Exploring Structure-Property Relationships in a Bio-Inspired Family of Bipodal and Electronically-Coupled Bistriphenylamine Dyes for Dye-Sensitized Solar Cell Applications
Abstract
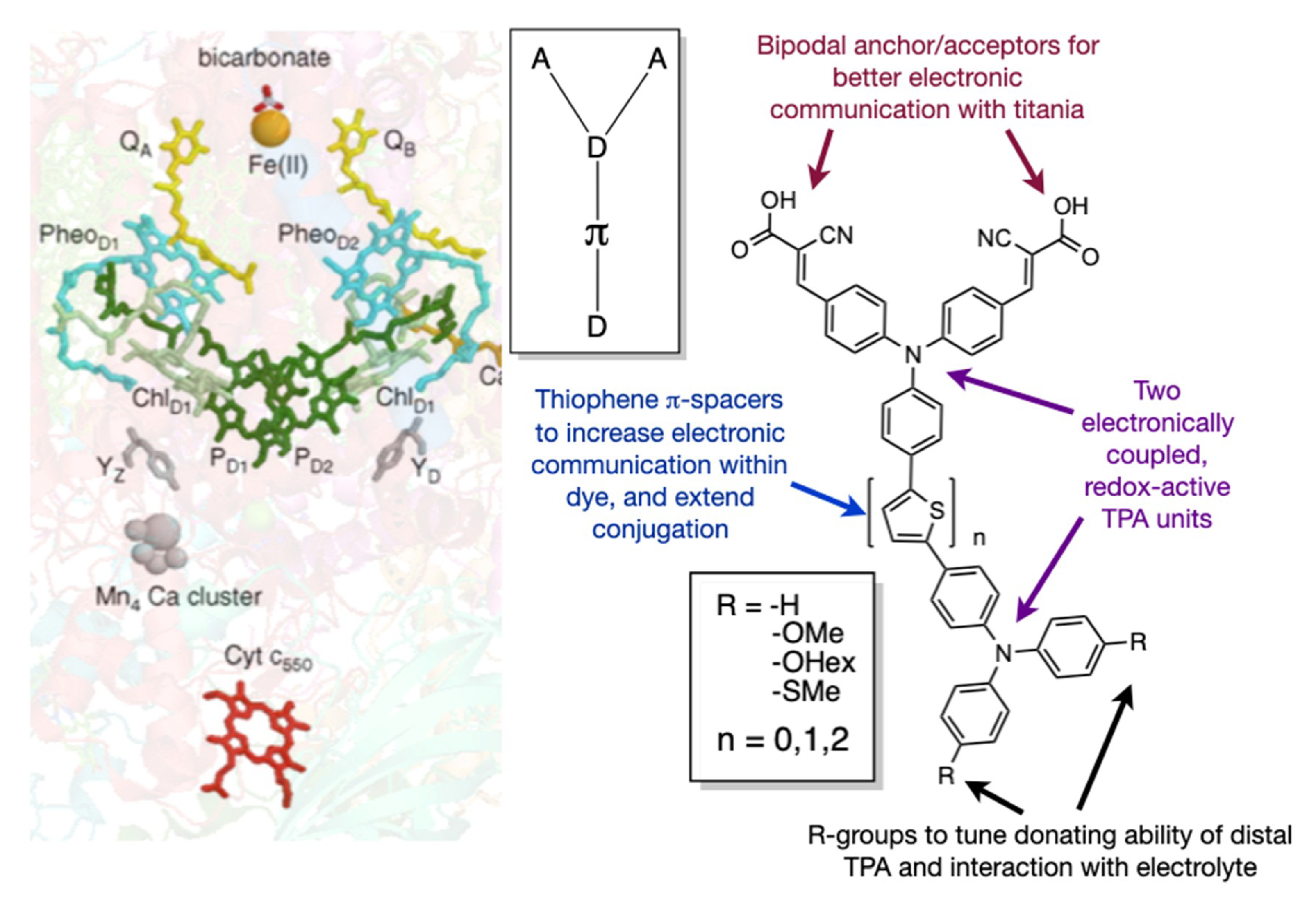
1. Introduction
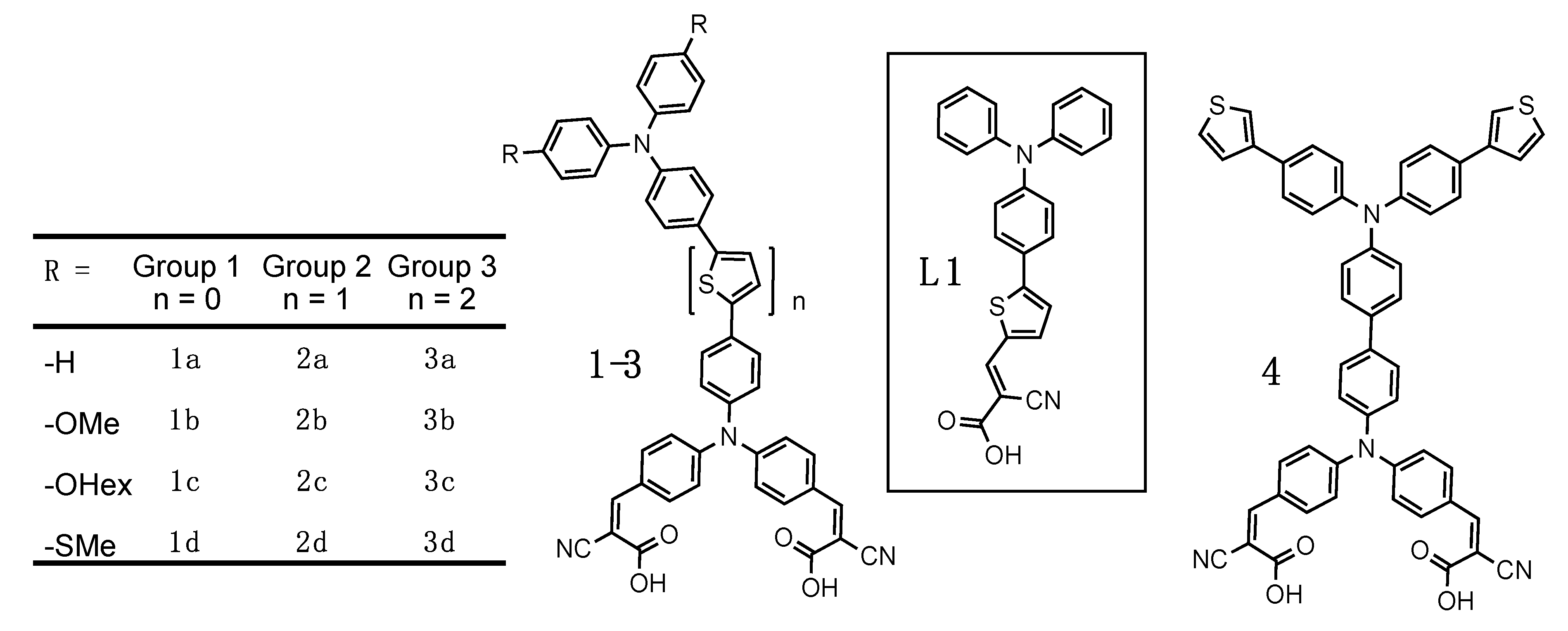
2. Dye Design and Synthesis
3. Results and Discussion
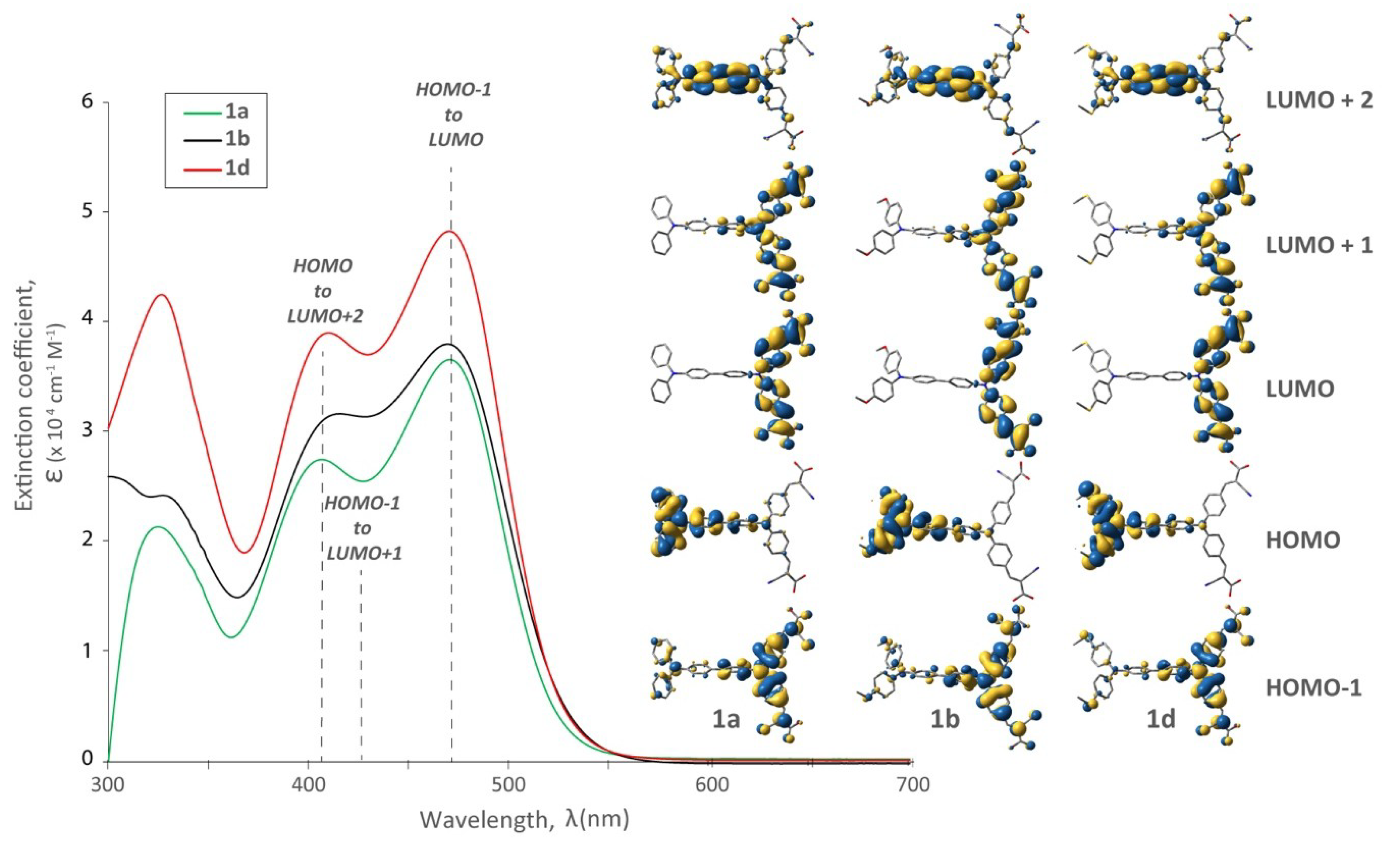
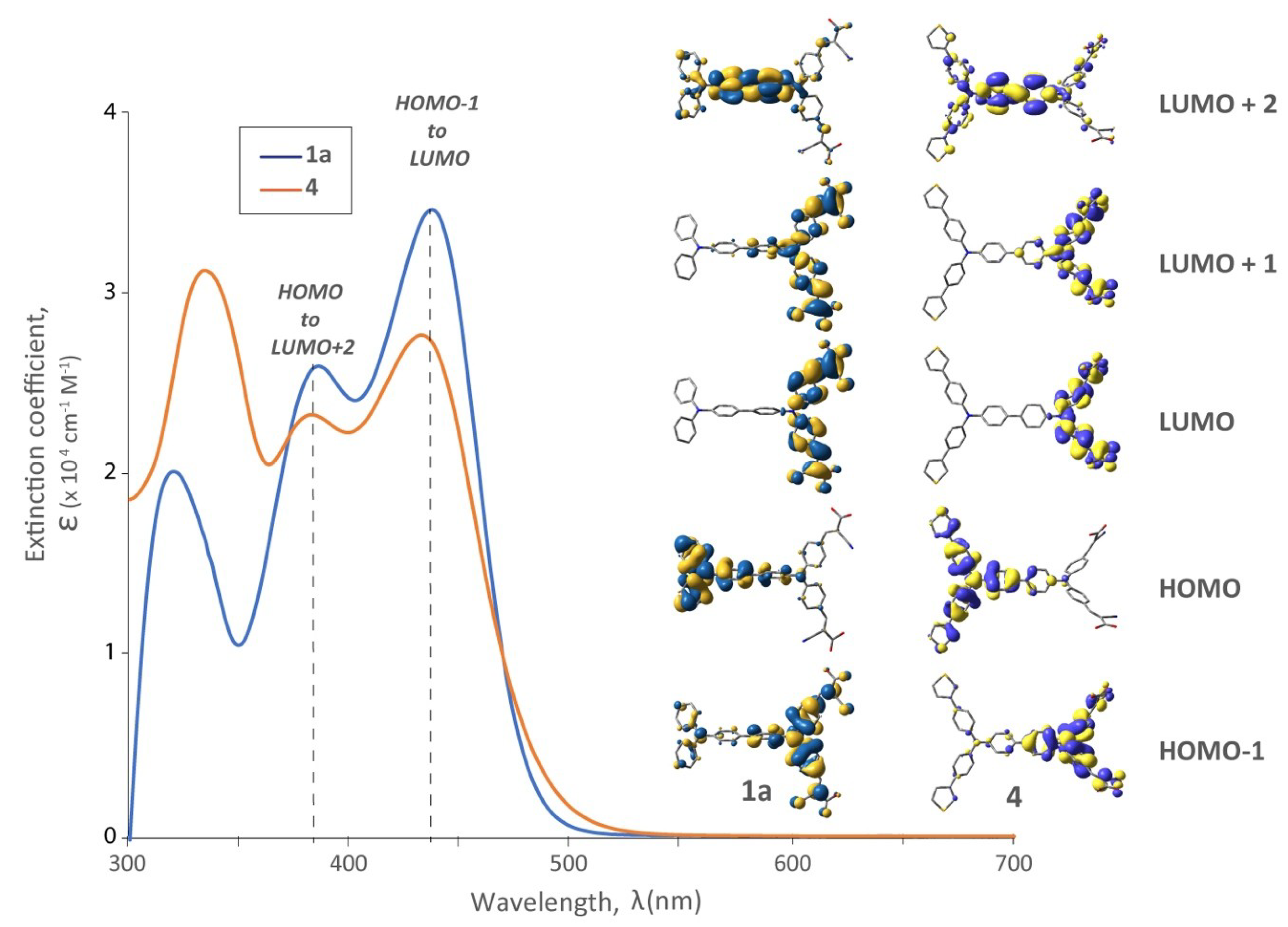
3.1. Optical Behaviour
3.2. Electrochemical Behaviour
3.3. Photovoltaic Performance within the DSSC
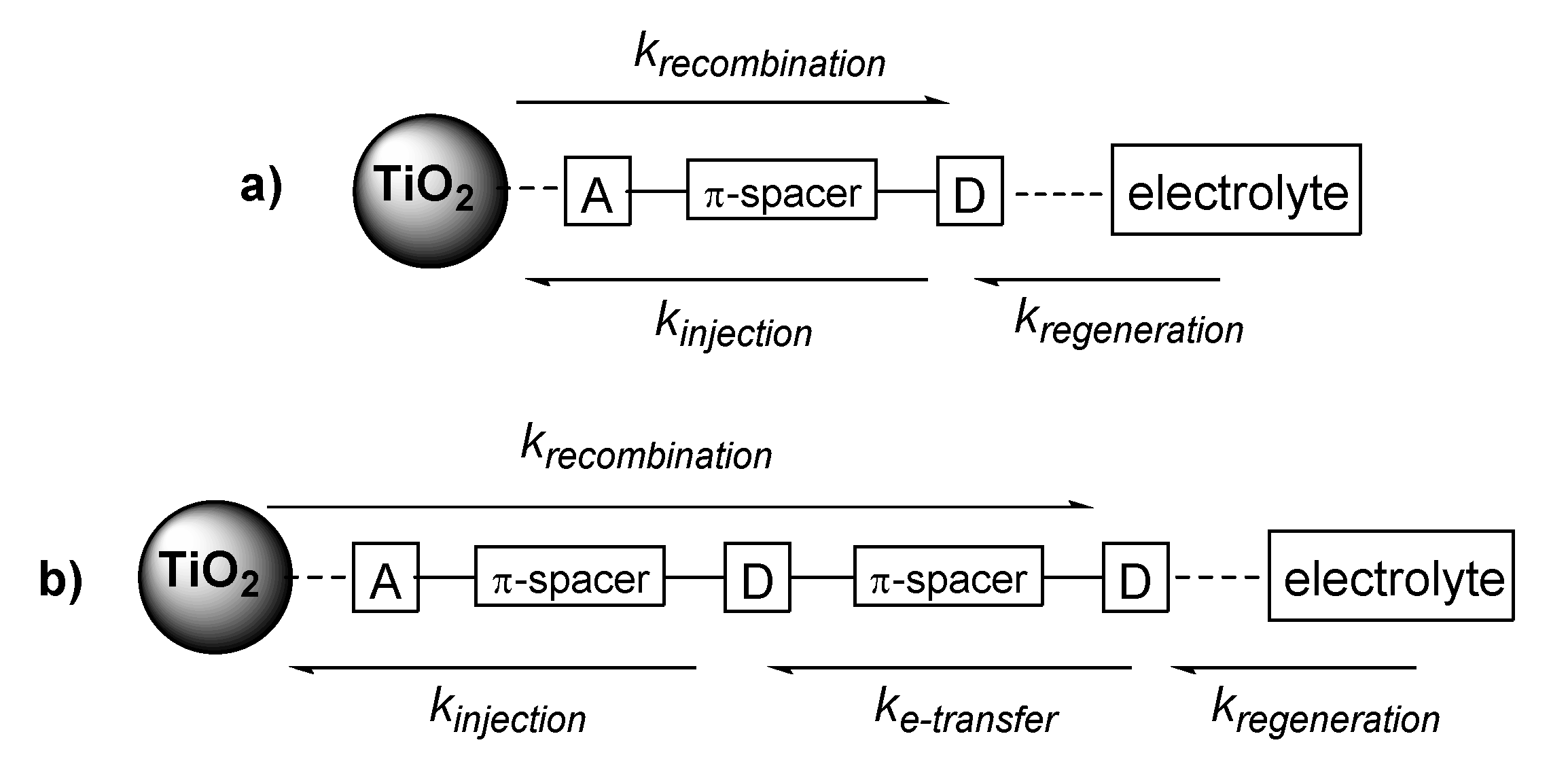
3.4. The Effect of the π-Spacer
3.5. The Effect of Distal TPA Modification
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chung, I.; Lee, B.; He, J.Q.; Chang, R.P.H.; Kanatzidis, M.G. All-solid-state dye-sensitized solar cells with high efficiency. Nature 2012, 485, 486–489. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zheng, D.; Wang, M.; Lin, C.; Lin, Z. High efficiency perovskite solar cells: From complex nanostructure to planar heterojunction. J. Mater. Chem. A 2014, 2, 5994–6003. [Google Scholar] [CrossRef]
- Jung, E.H.; Jeon, N.J.; Park, E.Y.; Moon, C.S.; Shin, T.J.; Yang, T.Y.; Noh, J.H.; Seo, J. Efficient, stable and scalable perovskite solar cells using poly(3-hexylthiophene). Nature 2019, 567, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Assadi, M.K.; Bakhoda, S.; Saidur, R.; Hanaei, H. Recent progress in perovskite solar cells. Renew. Sustain. Energy Rev. 2018, 81, 2812–2822. [Google Scholar] [CrossRef]
- Green, M.A.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Yoshita, M.; Ho-Baillie, A.W.Y. Solar cell efficiency tables (Version 55). Prog. Photovolt. 2020, 28, 3–15. [Google Scholar] [CrossRef]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef]
- Bella, F.; Gerbaldi, C.; Barolo, C.; Grätzel, M. Aqueous dye-sensitized solar cells. Chem. Soc. Rev. 2015, 44, 3431–3473. [Google Scholar] [CrossRef]
- Bella, F.; Porcarelli, L.; Mantione, D.; Gerbaldi, C.; Barolo, C.; Grätzel, M.; Mecerreyes, D. A water-based and metal-free dye solar cell exceeding 7% efficiency using a cationic poly(3,4-ethylenedioxythiophene) derivative. Chem. Sci. 2020, 11, 1485–1493. [Google Scholar] [CrossRef]
- D’Ercole, D.; Dominici, L.; Brown, T.M.; Michelotti, F.; Reale, A.; Di Carlo, A. Angular response of dye solar cells to solar and spectrally resolved light. Appl. Phys. Lett. 2011, 99, 213301. [Google Scholar] [CrossRef]
- Goncalves, L.M.; Bermudez, V.D.; Ribeiro, H.A.; Mendes, A.M. Dye-sensitized solar cells: A safe bet for the future. Energy Environ. Sci. 2008, 1, 655–667. [Google Scholar] [CrossRef]
- Escalante, R.; Pourjafari, D.; Reyes-Coronado, D.; Oskam, G. Dye-sensitized solar cell scale-up: Influence of substrate resistance. J. Renew. Sustain. Energy 2016, 8. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Jose, R.; Brown, T.M.; Fabregat-Santiago, F.; Bisquert, J. A perspective on the production of dye-sensitized solar modules. Energy Environ. Sci. 2014, 7, 3952–3981. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Y.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Direct Contact of Selective Charge Extraction Layers Enables High-Efficiency Molecular Photovoltaics. Joule 2018, 2, 1108–1117. [Google Scholar] [CrossRef]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.E.; Gratzel, M.; et al. Dye-sensitized solar cells for efficient power generation under ambient lighting. Nat. Photonics 2017, 11, 372. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; CurchodBasile, F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; NazeeruddinMd, K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef]
- Mishra, A.; Fischer, M.K.R.; Bauerle, P. Metal-Free Organic Dyes for Dye-Sensitized Solar Cells: From Structure: Property Relationships to Design Rules. Angew. Chem. Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef]
- Ooyama, Y.; Harima, Y. Molecular Designs and Syntheses of Organic Dyes for Dye-Sensitized Solar Cells. Eur. J. Org. Chem. 2009, 18, 2903–2934. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Wang, W.; Gurzadyan, G.G.; Li, J.; Li, X.; An, J.; Yu, Z.; Wang, H.; Cai, B.; et al. 13.6% Efficient organic dye-sensitized solar cells by minimizing energy losses of the excited state. ACS Energy Lett. 2019, 4, 943–951. [Google Scholar] [CrossRef]
- Ning, Z.; Tian, H. Triarylamine: A promising core unit for efficient photovoltaic materials. Chem. Commun. 2009, 37, 5483–5495. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Peng, B.; Chen, J.; Liang, M.; Cai, F. New triphenylamine-based dyes for dye-sensitized solar cells. J. Phys. Chem. C 2008, 112, 874–880. [Google Scholar] [CrossRef]
- Liang, M.; Xu, W.; Cai, F.S.; Chen, P.Q.; Peng, B.; Chen, J.; Li, Z.M. New triphenylamine-based organic dyes for efficient dye-sensitized solar cells. J. Phys. Chem. C 2007, 111, 4465–4472. [Google Scholar] [CrossRef]
- Liang, M.; Chen, J. Arylamine organic dyes for dye-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 3453–3488. [Google Scholar] [CrossRef] [PubMed]
- Robson, K.C.D.; Koivisto, B.D.; Gordon, T.J.; Baumgartner, T.; Berlinguette, C.P. Triphenylamine-modified ruthenium(II) terpyridine complexes: Enhancement of light absorption by conjugated bridging motifs. Inorg. Chem. 2010, 49, 5335–5337. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, P.; Kabra, D. A review on triphenylamine (TPA) based organic hole transport materials (HTMs) for dye sensitized solar cells (DSSCs) and perovskite solar cells (PSCs): Evolution and molecular engineering. J. Mater. Chem. A 2017, 5, 1348–1373. [Google Scholar] [CrossRef]
- Wang, J.; Liu, K.; Ma, L.; Zhan, X. Triarylamine: Versatile Platform for Organic, Dye-Sensitized, and Perovskite Solar Cells. Chem. Rev. 2016, 116, 14675–14725. [Google Scholar] [CrossRef]
- Marinado, T.; Nonomura, K.; Nissfolk, J.; Karlsson, M.K.; Hagberg, D.P.; Sun, L.C.; Mori, S.; Hagfeldt, A. How the Nature of Triphenylamine-Polyene Dyes in Dye-Sensitized Solar Cells Affects the Open-Circuit Voltage and Electron Lifetimes. Langmuir 2010, 26, 2592–2598. [Google Scholar] [CrossRef]
- Su, R.; Lyu, L.; Elmorsy, M.R.; El-Shafei, A. Novel metal-free organic dyes constructed with the D-D|A-π-A motif: Sensitization and co-sensitization study. Sol. Energy 2019, 194, 400–414. [Google Scholar] [CrossRef]
- Abdi, O.K.; Fischer, B.J.D.; Al-Faouri, T.; Buguis, F.L.; Devgan, H.S.; Schott, E.; Zarate, X.; Koivisto, B.D. Bipodal dyes with bichromic triphenylamine architectures for use in dye-sensitized solar cell applications. RSC Adv. 2018, 8, 42424–42428. [Google Scholar] [CrossRef]
- Hussein, B.A.; Huynh, J.T.; Prieto, P.L.; Barran, C.P.; Arnold, A.E.; Sarycheva, O.V.; Lough, A.J.; Koivisto, B.D. Molecular lemmings: Strategies to avoid when designing BODIPY ferrocene dyads for dye-sensitized solar cell applications. Dalton Trans. 2018, 47, 4916–4920. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.S.; Ramkumar, S.; Asiri, A.M.; Anandan, S. Bi-anchoring organic sensitizers of type D-(π-A)2 comprising thiophene-2-acetonitrile as π-spacer and malonic acid as electron acceptor for dye sensitized solar cell applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Berhe Desta, M.; Chaurasia, S.; Lin, J.T. Reversed Y-shape di-anchoring sensitizers for dye sensitized solar cells based on benzimidazole core. Dye. Pigment. 2017, 140, 441–451. [Google Scholar] [CrossRef]
- Pei, K.; Wu, Y.; Islam, A.; Zhang, Q.; Han, L.; Tian, H.; Zhu, W. Constructing high-efficiency D-A-π-A-featured solar cell sensitizers: A promising building block of 2,3-diphenylquinoxaline for antiaggregation and photostability. ACS Appl. Mater. Interfaces 2013, 5, 4986–4995. [Google Scholar] [CrossRef] [PubMed]
- Khanasa, T.; Jantasing, N.; Morada, S.; Leesakul, N.; Tarsang, R.; Namuangruk, S.; Kaewin, T.; Jungsuttiwong, S.; Sudyoadsuk, T.; Promarak, V. Synthesis and characterization of 2D-D-π-A-type organic dyes bearing bis(3,6-di-tert-butylcarbazol-9-ylphenyl)aniline as donor moiety for dye-sensitized solar cells. Eur. J. Org. Chem. 2013, 13, 2608–2620. [Google Scholar] [CrossRef]
- Preat, J.; Michaux, C.; Jacquemin, D.; Perpete, E.A. Enhanced Efficiency of Organic Dye-Sensitized Solar Cells: Triphenylamine Derivatives. J. Phys. Chem. C 2009, 113, 16821–16833. [Google Scholar] [CrossRef]
- Brigham, E.C.; Meyer, G.J. Ostwald Isolation to Determine the Reaction Order for TiO2(e(-))vertical bar S+ -> TiO2IS Charge Recombination at Sensitized TiO2 Interfaces. J. Phys. Chem. C 2014, 118, 7886–7893. [Google Scholar] [CrossRef]
- Haque, S.A.; Tachibana, Y.; Klug, D.R.; Durrant, J.R. Charge recombination kinetics in dye-sensitized nanocrystalline titanium dioxide films under externally applied bias. J. Phys. Chem. B 1998, 102, 1745–1749. [Google Scholar] [CrossRef]
- Hasselmann, G.M.; Meyer, G.J. Diffusion-limited interfacial electron transfer with large apparent driving forces. J. Phys. Chem. B 1999, 103, 7671–7675. [Google Scholar] [CrossRef]
- Sampaio, R.N.; Piechota, E.J.; Troian-Gautier, L.; Maurer, A.B.; Hu, K.; Schauer, P.A.; Blair, A.D.; Berlinguette, C.P.; Meyer, G.J. Kinetics teach that electronic coupling lowers the free-energy change that accompanies electron transfer. Proc. Natl. Acad. Sci. USA 2018, 115, 7248–7253. [Google Scholar] [CrossRef]
- Hu, K.; Robson, K.C.D.; Beauvilliers, E.E.; Schott, E.; Zarate, X.; Arratia-Perez, R.; Berlinguette, C.P.; Meyer, G.J. Intramolecular and Lateral Intermolecular Hole Transfer at the Sensitized TiO2 Interface. J. Am. Chem. Soc. 2014, 136, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Robson, K.C.D.; Johansson, P.G.; Berlinguette, C.P.; Meyer, G.J. Intramolecular Hole Transfer at Sensitized TiO2 Interfaces. J. Am. Chem. Soc. 2012, 134, 8352–8355. [Google Scholar] [CrossRef] [PubMed]
- Maggio, E.; Troisi, A. Theory of the Charge Recombination Reaction at the Semiconductor-Adsorbate Interface in the Presence of Defects. J. Phys. Chem. C 2013, 117, 24196–24205. [Google Scholar] [CrossRef]
- Hu, K.; Blair, A.D.; Piechota, E.J.; Schauer, P.A.; Sampaio, R.N.; Parlane, F.G.L.; Meyer, G.J.; Berlinguette, C.P. Kinetic pathway for interfacial electron transfer from a semiconductor to a molecule. Nat. Chem. 2016, 8, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhai, J.; Heng, L.; Wei, T.; Wen, L.; Jiang, L.; Zhao, X.; Zhang, X. Bio-inspired multi-scale structures in dye-sensitized solar cell. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 149–158. [Google Scholar] [CrossRef]
- Fang, Y.; Huang, H.; Jiang, P.; Li, H.; Shen, P.; Tian, Z.; Zhao, B.; Tan, S. Phenylenevinylene copolymers of dihexylthienylbenzothiadiazole and triphenylamine or tetraphenylbenzidine: Synthesis, characterization and photovoltaic properties. J. Mater. Sci. 2012, 47, 5706–5714. [Google Scholar] [CrossRef]
- Amthor, S.; Lambert, C. [2.2]Paracyclophane-bridged mixed-valence compounds: Application of a generalized mulliken-hush three-level model. J. Phys. Chem. A 2006, 110, 1177–1189. [Google Scholar] [CrossRef]
- Ferdowsi, P.; Saygili, Y.; Zhang, W.; Edvinson, T.; Kavan, L.; Mokhtari, J.; Zakeeruddin, S.M.; Grätzel, M.; Hagfeldt, A. Molecular Design of Efficient Organic D–A–Π –A Dye Featuring Triphenylamine as Donor Fragment for Application in Dye-Sensitized Solar Cells. ChemSusChem 2018, 11, 494–502. [Google Scholar] [CrossRef]
- Yin, X.; Wang, C.; Zhao, D.; Shrestha, N.; Grice, C.R.; Guan, L.; Song, Z.; Chen, C.; Li, C.; Chi, G.; et al. Binary hole transport materials blending to linearly tune HOMO level for high efficiency and stable perovskite solar cells. Nano Energy 2018, 51, 680–687. [Google Scholar] [CrossRef]
- Dienes, Y.; Durben, S.; Kárpáti, T.; Neumann, T.; Englert, U.; Nyulászi, L.; Baumgartner, T. Selective tuning of the band gap of π-conjugated dithieno [3,2-6: 2′,3′-d]phospholes toward different emission colors. Chem. A Eur. J. 2007, 13, 7487–7500. [Google Scholar] [CrossRef]
- Bonnier, C.; Machin, D.D.; Abdi, O.K.; Robson, K.C.D.; Koivisto, B.D. The effect of donor-modification in organic light-harvesting motifs: Triphenylamine donors appended with polymerisable thienyl subunits. Org. Biomol. Chem. 2013, 11, 7011–7015. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hagberg, D.P.; Marinado, T.; Karlsson, K.M.; Nonomura, K.; Qin, P.; Boschloo, G.; Brinck, T.; Hagfeldt, A.; Sun, L. Tuning the HOMO and LUMO energy levels of organic chromophores for dye sensitized solar cells. J. Org. Chem. 2007, 72, 9550–9556. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Severin, H.A.; Koivisto, B.D.; Robson, K.C.D.; Schott, E.; Arratia-Perez, R.; Meyer, G.J.; Berlinguette, C.P. Direct spectroscopic evidence for constituent heteroatoms enhancing charge recombination at a TiO2-ruthenium dye interface. J. Phys. Chem. C 2014, 118, 17079–17089. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, J.M. Dye aggregation in dye-sensitized solar cells. J. Mater. Chem. A 2017, 5, 19541–19559. [Google Scholar] [CrossRef]
- Heng, P.; Xu, J.; Mao, L.; Wang, L.; Wu, W.; Zhang, J. Rational design of D-π-A organic dyes to prevent “trade off” effect in dye-sensitized solar cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 221, 117167. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision C.01. Gaussian. Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Robson, K.C.D.; Sporinova, B.; Koivisto, B.D.; Schott, E.; Brown, D.G.; Berlinguette, C.P. Systematic modulation of a bichromic cyclometalated ruthenium(II) scaffold bearing a redox-active triphenylamine constituent. Inorg. Chem. 2011, 50, 6019–6028. [Google Scholar] [CrossRef]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Robson, K.C.D.; Hu, K.; Meyer, G.J.; Berlinguette, C.P. Atomic Level Resolution of Dye Regeneration in the Dye-Sensitized Solar Cell. J. Am. Chem. Soc. 2013, 135, 1961–1971. [Google Scholar] [CrossRef]
Sample Availability: Samples (5–10 mg) of the compounds are available from the authors. |

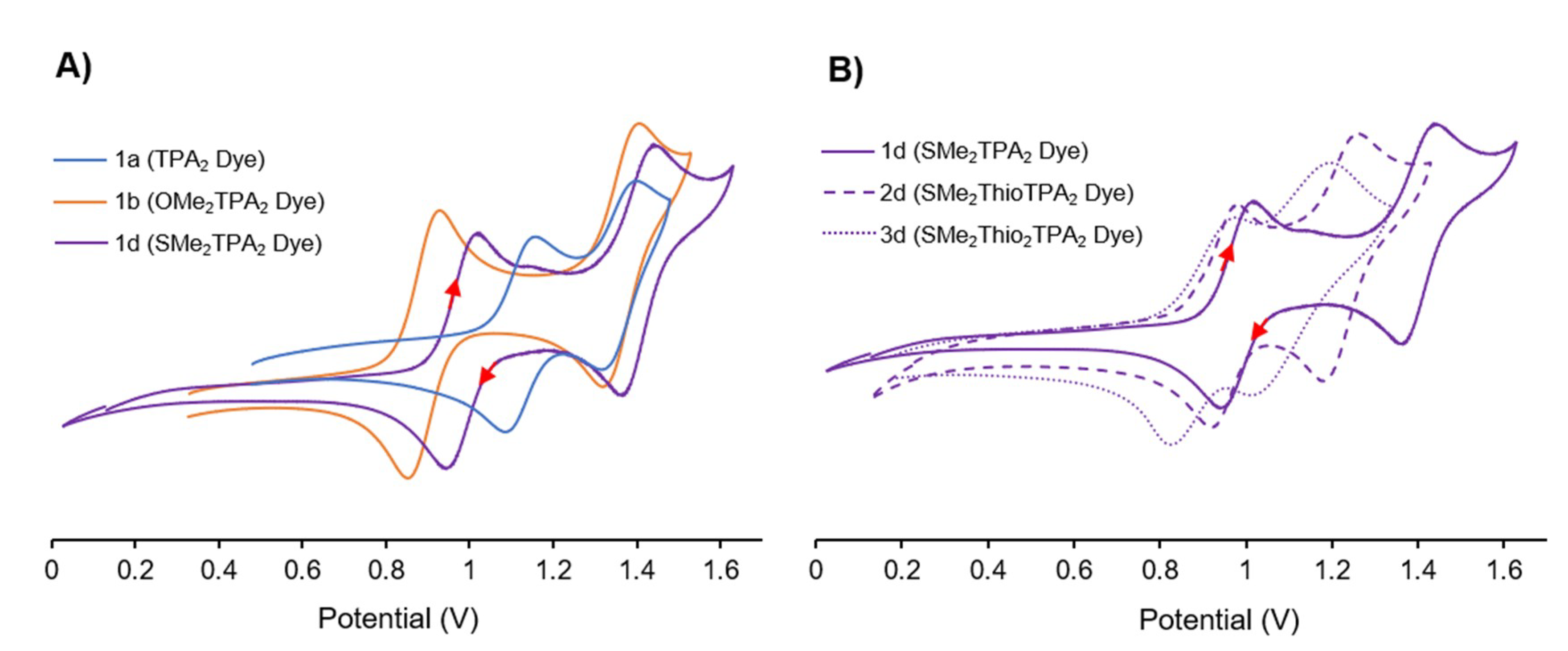
| Dye | UV-Vis λmax nm (ε × 104 M−1cm−1) | E1/2 a (V vs. NHE) | ||
|---|---|---|---|---|
| L1 | 483 (3.2) b | 1.23 | ||
| 1a | 471 (3.6) | 407 (2.7) | 1.12 | 1.36 |
| 1b | 470 (3.8) | 416 (3.2) | 0.89 | 1.36 |
| 1c | 423 (4.9) | 302 (2.8) | 0.87 | 1.34 |
| 1d | 471 (4.8) | 410 (3.9), 328 (4.2) | 0.98 | 1.39 |
| 2a | 421 (3.3) | 1.04 | 1.19 | |
| 2b | 433 (6.2) | 0.86 | 1.19 | |
| 2c | 415 (3.4) | 0.84 | 1.14 | |
| 2d | 428 (6.0) | 322 (3.5) | 0.95 | 1.22 |
| 3a | 436 (1.5) | 0.98 | 1.14 | |
| 3b | 434 (0.7) | 0.86 | 1.10 | |
| 3c | 437 (3.1) | 0.84 | 1.10 | |
| 3d | 438 (5.2) | 326 (2.4) | 0.93 | 1.13 |
| 4 | 344 (3.3) | 469(2.9), 399 (2.4) | 1.07 | 1.43 |
| Dye | VOC (V) | JSC (mA/cm2) | FF | η (%) | N |
|---|---|---|---|---|---|
| L1 | 0.51 ± 0.02 | 3.37 ± 0.32 | 0.58 ± 0.04 | 1.08 ± 0.13 | 7 |
| 1a | 0.65 ± 0.02 | 5.58 ± 0.42 | 0.71 ± 0.02 | 2.77 ± 0.26 | 8 |
| 1b | 0.64 ± 0.01 | 3.20 ± 0.64 | 0.70 ± 0.03 | 2.00 ± 0.35 | 7 |
| 1c | 0.61 ± 0.03 | 4.57 ± 0.69 | 0.64 ± 0.05 | 1.90 ± 0.41 | 8 |
| 1d | 0.66 ± 0.03 | 5.67 ± 1.00 | 0.66 ± 0.09 | 2.66 ± 0.68 | 8 |
| 2a | 0.61 ± 0.01 | 5.16 ± 0.53 | 0.68 ± 0.03 | 2.31 ± 0.34 | 8 |
| 2b | 0.68 ± 0.01 | 3.30 ± 0.43 | 0.67 ± 0.05 | 2.20 ± 0.23 | 7 |
| 2c | 0.58 ± 0.02 | 4.30 ± 0.19 | 0.70 ± 0.03 | 1.90 ± 0.10 | 6 |
| 2d | 0.71 ± 0.01 | 5.71 ± 0.84 | 0.72 ± 0.06 | 3.18 ± 0.63 | 9 |
| 3a | 0.59 ± 0.02 | 4.38 ± 0.49 | 0.69 ± 0.03 | 1.90 ± 0.30 | 8 |
| 3b | 0.54 ± 0.02 | 3.21 ± 0.56 | 0.64 ± 0.05 | 1.20 ± 0.21 | 8 |
| 3c | 0.55 ± 0.03 | 3.26 ± 0.57 | 0.65 ± 0.05 | 1.29 ± 0.30 | 8 |
| 3d | 0.66 ± 0.02 | 5.96 ± 0.73 | 0.66 ± 0.05 | 2.88 ± 0.21 | 8 |
| 4 | 0.63 ± 0.01 | 6.48 ± 0.75 | 0.65 ± 0.04 | 2.86 ± 0.41 | 7 |
| π-Spacer | -H (η%) | -OMe (η%) | -OHex (η%) | -SMe (η%) | Dye 4 (η%) |
|---|---|---|---|---|---|
| No thiophene spacer | 1a 2.77 | 1b 2.00 | 1c 1.90 | 1d 2.66 | 4 2.86 |
 | 2a 2.31 | 2b 2.20 | 2c 1.90 | 2d 3.18 | - |
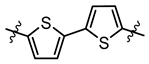 | 3a 1.90 | 3b 1.20 | 3c 1.29 | 3d 2.88 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Faouri, T.; Buguis, F.L.; Azizi Soldouz, S.; Sarycheva, O.V.; Hussein, B.A.; Mahmood, R.; Koivisto, B.D. Exploring Structure-Property Relationships in a Bio-Inspired Family of Bipodal and Electronically-Coupled Bistriphenylamine Dyes for Dye-Sensitized Solar Cell Applications. Molecules 2020, 25, 2260. https://doi.org/10.3390/molecules25092260
Al-Faouri T, Buguis FL, Azizi Soldouz S, Sarycheva OV, Hussein BA, Mahmood R, Koivisto BD. Exploring Structure-Property Relationships in a Bio-Inspired Family of Bipodal and Electronically-Coupled Bistriphenylamine Dyes for Dye-Sensitized Solar Cell Applications. Molecules. 2020; 25(9):2260. https://doi.org/10.3390/molecules25092260
Chicago/Turabian StyleAl-Faouri, Tamara, Francis L. Buguis, Saba Azizi Soldouz, Olga V. Sarycheva, Burhan A. Hussein, Reeda Mahmood, and Bryan D. Koivisto. 2020. "Exploring Structure-Property Relationships in a Bio-Inspired Family of Bipodal and Electronically-Coupled Bistriphenylamine Dyes for Dye-Sensitized Solar Cell Applications" Molecules 25, no. 9: 2260. https://doi.org/10.3390/molecules25092260
APA StyleAl-Faouri, T., Buguis, F. L., Azizi Soldouz, S., Sarycheva, O. V., Hussein, B. A., Mahmood, R., & Koivisto, B. D. (2020). Exploring Structure-Property Relationships in a Bio-Inspired Family of Bipodal and Electronically-Coupled Bistriphenylamine Dyes for Dye-Sensitized Solar Cell Applications. Molecules, 25(9), 2260. https://doi.org/10.3390/molecules25092260







