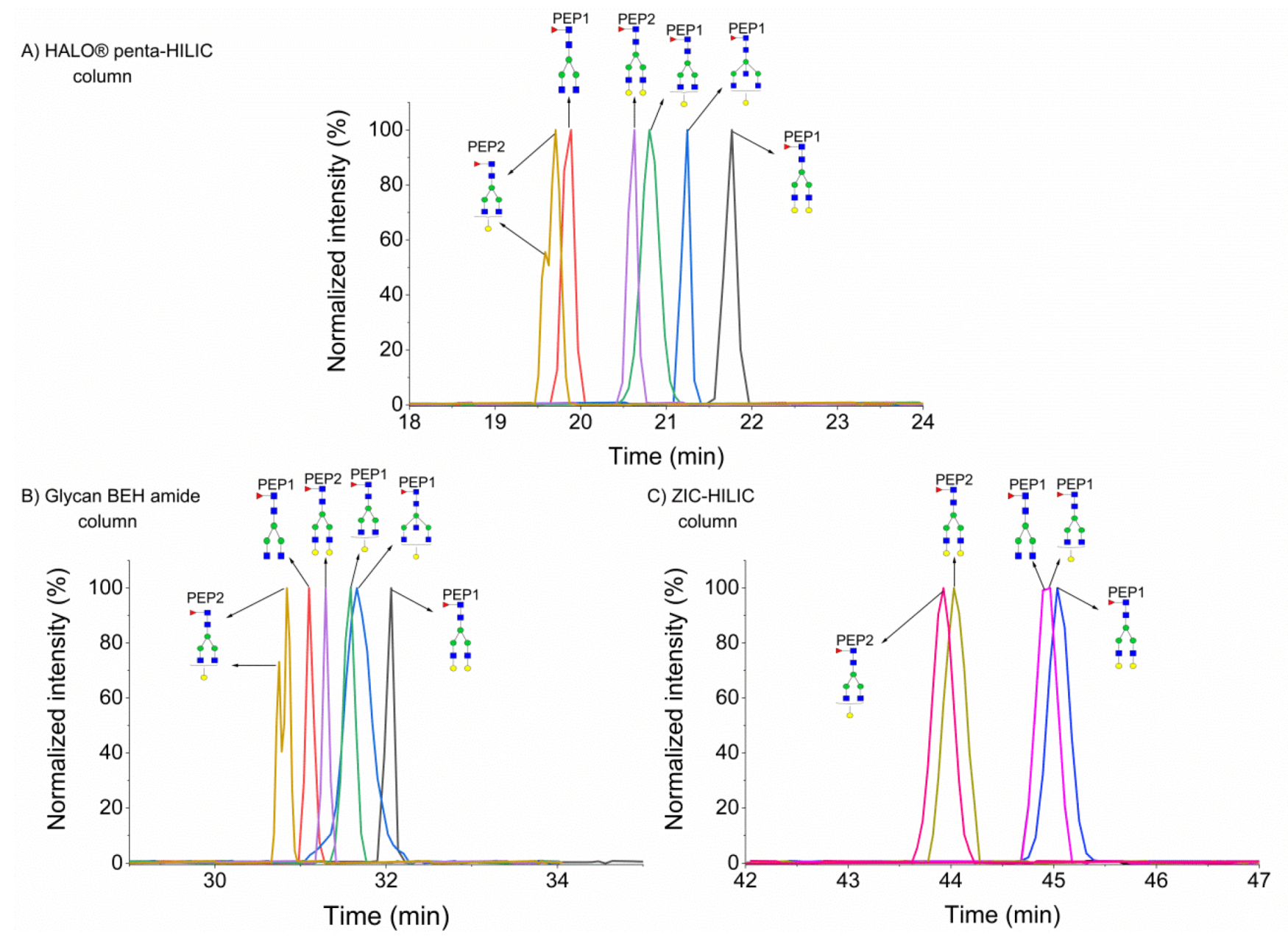
2.1. Separation of Glycopeptides by HILIC
2.1.1. Separation of Fucosylated Glycopeptides
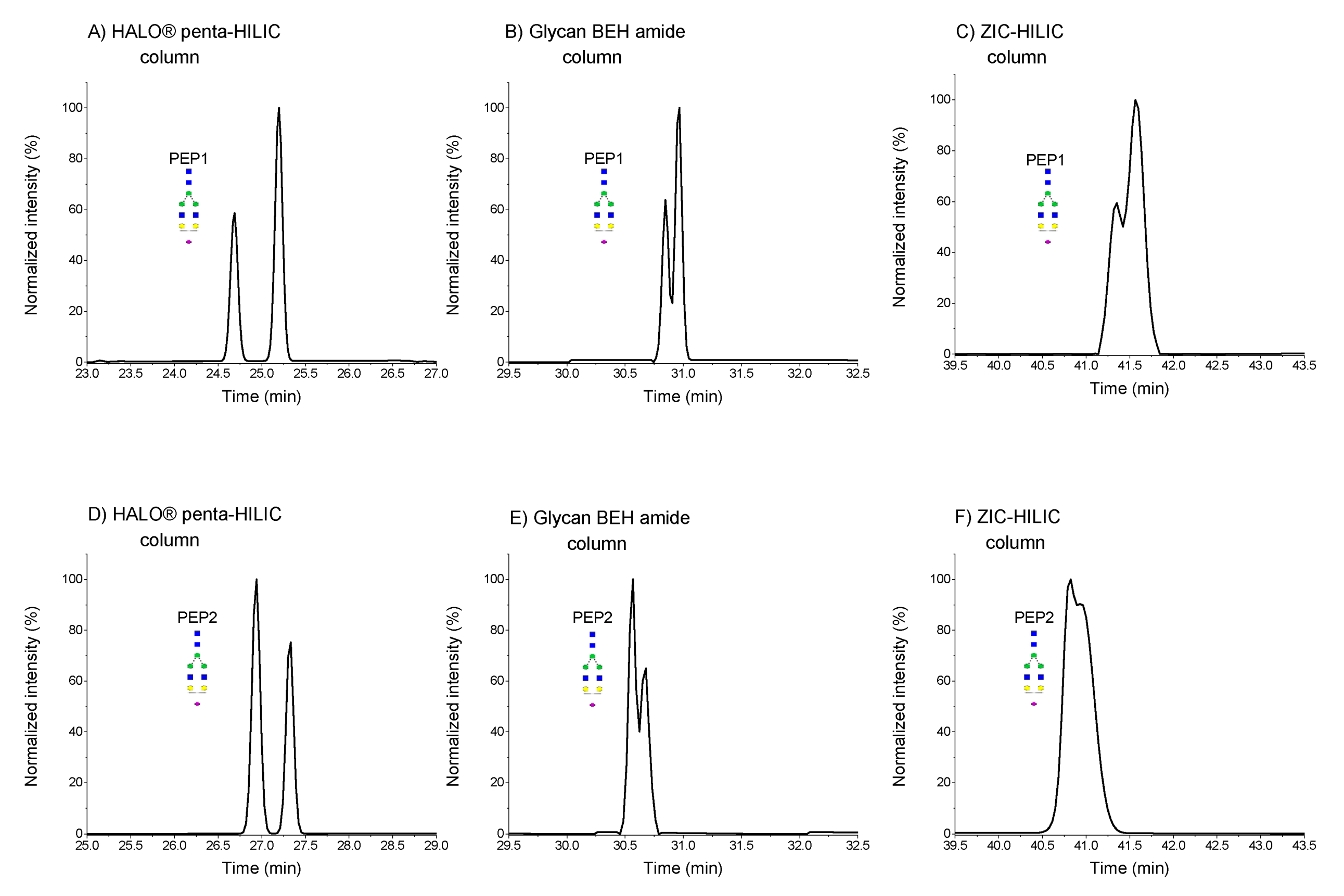
2.1.2. Separation of Sialylated Glycopeptides
2.2. Column Temperature Effect on the Separation of Glycopeptides Isomers
3. Materials and Methods
3.1. Chemicals
3.2. Sample Preparation
3.3. Instrumentation and Experimental Conditions
3.4. Mass Spectrometry Settings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H. Essentials of Glycobiology, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017. [Google Scholar]
- Spiro, R.G. Protein glycosylation: Nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology 2002, 12, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, E.; Jaskiewicz, E. Protein Glycosylation, an overview. eLS 2012. [Google Scholar] [CrossRef]
- Nilsson, J.; Rüetschi, U.; Halim, A.; Hesse, C.; Carlsohn, E.; Brinkmalm, G.; Larson, G. Enrichment of glycopeptides for glycan structure and attachment site identification. Nat. Methods 2009, 11, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Kailemia, M.J.; Park, D.; Lebrilla, C.B. Glycans and glycoproteins as specific biomarkers for cancer. Anal. Bioanal. Chem. 2017, 409, 395–410. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, N.; Wan, D.; Cui, M.; Liu, Z.; Liu, S. Mass spectrometry-based analysis of glycoproteins and its clinical applications in cancer biomarker discovery. Clin. Proteom. 2014, 11, 14. [Google Scholar] [CrossRef]
- Tian, Y.; Esteva, F.J.; Song, J.; Zhang, H. Altered Expression of Sialylated Glycoproteins in Breast Cancer Using Hydrazide Chemistry and Mass Spectrometry. Mol. Cell. Proteom. 2012, 11. [Google Scholar] [CrossRef]
- Stowell, S.R.; Ju, T.; Cummings, R.D. Protein Glycosylation in Cancer. Annu. Rev. Pathol. 2015, 10, 473–510. [Google Scholar] [CrossRef]
- Darebna, P.; Novak, P.; Kucera, R.; Topolcan, O.; Sanda, M.; Goldman, R.; Pompach, P. Changes in the expression of N- and O-glycopeptides in patients with colorectal cancer and hepatocellular carcinoma quantified by full-MS scan FT-ICR and multiple reaction monitoring. J. Proteom. 2017, 153, 44–52. [Google Scholar] [CrossRef]
- Badgett, M.J.; Boyes, B.; Orlando, R. Predicting the Retention Behavior of Specific O-Linked Glycopeptides. J. Biomol. Tech. 2017, 28, 122–126. [Google Scholar] [CrossRef]
- Bruderer, R.; Bernhardt, O.M.; Gandhi, T.; Reiter, L. High-precision iRT prediction in the targeted analysis of data-independent acquisition and its impact on identification and quantitation. Proteomics 2016, 16, 2246–2256. [Google Scholar] [CrossRef]
- Furuki, K.; Toyo’oka, T. Retention of glycopeptides analyzed using hydrophilic interaction chromatography is influenced by charge and carbon chain length of ion-pairing reagent for mobile phase. Biomed. Chromatogr. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Huang, Y.; Zhao, J.; Zhong, J.; Mechref, Y. Isomeric Separation of N-Glycopeptides Derived from Glycoproteins by Porous Graphitic Carbon (PGC) LC-MS/MS. Anal. Chem. 2020, 92, 9556–9565. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Goli, M.; Mirzaei, P.; Mechref, Y. Revealing the Biological Attributes of N-Glycan Isomers in Breast Cancer Brain Metastasis Using Porous Graphitic Carbon (PGC) Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). J. Proteom. Res. 2019, 18, 3731–3740. [Google Scholar] [CrossRef] [PubMed]
- Kozlik, P.; Sanda, M.; Goldman, R. Nano reversed phase versus nano hydrophilic interaction liquid chromatography on a chip in the analysis of hemopexin glycopeptides. J. Chromatogr. A 2017, 1519, 152–155. [Google Scholar] [CrossRef]
- Kozlik, P.; Goldman, R.; Sanda, M. Hydrophilic interaction liquid chromatography in the separation of glycopeptides and their isomers. Anal. Bioanal. Chem. 2018, 410, 5001–5008. [Google Scholar] [CrossRef]
- Tian, Y.; Tang, R.; Liu, L.; Yu, Y.; Ma, S.; Gong, B.; Ou, J. Glutathione-modified ordered mesoporous silicas for enrichment of N-linked glycopeptides by hydrophilic interaction chromatography. Talanta 2020, 217, 121082. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Yu, Y.; Xie, R.; Liao, H.; Zhang, B.; Chen, J. Coupling hydrophilic interaction chromatography materials with immobilized Fe3+ for phosphopeptide and glycopeptide enrichment and separation. RSC Adv. 2020, 10, 22176–22182. [Google Scholar] [CrossRef]
- Huang, Y.; Nie, Y.; Boyes, B.; Orlando, R. Resolving Isomeric Glycopeptide Glycoforms with Hydrophilic Interaction Chromatography (HILIC). J. Biomol. Tech. 2016, 27, 98–104. [Google Scholar] [CrossRef]
- Tao, S.; Huang, Y.; Boyes, B.E.; Orlando, R. Liquid Chromatography-Selected Reaction Monitoring (LC-SRM) Approach for the Separation and Quantitation of Sialylated N-Glycans Linkage Isomers. Anal. Chem. 2014, 86, 10584–10590. [Google Scholar] [CrossRef]
- Van der Burgt, Y.E.; Siliakus, K.M.; Cobbaert, C.M.; Ruhaak, L.R. HILIC–MRM–MS for Linkage-Specific Separation of Sialylated Glycopeptides to Quantify Prostate-Specific Antigen Proteoforms. J. Proteome Res. 2020, 19, 2708–2716. [Google Scholar] [CrossRef]
- Alley, W.R., Jr.; Madera, M.; Mechref, Y.; Novotny, M.V. Chip-based Reversed-phase Liquid Chromatography−Mass Spectrometry of Permethylated N-Linked Glycans: A Potential Methodology for Cancer-biomarker Discovery. Anal. Chem. 2010, 82, 5095–5106. [Google Scholar] [CrossRef]
- Tengattini, S.; Dominguez-Vega, E.; Temporini, C.; Bavaro, T.; Rinaldi, F.; Piubelli, L.; Pollegioni, L.; Massolini, G.; Somsen, G.W. Hydrophilic interaction liquid chromatography-mass spectrometry as a new tool for the characterization of intact semi-synthetic glycoproteins. Anal. Chim. Acta 2017, 981, 94–105. [Google Scholar] [CrossRef]
- Lauber, M.A.; Yu, Y.Q.; Brousmiche, D.W.; Hua, Z.; Koza, S.M.; Magnelli, P.; Guthrie, E.; Taron, C.H.; Fountain, K.J. Rapid Preparation of Released N -Glycans for HILIC Analysis Using a Labeling Reagent that Facilitates Sensitive Fluorescence and ESI-MS Detection. Anal. Chem. 2015, 87, 5401–5409. [Google Scholar] [CrossRef] [PubMed]
- Pedrali, A.; Tengattini, S.; Marrubini, G.; Bavaro, T.; Hemstrom, P.; Massolini, G.; Terreni, M.; Temporini, C. Characterization of Intact Neo-Glycoproteins by Hydrophilic Interaction Liquid Chromatography. Molecules 2014, 19, 9070–9088. [Google Scholar] [CrossRef]
- Takegawa, Y.; Deguchi, K.; Ito, H.; Keira, T.; Nakagawa, H.; Nishimura, S.I. Simple separation of isomeric sialylated N-glycopeptides by a zwitterionic type of hydrophilic interaction chromatography. J. Sep. Sci. 2006, 29, 2533–2540. [Google Scholar] [CrossRef]
- Yin, H.; Zhu, J.; Wu, J.; Tan, Z.; An, M.; Zhou, S.; Mechref, Y.; Lubman, D.M. A procedure for the analysis of site-specific and structure-specific fucosylation in alpha-1-antitrypsin. Electrophoresis 2016, 37, 2624–2632. [Google Scholar] [CrossRef] [PubMed]
- Gilar, M.; Yu, Y.Q.; Ahn, J.; Xie, H.; Han, H.; Ying, W.; Qian, X. Characterization of glycoprotein digests with hydrophilic interaction chromatography and mass spectrometry. Anal. Biochem. 2011, 417, 80–88. [Google Scholar] [CrossRef]
- Xue, Y.; Xie, J.; Fang, P.; Yao, J.; Yan, G.; Shen, H.; Yang, P. Study on behaviors and performances of universal N -glycopeptide enrichment methods. Analyst 2018, 143, 1870–1880. [Google Scholar] [CrossRef]
- Neue, K.; Mormann, M.; Peter-Katalinić, J.; Pohlentz, G. Elucidation of Glycoprotein Structures by Unspecific Proteolysis and Direct nanoESI Mass Spectrometric Analysis of ZIC-HILIC-Enriched Glycopeptides. J. Proteome Res. 2011, 10, 2248–2260. [Google Scholar] [CrossRef]
- Alagesan, K.; Khilji, S.K.; Kolarich, D. It is all about the solvent: On the importance of the mobile phase for ZIC-HILIC glycopeptide enrichment. Anal. Bioanal. Chem. 2017, 409, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Hernandez, O.; Quintanilla-Lopez, J.E.; Lebron-Aguilar, R.; Sanz, M.L.; Moreno, F.J. Characterization of post-translationally modified peptides by hydrophilic interaction and reverse phase liquid chromatography coupled to quadrupole-time-of-flight mass spectrometry. J. Chromatogr. A 2016, 1428, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Zauner, G.; Koeleman, C.A.; Deelder, A.M.; Wuhrer, M. Protein glycosylation analysis by HILIC-LC-MS of Proteinase K-generated N- and O-glycopeptides. J. Sep. Sci. 2010, 33, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, G.; Larsen, M.R.; Packer, N.H.; Thaysen-Andersen, M. Structural analysis of glycoprotein sialylation—part II: LC-MS based detection. RSC Adv. 2013, 3, 22706–22726. [Google Scholar] [CrossRef]
- Pompach, P.; Ashline, D.J.; Brnakova, Z.; Benicky, J.; Sanda, M.; Goldman, R. Protein and Site Specificity of Fucosylation in Liver-Secreted Glycoproteins. J. Proteome Res. 2014, 13, 5561–5569. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sanda, M.; Wei, R.; Zhang, L.; Goldman, R. Quantitative analysis of core fucosylation of serum proteins in liver diseases by LC-MS-MRM. J. Proteom. 2018, 189, 67–74. [Google Scholar] [CrossRef]
- Benicky, J.; Sanda, M.; Pompach, P.; Wu, J.; Goldman, R. Quantification of Fucosylated Hemopexin and Complement Factor H in Plasma of Patients with Liver Disease. Anal. Chem. 2014, 86, 10716–10723. [Google Scholar] [CrossRef]
- Kozlik, P.; Goldman, R.; Sanda, M. Study of structure-dependent chromatographic behavior of glycopeptides using reversed phase nanoLC. Electrophoresis 2017, 38, 2193–2199. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (hemopexin and IgG) are available from the authors. |


 , N-acetylglucosamine (GlcNAc);
, N-acetylglucosamine (GlcNAc);  , Mannose (Man);
, Mannose (Man);  , Galactose (Gal);
, Galactose (Gal);  , Fucose (Fuc);
, Fucose (Fuc);  , Sialic acid.
, Sialic acid.
 , N-acetylglucosamine (GlcNAc);
, N-acetylglucosamine (GlcNAc);  , Mannose (Man);
, Mannose (Man);  , Galactose (Gal);
, Galactose (Gal);  , Fucose (Fuc);
, Fucose (Fuc);  , Sialic acid.
, Sialic acid.| Hemopexin | |||||
|---|---|---|---|---|---|
| SWPAVGN187CSSALR |  A2G2 |  A2G2F1 |  A2G2S1 |  A2G2S2 | 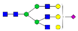 A3G3S1 |
| ALPQPQN453VTSLLGCTH |  A2G2 | 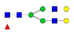 A2G2F1 |  A2G2S1 | 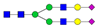 A2G2S2 | |
| IgG | |||||
| EEQYN180STYR (IgG1) | 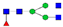 A2F1 | 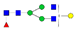 A2G1F | 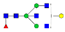 G1A3F1 | 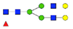 A2G2F1 | |
| EEQFN176STFR (IgG2) | 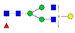 A2G1F | 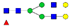 A2G2F1 | |||
| HALO® Penta-HILIC | Glycan BEH Amide | ZIC-HILIC | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Column Temperature | ||||||||||||||||||
| 40 °C | 50 °C | 60 °C | 40 °C | 50 °C | 60 °C | 40 °C | 50 °C | 60 °C | ||||||||||
| Hemopexin SWPAVGN187CSSALR | tR | R | tR | R | tR | R | tR | R | tR | R | tR | R | tR | R | tR | R | tR | R r |
| A2G2F1 (core) | 17.8 | 1.99 | 17.5 | 1.85 | 17.1 | 1.81 | 30.6 | 1.71 | 30.5 | 2.34 | 30.3 | 1.82 | 43.5 | − | 43.1 | − | 43.0 | − |
| A2G2F1 (outer arm) | 18.2 | 17.8 | 17.5 | 30.9 | 30.8 | 30.6 | ||||||||||||
| A2G2S1 (isomer 1) | 24.7 | 3.09 | 24.4 | 2.89 | 24.1 | 3.33 | 30.8 | 1.02 | 30.7 | 1.05 | 30.6 | 1.12 | 41.3 | 0.74 | 41.0 | 0.86 | 41.1 | 0.89 |
| A2G2S1 (isomer 2) | 25.2 | 24.9 | 24.7 | 30.9 | 30.9 | 30.7 | 41.6 | 41.3 | 41.4 | |||||||||
| A3G3S1 (isomer 1) | 25.9 | 1.28 1.80 | 25.7 | 0.39 0.37 | 25.4 | 1.33 | 31.3 | 1.23 | 31.3 | 0.74 | 31.1 | 0.73 | 41.8 | − | 41.7 | ND | ||
| A3G3S1 (isomer 2) | 26.2 | 26.0 | 25.8 | 31.5 | 31.4 | 31.2 | ||||||||||||
| A3G3S1 (isomer 3) | 26.4 | 26.1 | ND | ND | ND | ND | ND | ND | ND | |||||||||
| ALPQPQN453VTSLLGCTH | ||||||||||||||||||
| A2G2F1 (core) | 19.7 | 1.36 | 19.3 | 1.09 | 18.9 | 1.07 | 30.6 | − | 30.5 | − | 30.2 | − | 42.7 | − | ND | ND | ||
| A2G2F1 (outer arm) | 19.9 | 19.6 | 19.2 | |||||||||||||||
| A2G2S1 (isomer 1) | 26.9 | 2.24 | 26.7 | 2.72 | 26.3 | 3.11 | 30.5 | 0.82 | 30.4 | 1.01 | 30.3 | 0.99 | 40.8 | 0.43 | 40.6 | 0.50 | 40.5 | 0.61 |
| A2G2S1 (isomer 2) | 27.3 | 27.1 | 26.8 | 30.6 | 30.6 | 30.4 | 40.9 | 40.7 | 40.7 | |||||||||
| IgG2 EEQFN176STFR | ||||||||||||||||||
| A2G1F (isomer 1) | 19.5 | 0.74 | 19.4 | 0.80 | 19.2 | 0.73 | 30.8 | 0.90 | 30.7 | 0.97 | 30.5 | 1.02 | 43.9 | − | 43.7 | − | 43.5 | − |
| A2G1F (isomer 2) | 19.7 | 19.6 | 19.4 | 30.9 | 30.8 | 30.6 | ||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnarova, K.; Kozlík, P. Comparison of Different HILIC Stationary Phases in the Separation of Hemopexin and Immunoglobulin G Glycopeptides and Their Isomers. Molecules 2020, 25, 4655. https://doi.org/10.3390/molecules25204655
Molnarova K, Kozlík P. Comparison of Different HILIC Stationary Phases in the Separation of Hemopexin and Immunoglobulin G Glycopeptides and Their Isomers. Molecules. 2020; 25(20):4655. https://doi.org/10.3390/molecules25204655
Chicago/Turabian StyleMolnarova, Katarina, and Petr Kozlík. 2020. "Comparison of Different HILIC Stationary Phases in the Separation of Hemopexin and Immunoglobulin G Glycopeptides and Their Isomers" Molecules 25, no. 20: 4655. https://doi.org/10.3390/molecules25204655
APA StyleMolnarova, K., & Kozlík, P. (2020). Comparison of Different HILIC Stationary Phases in the Separation of Hemopexin and Immunoglobulin G Glycopeptides and Their Isomers. Molecules, 25(20), 4655. https://doi.org/10.3390/molecules25204655







