Freeze-Drying Technique for Microencapsulation of Elsholtzia ciliata Ethanolic Extract Using Different Coating Materials
Abstract
1. Introduction
2. Results and Discussion
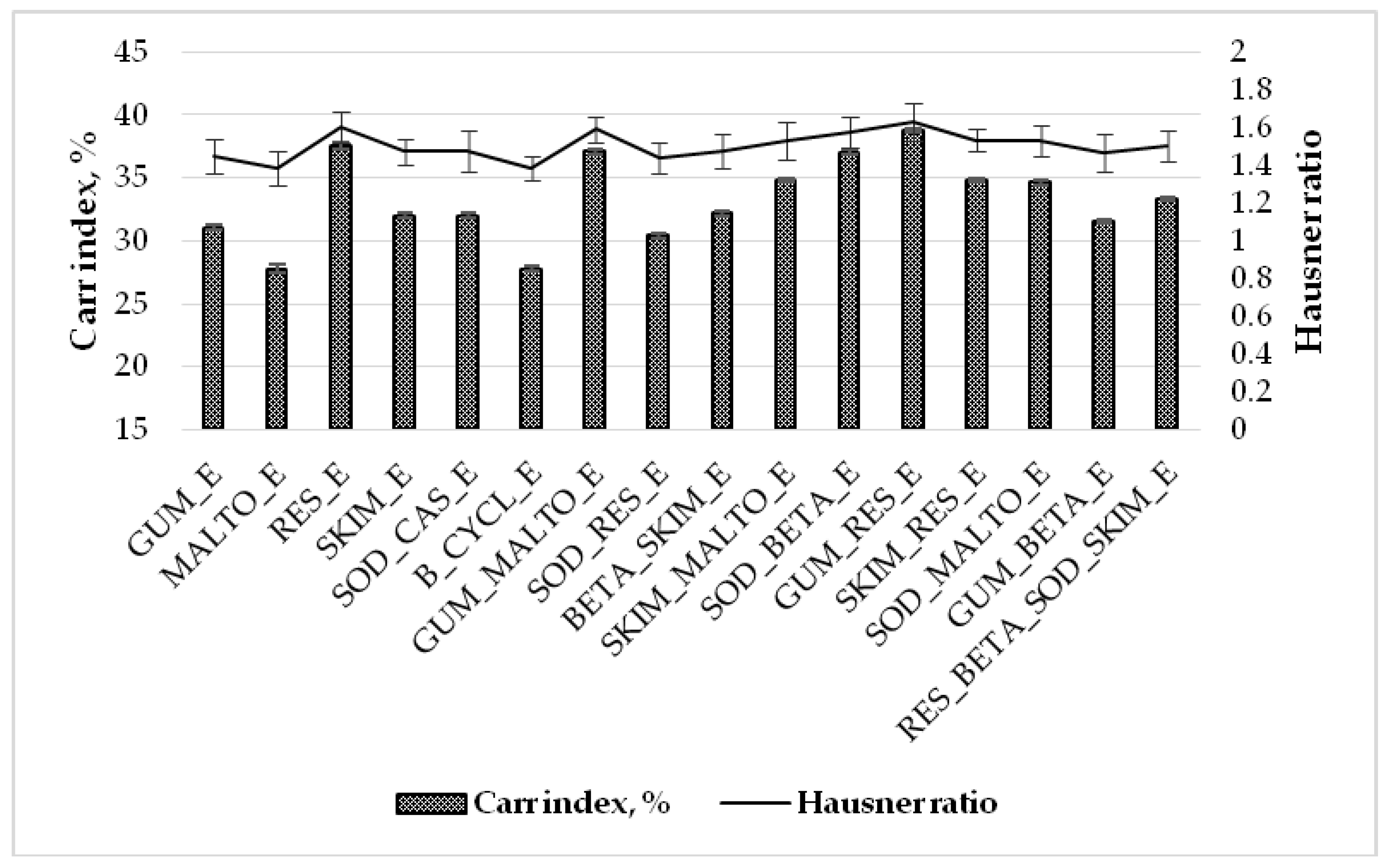
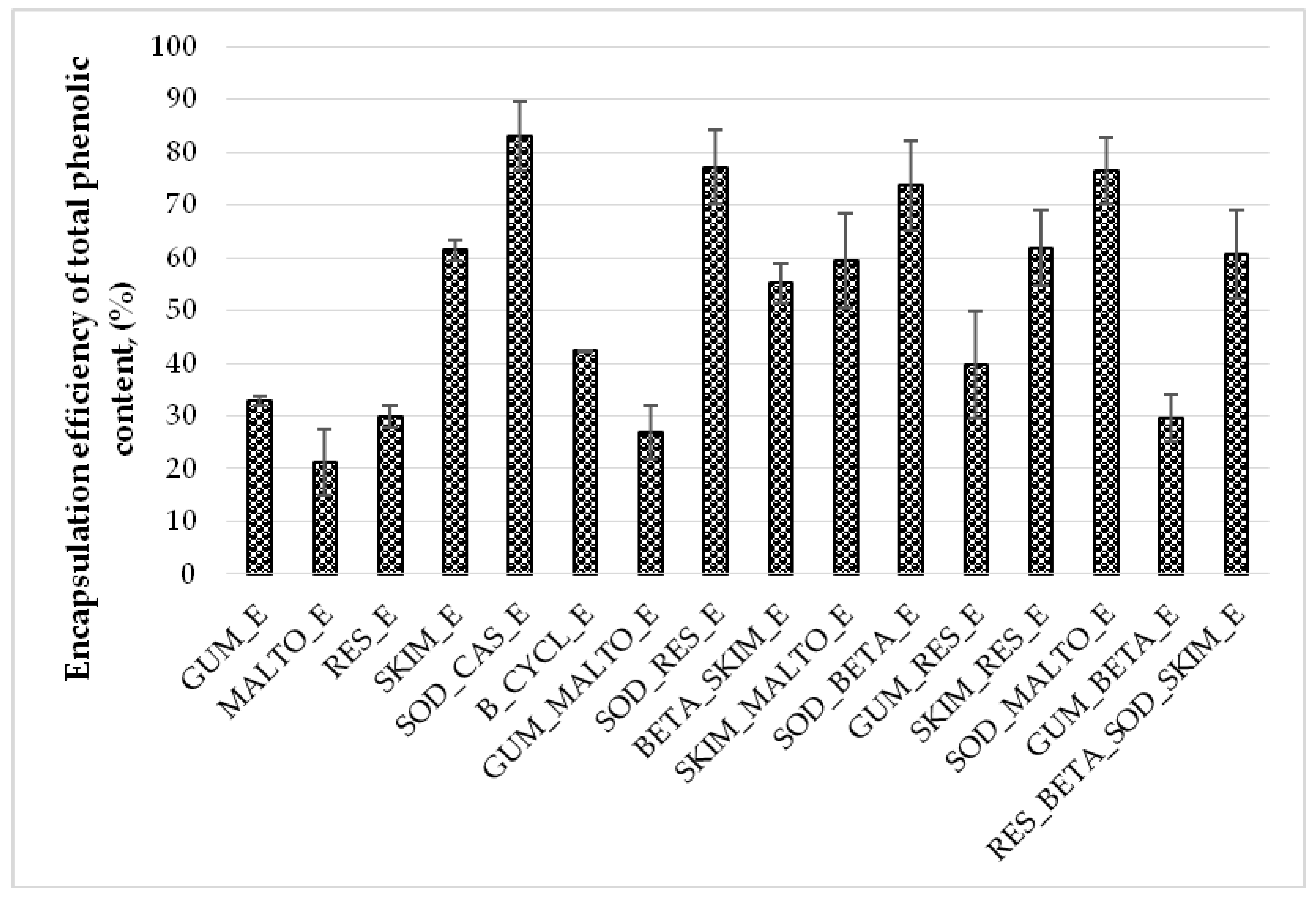
2.1. Influence of Wall Material Components on the Physicochemical Properties
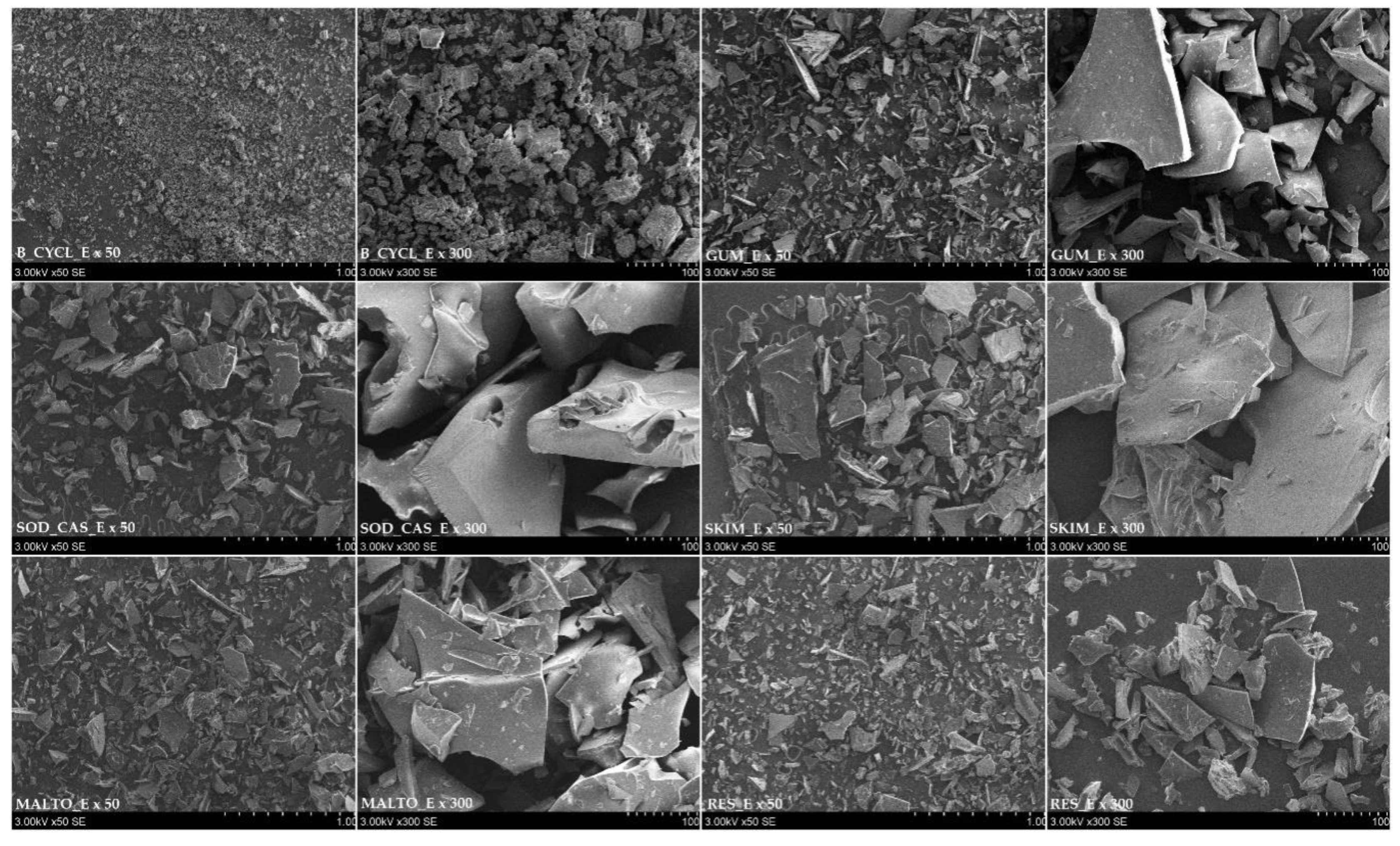
2.2. Morphology
2.3. Mucoadhesive Analysis
3. Materials and Methods
3.1. Materials
3.2. Microencapsulation of E. ciliata Ethanolic Extract
3.3. Moisture Content
3.4. Solubility
3.5. Bulk and Tapped Volumes
3.6. Total Phenolic Content (TPC) and Surface Phenolic Content (SPC) Determination
3.7. Morphology Analysis
3.8. Mucoadhesive Properties
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) Models. Front Microbiol. 2019, 18, 829. [Google Scholar] [CrossRef]
- El-Toumy, S.A.; Salib, J.Y.; El-Kashak, W.A.; Marty, C.; Bedoux, G.; Bourgougnon, N. Antiviral effect of polyphenol rich plant extracts on herpes simplex virus type 1. Food Sci. Hum. Wellness 2018, 7, 91–101. [Google Scholar] [CrossRef]
- Mileo, A.M.; Nisticò, P.; Miccadei, S. Polyphenols: Immunomodulatory and therapeutic implication in colorectal cancer. Front. Immunol. 2019, 10, 729. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.F.M.; Pogačnik, L. Food, polyphenols and neuroprotection. Neural Regen. Res. 2017, 12, 582–583. [Google Scholar] [CrossRef]
- Shah, S.M.A.; Akram, M.; Riaz, M.; Munir, N.; Rasool, G. Cardioprotective Potential of Plant-Derived Molecules: A Scientific and Medicinal Approach. Dose-Response 2019, 17. [Google Scholar] [CrossRef]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of grape (Vitis labrusca var. Bordo) skin phenolic extract using gum Arabic, polydextrose, and partially hydrolyzed guar gum as encapsulating agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef]
- Šaponjac, V.T.; Ćetković, G.; Čanadanović-Brunet, J.; Dilas, S.; Pajin, B.; Petrović, J.; Stajčić, S.; Vulić, J. Encapsulation of sour cherry pomace extract by freeze drying: Characterization and storage stability. Acta Chim. Slov. 2017, 64, 283–289. [Google Scholar] [CrossRef]
- Šturm, L.; Osojnik Črnivec, I.G.; Istenič, K.; Ota, A.; Megušar, P.; Slukan, A.; Humar, M.; Levic, S.; Nedović, V.; Kopinč, R.; et al. Encapsulation of non-dewaxed propolis by freeze-drying and spray-drying using gum Arabic, maltodextrin and inulin as coating materials. Food Bioprod. Process. 2019, 116, 196–211. [Google Scholar] [CrossRef]
- Wilkowska, A.; Ambroziak, W.; Czyzowska, A.; Adamiec, J. Effect of Microencapsulation by Spray-Drying and Freeze-Drying Technique on the Antioxidant Properties of Blueberry (Vaccinium myrtillus) Juice Polyphenolic Compounds. Pol. J. Food Nutr. Sci. 2016, 66, 11–16. [Google Scholar] [CrossRef]
- Papoutsis, K.; Golding, J.; Vuong, Q.; Pristijono, P.; Stathopoulos, C.; Scarlett, C.; Bowyer, M. Encapsulation of Citrus By-Product Extracts by Spray-Drying and Freeze-Drying Using Combinations of Maltodextrin with Soybean Protein and ι-Carrageenan. Foods 2018, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: A review. Dry. Technol. 2019, 38, 235–258. [Google Scholar] [CrossRef]
- Jeyakumari, A. Microencapsulation of Bioactive Food Ingredients and Controlled Release—A Review. MOJ Food Process. Technol. 2016, 2, 214–224. [Google Scholar]
- Cakrawati, D.; Handayani, M.N.; Noor, E.; Sunarti, T.C. Microencapsulation by freeze drying of limonin using β-cyclodextrin and its stability in different ph solution. J. Eng. Sci. Technol. 2018, 13, 2287–2298. [Google Scholar]
- Juárez Tomás, M.S.; De Gregorio, P.R.; Leccese Terraf, M.C.; Nader-Macías, M.E.F. Encapsulation and subsequent freeze-drying of Lactobacillus reuteri CRL 1324 for its potential inclusion in vaginal probiotic formulations. Eur. J. Pharm. Sci. 2015, 79, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Bar-Zeev, M.; Kelmansky, D.; Assaraf, Y.G.; Livney, Y.D. β-Casein micelles for oral delivery of SN-38 and elacridar to overcome BCRP-mediated multidrug resistance in gastric cancer. Eur. J. Pharm. Biopharm. 2018, 133, 240–249. [Google Scholar] [CrossRef]
- Cano-Higuita, D.M.; Vélez, H.A.V.; Telis, V.R.N. Microencapsulation of Turmeric Oleoresin in Binary and Ternary Blends of Gum Arabic, Maltodextrin and Modified Starch. Ciência e Agrotecnologia 2015, 39, 173–182. [Google Scholar] [CrossRef]
- Chranioti, C.; Tzia, C. Arabic Gum Mixtures as Encapsulating Agents of Freeze-Dried Fennel Oleoresin Products. Food Bioprocess Technol. 2014, 7, 1057–1065. [Google Scholar] [CrossRef]
- Watanabe, N.; Suzuki, M.; Yamaguchi, Y.; Egashira, Y. Effects of resistant maltodextrin on bowel movements: A systematic review and meta-analysis. Clin. Exp. Gastroenterol. 2018, 11, 85–96. [Google Scholar] [CrossRef]
- Astina, J.; Sapwarobol, S. Resistant Maltodextrin and Metabolic Syndrome: A Review. J. Am. Coll. Nutr. 2019, 38, 380–385. [Google Scholar] [CrossRef]
- Chen, Q.; Zhong, F.; Wen, J.; McGillivray, D.; Quek, S.Y. Properties and Stability of Spray-Dried and Freeze-Dried Microcapsules Co-Encapsulated with Fish Oil, Phytosterol Esters, and Limonene. Dry. Technol. 2013, 31, 707–716. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Marksa, M.; Jakstas, V.; Ivanauskas, L.; Kopustinskiene, D.M.; Bernatoniene, J. Microencapsulation of Elsholtzia ciliata Herb Ethanolic Extract by Spray-Drying: Impact of resistant-maltodextrin complemented with sodium caseinate, skim milk, and beta-cyclodextrin on the quality of spray-dried powders. Molecules 2019, 24, 1461. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, Z.; Wang, X.; Liu, W.; Jiang, R.; Cheng, R.; She, G. Elsholtzia: Phytochemistry and biological activities. Chem. Cent. J. 2012, 6, 147. [Google Scholar] [CrossRef] [PubMed]
- Pudziuvelyte, L.; Stankevicius, M.; Maruska, A.; Petrikaite, V.; Ragazinskiene, O.; Draksiene, G.; Bernatoniene, J. Chemical Composition and Anticancer Activity of Elsholtzia Ciliata Essential Oils and Extracts Prepared by Different Methods. Ind. Crop. Prod. 2017, 107, 90–96. [Google Scholar] [CrossRef]
- Raja, R.R. Medicinally Potential Plants of Labiatae (Lamiaceae) Family: An Overview. Res. J. Med. Plant 2012, 6, 203–213. [Google Scholar] [CrossRef]
- Petek, M.; Pintar, J.; Satovic, Z. Medicinal Plants of the Family Lamiaceae as Functional Foods—A Review. Czech J. Food Sci. 2016, 34, 377–390. [Google Scholar]
- Tzima, K.; Brunton, N.; Rai, D. Qualitative and Quantitative Analysis of Polyphenols in Lamiaceae Plants—A Review. Plants 2018, 7, 25. [Google Scholar] [CrossRef]
- Wang, X.; Gong, L.; Jiang, H. Study on the Difference between Volatile Constituents of the Different Parts from Elsholtzia ciliata by SHS-GC-MS. Am. J. Anal. Chem. 2017, 8, 625–635. [Google Scholar] [CrossRef]
- Kim, T.W.; Kim, Y.J.; Seo, C.S.; Kim, H.T.; Park, S.R.; Lee, M.Y.; Jung, J.Y. Elsholtzia ciliata (Thunb.) Hylander attenuates renal inflammation and interstitial fibrosis via regulation of TGF-ß and Smad3 expression on unilateral ureteral obstruction rat model. Phytomedicine 2016, 23, 331–339. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Liaudanskas, M.; Jekabsone, A.; Sadauskiene, I.; Bernatoniene, J. Elsholtzia ciliata (Thunb.) Hyl. Extracts from Different Plant Parts: Phenolic Composition, Antioxidant, and Anti-Inflammatory Activities. Molecules 2020, 25, 1153. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Jakstas, V.; Ivanauskas, L.; Laukeviciene, A.; Ibe, D.F.C.; Kursvietiene, L.; Bernatoniene, J. Different extraction methods for phenolic and volatile compounds recovery from Elsholtzia ciliata fresh and dried herbal materials. Ind. Crop. Prod. 2018, 120, 286–294. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Indrani, D.; Jena, B.S.; Anandharamakrishnan, C. Freeze drying technique for microencapsulation of Garcinia fruit extract and its effect on bread quality. J. Food Eng. 2013, 117, 513–520. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of grape polyphenols using maltodextrin and gum Arabic as two alternative coating materials: Development and characterization. J. Biotechnol. 2016, 239, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Hameed, A.; Nazir, Y.; Naz, T.; Wu, Y.; Suleria, H.; Song, Y. Microencapsulation and the Characterization of Polyherbal Formulation (PHF) Rich in Natural Polyphenolic Compounds. Nutrients 2018, 10, 843. [Google Scholar] [CrossRef]
- Szekalska, M.; Sosnowska, K.; Czajkowska-Kósnik, A.; Winnicka, K. Calcium chloride modified alginate microparticles formulated by the spray drying process: A strategy to prolong the release of freely soluble drugs. Materials 2018, 11, 1522. [Google Scholar] [CrossRef]
- Caliskan, G.; Dirim, S.N. The effect of different drying processes and the amounts of maltodextrin addition on the powder properties of sumac extract powders. Powder Technol. 2016, 287, 308–314. [Google Scholar] [CrossRef]
- Antonio, A.; Toledo, C.; Silva, E.K.; Cirillo, M.A. Matrix structure selection in the microparticles of essential oil oregano produced by spray dryer. J. Microencapsul. 2013, 30, 717–727. [Google Scholar]
Sample Availability: Samples of the freeze-dried powders are available from the authors. |



| Formulation | Kind of Adhesive Material | |||||
|---|---|---|---|---|---|---|
| Gelatin Disc | Porcine Buccal Mucosa | Porcine Stomach Mucosa | ||||
| Fmax (N) 1 | Wad (µJ) 2 | Fmax (N) 1 | Wad (µJ) 2 | Fmax (N) 1 | Wad (µJ) 2 | |
| Control 3 | 0.038 ± 0.003 | 0.007 ± 0.001 | 0.057 ± 0.006 | 0.016 ± 0.002 | 0.031 ± 0.003 | 0.005 ± 0.001 |
| SOD_BETA_E | 0.390 ± 0.01 | 0.049 ± 0.006 a | 0.444 ± 0.015 | 0.058 ± 0.004 a | 0.444 ± 0.01 | 0.058 ± 0.004 a |
| SOD_CAS_E | 0.251 ± 0.045 | 0.046 ± 0.004 a | 0.2687 ± 0.01 | 0.074 ± 0.003 b | 0.173 ± 0.02 | 0.047 ± 0.007 b |
| RES_BETA_SOD_SKIM_E | 0.147 ± 0.01 | 0.047 ± 0.008 a | 0.085 ± 0.007 | 0.086 ± 0.003 c | 0.273 ± 0.02 | 0.075 ± 0.007 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pudziuvelyte, L.; Marksa, M.; Sosnowska, K.; Winnicka, K.; Morkuniene, R.; Bernatoniene, J. Freeze-Drying Technique for Microencapsulation of Elsholtzia ciliata Ethanolic Extract Using Different Coating Materials. Molecules 2020, 25, 2237. https://doi.org/10.3390/molecules25092237
Pudziuvelyte L, Marksa M, Sosnowska K, Winnicka K, Morkuniene R, Bernatoniene J. Freeze-Drying Technique for Microencapsulation of Elsholtzia ciliata Ethanolic Extract Using Different Coating Materials. Molecules. 2020; 25(9):2237. https://doi.org/10.3390/molecules25092237
Chicago/Turabian StylePudziuvelyte, Lauryna, Mindaugas Marksa, Katarzyna Sosnowska, Katarzyna Winnicka, Ramune Morkuniene, and Jurga Bernatoniene. 2020. "Freeze-Drying Technique for Microencapsulation of Elsholtzia ciliata Ethanolic Extract Using Different Coating Materials" Molecules 25, no. 9: 2237. https://doi.org/10.3390/molecules25092237
APA StylePudziuvelyte, L., Marksa, M., Sosnowska, K., Winnicka, K., Morkuniene, R., & Bernatoniene, J. (2020). Freeze-Drying Technique for Microencapsulation of Elsholtzia ciliata Ethanolic Extract Using Different Coating Materials. Molecules, 25(9), 2237. https://doi.org/10.3390/molecules25092237











