Synthesis of Novel 3,6-Dithienyl Diketopyrrolopyrrole Dyes by Direct C-H Arylation
Abstract
1. Introduction
2. Results and Discussion
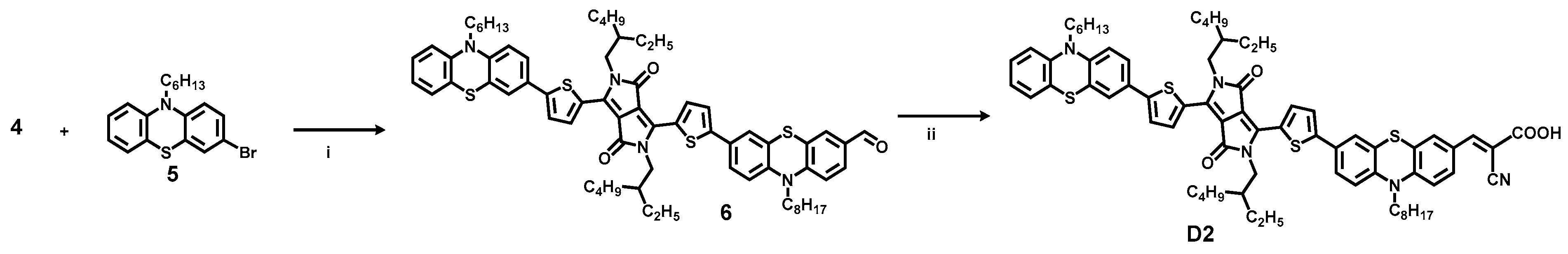
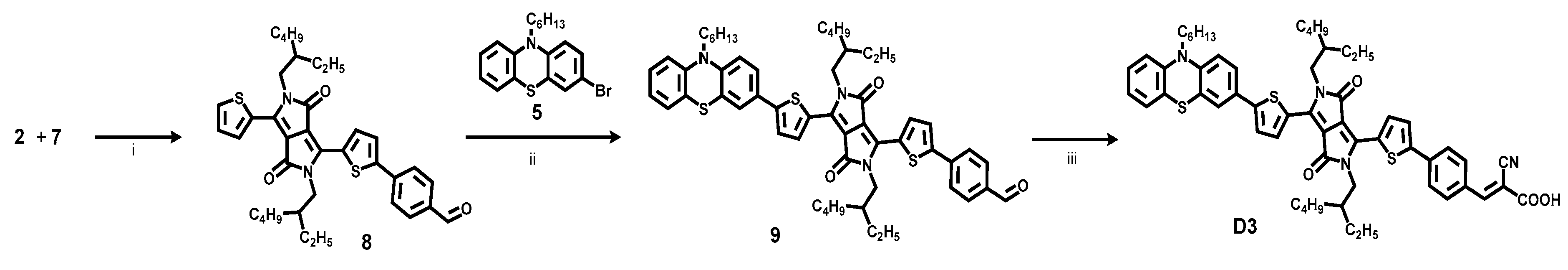
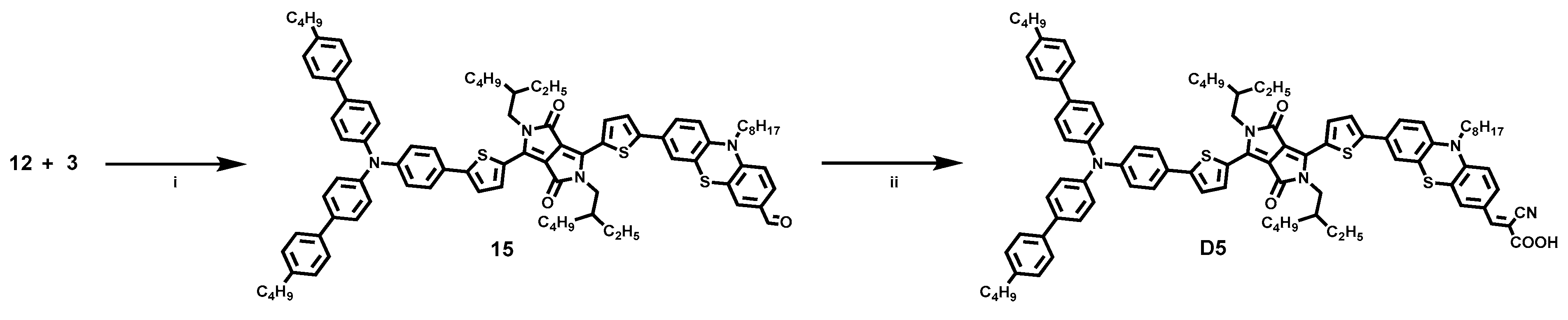
2.1. Dye Synthesis by Direct C-H Arylations
2.2. Photophysical and Electrochemical Properties
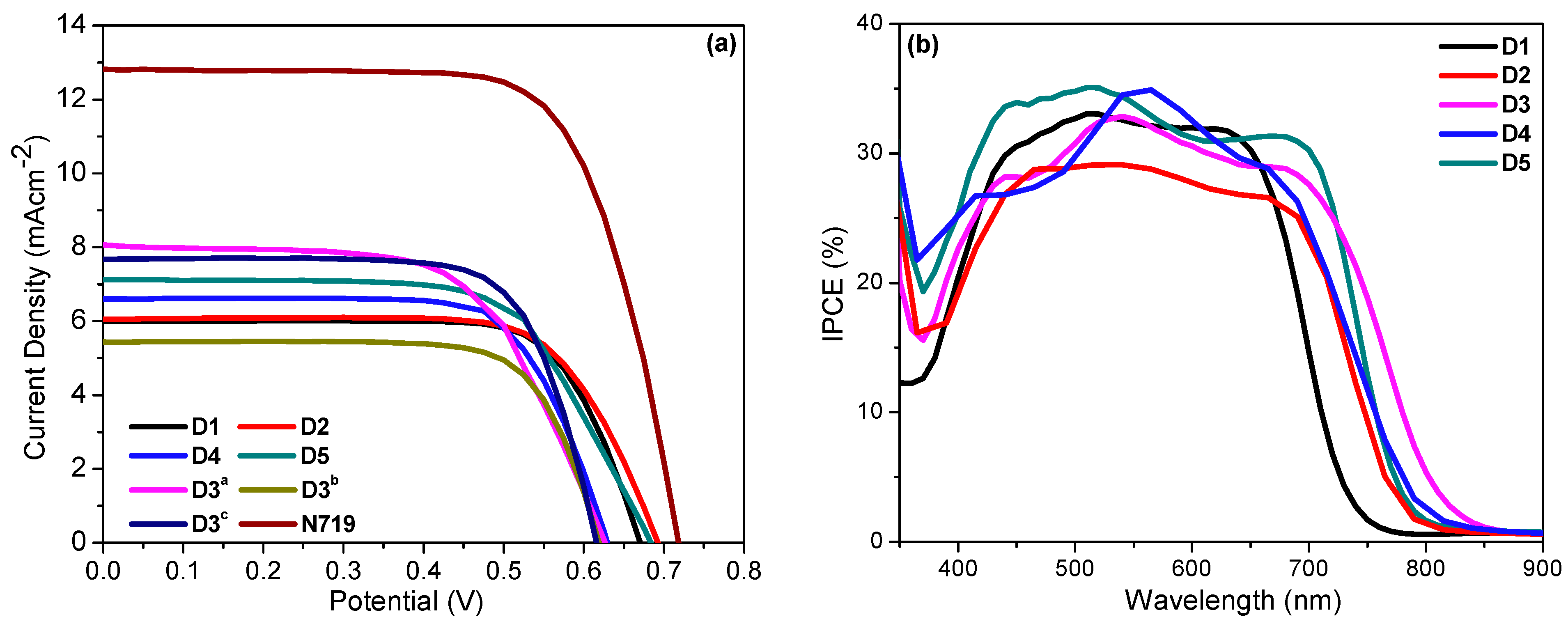
2.3. Photovoltaic Properties
3. Materials and Methods
3.1. General
3.2. Synthesis of Dye D1
3.2.1. 5-Diethylhexyl-3,6-dithiophen-2-ylpyrrolo-[3,4-c]pyrrole-1,4-dione (2)
3.2.2. 7-(5-(2,5-Bis(2-ethylhexyl)-3,6-dioxo-4-(thiophen-2-yl)-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrol-1-yl)-thiophen-2-yl)-10-octyl-10H-phenothiazine-3-carbaldehyde (4)
3.2.3. (E)-3-(7-(5-(2,5-Bis(2-ethylhexyl)-3,6-dioxo-4-(thiophen-2-yl)-2,3,5,6-tetrahydropyrrolo-[3,4-c]pyrrol-1-yl)thiophen-2-yl)-10-octyl-10H-phenothiazin-3-yl)-2-cyanoacrylic Acid (D1)
3.3. Synthesis of Dye D2
3.3.1. 7-(5-(2,5-Bis(2-ethylhexyl)-4-(5-(10-hexyl-10H-phenothiazin-3-yl)thiophen-2-yl)-3,6-dioxo-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrol-1-yl)thiophen-2-yl)-10-octyl-10H-phenothiazine-3-carbaldehyde (6)
3.3.2. (E)-3-(7-(5-(2,5-Bis(2-ethylhexyl)-4-(5-(10-hexyl-10H-phenothiazin-3-yl)thiophen-2-yl)-3,6-dioxo-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrol-1-yl)thiophen-2-yl)-10-octyl-10H-phenothiazin-3-yl)-2-cyanoacrylic Acid (D2)
3.4. Synthesis of Dye D3
3.4.1. 4-(5-(2,5-Bis(2-ethylhexyl)-3,6-dioxo-4-(thiophen-2-yl)-2,3,5,6-tetrahydropyrrolo[3,4-c] pyrrol-1-yl)thiophen-2-yl)benzaldehyde (8)
3.4.2. 4-(5-(2,5-Bis(2-ethylhexyl)-4-(5-(10-hexyl-10H-phenothiazin-3-yl)thiophen-2-yl)-3,6-dioxo-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrol-1-yl)thiophen-2-yl)benzaldehyde (9)
3.4.3. (E)-3-(4-(5-(2,5-Bis(2-ethylhexyl)-4-(5-(10-hexyl-10H-phenothiazin-3-yl)thiophen-2-yl)-3,6-dioxo-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrol-1-yl)thiophen-2-yl)phenyl)-2-cyanoacrylic Acid (D3)
3.5. Synthesis of Dye D4
3.5.1. 3-(5-Bromothiophen-2-yl)-2,5-bis(2-ethylhexyl)-6-(thiophen-2-yl)-2,5-dihydropyrrolo [3,4-c]pyrrole-1,4-dione (10)
3.5.2. 3-(5-(4-(Bis(4′-butyl-[1,1′-biphenyl]-4-yl)amino)phenyl)thiophen-2-yl)-2,5-bis (2-ethylhexyl)-6-(thiophen-2-yl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (12)
3.5.3. 4-(5-(4-(5-(4-(Bis(4′-butyl-[1,1′-biphenyl]-4-yl)amino)phenyl)thiophen-2-yl)-2,5-bis (2-ethylhexyl)-3,6-dioxo-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrol-1-yl)thiophen-2-yl)benzaldehyde (13)
3.5.4. (E)-3-(4-(5-(4-(5-(4-(Bis(4′-butyl-[1,1′-biphenyl]-4-yl)amino)phenyl)thiophen-2-yl)-2,5-bis (2-ethylhexyl)-3,6-dioxo-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrol-1-yl)thiophen-2-yl)phenyl)-2-cyanoacrylic Acid (D4)
3.6. Synthesis of Dye D5
3.6.1. 7-(5-(4-(5-(4-(Bis(4′-butyl-[1,1′-biphenyl]-4-yl)amino)phenyl)thiophen-2-yl)-2,5-bis (2-ethylhexyl)-3,6-dioxo-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrol-1-yl)thiophen-2-yl)-10-octyl-10H-phenothiazine-3-carbaldehyde (15)
3.6.2. (E)-3-(7-(5-(4-(5-(4-(Bis(4′-butyl-[1,1′-biphenyl]-4-yl)amino)phenyl)thiophen-2-yl)-2,5 -bis(2-ethylhexyl)-3,6-dioxo-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrol-1-yl)thiophen-2-yl)-10-octyl-10H-phenothiazin-3-yl)-2-cyanoacrylic Acid (D5)
3.7. DSSC Fabrication
3.8. Device Characterization
3.9. Electrochemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.P.; Lin, R.Y.-Y.; Lin, L.Y.; Li, C.T.; Chu, T.C.; Sun, S.S.; Lin, J.T.; Ho, K.C. Recent progress in organic sensitizers for dye-sensitized solar cells. RSC Adv. 2015, 5, 23810–23825. [Google Scholar] [CrossRef]
- Mishra, A.; Fischer, M.K.R.; Bauerle, P. Metal-free organic dyes for dye-sensitized solar cells: From structure: Property relationships to design rules. Angew. Chem. Int. Ed. Engl. 2009, 48, 2474–2499. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Suzuki, A. Carbon-carbon bonding made easy. Chem. Commun. 2005, 14, 4759–4763. [Google Scholar] [CrossRef]
- Cordovilla, C.; Bartolomé, C.; Martínez-Ilarduya, J.M.; Espinet, P. The Stille Reaction, 38 Years Later. ACS Catal. 2015, 5, 3040–3053. [Google Scholar] [CrossRef]
- Milstein, D.; Stille, J.K. A general, selective, and facile method for ketone synthesis from acid chlorides and organotin compounds catalyzed by palladium. J. Am. Chem. Soc. 1978, 100, 3636–3638. [Google Scholar] [CrossRef]
- Milstein, D.; Stille, J.K. Mechanism of reductive elimination. Reaction of alkylpalladium(II) complexes with tetraorganotin, organolithium, and Grignard reagents. Evidence for palladium(IV) intermediacy. J. Am. Chem. Soc. 1979, 101, 4981–4991. [Google Scholar] [CrossRef]
- Bohra, H.; Wang, M. Direct C-H arylation: A “Greener” approach towards facile synthesis of organic semiconducting molecules and polymers. J. Mater. Chem. A 2017, 5, 11550–11571. [Google Scholar] [CrossRef]
- Efrem, A.; Wang, K.; Wang, M. Facile synthesis of a narrow-bandgap strong-donor-alt-strong-acceptor copolymer of poly(5,6-difluorobenzo-[c][1,2,5]-thiadiazole-alt-5H-dithieno[3,2-b:2′,3′-d]pyran) via direct C-H arylation polymerization. Dyes Pigment. 2017, 145, 331–338. [Google Scholar] [CrossRef]
- Mercier, L.G.; Leclerc, M. Direct (hetero)arylation: A new tool for polymer chemists. Acc. Chem. Res. 2013, 46, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, W.; Rojas, A.J.; Jucov, E.V.; Timofeeva, T.V.; Parker, T.C.; Barlow, S.; Marder, S.R. Controllable direct arylation: Fast route to symmetrical and unsymmetrical 4,7-diaryl-5,6-difluoro-2,1,3-benzothiadiazole derivatives for organic optoelectronic materials. J. Am. Chem. Soc. 2013, 135, 16376–16379. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Cao, Q.; Wang, J.; Chai, Z.; Cai, G.; Ma, Z.; Han, H.; Li, Q.; Li, Z.; Chen, H. Novel D-A-π-A-type organic dyes containing a ladderlike dithienocyclopentacarbazole donor for effective dye-sensitized solar cells. ACS Omega 2017, 2, 7048–7056. [Google Scholar] [CrossRef]
- Kang, X.; Zhang, J.; O’Neil, D.; Rojas, A.J.; Chen, W.; Szymanski, P.; Marder, S.R.; El-Sayed, M.A. Effect of molecular structure perturbations on the performance of the D-A-π-A dye sensitized solar cells. Chem. Mater. 2014, 26, 4486–4493. [Google Scholar] [CrossRef]
- Lin, P.-H.; Lu, T.-J.; Cai, D.-J.; Lee, K.-M.; Liu, C.-Y. Connecting direct C-H arylation reactions with dye-sensitized solar cells: A shortcut to D-A-π-A organic dyes. ChemSusChem 2015, 8, 3222–3227. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-H.; Lin, P.-H.; Li, W.-M.; Lee, K.-M.; Liu, C.-Y. Sn- and Pd-free synthesis of D-π-A organic sensitizers for dye-sensitized solar cells by Cu-catalyzed direct arylation. ChemSusChem 2017, 10, 2284–2290. [Google Scholar] [CrossRef]
- Sil, M.C.; Sudhakar, V.; Mele Kavungathodi, M.F.; Punitharasu, V.; Nithyanandhan, J. Orthogonally functionalized donor/acceptor homo- and heterodimeric dyes for dye-sensitized solar cells: An approach to introduce panchromaticity and control the charge recombination. ACS Appl. Mater. Interfaces 2017, 9, 34875–34890. [Google Scholar] [CrossRef]
- Hao, Y.; Saygili, Y.; Cong, J.; Eriksson, A.; Yang, W.; Zhang, J.; Polanski, E.; Nonomura, K.; Zakeeruddin, S.M.; Graetzel, M.; et al. Novel blue organic dye for dye-sensitized solar cells achieving high efficiency in cobalt-based electrolytes and by co-sensitization. ACS Appl. Mater. Interfaces 2016, 8, 32797–32804. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Z.; Jiang, W.; Wu, W.; Wang, Z.; Tian, H. New D-A-π-A organic sensitizers for efficient dye-sensitized solar cells. Chem. Commun. 2015, 51, 3590–3592. [Google Scholar] [CrossRef]
- Qu, S.; Wu, W.; Hua, J.; Kong, C.; Long, Y.; Tian, H. New diketopyrrolopyrrole (DPP) dyes for efficient dye-sensitized solar cells. J. Phys. Chem. C 2010, 114, 1343–1349. [Google Scholar] [CrossRef]
- Singh, S.P.; Roy, M.S.; Thomas, A.; Bhanuprakash, K.; Sharma, G.D. Effect of linker used in D-A-π-A metal free dyes with different π-spacers for dye sensitized solar cells. Org. Electron. 2012, 13, 3108–3117. [Google Scholar] [CrossRef]
- Duvva, N.; Raptis, D.; Kumar, C.V.; Koukaras, E.N.; Giribabu, L.; Lianos, P. Design of diketopyrrolopyrrole chromophores applicable as sensitizers in dye-sensitized photovoltaic windows for greenhouses. Dyes Pigment. 2016, 134, 472–479. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Li, X.; Agren, H.; Zhu, W.; Tian, H.; Xie, Y. Cosensitizers for simultaneous filling up of both absorption valleys of porphyrins: A novel approach for developing efficient panchromatic dye-sensitized solar cells. Chem. Commun. 2014, 50, 15609–15612. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Cai, S.; Li, X.; Agren, H.; Wang, Q.; Huang, J.; Su, J. A new D-A-π-A type organic sensitizer based on substituted dihydroindolo [2,3-b] carbazole and DPP unit with a bulky branched alkyl chain for highly efficient DSCs. J. Mater. Chem. A 2015, 3, 3777–3784. [Google Scholar] [CrossRef]
- Zang, X.-F.; Huang, Z.-S.; Wu, H.-L.; Iqbal, Z.; Wang, L.; Meier, H.; Cao, D. Molecular design of the diketopyrrolopyrrole-based dyes with varied donor units for efficient dye-sensitized solar cells. J. Power Sources 2014, 271, 455–464. [Google Scholar] [CrossRef]
- Holcombe, T.W.; Yum, J.-H.; Yoon, J.; Gao, P.; Marszalek, M.; Censo, D.D.; Rakstys, K.; Nazeeruddin, M.K.; Graetzel, M. A structural study of DPP-based sensitizers for DSC applications. Chem. Commun. 2012, 48, 10724–10726. [Google Scholar] [CrossRef]
- Punzi, A.; Zappimbulso, N.; Farinola, G.M. Synthesis of novel diketopyrrolopyrrole-based dyes. Mon. Chem. 2019, 150, 59–66. [Google Scholar] [CrossRef]
- Yum, J.-H.; Holcombe, T.W.; Kim, Y.; Rakstys, K.; Moehl, T.; Teuscher, J.; Delcamp, J.H.; Nazeeruddin, M.K.; Gratzel, M. Blue-coloured highly efficient dye-sensitized solar cells by implementing the diketopyrrolopyrrole chromophore. Sci. Rep. 2013, 3, 2446. [Google Scholar] [CrossRef]
- Yum, J.-H.; Holcombe, T.W.; Kim, Y.; Yoon, J.; Rakstys, K.; Nazeeruddin, M.K.; Grätzel, M. Towards high-performance DPP-based sensitizers for DSC applications. Chem. Commun. 2012, 48, 10727–10729. [Google Scholar] [CrossRef]
- Aouine, Y.; Alami, A.; El Hallaoui, A.; Elachqar, A.; Zouihri, H. 10-(Prop-2-ynyl)-10H-phenothiazine. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, 2830. [Google Scholar] [CrossRef]
- McDowell, J.J.H. The crystal and molecular structure of phenothiazine. Acta Crystallogr. Sect. B 1976, 32, 5–10. [Google Scholar] [CrossRef]
- Frebort, S.; Elias, Z.; Lycka, A.; Lunak, S., Jr.; Vynuchal, J.; Kubac, L.; Hrdina, R.; Burgert, L. O- and N-alkylated diketopyrrolopyrrole derivatives. Tetrahedron Lett. 2011, 52, 5769–5773. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, K.; Xue, F.; Ouyang, J. Isomers of dialkyl diketo-pyrrolo-pyrrole: Electron-deficient units for organic semiconductors. Org. Electron. 2012, 13, 2516–2524. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Pechy, P.; Renouard, T.; Zakeeruddin, S.M.; Humphry-Baker, R.; Comte, P.; Liska, P.; Cevey, L.; Costa, E.; Shklover, V.; et al. Engineering of efficient panchromatic sensitizers for nanocrystalline TiO2-based solar cells. J. Am. Chem. Soc. 2001, 123, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cole, J.M. Dye aggregation in dye-sensitized solar cells. J. Mater. Chem. A 2017, 5, 19541–19559. [Google Scholar] [CrossRef]
- Espinoza, E.M.; Clark, J.A.; Soliman, J.; Derr, J.B.; Morales, M.; Vullev, V.I. Practical aspects of cyclic voltammetry: How to estimate reduction potentials when irreversibility prevails. J. Electrochem. Soc. 2019, 166, H3175–H3187. [Google Scholar] [CrossRef]
- Huang, J.-H.; Jiang, K.-J.; Zhang, F.; Wu, W.; Li, S.-G.; Yang, L.-M.; Song, Y.-L. Engineering diketopyrrolopyrrole sensitizers for highly efficient dye-sensitized solar cells: Enhanced light harvesting and intramolecular charge transfer. RSC Adv. 2014, 4, 16906–16912. [Google Scholar] [CrossRef]
- Buene, A.F.; Ose, E.E.; Zakariassen, A.G.; Hagfeldt, A.; Hoff, B.H. Auxiliary donors for phenothiazine sensitizers for dye-sensitized solar cells—How important are they really? J. Mater. Chem. A 2019, 7, 7581–7590. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Lan, J.; Wu, D.; Wang, R.; You, J. Construction of 3,7-Dithienyl Phenothiazine-Based Organic Dyes via Multistep Direct C-H Arylation Reactions. J. Org. Chem. 2018, 83, 8114–8126. [Google Scholar] [CrossRef]
- Huo, L.; Hou, J.; Chen, H.-Y.; Zhang, S.; Jiang, Y.; Chen, T.L.; Yang, Y. Bandgap and Molecular Level Control of the Low-Bandgap Polymers Based on 3,6-Dithiophen-2-yl-2,5-dihydropyrrolo[3,4-c] pyrrole-1,4-dione toward Highly Efficient Polymer Solar Cells. Macromolecules 2009, 42, 6564–6571. [Google Scholar] [CrossRef]
- Harschneck, T.; Zhou, N.; Manley, E.F.; Lou, S.J.; Yu, X.; Butler, M.R.; Timalsina, A.; Turrisi, R.; Ratner, M.A.; Chen, L.X.; et al. Substantial photovoltaic response and morphology tuning in benzo[1,2-b:6,5-b′]dithiophene (bBDT) molecular donors. Chem. Commun. 2014, 50, 4099–4101. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of intermediates might be available from the authors. |



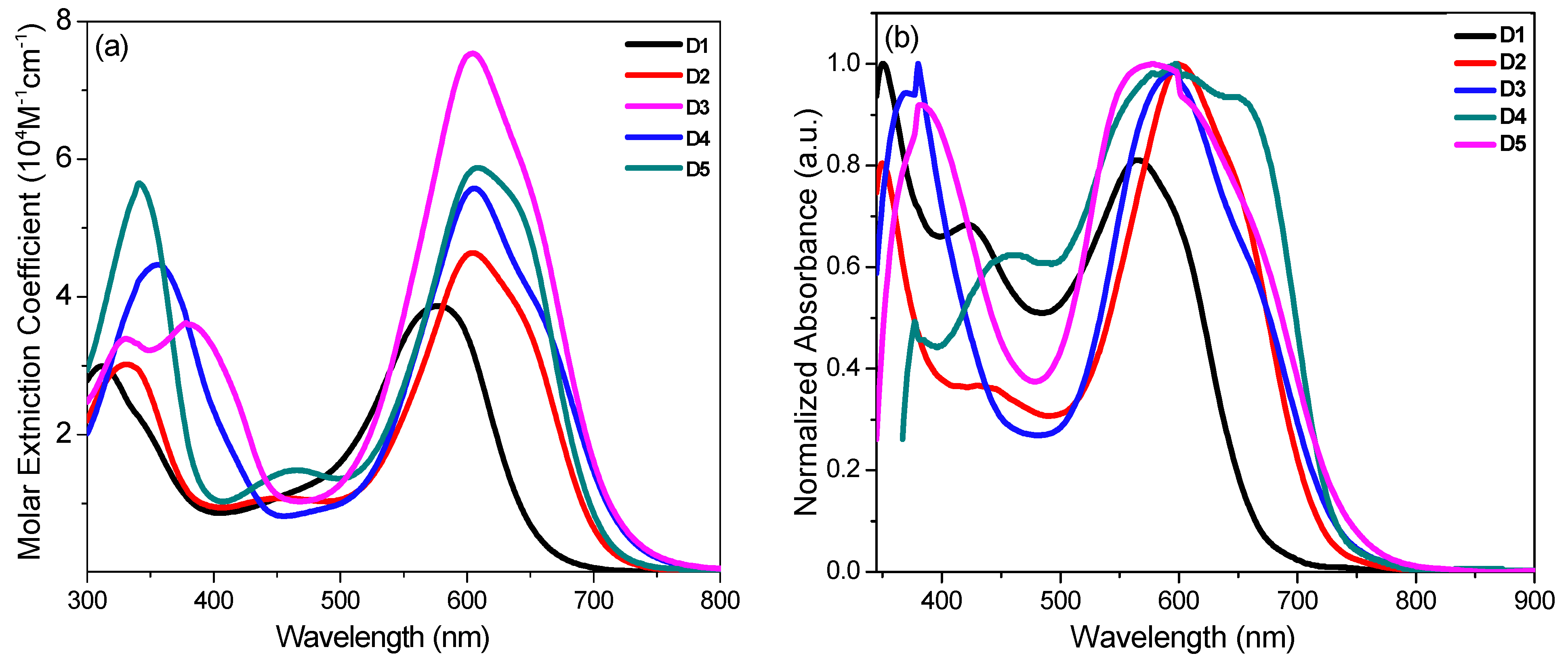

| Dye | λmax (nm) a | ε (104 M−1 cm−1) a | λmax TiO2 (nm) b | λint. (nm) c | E0−0, (eV) d | EHOMO (V) e | ELUMO (V) f |
|---|---|---|---|---|---|---|---|
| D1 | 312, 576 | 2.995, 3.589 | 350, 565 | 611 | 2.03 | 1.12 | −0.91 |
| D2 | 331, 604 | 3.020, 4.643 | 350, 599 | 649 | 1.91 | 1.02 | −0.89 |
| D3 | 331, 378, 604 | 3.390, 3.620, 7.548 | 381, 578 | 667 | 1.86 | 0.98 | −0.88 |
| D4 | 356, 606 | 4.465, 5.603 | 380, 595 | 664 | 1.86 | 1.04 | −0.82 |
| D5 | 341, 608 | 5.560, 5.687 | 597 | 662 | 1.87 | 1.02 | −0.85 |
| Dye | Donor | π-Linker | Jsc (mAcm−2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|---|
| D1 a | Thiophene | Phenoth. | 5.97 ± 0.05 | 0.67 ± 0.00 | 73.3 ± 0.00 | 2.94 ± 0.03 |
| D2 a | Phenoth. | Phenoth. | 5.62 ± 0.68 | 0.70 ± 0.00 | 72.6 ± 0.03 | 2.83 ± 0.22 |
| D3 a | Phenoth. | Phenyl | 7.55 ± 0.73 | 0.63 ± 0.00 | 61.5 ± 0.00 | 2.94 ± 0.26 |
| D4 a | Triaryl | Phenyl | 6.15 ± 0.67 | 0.63 ± 0.00 | 69.8 ± 0.02 | 2.72 ± 0.37 |
| D5 a | Triaryl | Phenoth. | 6.97 ± 0.21 | 0.69 ± 0.01 | 65.9 ± 0.01 | 3.16 ± 0.02 |
| D3 b | Phenoth. | Phenyl | 5.24 ± 0.30 | 0.63 ± 0.00 | 68.2 ± 0.05 | 2.27 ± 0.29 |
| D3 c | Phenoth. | Phenyl | 7.46 ± 0.35 | 0.63 ± 0.00 | 71.4 ± 0.01 | 3.35 ± 0.09 |
| N719 d | 13.4 ± 0.83 | 0.71 ± 0.01 | 68.3 ± 0.03 | 6.48 ± 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yemene, A.E.; Venkatraman, V.; Moe Almenningen, D.; Hoff, B.H.; Gautun, O.R. Synthesis of Novel 3,6-Dithienyl Diketopyrrolopyrrole Dyes by Direct C-H Arylation. Molecules 2020, 25, 2349. https://doi.org/10.3390/molecules25102349
Yemene AE, Venkatraman V, Moe Almenningen D, Hoff BH, Gautun OR. Synthesis of Novel 3,6-Dithienyl Diketopyrrolopyrrole Dyes by Direct C-H Arylation. Molecules. 2020; 25(10):2349. https://doi.org/10.3390/molecules25102349
Chicago/Turabian StyleYemene, Amsalu Efrem, Vishwesh Venkatraman, David Moe Almenningen, Bård Helge Hoff, and Odd Reidar Gautun. 2020. "Synthesis of Novel 3,6-Dithienyl Diketopyrrolopyrrole Dyes by Direct C-H Arylation" Molecules 25, no. 10: 2349. https://doi.org/10.3390/molecules25102349
APA StyleYemene, A. E., Venkatraman, V., Moe Almenningen, D., Hoff, B. H., & Gautun, O. R. (2020). Synthesis of Novel 3,6-Dithienyl Diketopyrrolopyrrole Dyes by Direct C-H Arylation. Molecules, 25(10), 2349. https://doi.org/10.3390/molecules25102349







