Strawberry Decreases Intraluminal and Intestinal Wall Hydrolysis of Testosterone Undecanoate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Total Phenolics in STW
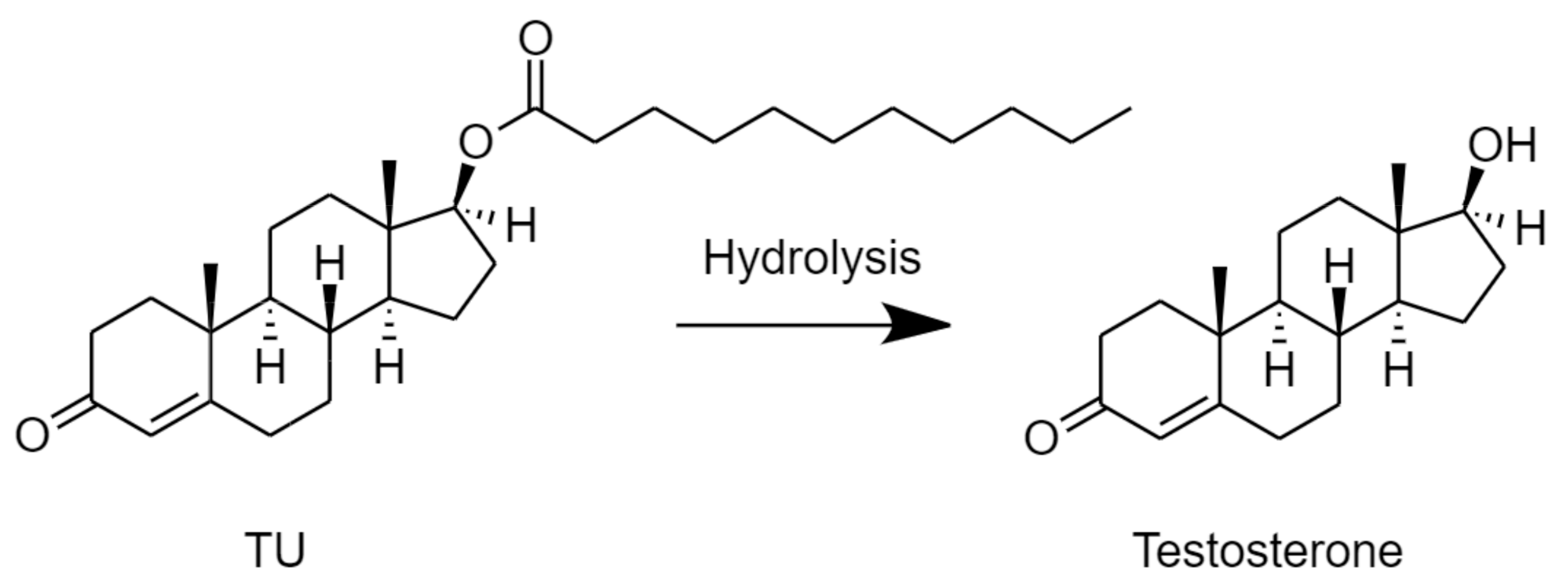
2.3. Stability of TU in Fasted State Simulated Intestinal Fluid with Added Esterase Activity (FaSSIF/ES)
2.4. Stability of TU in Caco-2 Cell Homogenates
2.5. Assessment of Intestinal Lymphatic Transport Potential
2.6. Analytical Methods
2.7. Statistical Analysis
3. Results
3.1. Phenolic Contents in Freeze-Dried STW and Fresh STW Fruit
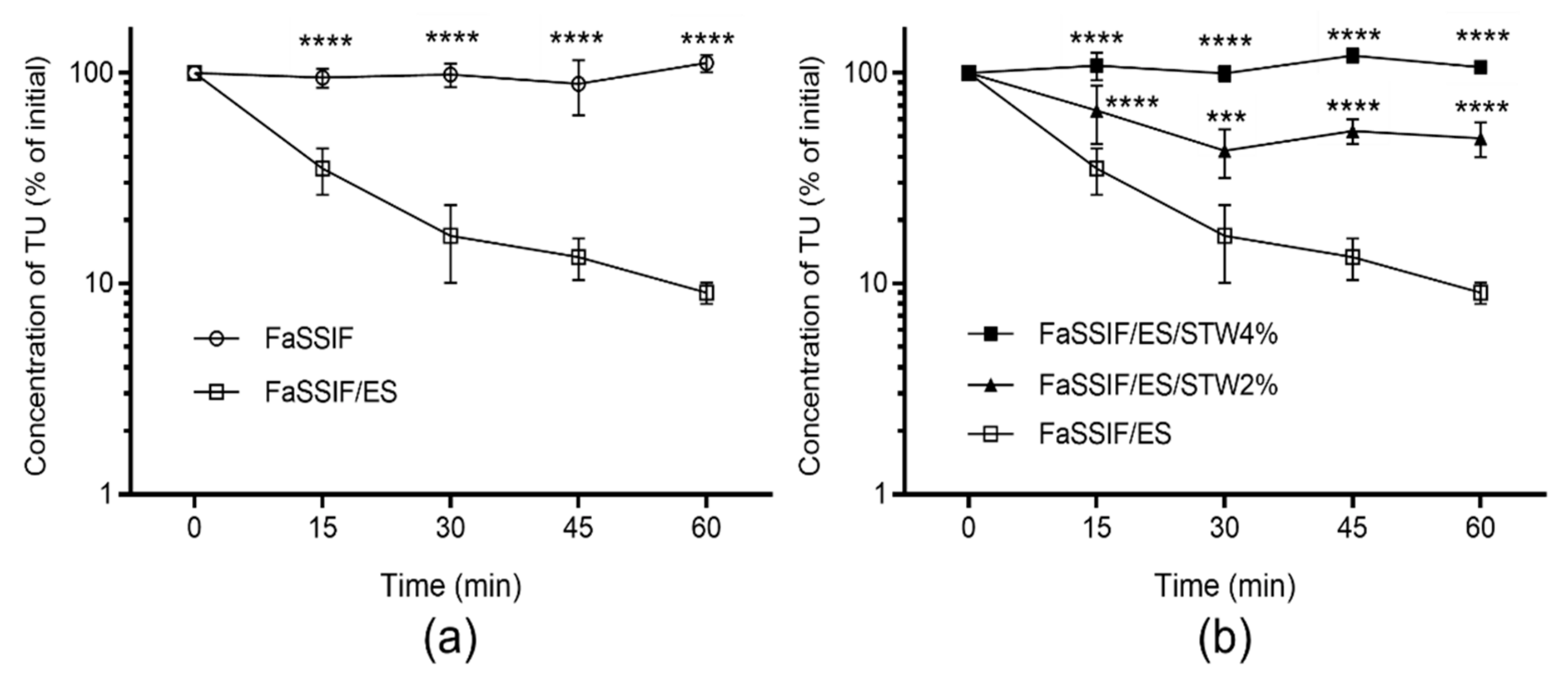
3.2. Stability of TU in FaSSIF/ES
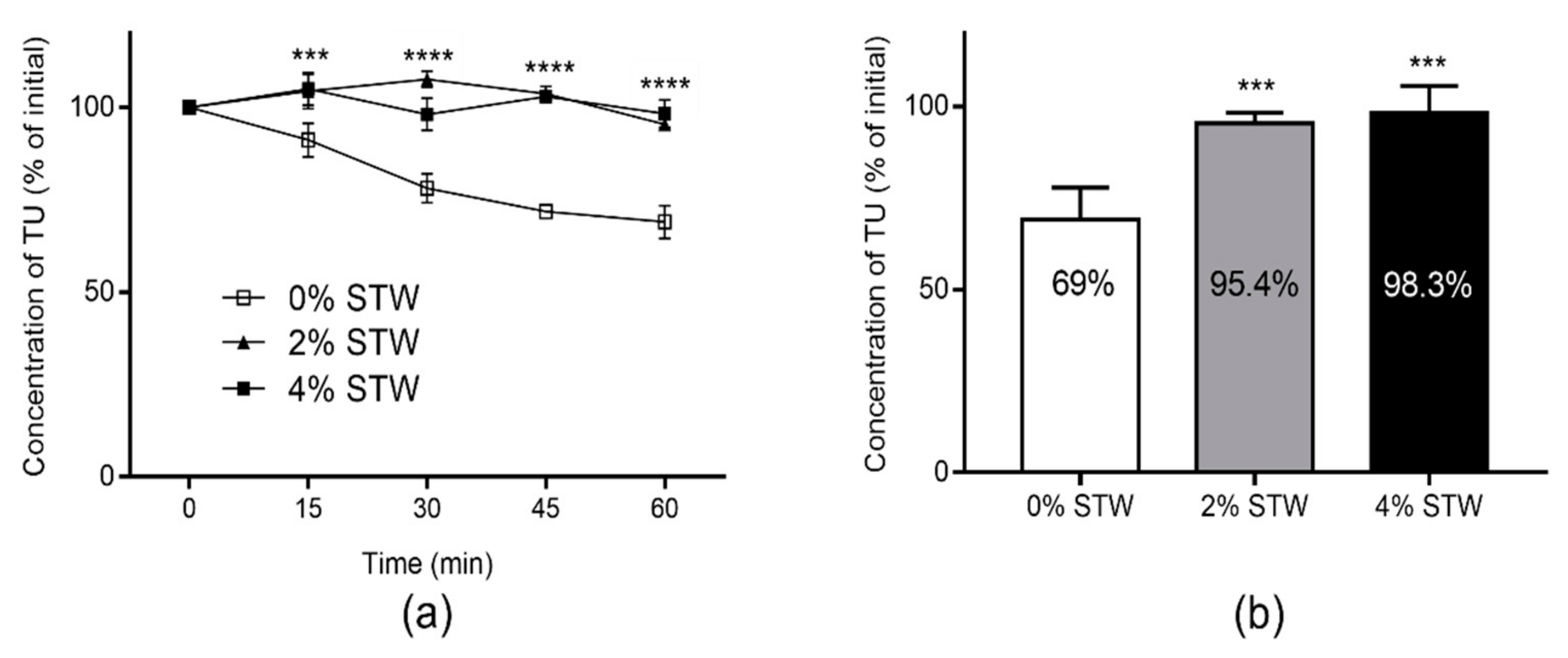
3.3. Stability of TU in Caco-2 Cell Homogenate
3.4. Intestinal Lymphatic Transport Potential of TU
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nieschlag, E.; Behre, H.M.; Nieschlag, S. Testosterone: Action, Deficiency, Substitution, 4th ed.; Cambridge University Press: Cambridge, UK, 2012; ISBN 9781139003353. [Google Scholar]
- Oettel, M. The endocrine pharmacology of testosterone therapy in men. Naturwissenschaften 2004, 91, 66–76. [Google Scholar] [CrossRef]
- Tsametis, C.P.; Isidori, A.M. Testosterone replacement therapy: For whom, when and how? Metabolism 2018, 86, 69–78. [Google Scholar] [CrossRef]
- Yin, A.; Alfadhli, E.; Htun, M.; Dudley, R.; Faulkner, S.; Hull, L.; Leung, A.; Bross, R.; Longstreth, J.; Swerdloff, R.; et al. Dietary fat modulates the testosterone pharmacokinetics of a new self-emulsifying formulation of oral testosterone undecanoate in hypogonadal men. J. Androl. 2012, 36, 1282–1290. [Google Scholar] [CrossRef]
- Hoberman, J. Testosterone dreams: Rejuvenation, Aphrodisia, Doping; University of California Press: Los Angeles, CA, USA, 2005; ISBN 9780520939783. [Google Scholar]
- Lachance, S.; Dhingra, O.; Bernstein, J.; Gagnon, S.; Savard, C.; Pelletier, N.; Boudreau, N.; Lévesque, A. Importance of measuring testosterone in enzyme-inhibited plasma for oral testosterone undecanoate androgen replacement therapy clinical trials. Futur. Sci. OA 2015. [Google Scholar] [CrossRef]
- Coert, A.; Geelen, J.; De Visser, J.; Van Der Vies, J. The pharmacology and metabolism of testosterone undecanoate (TU), a new orally active androgen. Acta Endocrinol. (Copenh.) 1975, 79, 789–800. [Google Scholar] [CrossRef]
- Shackleford, D.M.; Faassen, W.A.; Houwing, N.; Lass, H.; Edwards, G.A.; Porter, C.J.H.; Charman, W.N. Contribution of lymphatically transported testosterone undecanoate to the systemic exposure of testosterone after oral administration of two andriol formulations in conscious lymph duct-cannulated dogs. J. Pharmacol. Exp. Ther. 2003, 306, 925–933. [Google Scholar] [CrossRef]
- Horst, H.J.; Höltje, W.J.; Dennis, M.; Coert, A.; Geelen, J.; Voigt, K.D. Lymphatic absorption and metabolism of orally administered testosterone undecanoate in man. Klin. Wochenschr. 1976, 54, 875–879. [Google Scholar] [CrossRef]
- Täuber, U.; Schröder, K.; Düsterberg, B.; Matthes, H. Absolute bioavailability of testosterone after oral administration of testosterone-undecanoate and testosterone. Eur. J. Drug Metab. Pharmacokinet. 1986, 11, 145–149. [Google Scholar] [CrossRef]
- Geelen, J.; Coert, A.; Meijer, R.; Van Der Vies, J. Comparison of the metabolism of testosterone undecanoate and testosterone in the gastrointestinal wall of the rat in vitro and in vivo. Acta Endocrinol. (Copenh.) 1977, 86, 216–224. [Google Scholar] [CrossRef]
- Zgair, A.; Dawood, Y.; Ibrahem, S.M.; Back, H.; Kagan, L.; Gershkovich, P.; Lee, J.B. Predicting Intestinal and Hepatic First-Pass Metabolism of Orally Administered Testosterone Undecanoate. Appl. Sci. 2020, 10, 7283. [Google Scholar] [CrossRef]
- Van Gelder, J.; Annaert, P.; Naesens, L.; De Clercq, E.; Van den Mooter, G.; Kinget, R.; Augustijns, P. Inhibition of intestinal metabolism of the antiviral ester prodrug bis (POC)-PMPA by nature-identical fruit extracts as a strategy to enhance its oral absorption: An in vitro study. Pharm. Res. 1999, 16, 1035–1040. [Google Scholar] [CrossRef]
- Augustijns, P.; Van Gelder, J.; Deferme, S.; Annaert, P.; Naesens, L.; De Clercq, E.; Van Den Mooter, G.; Kinget, R. Increased absorption of the antiviral ester prodrug tenofovir disoproxil in rat ileum by inhibiting its intestinal metabolism. Drug Metab. Dispos. 2000, 28, 1394–1396. [Google Scholar]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Lester, G.E.; Lewers, K.S.; Medina, M.B.; Saftner, R.A. Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin-Ciocalteu: Assay interference by ascorbic acid. J. Food Compos. Anal. 2012, 27, 102–107. [Google Scholar] [CrossRef]
- Merchant, H.A.; Afonso-Pereira, F.; Rabbie, S.C.; Youssef, S.A.; Basit, A.W. Gastrointestinal characterisation and drug solubility determination in animals. J. Pharm. Pharmacol. 2015, 67, 630–639. [Google Scholar] [CrossRef]
- Lee, J.B.; Zgair, A.; Malec, J.; Kim, T.H.; Kim, M.G.; Ali, J.; Qin, C.; Feng, W.; Chiang, M.; Gao, X.; et al. Lipophilic activated ester prodrug approach for drug delivery to the intestinal lymphatic system. J. Control. Release 2018, 286, 10–19. [Google Scholar] [CrossRef]
- Qin, C.; Chu, Y.; Feng, W.; Fromont, C.; He, S.; Ali, J.; Lee, J.B.; Zgair, A.; Berton, M.; Bettonte, S.; et al. Targeted delivery of lopinavir to HIV reservoirs in the mesenteric lymphatic system by lipophilic ester prodrug approach. J. Control. Release 2020. [Google Scholar] [CrossRef]
- Tsume, Y.; Amidon, G.L. Selection of Suitable Prodrug Candidates for in vivo Studies via in vitro Studies; The correlation of Prodrug Stability in Between Cell Culture Homogenates and Human Tissue Homogenates. J. Pharm. Pharm. Sci. 2012, 15, 433. [Google Scholar] [CrossRef]
- Zgair, A.; Wong, J.C.M.; Lee, J.B.; Mistry, J.; Sivak, O.; Wasan, K.M.; Hennig, I.M.; Barrett, D.A.; Constantinescu, C.S.; Fischer, P.M.; et al. Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicines. Am. J. Transl. Res. 2016, 8, 3448. [Google Scholar]
- Gershkovich, P.; Hoffman, A. Uptake of lipophilic drugs by plasma derived isolated chylomicrons: Linear correlation with intestinal lymphatic bioavailability. Eur. J. Pharm. Sci. 2005, 26, 394–404. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research. Bioanalytical Method Validation: Guidance for Industry; U.S. Department of Health and Human Services: Silver Spring, MD, USA, 2018.
- Zgair, A.; Man Wong, J.C.; Gershkovich, P. Targeting Immunomodulatory Agents to the Gut-Associated Lymphoid Tissue; Springer Nature: Cham, Switzerland, 2016; ISBN 9783319286099. [Google Scholar]
- Geboers, S.; Haenen, S.; Mols, R.; Brouwers, J.; Tack, J.; Annaert, P.; Augustijns, P. Intestinal behavior of the ester prodrug tenofovir DF in humans. Int. J. Pharm. 2015, 485, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Ohura, K.; Soejima, T.; Nogata, R.; Adachi, Y.; Ninomiya, S.I.; Imai, T. Effect of intestinal first-pass hydrolysis on the oral bioavailability of an ester prodrug of fexofenadine. J. Pharm. Sci. 2012, 101, 3264–3274. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration, Health and Human Services (H.H.S.). Food Labeling: Serving Sizes of Foods That Can Reasonably Be Consumed at One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendmen. Fed. Regist. 2016, 81, 34000. [Google Scholar]
- Zgair, A.; Lee, J.B.; Wong, J.C.M.; Taha, D.A.; Aram, J.; Di Virgilio, D.; McArthur, J.W.; Cheng, Y.-K.; Hennig, I.M.; Barrett, D.A.; et al. Oral administration of cannabis with lipids leads to high levels of cannabinoids in the intestinal lymphatic system and prominent immunomodulation. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Charman, W.N.A.; Stella, V.J. Lymphatic appearance of DDT in thoracic or mesenteric lymph duct cannulated rats. Int. J. Pharm. 1985, 24, 185–192. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zgair, A.; Dawood, Y.; Ibrahem, S.M.; Lee, J.B.; Feng, W.; Fischer, P.M.; Gershkovich, P. Strawberry Decreases Intraluminal and Intestinal Wall Hydrolysis of Testosterone Undecanoate. Molecules 2021, 26, 233. https://doi.org/10.3390/molecules26010233
Zgair A, Dawood Y, Ibrahem SM, Lee JB, Feng W, Fischer PM, Gershkovich P. Strawberry Decreases Intraluminal and Intestinal Wall Hydrolysis of Testosterone Undecanoate. Molecules. 2021; 26(1):233. https://doi.org/10.3390/molecules26010233
Chicago/Turabian StyleZgair, Atheer, Yousaf Dawood, Suhaib M. Ibrahem, Jong Bong Lee, Wanshan Feng, Peter M. Fischer, and Pavel Gershkovich. 2021. "Strawberry Decreases Intraluminal and Intestinal Wall Hydrolysis of Testosterone Undecanoate" Molecules 26, no. 1: 233. https://doi.org/10.3390/molecules26010233
APA StyleZgair, A., Dawood, Y., Ibrahem, S. M., Lee, J. B., Feng, W., Fischer, P. M., & Gershkovich, P. (2021). Strawberry Decreases Intraluminal and Intestinal Wall Hydrolysis of Testosterone Undecanoate. Molecules, 26(1), 233. https://doi.org/10.3390/molecules26010233






