Abstract
Guava (Psidium guajava) leaves are commonly used in the treatment of diseases. They are considered a waste product resulting from guava cultivation. The leaves are very rich in essential oils (EOs) and volatiles. This work represents the detailed comparative chemical profiles of EOs derived from the leaves of six guava varieties cultivated in Egypt, including Red Malaysian (RM), El-Qanater (EQ), White Indian (WI), Early (E), El-Sabahya El-Gedida (ESEG), and Red Indian (RI), cultivated on the same farm in Egypt. The EOs from the leaves of guava varieties were extracted by hydro-distillation and analyzed with GC-MS. The EOs were categorized in a holistic manner using chemometric tools. The hydro-distillation of the samples yielded 0.11–0.48% of the EO (v/w). The GC-MS analysis of the extracted EOs showed the presence of 38 identified compounds from the six varieties. The sesquiterpene compounds were recorded as main compounds of E, EQ, ESEG, RI, and WI varieties, while the RM variety attained the highest content of monoterpenes (56.87%). The sesquiterpenes, β-caryophyllene (11.21–43.20%), and globulol (76.17–26.42%) were detected as the major compounds of all studied guava varieties, while trans-nerolidol (0.53–10.14) was reported as a plentiful compound in all of the varieties except for the RM variety. A high concentration of D-limonene was detected in the EOs of the RM (33.96%), WI (27.04%), and ESEG (9.10%) varieties. These major compounds were consistent with those reported for other genotypes from different countries. Overall, the EOs’ composition and the chemometric analysis revealed substantial variations among the studied varieties that might be ascribed to genetic variability, considering the stability of the cultivation and climate conditions. Therefore, this chemical polymorphism of the studied varieties supports that these varieties could be considered as genotypes of P. guajava. It is worth mentioning here that the EOs, derived from leaves considered to be agricultural waste, of the studied varieties showed that they are rich in biologically active compounds, particularly β-caryophyllene, trans-nerolidol, globulol, and D-limonene. These could be considered as added value for pharmacological and industrial applications. Further study is recommended to confirm the chemical variations of the studied varieties at a molecular level, as well as their possible medicinal and industrial uses.
1. Introduction
Since the beginning of humanity, plants have been used as the main resources of foods, medicines, clothing, and other goods [1]. Many pharmaceutical drugs are derived from plant resources with potent biological activities, along with the low side effects and costs [2]. There are more than 250,000 identified plant species worldwide; among them, 7000 species are cultivated plants that are used in various human activities [3] to provide a myriad of bioactive components, i.e., dietary fiber, minerals, vitamins, and diverse amounts of phytochemicals or secondary metabolites [4].
The guava tree (Psidium guajava; Family: Myrtaceae) is cultivated for its nutritive fruit characterized by high contents of minerals and vitamins [5]. However, other parts (the leaves, bark, and root) of the guava tree are used in traditional medicines to treat several diseases. The guava tree produces a large quantity of biomass that results from the continuous pruning process. This biomass—a waste or byproduct—can be considered as an added value where it can be integrated into the production of various bioactive compounds with pharmacological and industrial application [6]. Different extracts from the guava leaf exhibit potent biological activities, such as anti-inflammatory, antipyretic, neuroprotective, antihypertensive, hypolipidemic, anti-obesity, cardioprotective, antioxidant, hepatoprotective, antidiarrheal, anticancer, immune-strengthening, anti-osteo-renal, antimicrobial, antivirus, and antiplatelet aggregation activities [5,7,8,9,10,11]. In addition, several chemical investigations described the identification of several vitamins (A, C, B, E, and K), carbohydrates, tannins, triterpenoids, flavonoids, benzophenones, and phenolics [8,12,13,14].
The biological activities of P. guajava leaves usually correlate to its essential oils (EOs) and volatiles that represent the main constituents of the leaves. Many compounds can be characterized from the EOs that are extracted from guava leaves around the world, especially the terpenoids, such as limonene, α-pinene, eucalyptol, caryophyllene isomers, α-humulene, γ-murolene, selinene isomers, β-bisabolene, caryophyllene oxide, and epi-β-cubenol [5,6,7,15,16]. The EOs’ composition is reported to be affected by various exogenous factors, such as precipitation, light, season, altitude, and soil characteristics. In addition, various endogenous factors such as anatomical, physiological, and genetic characteristics can modify either the qualitative or quantitative amounts of the EOs’ chemical compounds [17,18,19]. Chemical polymorphism is a phenomenon wherein the same species show variation in the chemical composition of the bioactive compounds [20,21]. This phenomenon is well known in the EOs of various plants [20,22,23,24]. The study of the plants’ variations in chemotypes is essential from a taxonomic point of view, as well as for agronomic and pharmacological applications [6,25]. The chemical polymorphism of the EOs from 22 genotypes of P. guajava grown in two Brazilian environments was observed by de Souza et al. [6]. However, the chemical polymorphism in P. guajava that grows in Egypt is not well studied. Therefore, the present work aims to (i) construct the chemical profiles of EOs extracted from the leaves of six cultivated varieties of P. guajava growing under similar environmental conditions in Egypt, and (ii) to establish a chemical-based relationship among the six varieties using chemometric analysis.
2. Results and Discussion
2.1. Chemical Profiles of the EOs from Different Varieties of P. guajava
The EOs were extracted via hydro-distillation from the leaves of six varieties of guava: Red Malaysian (RM), El-Qanater (EQ), White Indian (WI), Early (E), El-Sabahya El-Gedida (ESEG), and Red Indian (RI). The extracted EOs showed considerable variation in the yields, wherein they produced 0.48, 0.25, 0.21, 0.19, 0.18, 0.15, and 0.11% (v/w) for ESEG, RI, E, RM, WI, EQ, and RT, respectively. The oil obtained from the ESEG guava variety was comparable to that extracted from varieties of P. guajava cultivated in Pakistan (0.60%) [15], Tunisia (0.66%) [26], Brazil (0.40%) [6], and Oman (0.38%) [16]. In contrast, other studied varieties attained lower yields compared to other investigated varieties (Brazilian, Tunisian, and Omani). These variations could be related to seasonal variations, climatic conditions, or habitat [27,28,29,30,31]. The high yield in the ESEG variety showed that it is a premium variety for the production of guava essential oil.
The GC-MS chromatograms revealed substantial variations among the six different varieties (Figure 1). The GC-MS analysis revealed that the chemical compounds can be categorized under four classes (Figure 2). The sesquiterpenes of the E variety were classified into sesquiterpene hydrocarbons (62.15%) and oxygenated sesquiterpenes (37.15%). Meanwhile, the ESEG variety attained 60.95% as sesquiterpene hydrocarbons and 26.11% as oxygenated sesquiterpenes (Figure 2a).
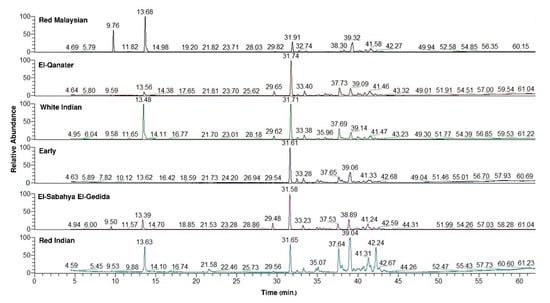
Figure 1.
Gas chromatography–mass spectrometry (GC-MS) chromatogram of the essential oils (EOs) from the leaves of the six studied varieties of Psidium guajava.
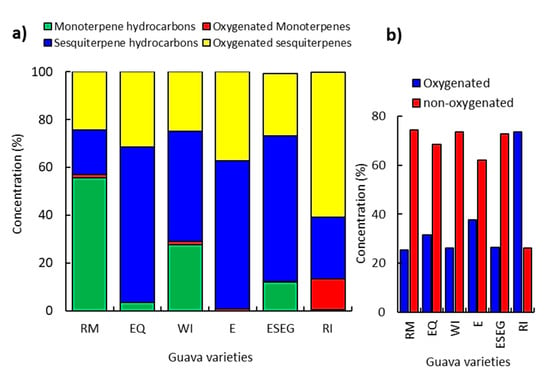
Figure 2.
Relative percentile levels of the different classes of the identified compounds of the six studied varieties of P. guajava; (a) the four specified classes, and (b) the oxygenated and non-oxygenated components.
On the other side, the RM variety attained the highest content of monoterpenes (56.87%), composed mostly of monoterpene hydrocarbons (55.72%), while oxygenated monoterpenes were minor (1.15%). The RI, E, EQ, WI, and ESEG varieties attained 28.88, 13.41, 12.22, 3.55, and 0.68% of the monoterpene compounds (Figure 2a). In general, RI attained the highest content of oxygenated compounds (73.72%), followed by E (37.83%), EQ (31.49%), ESEG (26.38%), WI (26.24%), and RM (25.51%). In contrast, the RM, WI, and ESEG varieties exhibited 74.48, 73.72, and 72.90% as non-oxygenated compounds, which suggested that terpene hydrocarbon biosynthesis is more activated in these varieties (Figure 2b).
The observed variations among the different varieties could be ascribed to genetic variability [32]. Therefore, this chemical polymorphism of the studied varieties supports that these varieties could be considered as genotypes of P. guajava. This phenomenon is known to exist for other species such as Thymus carnosus [23], Salvia fruticose [20], Calotropis procera [17], Origanum libanoticum [24], Origanum syriacum [20], and Cinnamomum osmophloeum [22]. The exogenous factors such as precipitation, light, season, altitude, and soil characteristics can modify either the qualitative or quantitative amounts of the chemical compounds in the EOs [18,19,29,33]. However, the samples of the different varieties in the present study were collected from the same location in the same period; therefore, the exogenous factors can be excluded as controlling factors.
The chemical profiles of the EOs of different P. guajava varieties are shown in Table 1. A total of 38 chemical compounds were identified in the EOs of the studied guava varieties. The WI variety attained the highest number of compounds (29), while RM, ESEG, RI, E, and EQ had 28, 26, 25, 23, and 20 compounds, respectively. This composition was relatively higher than those reported for the Tunisian variety [26].

Table 1.
Chemical compositions of the essential oils (EOs) extracted from the leaves of six varieties of P. guajava.
The sesquiterpenes β-caryophyllene and globulol were detected as major compounds of all studied guava varieties, while trans-nerolidol was reported as a major compound in all except for the RM variety (Table 1). The preponderance of caryophyllene in these varieties was in accordance with those reported by de Souza et al. [6], where they investigated 22 guava genotypes grown in two environments. However, globulol was not detected in the genotypes of de Souza et al.’s [6] study. Although Arain et al. [15] reported that P. guajava leaves collected from Pakistan present an excellent source of β-caryophyllene, our study revealed that the ESEG and E varieties of P. guajava had approximately twice the amount of β-caryophyllene compared to that of the Pakistani variety.
The EOs of the RM guava variety showed the presence of D-limonene, α-pinene, globulol, and β-caryophyllene, and they are represented by 33.96, 20.58, 14.13, and 11.21%, respectively. In the EQ variety, the main compounds detected were β-caryophyllene, globulol, trans-nerolidol, and α-copaene, recorded at 43.20, 10.57, 9.03, and 6.71%, respectively (Table 1). The EQ EO results were similar to the figures reported for the Pakistani variety [15]. According to these results, D-limonene and α-pinene might be assigned as a chemo-taxonomical fingerprint for the RM guava variety, while trans-nerolidol and α-copaene can be assigned for the EQ variety.
On the other side, β-caryophyllene (30.33%), D-limonene (27.04%), trans-nerolidol (8.27%), and globulol (6.17%) were reported as significant compounds in the WI variety. β-caryophyllene (43.12%), globulol (18.47%), and trans-nerolidol (5.81%) were the major constituents of the E variety of P. guajava.
The ESEG variety showed a high content of sesquiterpenes, exemplified by β-caryophyllene (38.42%), globulol (10.75%), α-copaene (7.00%), and trans-nerolidol (5.39%). Moreover, the RI variety was characterized by a preponderance of globulol (26.42%), β-caryophyllene (13.40%), eucalyptol (10.89%), eudesm-7(11)-en-4-ol (10.59%), and trans-nerolidol (10.14%). In our study, the six varieties were cultivated in the same garden with the same cultivation, soil, and climate conditions that directly affect the EO chemical compositions [18,19,29,33]. Thus, these observed variations in the chemical composition of the studied varieties confirmed the polymorphism phenomenon. Consequently, these varieties can be considered as different chemotypes of P. guajava. In addition, these variations could be attributed to endogenous factors such as individual genetic variability [32].
β-Caryophyllene is reported to possess anticancer, analgesic [34], anticonvulsant [35], anti-inflammatory [36], antioxidant, and antimicrobial activities [37], and its abundance, especially in the E, EQ, and ESEG varieties, makes them a possible source of this compounds. As a result, β-caryophyllene was described as a potential agent to treat several diseases due to its activity [38]. In addition, globulol, which was the highest compound in the RI variety, is reported to have various biological activities, such as antifungal [39] and antibacterial activities [40]. RI is also abundant in nerolidol and reported to possess antileishmanial [41], antiparasitic [42], antimalarial [43], and antimicrobial activities [44].
The biologically active monoterpenes α-pinene and limonene were found in the main compounds of the RM variety. α-pinene was described as the main component of most EOs derived from the plant kingdom [45,46]. This compound is integrated as a basic intermediate in bakery and chilled dairy products [47]. Several studies report that the isomers of pinenes, especially α-pinene, have various biological potentialities such as anti-inflammatory, antimicrobial, anticancer, antiviral, flavor, fragrance, antiallergy, and fungicidal activities [45]. On the other hand, D-limonene was reported as a safe anticancer agent, particularly for breast cancer [48]. As a result, the guava leaves’ high biomass yield could be considered a rich resource for these effective and sustainable bioactive compounds.
2.2. Multivariate Data Analysis of the EOs GC-MS Dataset
Although differences in chromatographic patterns were observed among essential oil specimens, we attempted to categorize them in a holistic manner using chemometric tools. Principal component multivariate data analysis (PCA) was applied to model the EO compounds dataset (Figure 3A) and extracted using Metabolomics Ion-based Data Extraction Algorithm (MET-IDEA), and led to the detection of 867 Mass Spectral (MS) signals. The model accounted for 77% of the total variance described by principal components (PC1 and PC2). The PCA score plot (Figure 3A) revealed the distant separation of RM with positive score values along PC1 (right in PC1), whereas the ESEG variety was positioned on the other side with negative score values along with PC. On the other side, all other varieties were clustered together in the center of the PCA and had positive score values. Moreover, the examination of the loading plot revealed that α-pinene and limonene contributed the most to oil segregation and were more abundant in the RM variety. In contrast, the position of the ESEG variety was attributed to its abundance in sesquiterpenes, i.e., caryophyllene, α-copaene, and junipene. Hierarchical clustering analysis further confirmed the EOs segregation pattern, where RM and ESEG varieties were placed separately away in the dendrogram (Figure 3C).
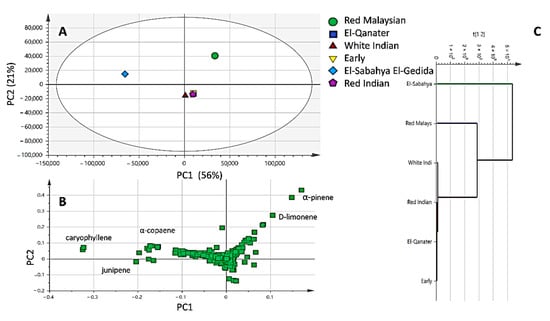
Figure 3.
Principal component analysis (A) score plot, loading plot (B), and hierarchical clustering analysis (C). The model explains 77% of the total variance prescribed by principal components (PC1 and PC2).
3. Materials and Methods
3.1. Plant Materials Collection and Preparation
The fresh, healthy, and well-developed (mature) leaves of the six guava (P. guajava) varieties were collected during the fruiting period (June 2019) from the same garden in Almansouria, Alharam, Egypt. These varieties were characterized as Red Malaysian (RM), El-Qanater (EQ), White Indian (WI), Early (E), El-Sabahya El-Gedida (ESEG), and Red Indian (RI). The garden is located in a semi-urban area, and the soil in the garden is loamy. The climate of the study area has an average temperature of 30–35 °C and average relative humidity of 60%. All of the collected varieties were authenticated by Mohamed El Gebaly, Professor of Taxonomy at the El-Orman Garden and National Research Center. The leaves were dried in the shade at room temperature (25 ± 3 °C) for two days before they were ground into a fine powder and packed in paper bags at −4 °C until further analysis [30]. Because air-drying aromatic plants at high temperature causes isoprenoid loss [49], leaf samples were dried in a shady place at room temperature, and all samples were treated with same procedures to avoid bias.
3.2. EOs, Extraction, GC-MS Analysis, and Components Characterization
The air-dried leaves (200 g) of the six varieties were subjected separately to hydro-distillation using Clevenger-type apparatuses (Shiva Scientific Glass Private Limited, New Delhi, India) for three hours. The oil layer was collected using hexane, dried with 0.5 g of sodium sulfate (anhydrous), and stored in glass vials until GC-MS analysis. The six extracted EOs were separately analyzed via the GC-MS technique using the GC-MS instrument (THERMO Scientific ™ Corporate, Waltham, MA, USA) at the Department of Medicinal and Aromatic Plants Research, National Research Center, Egypt [17]. The specifications of the used GC-MS instrument were adjusted according to the following conditions: TRACE GC Ultra Gas Chromatographs (THERMO Scientific™ Corporate, Waltham, MA, USA), lined with a Thermo Scientific ISQ™ EC single quadrupole mass spectrometer. The GC-MS system was equipped with a TR-5 MS column with dimensions of 30 m × 0.32 mm, i.d., 0.25 μm film thickness. Helium was used as carrier gas at a flow rate of 1.0 mL/min with a split ratio of 1:10 using the following temperature program: 60 °C for 1 min, rising at 4.0 °C/min to 240 °C, and held for 1 min. Both the injector and detector were held at 210 °C. An aliquot of 1 μL of diluted samples in hexane (1:10, v/v) was always injected. Mass spectra were recorded by electron ionization (EI) at 70 eV, using a spectral range of m/z 40–450.
Chemical constituents of the EOs under investigation were characterized by Automated Mass spectral Deconvolution and Identification (AMDIS) software, version 2.71 (Gaithersburg, MD, USA) (www.amdis.net), retention indices (relative to n-alkanes C8–C22), and comparison of the mass spectrum with authentic compounds (if available) from the Wiley spectral library collection and NIST library database (Gaithersburg, MD, USA; Wiley, Hoboken, NJ, USA).
3.3. GC-MS Multivariate Data Analyses
The chemical compounds of the identified EOs were extracted using MET-IDEA software with default parameter settings for GC-MS [50]. The aligned peak abundance data table was further exported to principal component analysis (PCA) using the SIMCA-P version 13.0 software package (Umetrics, Umeå, Sweden). All variables were mean-centered and scaled to the Pareto variance.
4. Conclusions
The GC-MS analysis of the six studied varieties of P. guajava revealed a substantial variation either in the quantity or quality of their EOs’ chemical composition. This variation reflects the chemical polymorphism phenomenon, and these varieties are considered to be different chemotypes. Based on the main compounds and the PCA analysis, it is evident that some main compounds such as β-caryophyllene and globulol were reported in all studied varieties, while trans-nerolidol was reported as a major compound in all varieties except for the RM variety. Other major compounds characterize specific varieties; for example, α-pinene and limonene characterize the RM variety, and caryophyllene, α-copaene, and junipene distinguish the ESEG variety. Therefore, these compounds could be used as a chemical fingerprint to identify these varieties. In practical terms, these major compounds are biologically effective compounds with various activities. The large biomass of guava trees that results from the pruning process, which is considered a waste or byproduct, can be a potential source for these important compounds. The characterization of chemotypes in cultivated plants is crucial for agricultural applications, chemistry purposes, and pharmacological uses. Further study is recommended to characterize the studied varieties at the molecular level to confirm their chemotaxonomic differences.
Author Contributions
Conceptualization, E.M.H., A.E.-N.G.E.G., A.M.A.-E., A.I.E., M.A.F., and E.A.O.; formal analysis, E.M.H., A.E.-N.G.E.G., A.I.E., M.A.F., and E.A.O.; investigation, E.M.H., A.E.-N.G.E.G., A.M.A.-E., A.I.E., M.A.F., S.F.A. and E.A.O.; writing—original draft preparation, A.M.A.-E., A.I.E. and M.A.F.; writing—review and editing, E.M.H., A.E.-N.G.E.G., A.M.A.-E., A.I.E., M.A.F., S.F.A. and E.A.O.; funding acquisition, S.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
Research was supported by project number (RSP 2020/241) King Saud University, Riyadh, Saudi Arabia, and the APC was also funded this project number at the King Saud University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not available.
Acknowledgments
The authors extend their appreciation to the researchers supporting project number (RSP 2020/241) at the King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Murphy, D.J. People, Plants and Genes. The Story of Crops and Humanity; Oxford University Press: Oxford, UK, 2008; Volume 44, p. 571. [Google Scholar]
- Mandal, S.C.; Mandal, V.; Konishi, T. Natural Products and Drug Discovery: An Integrated Approach; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Khoshbakht, K.; Hammer, K. How many plant species are cultivated? Genet. Resour. Crop Evol. 2008, 55, 925–928. [Google Scholar] [CrossRef]
- Bvenura, C.; Sivakumar, D. The role of wild fruits and vegetables in delivering a balanced and healthy diet. Food Res. Int. 2017, 99, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Vijaya Anand, A.; Velayuthaprabhu, S.; Rengarajan, R.L.; Sampathkumar, P.; Radhakrishnan, R. Bioactive Compounds of Guava (Psidium guajava L.). In Bioactive Compounds in Underutilized Fruits and Nuts; Murthy, H.N., Bapat, V.A., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 503–527. [Google Scholar]
- De Souza, T.D.S.; da Silva Ferreira, M.F.; Menini, L.; de Lima Souza, J.R.C.; Parreira, L.A.; Cecon, P.R.; Ferreira, A. Essential oil of Psidium guajava: Influence of genotypes and environment. Sci. Hortic. 2017, 216, 38–44. [Google Scholar] [CrossRef]
- Qin, X.-J.; Yu, Q.; Yan, H.; Khan, A.; Feng, M.-Y.; Li, P.-P.; Hao, X.-J.; An, L.-K.; Liu, H.-Y. Meroterpenoids with antitumor activities from guava (Psidium guajava). J. Agric. Food Chem. 2017, 65, 4993–4999. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Yin, M.-C. Renal protective effects of extracts from guava fruit (Psidium guajava L.) in diabetic mice. Plant Foods Hum. Nutr. 2012, 67, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-F.; Kuo, Y.-T.; Chen, T.-Y.; Chien, C.-T. Quercetin-rich guava (Psidium guajava) juice in combination with trehalose reduces autophagy, apoptosis and pyroptosis formation in the kidney and pancreas of type II diabetic rats. Molecules 2016, 21, 334. [Google Scholar] [CrossRef]
- Rasheed, H.M.; Khan, T.; Wahid, F.; Khan, R.; Shah, A.J. Chemical composition and vascular and intestinal smooth muscle relaxant effects of the essential oil from Psidium guajava fruit. Pharm. Biol. 2016, 54, 2679–2684. [Google Scholar] [CrossRef]
- De Oliveira, S.D.; Araújo, C.M.; Borges, G.D.S.C.; dos Santos Lima, M.; Viera, V.B.; Garcia, E.F.; de Souza, E.L.; de Oliveira, M.E.G. Improvement in physicochemical characteristics, bioactive compounds and antioxidant activity of acerola (Malpighia emarginata DC) and guava (Psidium guajava L.) fruit by-products fermented with potentially probiotic lactobacilli. LWT 2020, 134, 110200. [Google Scholar] [CrossRef]
- Alquezar, B.; Rodrigo, M.J.; Zacarías, L. Regulation of carotenoid biosynthesis during fruit maturation in the red-fleshed orange mutant Cara Cara. Phytochemistry 2008, 69, 1997–2007. [Google Scholar] [CrossRef]
- Metwally, A.; Omar, A.; Harraz, F.; El Sohafy, S. Phytochemical investigation and antimicrobial activity of Psidium guajava L. leaves. Pharmacogn. Mag. 2010, 6, 212. [Google Scholar]
- Tambe, R.; Singhal, R.; Bhise, K.; Kulkarni, M. Phytochemical screening and HPTLC fingerprinting of leaf extracts of Psidium guajava Linn. J. Pharmacogn. Phytochem. 2014, 3, 52–56. [Google Scholar]
- Arain, A.; Hussain Sherazi, S.T.; Mahesar, S.A.; Sirajuddin. Essential oil from Psidium guajava leaves: An excellent source of β-caryophyllene. Nat. Prod. Commun. 2019, 14, 1–5. [Google Scholar] [CrossRef]
- Weli, A.; Al-Kaabi, A.; Al-Sabahi, J.; Said, S.; Hossain, M.A.; Al-Riyami, S. Chemical composition and biological activities of the essential oils of Psidium guajava leaf. J. King Saud Univ. Sci. 2019, 31, 993–998. [Google Scholar] [CrossRef]
- Al-Rowaily, S.L.; Abd-ElGawad, A.M.; Assaeed, A.M.; Elgamal, A.M.; Gendy, A.E.-N.G.E.; Mohamed, T.A.; Dar, B.A.; Mohamed, T.K.; Elshamy, A.I. Essential oil of Calotropis procera: Comparative chemical profiles, antimicrobial activity, and allelopathic potential on weeds. Molecules 2020, 25, 5203. [Google Scholar] [CrossRef] [PubMed]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef]
- Elshamy, A.; Abd-ElGawad, A.M.; El-Amier, Y.A.; El Gendy, A.; Al-Rowaily, S. Interspecific variation, antioxidant and allelopathic activity of the essential oil from three Launaea species growing naturally in heterogeneous habitats in Egypt. Flavour Fragr. J. 2019, 34, 316–328. [Google Scholar] [CrossRef]
- El-Alam, I.; Zgheib, R.; Iriti, M.; El Beyrouthy, M.; Hattouny, P.; Verdin, A.; Fontaine, J.; Chahine, R.; Lounès-Hadj Sahraoui, A.; Makhlouf, H. Origanum syriacum essential oil chemical polymorphism according to soil type. Foods 2019, 8, 90. [Google Scholar] [CrossRef]
- Zgheib, R.; Yassine, C.; Azzi-Achkhouty, S.; Beyrouthy, M.E. Investigation of essential oil chemical polymorphism of Salvia fruticosa naturally growing in Lebanon. J. Essent. Oil Bear. Plants 2019, 22, 408–430. [Google Scholar] [CrossRef]
- Cheng, S.-S.; Liu, J.-Y.; Hsui, Y.-R.; Chang, S.-T. Chemical polymorphism and antifungal activity of essential oils from leaves of different provenances of indigenous cinnamon (Cinnamomum osmophloeum). Bioresour. Technol. 2006, 97, 306–312. [Google Scholar] [CrossRef]
- Salgueiro, L.; Vila, R.; Tomas, X.; Tomi, F.; Cañigueral, S.; Casanova, J.; da Cunha, A.P.; Adzet, T. Chemical polymorphism of the essential oil of Thymus carnosus from Portugal. Phytochemistry 1995, 38, 391–396. [Google Scholar] [CrossRef]
- Zgheib, R.; Chaillou, S.; Ouaini, N.; Rutledge, D.N.; Stien, D.; Kassouf, A.; Beyrouthy, M.E. Investigation of Origanum libanoticum essential oils chemical polymorphism by independent components analysis (ICA). Nat. Prod. Commun. 2018, 13, 1731–1740. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Khadhri, A.; El Mokni, R.; Almeida, C.; Nogueira, J.; Araújo, M.E.M. Chemical composition of essential oil of Psidium guajava L. growing in Tunisia. Ind. Crops Prod. 2014, 52, 29–31. [Google Scholar] [CrossRef]
- Parki, A.; Chaubey, P.; Prakash, O.; Kumar, R.; Pant, A.K. Seasonal variation in essential oil compositions and antioxidant properties of Acorus calamus L. accessions. Medicines 2017, 4, 81. [Google Scholar] [CrossRef] [PubMed]
- Melito, S.; Petretto, G.L.; Chahine, S.; Pintore, G.; Chessa, M. Seasonal variation of essential oil in Rosmarinus officinalis leaves in sardinia. Nat. Prod. Commun. 2019, 14, 1934578X19864005. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Elshamy, A.I.; Al-Rowaily, S.L.; El-Amier, Y.A. Habitat affects the chemical profile, allelopathy, and antioxidant properties of essential oils and phenolic enriched extracts of the invasive plant Heliotropium curassavicum. Plants 2019, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Gawad, A.M. Chemical constituents, antioxidant and potential allelopathic effect of the essential oil from the aerial parts of Cullen plicata. Ind. Crops Prod. 2016, 80, 36–41. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M.; El-Amier, Y.A.; Bonanomi, G. Essential oil composition, antioxidant and allelopathic activities of Cleome droserifolia (Forssk.) Delile. Chem. Biodivers. 2018, 15, e1800392. [Google Scholar] [CrossRef]
- Vogel, H.; Razmilic, I.; Muñoz, M.; Doll, U.; San Martin, J. Studies of genetic variation of essential oil and alkaloid content in boldo (Peumus boldus). Planta Med. 1999, 65, 90–91. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M.; Elshamy, A.I.; El Gendy, A.E.-N.; Gaara, A.; Assaeed, A.M. Volatiles profiling, allelopathic activity, and antioxidant potentiality of Xanthium strumarium leaves essential oil from Egypt: Evidence from chemometrics analysis. Molecules 2019, 24, 584. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—Natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.C.; de Oliveira, C.V.; Grigoletto, J.; Ribeiro, L.R.; Funck, V.R.; Grauncke, A.C.B.; de Souza, T.L.; Souto, N.S.; Furian, A.F.; Menezes, I.R.A. Anticonvulsant activity of β-caryophyllene against pentylenetetrazol-induced seizures. Epilepsy Behav. 2016, 56, 26–31. [Google Scholar] [CrossRef]
- Brito, L.F.; Oliveira, H.B.M.; das Neves Selis, N.; e Souza, C.L.S.; Júnior, M.N.S.; de Souza, E.P.; Silva, L.S.C.d.; de Souza Nascimento, F.; Amorim, A.T.; Campos, G.B. Anti-inflammatory activity of β-caryophyllene combined with docosahexaenoic acid in a model of sepsis induced by Staphylococcus aureus in mice. J. Sci. Food Agric. 2019, 99, 5870–5880. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Al Kaabi, M.J.; Nurulain, S.M.; Goyal, S.N.; Amjad Kamal, M.; Ojha, S. Polypharmacological properties and therapeutic potential of β-caryophyllene: A dietary phytocannabinoid of pharmaceutical promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Zhou, L.; Huang, Y.; Wang, Y.; Hao, X.; Wang, J. Antimicrobial activity of globulol isolated from the fruits of Eucalyptus globulus Labill. Nat. Prod. Res. 2008, 22, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.; de Paula, R.; Costa, R.B.; dos Anjos, J.; da Silva, M.; Correia, M. Bioprospection of Libidibia ferrea var. ferrea: Phytochemical properties and antibacterial activity. S. Afr. J. Bot. 2020, 130, 103–108. [Google Scholar] [CrossRef]
- Silva, M.P.; Oliveira, G.L.; De Carvalho, R.B.; De Sousa, D.P.; Freitas, R.M.; Pinto, P.L.; Moraes, J.D. Antischistosomal activity of the terpene nerolidol. Molecules 2014, 19, 3793–3803. [Google Scholar] [CrossRef]
- Silva, M.P.; de Oliveira, R.N.; Mengarda, A.C.; Roquini, D.B.; Allegretti, S.M.; Salvadori, M.C.; Teixeira, F.S.; de Sousa, D.P.; Pinto, P.L.; da Silva Filho, A.A. Antiparasitic activity of nerolidol in a mouse model of schistosomiasis. Int. J. Antimicrob. Agents 2017, 50, 467–472. [Google Scholar] [CrossRef]
- Saito, A.Y.; Rodriguez, A.A.M.; Vega, D.S.M.; Sussmann, R.A.; Kimura, E.A.; Katzin, A.M. Antimalarial activity of the terpene nerolidol. Int. J. Antimicrob. Agents 2016, 48, 641–646. [Google Scholar] [CrossRef]
- Krist, S.; Banovac, D.; Tabanca, N.; Wedge, D.E.; Gochev, V.K.; Wanner, J.; Schmidt, E.; Jirovetz, L. Antimicrobial activity of nerolidol and its derivatives against airborne microbes and further biological activities. Nat. Prod. Commun. 2015, 10, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; LD Jayaweera, S.; A Dias, D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N. Therapeutic potential of α-and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Gawad, A.; El Gendy, A.; Elshamy, A.; Omer, E. Chemical composition of the essential oil of Trianthema portulacastrum L. Aerial parts and potential antimicrobial and phytotoxic activities of its extract. J. Essent. Oil Bear. Plants 2016, 19, 1684–1692. [Google Scholar] [CrossRef]
- Vespermann, K.A.; Paulino, B.N.; Barcelos, M.C.; Pessôa, M.G.; Pastore, G.M.; Molina, G. Biotransformation of α-and β-pinene into flavor compounds. Appl. Microbiol. Biotechnol. 2017, 101, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Sun, J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259. [Google Scholar] [PubMed]
- Portillo-Estrada, M.; Copolovici, L.; Niinemets, Ü. Bias in leaf dry mass estimation after oven-drying isoprenoid-storing leaves. Trees 2015, 29, 1805–1816. [Google Scholar] [CrossRef]
- Ramadan, N.S.; Wessjohann, L.A.; Mocan, A.; Vodnar, D.C.; El-Sayed, N.H.; El-Toumy, S.A.; Mohamed, D.A.; Aziz, Z.A.; Ehrlich, A.; Farag, M.A. Nutrient and sensory metabolites profiling of Averrhoa carambola L. (Starfruit) in the context of its origin and ripening stage by GC/MS and chemometric analysis. Molecules 2020, 25, 2423. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).