Natural Ingredient-Based Polymeric Nanoparticles for Cancer Treatment
Abstract
1. Introduction
2. Natural Polymers
2.1. Polysaccharides
2.1.1. Chitosan
2.1.2. Hyaluronic Acid
2.1.3. Alginates
2.1.4. Dextran
2.2. Protein-Based Polymers
2.2.1. Collagen
2.2.2. Gelatin
2.2.3. Albumin
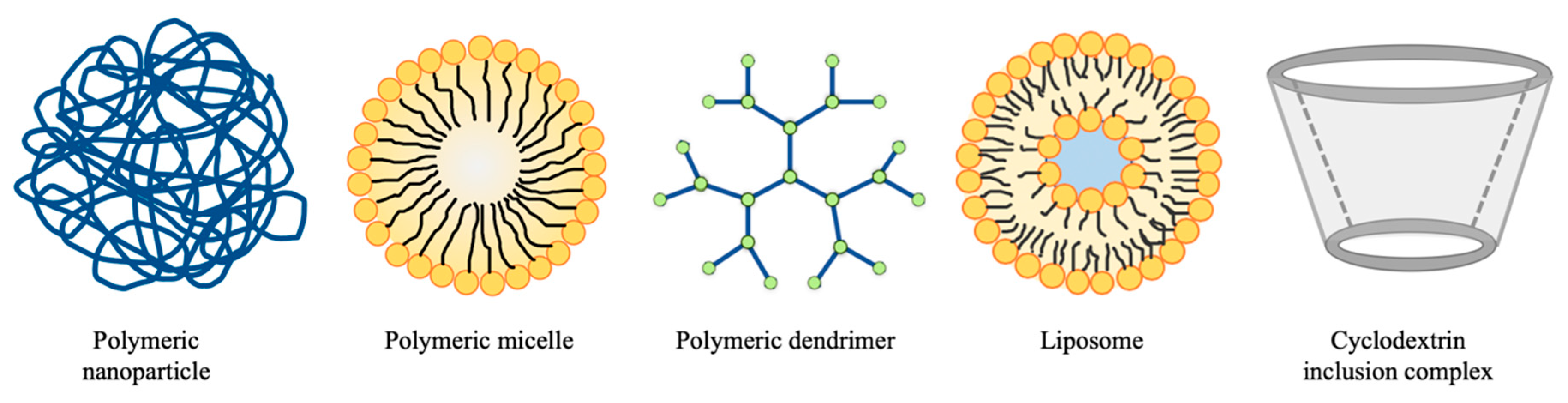
3. Drug Delivery Systems for Cancer Treatment
3.1. Nanoparticles
3.1.1. Chitosan-Based Nanoparticles for Cancer Therapy
3.1.2. Hyaluronic Acid-Based Nanoparticles for Cancer Therapy
3.1.3. Alginate-Based Nanoparticles for Cancer Therapy
3.1.4. Dextran-Based Nanoparticles for Cancer Therapy
| Materials | Composition of Nanoparticles | Significances | Ref. |
|---|---|---|---|
| Chitosan | Ascorbic acid, pentasodium tripolyphosphate | Antioxidative; reduced viability of cervical cancer cells; nontoxic to human normal cells | [40] |
| EGFR binding peptide, PEG2000, Mad2 siRNA | Selective uptake by NSCLC cells; stronger tumor inhibition in a drug-resistant model | [41,42] | |
| Folate, curcumin | Targeted folate receptors; enhanced toxicity to breast cancer cells; controlled release in acidic environments | [43] | |
| Glycyrrhetinic acid, doxorubicin | Enhanced cellular uptake and cytotoxicity of doxorubicin | [45] | |
| PNVCL, cell-penetrating peptide, doxorubicin | Controlled in acidic and hyperpyrexic conditions; selective cellular uptake; stronger tumor inhibition and lower systemic toxicity | [47] | |
| Hyaluronic acid | Cisplatin, siRNA, near IR dye indocyanine green (ICG), various fatty amines or cationic polyamines | Targeted CD44 receptors; effective in combination treatments against resistant cancers | [48,49] |
| L-lysine methyl ester, lipoic acid, doxorubicin | Controlled release of doxorubicin triggered by GSH; targeted CD44 receptors | [50] | |
| PEGylated cationic quaternary amine, n-octyl acrylate segments, doxorubicin | Controlled release in acidic environments; antibacterial; overcame bacteria-induced tumor resistance | [51] | |
| Glycyrrhetinic acid, L-histidine, doxorubicin | Controlled release in acidic environments; improved antitumor efficacy of doxorubicin | [52] | |
| Polycaprolactone, 2-(Pyridyldithio)-ethylamine, doxorubicin | Improved performance of doxorubicin; targeted delivery; controlled release in acidic environments | [53] | |
| Dodecylamide, docetaxel | Inhibited the growth of A549 cells; stable in human plasma | [54] | |
| PLGA, PEI, docetaxel, α-naphthoflavone | Overcame the multidrug resistance; improved bioavailability of docetaxel | [55] | |
| Alginate | Thiolated sodium alginate, fluorescein-labeled wheat germ agglutinin (fWGA), docetaxel | Selective uptake by cancer cells; stronger cytotoxicity toward HT-29 cells; degraded by GSH | [62] |
| Disulfide crosslinked alginate, doxorubicin | Improved safety profile of doxorubicin; selective uptake by cancer cells; | [64] | |
| Poly(allylamine hydrochloride), poly(4-styrenesulfonic acid-co-maleic acid) sodium salt, paclitaxel | Selective uptake by HT-29 cells; induced cell death to the cancer cells | [65] | |
| pheophorbide A, doxorubicin | GSH dose-dependent release manner of payloads; accumulated in the tumor site; combination of chemotherapy and photodynamic therapy | [66] | |
| Dextran | Carboxymethyl dextran, lithocholic acid, doxorubicin | Release triggered by GSH; improved therapeutic efficacy and biodistribution profile of doxorubicin | [68] |
| Curcumin, methotrexate | Sustained release; synergistic effect in treating MCF-7 cells. | [72] | |
| Chlorin e6, gold nanoparticles | Efficient cellular uptake; no leakage; accumulation of chlorin e6 at tumor site | [73] | |
| Dextran acrylate, stearyl amine microRNAs | Stabilized and delivered microRNAs into the carcinoma cells; suppressed osteosarcoma cell proliferation | [74] | |
| PEGylated dextran, siRNA | Changed biodistribution and cellular uptake without affecting cytotoxicity | [75] | |
| Folic acid, doxorubicin | Enhanced tumor inhibition; targeting folate receptors | [76] |
3.1.5. Albumin-Based Nanoparticles for Cancer Therapy
3.1.6. Gelatin-Based Nanoparticles for Cancer Therapy
| Materials | Composition of Nanoparticles | Significances | Ref. |
|---|---|---|---|
| Albumin | Bovine serum albumin, piceatannol, glutaraldehyde | Improved anticancer activity of piceatannol | [77] |
| Bovine serum albumin, curcumin | Enhanced dissolution rate, solubility, bioavailability and antitumor activity of curcumin | [80] | |
| Bovine serum albumin, curcumin, doxorubicin | Controlled release in an acidic environment; decreased the adaptive treatment tolerance effect | [81] | |
| Bovine serum albumin, doxorubicin, cyclopamine | Reversed drug resistance in cancer cells model; distributed at the tumor sites; reduced distant metastasis | [84] | |
| Human serum albumin, doxorubicin | Increased the anticancer activity of doxorubicin in the drug-resistant cell lines | [86] | |
| Human serum albumin, doxorubicin | Weaker cytotoxicity in vitro; opposite in vivo results; suppressed tumor metastasis | [88] | |
| Human serum albumin, doxorubicin prodrug | Stronger cellular uptake; improved cytotoxicity in vitro; lower cardiotoxicity | [89] | |
| Human serum albumin, derivative of maytansine | Improved safety profile; controllable release; protected drug molecules from body clearance | [90] | |
| Human serum albumin, paclitaxel | Could be lyophilized and rehydrated prior to use; better therapeutic efficacy than Abraxane®; prolonged the circulation time | [91] | |
| Human serum albumin, PEI, morphogenetic protein-2 | Improved cellular uptake and cytotoxicity in breast cancer therapy | [92] | |
| Gelatin | Gelatin, paclitaxel | Protected paclitaxel from dilution by urine; drug targeted and accumulated in bladder tissues, with pharmacologically active concentration for at least 1 week | [94] |
| Gelatin, doxorubicin, 3-carboxyphenylboronic acid | Controlled release in acidic environments; higher tumor accumulation and antitumor activity | [95] | |
| Gelatin, dendritic poly-L-lysine, doxorubicin | Hydrolyzed by MMP-2 to release the small doxorubicin/DGL conjugates; facilitated deep penetration of doxorubicin | [96] | |
| Doxorubicin, 5-ALA | Release triggered by MMP-2; synergistic effects from chemotherapy and photodynamic therapy | [99] | |
| Gelatin, resveratrol | Sustained release; rapid cellular uptake; improved antitumor activity as compared to the free drug | [100,101] | |
| Gelatin, phytohemagglutinin erythroagglutinating, gemcitabine | Targeted EGFR; inhibited cancer cell growth by mediating EGFR phosphorylation and causing cell apoptosis | [102] | |
| Gelatin, iron oxide, gemcitabine | Controllable and pH-dependent release manner; sustained release | [103] | |
| ERFR-targeted thiolated targeted gelatin, gemcitabine, wt-p53 plasmid | Efficient antitumor activity in human pancreatic adenocarcinoma-bearing mice; synergistic effect for combination therapy | [104,105] |
3.1.7. Co-Natural Polymer-Based Nanoparticles for Cancer Therapy
3.2. Other Natural Ingredient-Based Drug Delivery Systems
3.2.1. Polymeric Gel
3.2.2. Polymeric Micelles
3.2.3. Liposomes
3.2.4. Cell Membrane-Based Drug Delivery Systems
3.2.5. Cyclodextrin Inclusion Complex
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Goding-Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Gianfaldoni, S.; Gianfaldoni, R.; Wollina, U.; Lotti, J.; Tchernev, G.; Lotti, T. An overview on radiotherapy: From its history to its current applications in dermatology. J. Med. Sci. 2017, 5, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Alatise, O.I.; Anderson, B.O.; Audisio, R.; Autier, P.; Aggarwal, A.; Balch, C.; Brennan, M.; Dare, A.; D’Cruz, A.; et al. Global cancer surgery: Delivering safe, affordable, and timely cancer surgery. Lancet Oncol. 2015, 16, 1193–1224. [Google Scholar] [CrossRef]
- Christofi, T.; Baritaki, S.; Falzone, L.; Libra, M.; Zaravinos, A. Current perspectives in cancer immunotherapy. Cancers 2019, 11, 1472. [Google Scholar] [CrossRef] [PubMed]
- Baudino, T.A. Targeted cancer therapy: The next generation of cancer treatment. Curr. Drug Discov. Technol. 2015, 12, 3–20. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2018, 15, 1–18. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nano. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hola, K.; Subr, V.; Bakandritsos, A.; Tucek, J.; Zboril, R. Targeted drug delivery with polymers and magnetic nanoparticles: Covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Ige, O.O.; Umoru, L.E.; Aribo, S. Natural products: A minefield of biomaterials. ISRN Mater. Sci. 2012, 2012, 983062. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V. Polysaccharides constructed hydrogels as vehicles for proteins and peptides. A review. Carbohydr. Polym. 2019, 225, 115210. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R. Scaffold: A novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Pandey, J.; Raj, V.; Kumar, P. A Review on the modification of polysaccharide through graft copolymerization for various potential applications. Open Med. Chem. J. 2017, 11, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Hamman, J.H. Composition and applications of Aloe vera leaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Motiei, M.; Kashanian, S.; Lucia, L.A.; Khazaei, M. Intrinsic parameters for the synthesis and tuned properties of amphiphilic chitosan drug delivery nanocarriers. J. Control. Release 2017, 260, 213–225. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Alburquenque, C.; Bucarey, S.A.; Neira-Carrillo, A.; Urzúa, B.; Hermosilla, G.; Tapia, C.V. Antifungal activity of low molecular weight chitosan against clinical isolates of Candida spp. Med. Mycol. 2010, 48, 1018–1023. [Google Scholar] [CrossRef]
- Argüelles-Monal, W.M.; Lizardi-Mendoza, J.; Fernández-Quiroz, D.; Recillas-Mota, M.T.; Montiel-Herrera, M. Chitosan derivatives: Introducing new functionalities with a controlled molecular architecture for innovative materials. Polymers 2018, 10, 342. [Google Scholar] [CrossRef]
- Misra, S.; Heldin, P.; Hascall, V.C.; Karamanos, N.K.; Skandalis, S.S.; Markwald, R.R.; Ghatak, S. Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J. 2011, 278, 1429–1443. [Google Scholar] [CrossRef]
- McAtee, C.O.; Barycki, J.J.; Simpson, M.A. Emerging roles for hyaluronidase in cancer metastasis and therapy. Adv. Cancer Res. 2014, 123, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwar, V.B.; Rubinowicz, D.; Schroeder, G.L.; Forgacs, E.; Minna, J.D.; Block, N.L.; Nadji, M.; Lokeshwar, B.L. Stromal and epithelial expression of tumor markers hyaluronic acid and HYAL1 hyaluronidase in prostate cancer. J. Biol. Chem. 2001, 276, 11922–11932. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, M.; Zhang, M.; Wang, S.; Xu, L.; Li, C. Interfering with lactate-fueled respiration for enhanced photodynamic tumor therapy by a porphyrinic MOF nanoplatform. Adv. Funct. Mater. 2018, 28, 1803498. [Google Scholar] [CrossRef]
- Rehm, B.H.; Valla, S. Bacterial alginates: Biosynthesis and applications. Appl. Microbiol. Biotechnol. 1997, 48, 281–288. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef]
- Tonnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Khalikova, E.; Susi, P.; Korpela, T. Microbial dextran-hydrolyzing enzymes: Fundamentals and applications. Microbiol. Mol. Biol. Rev. 2005, 69, 306–325. [Google Scholar] [CrossRef]
- Yee, E.M.H.; Cirillo, G.; Brand, M.B.; Black, D.S.; Vittorio, O.; Kumar, N. Synthesis of dextran-phenoxodiol and evaluation of its physical stability and biological activity. Front. Bioeng. Biotechnol. 2019, 7, 183. [Google Scholar] [CrossRef]
- DeFrates, K.; Markiewicz, T.; Gallo, P.; Rack, A.; Weyhmiller, A.; Jarmusik, B.; Hu, X. Protein polymer-based nanoparticles: Fabrication and medical applications. Int. J. Mol. Sci. 2018, 19, 1717. [Google Scholar] [CrossRef]
- Chaubaroux, C.; Vrana, E.; Debry, C.; Schaaf, P.; Senger, B.; Voegel, J.C.; Haikel, Y.; Ringwald, C.; Hemmerlé, J.; Lavalle, P.; et al. Collagen-based fibrillar multilayer films cross-linked by a natural agent. Biomacromolecules 2012, 13, 2128–3235. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kozłowska, J. Properties and modification of porous 3-D collagen/hydroxyapatite composites. Int. J. Biol. Macromol. 2013, 52, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Mad-Ali, S.; Benjakul, S.; Prodpran, T.; Maqsood, S. Characteristics and gelling properties of gelatin from goat skin as affected by drying methods. J. Food Sci. Technol. 2017, 54, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, D.; Guo, H.; Hao, L.; Zheng, D.; Liu, G.; Shen, J.; Tian, X.; Zhang, Q. Preparation and characterization of galactosylated bovine serum albumin nanoparticles for liver-targeted delivery of oridonin. Int. J. Pharm. 2013, 448, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Adabi, E.; Saebi, F.; Moradi Hasan-Abad, A.; Teimoori-Toolabi, L.; Kardar, G.A. Evaluation of an Albumin-binding domain protein fused to recombinant human IL-2 and its effects on the bioactivity and serum half-life of the cytokine. Iran. Biomed. J. 2017, 21, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhao, P.; Jiang, Y.; Tang, Y.; Jin, H.; Pan, Z.; He, H.; Yang, V.C.; Huang, Y. Blood-brain-barrier-penetrating albumin nanoparticles for biomimetic drug delivery via albumin-binding protein pathways for antiglioma therapy. ACS Nano 2016, 10, 9999–10012. [Google Scholar] [CrossRef] [PubMed]
- Thai, H.; Thuy Nguyen, C.; Thi Thach, L.; Thi Tran, M.; Duc Mai, H.; Thi Thu Nguyen, T.; Duc Le, G.; Van Can, M.; Dai Tran, L.; Long Bach, G.; et al. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci. Rep. 2020, 10, 909. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Sekar, V.; Rajendran, K.; Vallinayagam, S.; Deepak, V.; Mahadevan, S. Synthesis and characterization of chitosan ascorbate nanoparticles for therapeutic inhibition for cervical cancer and their in silico modeling. J. Ind. Eng. Chem. 2018, 62, 239–249. [Google Scholar] [CrossRef]
- Nascimento, A.V.; Singh, A.; Bousbaa, H.; Ferreira, D.; Sarmento, B.; Amiji, M.M. Mad2 checkpoint gene silencing using epidermal growth factor receptor-targeted chitosan nanoparticles in non-small cell lung cancer model. Mol. Pharm. 2014, 11, 3515–3527. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.V.; Singh, A.; Bousbaa, H.; Ferreira, D.; Sarmento, B.; Amiji, M.M. Overcoming cisplatin resistance in non-small cell lung cancer with Mad2 silencing siRNA delivered systemically using EGFR-targeted chitosan nanoparticles. Acta Biomater. 2017, 47, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Esfandiarpour-Boroujeni, S.; Bagheri-Khoulenjani, S.; Mirzadeh, H.; Amanpour, S. Fabrication and study of curcumin loaded nanoparticles based on folate-chitosan for breast cancer therapy application. Carbohydr. Polym. 2017, 168, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Vivek, R.; Nipun-Babu, V.; Thangam, R.; Subramanian, K.S.; Kannan, S. pH-responsive drug delivery of chitosan nanoparticles as Tamoxifen carriers for effective anti-tumor activity in breast cancer cells. Colloids Surf. B 2013, 111, 117–123. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, C.N.; Wang, X.H.; Wang, W.; Huang, W.; Cha, R.T.; Wang, C.H.; Yuan, Z.; Liu, M.; Wan, H.Y.; et al. Glycyrrhetinic acid-modified chitosan/poly(ethylene glycol) nanoparticles for liver-targeted delivery. Biomaterials 2010, 31, 4748–4756. [Google Scholar] [CrossRef]
- Mao, S.J.; Bi, Y.Q.; Jin, H.; Wei, D.P.; He, R.; Hou, S.X. Preparation, characterization and uptake by primary cultured rat hepatocytes of liposomes surface-modified with glycyrrhetinic acid. Pharmazie 2007, 62, 614–619. [Google Scholar] [CrossRef]
- Niu, S.; Williams, G.R.; Wu, J.; Wu, J.; Zhang, X.; Chen, X.; Li, S.; Jiao, J.; Zhu, L.M. A chitosan-based cascade-responsive drug delivery system for triple-negative breast cancer therapy. J. Nanobiotechnol. 2019, 17, 95. [Google Scholar] [CrossRef]
- Ganesh, S.; Iyer, A.K.; Morrissey, D.V.; Amiji, M.M. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials 2013, 34, 3489–3502. [Google Scholar] [CrossRef]
- Ganesh, S.; Iyer, A.K.; Gattacceca, F.; Morrissey, D.V.; Amiji, M.M. In vivo biodistribution of siRNA and cisplatin administered using CD44-targeted hyaluronic acid nanoparticles. J. Control. Release 2013, 172, 699–706. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, J.; Cheng, R.; Deng, C.; Meng, F.; Xie, F.; Zhong, Z. Reversibly crosslinked hyaluronic acid nanoparticles for active targeting and intelligent delivery of doxorubicin to drug-resistant CD44+ human breast tumor xenografts. J. Control. Release 2015, 205, 144–154. [Google Scholar] [CrossRef]
- Yan, X.; Chen, Q.; An, J.; Liu, D.-E.; Huang, Y.; Yang, R.; Li, W.; Chen, L.; Gao, H. Hyaluronic acid/PEGylated amphiphilic nanoparticles for pursuit of selective intracellular doxorubicin release. J. Mater. Chem. B 2019, 7, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Sun, X.; Bai, J.; Dong, J.; Zhang, B.; Gao, Z.; Wu, J. Doxorubicin-loaded dual-functional hyaluronic acid nanoparticles: Preparation, characterization and antitumor efficacy in vitro and in vivo. Mol. Med. Rep. 2019, 19, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Thambi, T.; Choi, K.Y.; Son, S.; Ko, H.; Lee, M.C.; Jo, D.G.; Chae, Y.S.; Kang, Y.M.; Lee, J.V.; et al. Bioreducible shell-cross-linked hyaluronic acid nanoparticles for tumor-targeted drug delivery. Biomacromolecules 2015, 16, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Cadete, A.; Olivera, A.; Besev, M.; Dhal, P.K.; Gonçalves, L.; Almeida, A.J.; Bastiat, G.; Benoit, J.-P.; de la Fuente, M.; Garcia-Fuentes, M.; et al. Self-assembled hyaluronan nanocapsules for the intracellular delivery of anticancer drugs. Sci. Rep. 2019, 9, 11565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, J.; Liang, X.; Yin, Y.; Zuo, T.; Chen, D.; Shen, Q. Hyaluronic acid-modified cationic nanoparticles overcome enzyme CYP1B1-mediated breast cancer multidrug resistance. Nanomedicine 2019, 14, 447–464. [Google Scholar] [CrossRef]
- Lee, D.; Beack, S.; Yoo, J.; Kim, S.-K.; Lee, C.; Kwon, W.; Hahn, S.K.; Kim, C. In vivo photoacoustic imaging of livers using biodegradable hyaluronic acid-conjugated silica nanoparticles. Adv. Funct. Mater. 2018, 28, 1800941. [Google Scholar] [CrossRef]
- Cao, F.; Yan, M.; Liu, Y.; Liu, L.; Ma, G. Photothermally controlled MHC class I restricted CD8+ T-Cell responses elicited by hyaluronic acid decorated gold nanoparticles as a vaccine for cancer immunotherapy. Adv. Healthc. Mater. 2018, 7, e1701439. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, J.; Zhu, W.; Sun, C.; Di, D.; Zhang, Y.; Wang, P.; Wang, Z.; Wang, S. Dual-stimuli responsive hyaluronic acid-conjugated mesoporous silica for targeted delivery to CD44-overexpressing cancer cells. Acta Biomater. 2015, 23, 147–156. [Google Scholar] [CrossRef]
- Shu, F.; Lv, D.; Song, X.-L.; Huang, B.; Wang, C.; Yu, Y.; Zhao, S.C. Fabrication of a hyaluronic acid conjugated metal–organic framework for targeted drug delivery and magnetic resonance imaging. RSC Adv. 2018, 8, 6581–6589. [Google Scholar] [CrossRef]
- Xu, W.; Qian, J.; Hou, G.; Suo, A.; Wang, Y. Hyaluronic acid-functionalized gold nanorods with pH/NIR dual-responsive drug release for synergetic targeted photothermal chemotherapy of breast cancer. ACS Appl. Mater. Interfaces 2017, 9, 36533–36547. [Google Scholar] [CrossRef]
- Shi, X.L.; Li, Y.; Zhao, L.M.; Su, L.W.; Ding, G. Delivery of MTH1 inhibitor (TH287) and MDR1 siRNA via hyaluronic acid-based mesoporous silica nanoparticles for oral cancers treatment. Colloids Surf. B Biointerfaces 2019, 173, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.I.; Ayub, A.D.; Mat Yusuf, S.N.A.; Yahaya, N.; Abbd Kadir, E.; Lim, V. Docetaxel-loaded disulfide cross-linked nanoparticles derived from thiolated sodium alginate for colon cancer drug delivery. Pharmaceutics 2020, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Mukherjee, D.; Mishra, R.; Kundu, P.P. Development of pH sensitive polyurethane-alginate nanoparticles for safe and efficient oral insulin delivery in animal models. RSC Adv. 2016, 6, 41835–41846. [Google Scholar] [CrossRef]
- Gao, C.; Tang, F.; Zhang, J.; Lee, S.M.Y.; Wang, R. Glutathione-responsive nanoparticles based on a sodium alginate derivative for selective release of doxorubicin in tumor cells. J. Mater. Chem. B 2017, 5, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Ayub, A.D.; Chiu, H.I.; Mat Yusuf, S.N.A.; Abd Kadir, E.; Ngalim, S.H.; Lim, V. Biocompatible disulphide cross-linked sodium alginate derivative nanoparticles for oral colon-targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, G.; Zhang, J.; Niu, J.; Huang, P.; Wang, Z.; Wang, Y.; Wang, W.; Li, C.; Kong, D. Redox- and light-responsive alginate nanoparticles as effective drug carriers for combinational anticancer therapy. Nanoscale 2017, 9, 3304–3314. [Google Scholar] [CrossRef] [PubMed]
- Jayapal, J.J.; Dhanaraj, S. Exemestane loaded alginate nanoparticles for cancer treatment: Formulation and in vitro evaluation. Int. J. Biol. Macromol. 2017, 105, 416–421. [Google Scholar] [CrossRef]
- Thambi, T.; You, D.G.; Han, H.S.; Deepagan, V.G.; Jeon, S.M.; Suh, Y.D.; Choi, K.Y.; Kim, K.; Kwon, I.C.; Yi, G.R.; et al. Bioreducible carboxymethyl dextran nanoparticles for tumor-targeted drug delivery. Adv. Healthc. Mater. 2014, 3, 1829–1838. [Google Scholar] [CrossRef]
- Kumar, S.U.; Kumar, V.; Priyadarshi, R.; Gopinath, P.; Negi, Y.S. pH-responsive prodrug nanoparticles based on xylan-curcumin conjugate for the efficient delivery of curcumin in cancer therapy. Carbohydr. Polym. 2018, 188, 252–259. [Google Scholar] [CrossRef]
- Kim, C.Y.; Bordenave, N.; Ferruzzi, M.G.; Safavy, A.; Kim, K.H. Modification of curcumin with polyethylene glycol enhances the delivery of curcumin in preadipocytes and its antiadipogenic property. J. Agric. Food Chem. 2011, 59, 1012–1019. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Cirillo, G.; Tucci, P.; Farfalla, A.; Bevacqua, E.; Vittorio, O.; Iemma, F.; Nicoletta, F.P. Dextran-curcumin nanoparticles as a methotrexate delivery vehicle: A step forward in breast cancer combination therapy. Pharmaceuticals 2019, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, H.; Vijayakameswara Rao, N.; Han, H.S.; Jeon, S.; Jeon, J.; Park, J.H. Gold-stabilized carboxymethyl dextran nanoparticles for image-guided photodynamic therapy of cancer. J. Mater. Chem. B 2017, 5, 7319–7327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lyer, A.K.; Yang, X.; Kobayashi, E.; Guo, Y.; Mankin, H.; Hornicek, F.J.; Amiji, M.M.; Duan, Z. Polymeric nanoparticle-based delivery of microRNA-199a-3p inhibits proliferation and growth of osteosarcoma cells. Int. J. Nanomed. 2015, 10, 2913–2924. [Google Scholar] [CrossRef]
- Foerster, F.; Bamberger, D.; Schupp, J.; Weilbächer, M.; Kaps, L.; Strobl, S.; Radi, L.; Diken, M.; Strand, D.; Tuettenberg, A.; et al. Dextran-based therapeutic nanoparticles for hepatic drug delivery. Nanomedicine 2016, 11, 2663–2677. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, Y.; Xu, R.; Li, S.; Hu, H.; Xiao, C.; Wu, H.; Zhu, L.; Ming, J.; Chu, Z.; et al. Self-assembly of folic acid dextran conjugates for cancer chemotherapy. Nanoscale 2018, 10, 17265–17274. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Bakshi, H.A.; Hakkim, F.L.; Haggag, Y.A.; Al-Batanyeh, K.M.; Zoubi, M.S.A.; Al-Trad, B.; Nasef, M.M.; Satija, S.; Mehta, M.; et al. Albumin nano-encapsulation of piceatannol enhances its anticancer potential in colon cancer via downregulation of nuclear p65 and HIF-1α. Cancers 2020, 12, 113. [Google Scholar] [CrossRef]
- Tambuwala, M.M.; Khan, M.N.; Thompson, P.; McCarron, P.A. Albumin nano-encapsulation of caffeic acid phenethyl ester and piceatannol potentiated its ability to modulate HIF and NF-kB pathways and improves therapeutic outcome in experimental colitis. Drug Deliv. Transl. Res. 2019, 9, 14–24. [Google Scholar] [CrossRef]
- Khan, M.N.; Haggag, Y.A.; Lane, M.E.; McCarron, P.A.; Tambuwala, M.M. Polymeric nano-encapsulation of curcumin enhances its anti-cancer activity in breast (MDA-MB231) and lung (A549) cancer cells through reduction in expression of HIF-1α and Nuclear p65 (Rel A). Curr. Drug Deliv. 2018, 15, 286–295. [Google Scholar] [CrossRef]
- Jithan, A.; Madhavi, K.; Madhavi, M.; Prabhakar, K. Preparation and characterization of albumin nanoparticles encapsulating curcumin intended for the treatment of breast cancer. Int. J. Pharm. Investig. 2011, 1, 119–125. [Google Scholar] [CrossRef]
- Motevalli, S.M.; Eltahan, A.S.; Liu, L.; Magrini, A.; Rosato, N.; Guo, W.; Bottini, M.; Liang, X.-J. Co-encapsulation of curcumin and doxorubicin in albumin nanoparticles blocks the adaptive treatment tolerance of cancer cells. Biophys. Rep. 2019, 5, 19–30. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, X.; Chen, J.; Zeng, J.; Cheng, Z.; Liu, Z. A self-assembled albumin-based nanoprobe for in vivo ratiometric photoacoustic pH imaging. Adv. Mater. 2015, 27, 6820–6827. [Google Scholar] [CrossRef]
- Neerati, P.; Sudhakar, Y.A.; Kanwar, J.R. Curcumin regulates colon cancer by inhibiting P-glycoprotein in in-situ cancerous colon perfusion rat model. J. Cancer Sci. Ther. 2013, 5, 313–319. [Google Scholar] [PubMed]
- Lu, Y.L.; Ma, Y.B.; Feng, C.; Zhu, D.; Liu, J.; Chen, L.; Liang, S.; Dong, C. Co-delivery of cyclopamine and doxorubicin mediated by bovine serum albumin nanoparticles reverses doxorubicin resistance in breast cancer by down-regulating P-glycoprotein expression. J. Cancer 2019, 10, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Malhi, V.; Colburn, D.; Williams, S.J.; Hop, C.E.C.A.; Dresser, M.J.; Chandra, P.; Graham, R.A. A clinical drug-drug interaction study to evaluate the effect of a proton-pump inhibitor, a combined P-glycoprotein/cytochrome 450 enzyme (CYP)3A4 inhibitor, and a CYP2C9 inhibitor on the pharmacokinetics of vismodegib. Cancer Chemother. Pharmacol. 2016, 78, 41–49. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Onafuye, H.; Pieper, S.; Mulac, D.; Cinatl, J., Jr.; Wass, M.N.; Langer, K.; Michaelis, M. Doxorubicin-loaded human serum albumin nanoparticles overcome transporter-mediated drug resistance in drug-adapted cancer cells. Beilstein J. Nanotechnol. 2019, 10, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Pieper, S.; Onafuye, H.; Mulac, D.; Cinatl, J., Jr.; Wass, M.N.; Michaelis, M.; Langer, K. Incorporation of doxorubicin in different polymer nanoparticles and their anticancer activity. Beilstein J. Nanotechnol. 2019, 10, 2062–2072. [Google Scholar] [CrossRef]
- Kimura, K.; Yamasaki, K.; Nishi, K.; Taguchi, K.; Otagiri, M. Investigation of anti-tumor effect of doxorubicin-loaded human serum albumin nanoparticles prepared by a desolvation technique. Cancer Chemother. Pharmacol. 2019, 83, 1113–1120. [Google Scholar] [CrossRef]
- Zhang, B.; Wan, S.; Peng, X.; Zhao, M.; Li, S.; Pu, Y.; He, B. Human serum albumin-based doxorubicin prodrug nanoparticles with tumor pH-responsive aggregation-enhanced retention and reduced cardiotoxicity. J. Mater. Chem. B 2020, 8, 3939–3948. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Xu, L.; Xie, K.; Chen, C.; Dong, Y. Albumin nanoparticle encapsulation of potent cytotoxic therapeutics shows sustained drug release and alleviates cancer drug toxicity. Chem. Commun. 2017, 53, 2618–2621. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, M.G.; Jang, Y.L.; Lee, M.S.; Kim, N.W.; Yin, Y.; Lee, J.H.; Lim, S.Y.; Park, J.W.; Kim, J.; et al. Self-assembled PEGylated albumin nanoparticles (SPAN) as a platform for cancer chemotherapy and imaging. Drug Deliv. 2018, 25, 1570–1578. [Google Scholar] [CrossRef]
- Abbasi, S.; Paul, A.; Shao, W.; Prakash, S. Cationic albumin nanoparticles for enhanced drug delivery to treat breast cancer: Preparation and in vitro assessment. J. Drug Deliv. 2012, 2012, 686108. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Siggers, K.; Zhang, S.; Jiang, H.; Xu, Z.; Zernicke, R.F.; Matyas, J.; Uludağ, H. Preparation of BMP-2 containing bovine serum albumin (BSA) nanoparticles stabilized by polymer coating. Pharm. Res. 2008, 25, 2896–2909. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Yeh, T.K.; Wang, J.; Chen, L.; Lyness, G. Paclitaxel gelatin nanoparticles for intravesical bladder cancer therapy. J. Urol. 2011, 185, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, B.; Cheng, X.; Wang, J.; Tang, R. Phenylboronic acid-decorated gelatin nanoparticles for enhanced tumor targeting and penetration. Nanotechnology 2016, 27, 385101. [Google Scholar] [CrossRef]
- Hu, G.; Wang, Y.; He, Q.; Gao, H. Multistage drug delivery system based on microenvironment-responsive dendrimer–gelatin nanoparticles for deep tumor penetration. RSC Adv. 2015, 5, 85933–85937. [Google Scholar] [CrossRef]
- Yevlampieva, N.; Dobrodumov, A.; Nazarova, O.; Okatova, O.; Cottet, H. Hydrodynamic behavior of dendrigraft polylysines in water and dimethylformamide. Polymers 2012, 4, 20–31. [Google Scholar] [CrossRef]
- Xu, J.H.; Gao, F.P.; Liu, X.F.; Zeng, Q.; Guo, S.S. Supramolecular gelatin nanoparticles as matrix metalloproteinase responsive cancer cell imaging probes. Chem. Commun. 2013, 49, 4462–4464. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Liu, X.; Huo, P.; Zhang, Y.; Chen, H.; Tian, Q.; Zhang, N. MMP-2-responsive gelatin nanoparticles for synergistic tumor therapy. Pharm. Dev. Technol. 2019, 24, 1002–1013. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Rajendra Prasad, N.; Ganamani, A.; Balamurugan, E. Anticancer activity of resveratrol-loaded gelatin nanoparticles on NCI-H460 non-small cell lung cancer cells. Biomed. Prev. Nutr. 2012, 3, 64–73. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Hoti, S.L.; Prasad, N.R. Resveratrol loaded gelatin nanoparticles synergistically inhibits cell cycle progression and constitutive NF-kappaB activation, and induces apoptosis in non-small cell lung cancer cells. Biomed. Pharmacother. 2015, 70, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Huang, J.Y.; Chen, M.H.; Chen, C.Y.; Shyong, Y.J. Development of gelatin nanoparticles conjugated with phytohemagglutinin erythroagglutinating loaded with gemcitabine for inducing apoptosis in non-small cell lung cancer cells. J. Mater. Chem. B 2016, 4, 2444–2454. [Google Scholar] [CrossRef] [PubMed]
- Hamarat Sanlıer, S.; Yasa, M.; Cihnioglu, A.O.; Abdulhayoglu, M.; Yılmaz, H. Development of gemcitabine-adsorbed magnetic gelatin nanoparticles for targeted drug delivery in lung cancer. Artif. Cells Nanomed. Biotechnol. 2016, 44, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Singh, A.; Amiji, M.M. Redox-responsive targeted gelatin nanoparticles for delivery of combination wt-p53 expressing plasmid DNA and gemcitabine in the treatment of pancreatic cancer. BMC Cancer 2014, 14, 75. [Google Scholar] [CrossRef]
- Xu, J.; Gattacceca, F.; Amiji, M. Biodistribution and pharmacokinetics of EGFR-targeted thiolated gelatin nanoparticles following systemic administration in pancreatic tumor-bearing mice. Mol. Pharm. 2013, 10, 2031–2044. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef]
- Shargh, V.H.; Hondermarck, H.; Liang, M. Gelatin-albumin hybrid nanoparticles as matrix metalloproteinases-degradable delivery systems for breast cancer therapy. Nanomedicine 2017, 12, 977–989. [Google Scholar] [CrossRef]
- Song, W.; Su, X.; Gregory, D.A.; Li, W.; Cai, Z.; Zhao, X. Magnetic alginate/chitosan nanoparticles for targeted delivery of curcumin into human breast cancer cells. Nanomaterials 2018, 8, 907. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Ratnatilaka Na Bhuket, P.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising approach for oral delivery of curcumin diglutaric acid for cancer treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 178–190. [Google Scholar] [CrossRef]
- Edelman, R.; Assaraf, Y.G.; Levitzky, I.; Shahar, T.; Livney, Y.D. Hyaluronic acid-serum albumin conjugate-based nanoparticles for targeted cancer therapy. Oncotarget 2017, 8, 24337–24353. [Google Scholar] [CrossRef]
- Rogovina, L.Z.; Vasilev, V.G.; Braudo, E.E. Definition of the concept of polymer gel. Polym. Sci. Ser. C 2008, 50, 85–92. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Zhu, Z.; Xu, X.; Li, X.; Zheng, D.; Sun, W. Efficient antitumor effect of co-drug-loaded nanoparticles with gelatin hydrogel by local implantation. Sci. Rep. 2016, 6, 26546. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Nishio, Y.; Makiura, R.; Nakahira, A.; Kojima, C. Paclitaxel-loaded hydroxyapatite/collagen hybrid gels as drug delivery systems for metastatic cancer cells. Int. J. Pharm. 2013, 446, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Bauleth-Ramos, T.; Shih, T.-Y.; Shahbazi, M.-A.; Najibi, A.J.; Mao, A.S.; Liu, D.; Granja, P.; Santos, H.A.; Sarmento, B.; Mooney, D.J. Acetalated dextran nanoparticles loaded into an injectable alginate cryogel for combined chemotherapy and cancer vaccination. Adv. Funct. Mater. 2019, 29, 1903686. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Li, S. Polymeric micelles: Nanocarriers for cancer-targeted drug delivery. AAPS PharmSciTech 2014, 15, 862–871. [Google Scholar] [CrossRef]
- Meng, L.; Huang, W.; Wang, D.; Huang, X.; Zhu, X.; Yan, D. Chitosan-based nanocarriers with pH and light dual response for anticancer drug delivery. Biomacromolecules 2013, 14, 2601–2610. [Google Scholar] [CrossRef]
- Lachowicz, D.; Karabasz, A.; Bzowska, M.; Szuwarzyński, M.; Karewicz, A.; Nowakowska, M. Blood-compatible, stable micelles of sodium alginate-curcumin bioconjugate for anti-cancer applications. Eur. Polym. J. 2019, 113, 208–219. [Google Scholar] [CrossRef]
- Karabasz, A.; Lachowicz, D.; Karewicz, A.; Mezyk-Kopec, R.; Stalinska, K.; Werner, E.; Cierniak, A.; Dyduch, G.; Bereta, J.; Bzowska, M. Analysis of toxicity and anticancer activity of micelles of sodium alginate-curcumin. Int. J. Nanomed. 2019, 14, 7249–7262. [Google Scholar] [CrossRef]
- Jin, R.; Guo, X.; Dong, L.; Xie, E.; Cao, A. Amphipathic dextran-doxorubicin prodrug micelles for solid tumor therapy. Colloids Surf. B Biointerfaces 2017, 158, 47–56. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Dan, Z.; He, X.; Zhang, Z.; Yu, H.; Yin, Q.; Li, Y. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano 2016, 10, 7738–7748. [Google Scholar] [CrossRef] [PubMed]
- AlQahtani, S.A.; Harisa, G.I.; Badran, M.M.; AlGhamdi, K.M.; Kumar, A.; Salem-Bekhit, M.M.; Ahmad, S.F.; Alanazi, F.K. Nano-erythrocyte membrane-chaperoned 5-fluorouracil liposomes as biomimetic delivery platforms to target hepatocellular carcinoma cell lines. Artif. Cells Nanomed. Biotechnol. 2019, 47, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Kuerban, K.; Gao, X.; Zhang, H.; Liu, J.; Dong, M.; Wu, L.; Ye, R.; Feng, M.; Ye, L. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-smallcell lung cancer. Acta Pharm. Sin. B 2020. [Google Scholar] [CrossRef]
- Kim, O.Y.; Park, H.T.; Dinh, N.T.H.; Choi, S.J.; Lee, J.; Kim, J.H.; Lee, S.-W.; Gho, Y.S. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat. Commun. 2017, 8, 626. [Google Scholar] [CrossRef]
- Peng, S.; Zou, L.; Liu, W.; Li, Z.; Liu, W.; Hu, X.; Chen, X.; Liu, C. Hybrid liposomes composed of amphiphilic chitosan and phospholipid: Preparation, stability and bioavailability as a carrier for curcumin. Carbohydr. Polym. 2017, 156, 322–332. [Google Scholar] [CrossRef]
- Nam, J.; Ha, Y.S.; Hwang, S.; Lee, W.; Song, J.; Yoo, J.; Kim, S. pH-responsive gold nanoparticles-in-liposome hybrid nanostructures for enhanced systemic tumor delivery. Nanoscale 2013, 5, 10175–10178. [Google Scholar] [CrossRef][Green Version]
- Zhai, Y.; Su, J.; Ran, W.; Zhang, P.; Yin, Q.; Zhang, Z.; Yu, H.; Li, Y. Preparation and application of cell membrane-camouflaged nanoparticles for cancer therapy. Theranostics 2017, 7, 2575–2592. [Google Scholar] [CrossRef]
- Tan, S.; Wu, T.; Zhang, D.; Zhang, Z. Cell or cell membrane-based drug delivery systems. Theranostics 2015, 5, 863–881. [Google Scholar] [CrossRef] [PubMed]
- Luk, B.T.; Fang, R.H.; Hu, C.M.; Copp, J.A.; Thamphiwatana, S.; Dehaini, D.; Gao, W.; Zhang, K.; Li, S.; Zhang, L. Safe and immunocompatible nanocarriers cloaked in RBC membranes for drug delivery to treat solid tumors. Theranostics 2016, 6, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Shao, J.; Dai, L.; He, Q.; Li, J. Macrophage cell membrane camouflaged mesoporous silica nanocapsules for in vivo cancer therapy. Adv. Healthc. Mater. 2015, 4, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Ben Mihoub, A.; Larue, L.; Moussaron, A.; Youssef, Z.; Colombeau, L.; Baros, F.; Colombeau, L.; Frochot, C.; Vanderesse, R.; Acherar, S. Use of cyclodextrins in anticancer photodynamic therapy treatment. Molecules 2018, 23, 1936. [Google Scholar] [CrossRef]
- Hu, S.C.; Lai, Y.C.; Lin, C.L.; Tzeng, W.S.; Yen, F.L. Inclusion complex of saikosaponin-d with hydroxypropyl-β-cyclodextrin: Improved physicochemical properties and anti-skin cancer activity. Phytomedicine 2019, 57, 174–182. [Google Scholar] [CrossRef]
- Doan, V.T.H.; Lee, J.H.; Takahashi, R.; Nguyen, P.T.M.; Nguyen, V.A.T.; Pham, H.T.T.; Fujii, S.; Sakurai, K. Cyclodextrin-based nanoparticles encapsulating α-mangostin and their drug release behavior: Potential carriers of α-mangostin for cancer therapy. Polym. J. 2020, 52, 457–466. [Google Scholar] [CrossRef]
- Gadade, D.D.; Pekamwar, S.S. Cyclodextrin based nanoparticles for drug delivery and theranostics. Adv. Pharm. Bull. 2020, 10, 166–183. [Google Scholar] [CrossRef]
- Kono, H.; Teshirogi, T. Cyclodextrin-grafted chitosan hydrogels for controlled drug delivery. Int. J. Biol. Macromol. 2015, 72, 299–308. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Oliveira, J.L.; Fraceto, L.F. Poly(ethylene glycol) and cyclodextrin-grafted chitosan: From methodologies to preparation and potential biotechnological applications. Front. Chem. 2017, 5, 93. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]

| Materials | Composition of Nanoparticles | Encapsulation Advantages | Ref. |
|---|---|---|---|
| Hyaluronic acid, chitosan | Hyaluronic acid, chitosan, microRNA-34a, doxorubicin | Achieved synergistic effects; reduced drug resistance and side effects of doxorubicin | [106] |
| Gelatin, albumin | Gelatin, albumin, GNF-5837 | Efficient cellular uptake; improved therapeutic efficacy of GNF-5837 | [107] |
| Alginate, chitosan | Alginate, chitosan, curcumin, iron oxide | Controllable release; targeted delivery with the aid of magnetic field; improved therapeutic performance of curcumin | [108] |
| Alginate, chitosan | Alginate, chitosan, curcumin diglutaric acid | Improved protection and controlled release; enhanced cellular uptake and anticancer activity | [109] |
| Hyaluronic acid, albumin | hyaluronic acid, albumin, paclitaxel | Prevented paclitaxel from aggregation and crystallization; stronger cytotoxicity due to selective internalization | [110] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, K.H.; Lu, A.; Chen, X.; Yang, Z. Natural Ingredient-Based Polymeric Nanoparticles for Cancer Treatment. Molecules 2020, 25, 3620. https://doi.org/10.3390/molecules25163620
Wong KH, Lu A, Chen X, Yang Z. Natural Ingredient-Based Polymeric Nanoparticles for Cancer Treatment. Molecules. 2020; 25(16):3620. https://doi.org/10.3390/molecules25163620
Chicago/Turabian StyleWong, Ka Hong, Aiping Lu, Xiaoyu Chen, and Zhijun Yang. 2020. "Natural Ingredient-Based Polymeric Nanoparticles for Cancer Treatment" Molecules 25, no. 16: 3620. https://doi.org/10.3390/molecules25163620
APA StyleWong, K. H., Lu, A., Chen, X., & Yang, Z. (2020). Natural Ingredient-Based Polymeric Nanoparticles for Cancer Treatment. Molecules, 25(16), 3620. https://doi.org/10.3390/molecules25163620






