Steric and Electronic Effect of Cp-Substituents on the Structure of the Ruthenocene Based Pincer Palladium Borohydrides
Abstract
1. Introduction
2. Results and Discussion
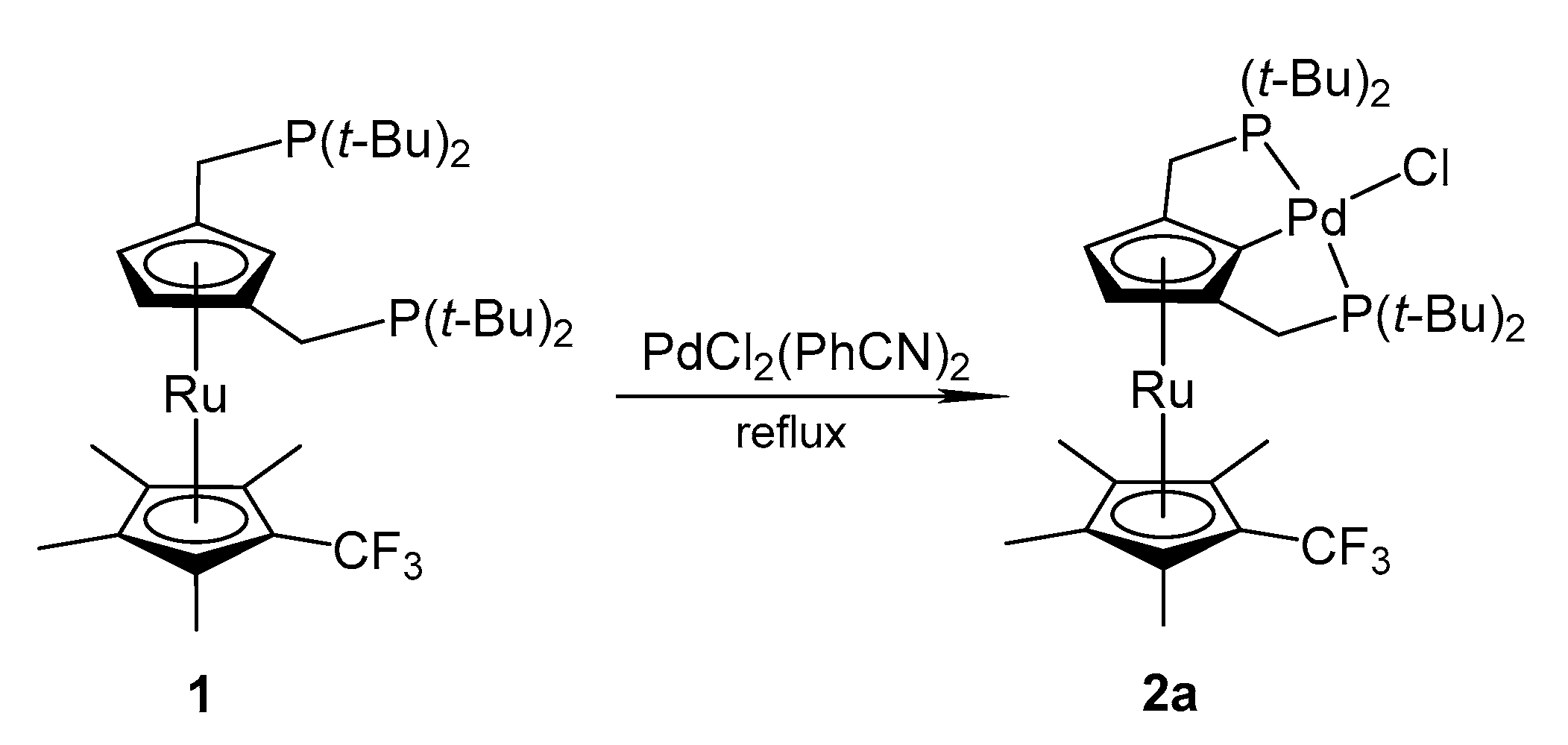
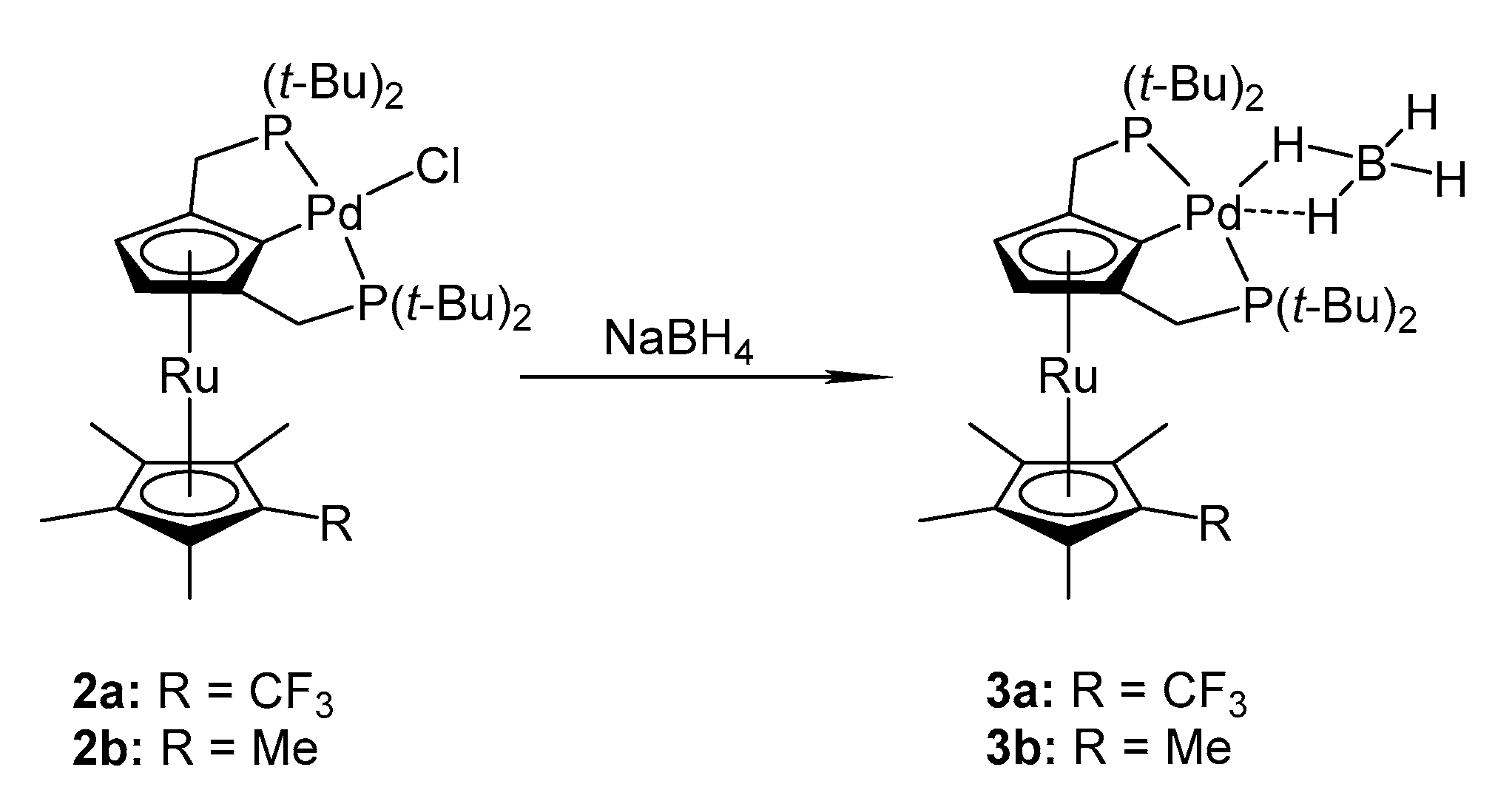
2.1. Synthesis
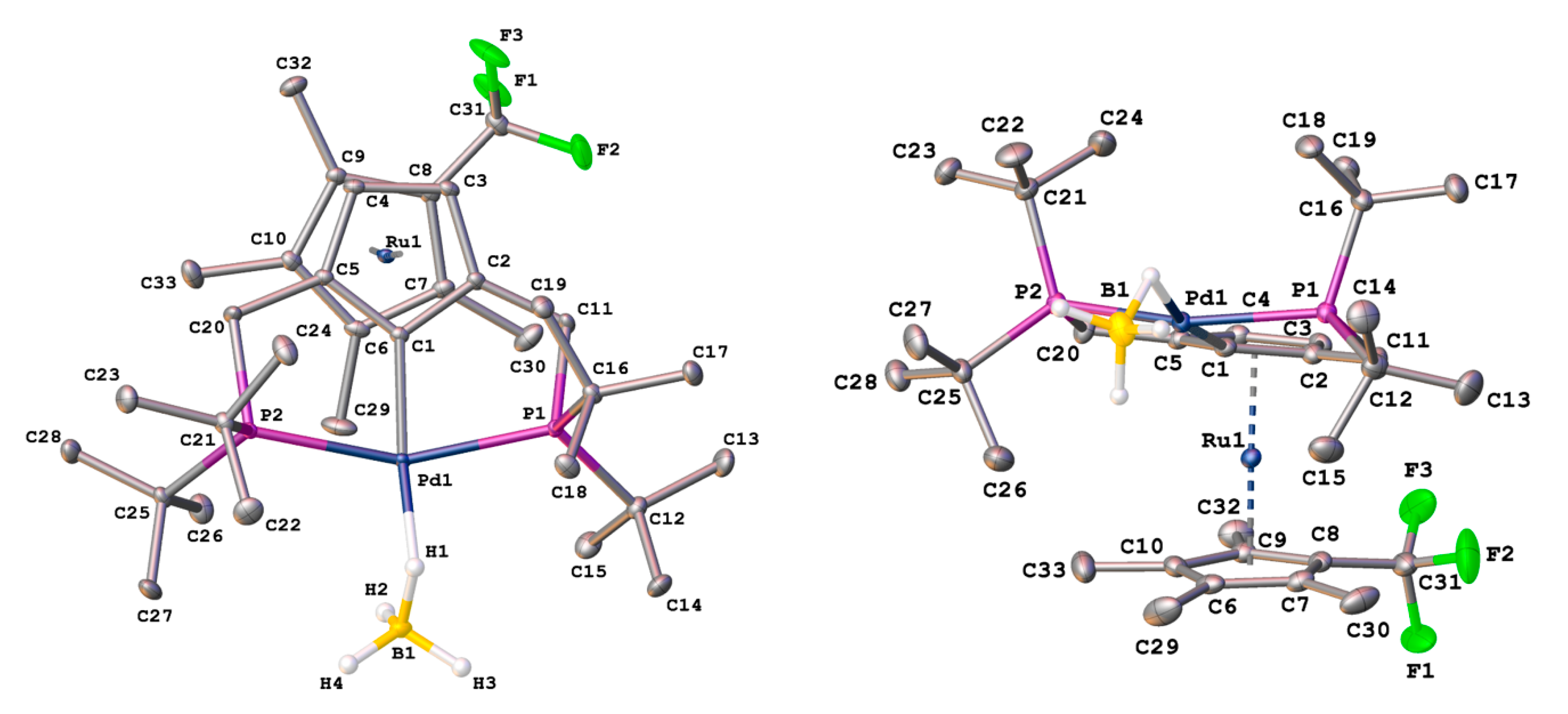
2.2. X-Ray Diffraction Study
2.3. IR Spectra
3. Materials and Methods
3.1. General Considerations
3.2. Crystallographic Data
3.3. Synthesis
3.3.1. Preparation of {2,5-Bis(di-tert-butylphosphinomethyl)-1′-(trifluoromethyl)-2′,3′,4′,5′-(tetramethyl)ruthenocen-1-yl]palladium(II) chloride PdCl[{2,5-(tBu2PCH2)2C5H2}Ru(C5Me4CF3)] (2a)
3.3.2. General Procedure for Preparation of Palladium Tetrahydroborate Complexes 3a and 3b
Preparation of {2,5-Bis(di-tert-butylphosphinomethyl)-1′-(trifluoromethyl)-2′,3′,4′,5′-(tetramethyl)-ruthenocen-1-yl]palladium(II) tetrahydroborate Pd(BH4)[{2,5-(tBu2PCH2)2C5H2}Ru(C5Me4CF3)] (3a)
Preparation of {2,5-Bis(di-tert-butylphosphinomethyl)-1′,2′,3′,4′,5′-(pentamethyl)ruthenocen-1-yl]palladium(II) tetrahydroborate Pd(BH4)[{2,5-(tBu2PCH2)2C5H2}Ru(C5Me5)] (3b)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moulton, C.J.; Shaw, B.L. Transition metal-carbon bonds. Part XLII. Complexes of nickel, palladium, platinum, rhodium and iridium with the tridentate ligand 2,6-bis[(di-t-butylphosphino)methyl]phenyl. J. Chem. Soc. Dalton Trans. 1976, 1020–1024. [Google Scholar] [CrossRef]
- van Koten, G.; Milstein, D. Organometallic Pincer Chemistry; Top. Organomet. Chem.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–356. [Google Scholar]
- van Koten, G.; Gossage, R.A. The Privileged Pincer-Metal Platform: Coordination Chemistry and Applications; Top. Organomet. Chem.; Springer: Heidelberg, Germany, 2016; pp. 1–374. [Google Scholar]
- Morales-Morales, D. Pincer Compounds; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 1–450. [Google Scholar]
- Peris, E.V.; Crabtree, R.H. Key factors in pincer ligand design. Chem. Soc. Rev. 2018, 47, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Polukeev, A.V.; Wendt, O.F. Iridium complexes with aliphatic, non-innocent pincer ligands. J. Organomet. Chem. 2018, 867, 33–50. [Google Scholar] [CrossRef]
- Asay, M.; Morales-Morales, D. Non-symmetric pincer ligands: Complexes and applications in catalysis. Dalton Trans. 2015, 44, 17432–17447. [Google Scholar] [CrossRef]
- Choi, J.; MacArthur, A.H.R.; Brookhart, M.; Goldman, A. Dehydrogenation and Related Reactions Catalyzed by Iridium Pincer Complexes. Chem. Rev. 2011, 111, 1761–1779. [Google Scholar] [CrossRef]
- Selander, N.; Szabó, K.J. Catalysis by Palladium Pincer Complexes. Chem. Rev. 2010, 111, 2048–2076. [Google Scholar] [CrossRef]
- Leis, W.; Mayer, H.; Kaska, W. Cycloheptatrienyl, alkyl and aryl PCP-pincer complexes: Ligand backbone effects and metal reactivity. Co-ord. Chem. Rev. 2008, 252, 1787–1797. [Google Scholar] [CrossRef]
- Van Der Boom, M.E.; Milstein, D. Cyclometalated Phosphine-Based Pincer Complexes: Mechanistic Insight in Catalysis, Coordination, and Bond Activation. Chem. Rev. 2003, 103, 1759–1792. [Google Scholar] [CrossRef]
- Albrecht, M.; Van Koten, G. Platinum Group Organometallics Based on “Pincer” Complexes: Sensors, Switches, and Catalysts. Angew. Chem. Int. Ed. 2001, 40, 3750–3781. [Google Scholar] [CrossRef]
- Koridze, A.A.; Sheloumov, A.M.; Kuklin, S.A.; Lagunova, V.Y.; Petukhova, I.I.; Dolgushin, F.M.; Ezernitskaya, M.G.; Petrovskii, P.V.; Macharashvili, A.A.; Chedia, R.V. PCP pincer ligands based on metallocenes. Crystal structure of the rhodium complex cis-RhCl2(CO)[{2,5-(Pri2PCH2)2C5H2}Fe(C5H5)]. Russ. Chem. Bull. 2002, 51, 1077–1078. [Google Scholar] [CrossRef]
- Farrington, E.J.; Viviente, E.M.; Williams, B.S.; Van Koten, G.; Brown, J.M. Synthesis and reactivity of a ferrocene-derived PCP-pincer ligand. Chem. Commun. 2002, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Koridze, A.A.; Kuklin, S.A.; Sheloumov, A.M.; Dolgushin, F.M.; Lagunova, V.Y.; Petukhova, I.I.; Ezernitskaya, M.G.; Peregudov, A.S.; Petrovskii, P.V.; Vorontsov, E.V.; et al. Ferrocene-Based Pincer Complexes of Palladium: Synthesis, Structures, and Spectroscopic and Electrochemical Properties. Organometallics 2004, 23, 4585–4593. [Google Scholar] [CrossRef]
- Kuklin, S.A.; Sheloumov, A.M.; Dolgushin, F.M.; Ezernitskaya, M.G.; Peregudov, A.S.; Petrovskii, P.V.; Koridze, A.A. Highly Active Iridium Catalysts for Alkane Dehydrogenation. Synthesis and Properties of Iridium Bis(phosphine) Pincer Complexes Based on Ferrocene and Ruthenocene. Organometallics 2006, 25, 5466–5476. [Google Scholar] [CrossRef]
- Kuklin, S.A.; Dolgushin, F.M.; Petrovskii, P.V.; Koridze, A.A. Synthesis and comparative X-ray diffraction study of first ruthenocene-based pincer palladium complexes, PdCl[{2,5-(But 2PCH2)2C5H2}Ru(Cp′)] (Cp′ = C5H5 or C5Me5). Russ. Chem. Bull. 2006, 55, 1950–1955. [Google Scholar] [CrossRef]
- Sheloumov, A.M.; Dolgushin, F.M.; Kondrashov, M.V.; Petrovskii, P.V.; Barbakadze, K.A.; Lekashvili, O.I.; Koridze, A.A. Ruthenium complexes with ferrocene-based P,C,P pincer ligand. Russ. Chem. Bull. 2007, 56, 1757–1764. [Google Scholar] [CrossRef]
- Sheloumov, A.M.; Tundo, P.; Dolgushin, F.M.; Koridze, A.A. Suzuki Aryl Coupling Catalysed by Palladium Bis(phosphane) Pincer Complexes Based on Ferrocene; X-ray Structure Determination of {PdCl[{2,5-(tBu2PCH2)2C5H2}Fe(C5H5)]}OTf. Eur. J. Inorg. Chem. 2008, 572–576. [Google Scholar] [CrossRef]
- Safronov, S.V.; Sheloumov, A.M.; Kreindlin, A.Z.; Kamyshova, A.A.; Dolgushin, F.M.; Smol’yakov, A.F.; Petrovskii, P.V.; Ezernitskaya, M.G.; Koridze, A.A. P,C,P-Pincer complexes of ruthenium based on ruthenocene and pentamethylruthenocene. Russ. Chem. Bull. 2010, 59, 1740–1744. [Google Scholar] [CrossRef]
- Koridze, A.A.; Polezhaev, A.V.; Safronov, S.V.; Sheloumov, A.M.; Dolgushin, F.M.; Ezernitskaya, M.G.; Lokshin, B.V.; Petrovskii, P.V.; Peregudov, A.S. Cationic Ruthenium Hydrido−Carbonyls Derived from Metallocene-Based Pincers: Unusual Rearrangements and H2Evolution with Formation of Cationic Ruthenium Metallocenylidenes. Organometallics 2010, 29, 4360–4368. [Google Scholar] [CrossRef]
- Polukeev, A.V.; Kuklin, S.A.; Petrovskii, P.V.; Peregudova, S.M.; Smol’yakov, A.F.; Dolgushin, F.M.; Koridze, A.A. Synthesis and characterization of fluorophenylpalladium pincer complexes: Electronic properties of some pincer ligands evaluated by multinuclear NMR spectroscopy and electrochemical studies. Dalton Trans. 2011, 40, 7201–7209. [Google Scholar] [CrossRef]
- Bonnet, S.; Lutz, M.; Spek, A.L.; Van Koten, G.; Klein Gebbink, R.J.M. η6-Coordination of a Ruthenium(II) Organometallic Fragment to the Arene Ring of N,C,N-Pincer Metal Complexes. Organometallics 2008, 27, 159–162. [Google Scholar] [CrossRef]
- Bonnet, S.; Van Lenthe, J.H.; Siegler, M.A.; Spek, A.L.; Van Koten, G.; Klein Gebbink, R.J.M. Bimetallic η6,η1 NCN-Pincer Ruthenium Palladium Complexes with η6-RuCp Coordination: Synthesis, X-ray Structures, and Catalytic Properties⊥. Organometallics 2009, 28, 2325–2333. [Google Scholar] [CrossRef]
- Bonnet, S.; Li, J.; Siegler, M.A.; Von Chrzanowski, L.S.; Spek, A.L.; Van Koten, G.; Klein Gebbink, R.J.M. Synthesis and Resolution of Planar-Chiral Ruthenium-Palladium Complexes with ECE′ Pincer Ligands. Chem.-A Eur. J. 2009, 15, 3340–3343. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Lutz, M.; Spek, A.L.; Van Koten, G.; Klein Gebbink, R.J.M. Bimetallic η6,η1 SCS- and PCP-Pincer Ruthenium Palladium Complexes: Synthesis, Structure, and Catalytic Activity. Organometallics 2010, 29, 1157–1167. [Google Scholar] [CrossRef]
- Walter, M.D.; White, P.S. [Cp′FeI]2 as convenient entry into iron-modified pincer complexes: Bimetallic η6,κ1-POCOP-pincer iron iridium compounds. New J. Chem. 2011, 35, 1842–1854. [Google Scholar] [CrossRef]
- Espinosa-Jalapa, N.Á.; Hernández-Ortega, S.; Morales-Morales, D.; Le Lagadec, R. Facile synthesis of heterobimetallic compounds from the cyclopentadienyl-ruthenium moiety and group 10 POCOP pincer complexes. J. Organomet. Chem. 2012, 716, 103–109. [Google Scholar] [CrossRef]
- Espinosa-Jalapa, N.Á.; Hernández-Ortega, S.; Le Goff, X.; Morales-Morales, D.; Djukic, J.-P.; Le Lagadec, R. Coordination of 12-Electron Organometallic Fragments to the Arene Ring of Nonsymmetric Group 10 POCOP Pincer Complexes. Organometallics 2013, 32, 2661–2673. [Google Scholar] [CrossRef]
- Espinosa-Jalapa, N.A.; Ramires, M.A.R.; Toscano, R.A.; Djukic, J.-P.; Le Lagadec, R. Preparative resolution of stable enantio-enriched POCOP-based planar chiral pincer complexes. J. Organomet. Chem. 2017, 845, 125–134. [Google Scholar] [CrossRef]
- Eberhardt, N.A.; Guan, H. Nickel Hydride Complexes. Chem. Rev. 2016, 116, 8373–8426. [Google Scholar] [CrossRef]
- Gassman, P.G.; Mickelson, J.W.; Sowa, J.R. 1,2,3,4-Tetramethyl-5-(trifluoromethyl)cyclopentadienide: A unique ligand with the steric properties of pentamethylcyclopentadienide and the electronic properties of cyclopentadienide. J. Am. Chem. Soc. 1992, 114, 6942–6944. [Google Scholar] [CrossRef]
- Gassman, P.G.; Sowa, J.R.; Hill, M.G.; Mann, K.R. Electrochemical Studies of Organometallic Complexes with Tetra-n-butylammonium Tetrakis [3,5-bis(trifluoromethyl)phenyl]borate as the Electrolyte. X-ray Crystal Structure of [C5(CF3)Me4]Fe(C5H5). Organometallics 1995, 14, 4879–4885. [Google Scholar] [CrossRef]
- Gusev, O.V.; A Ievlev, M.; A Peganova, T.; Peterleitner, M.G.; Petrovskii, P.V.; Oprunenko, Y.F.; Ustynyuk, N.A. Synthesis and reduction of trifluoromethyl-substituted arenecyclopentadienylruthenium sandwiches [Ru(η5-C5Me4CF3)(η6-C6R6]+ (R=H, Me) and [Ru(η5-C5Me5)(η6-C6H5CF3)]+. J. Organomet. Chem. 1998, 551, 93–100. [Google Scholar] [CrossRef]
- Safronov, S.V.; Sheloumov, A.M.; Petrovskii, P.V.; Ezernitskaya, M.G.; Koridze, A.A. (1,3-Diformylindenyl)cyclopentadienyl ruthenium derivatives. Russ. Chem. Bull. 2012, 61, 2065–2069. [Google Scholar] [CrossRef]
- Safronov, S.V.; Gutsul, E.I.; Golub, I.E.; Dolgushin, F.M.; Nelubina, Y.V.; Filippov, O.A.; Epstein, L.M.; Peregudov, A.S.; Belkova, N.V.; Shubina, E.S.; et al. Synthesis, structural properties and reactivity of ruthenocene-based pincer Pd(ii) tetrahydroborate. Dalton Trans. 2019, 48, 12720–12729. [Google Scholar] [CrossRef] [PubMed]
- Safronov, S.V.; Koridze, A.A. New ruthenocene-based ruthenium pincer complex bearing the C5Me4CF3 ligand. Russ. Chem. Bull. 2018, 67, 1247–1250. [Google Scholar] [CrossRef]
- Adhikary, A.; Schwartz, J.R.; Meadows, L.M.; Krause, J.A.; Guan, H. Interaction of alkynes with palladium POCOP-pincer hydride complexes and its unexpected relation to palladium-catalyzed hydrogenation of alkynes. Inorg. Chem. Front. 2014, 1, 71–82. [Google Scholar] [CrossRef]
- Marks, T.J.; Kolb, J.R. Covalent transition metal, lanthanide, and actinide tetrahydroborate complexes. Chem. Rev. 1977, 77, 263–293. [Google Scholar] [CrossRef]
- Johansson, R.; Wendt, O.F. Synthesis and Reactivity of (PCP) Palladium Hydroxy Carbonyl and Related Complexes toward CO2 and Phenylacetylene. Organometallics 2007, 26, 2426–2430. [Google Scholar] [CrossRef]
- Suh, H.-W.; Schmeier, T.J.; Hazari, N.; Kemp, R.A.; Takase, M.K. Experimental and Computational Studies of the Reaction of Carbon Dioxide with Pincer-Supported Nickel and Palladium Hydrides. Organometallics 2012, 31, 8225–8236. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, Y.; Ma, Q.-Q.; Cao, B.; Chang, J.; Li, S.; Chen, X. Reactions of POCOP pincer palladium benzylthiolate complexes with BH3·THF: Isolation and characterization of unstable POCOP-Pd(η1-HBH3) complexes. J. Organomet. Chem. 2019, 882, 50–57. [Google Scholar] [CrossRef]
- Ding, Y.; Ma, Q.-Q.; Kang, J.; Zhang, J.; Li, S.; Chen, X. Palladium(ii) complexes supported by PBP and POCOP pincer ligands: A comparison of their structure, properties and catalytic activity. Dalton Trans. 2019, 48, 17633–17643. [Google Scholar] [CrossRef]
- Makhaev, V.D. Structural and dynamic properties of tetrahydroborate complexes. Russ. Chem. Rev. 2000, 69, 727–746. [Google Scholar] [CrossRef]
- Dolomanov, O.; Bourhis, L.J.; Gildea, R.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


| 3a(KBr) | 3b(KBr) | 3c(KBr) [36] | 3a(CH2Cl2) | 3b(CH2Cl2) | |
|---|---|---|---|---|---|
| νas(B-Hterm) | 2388, 2363 | 2374 | 2364 | 2376 | 2372 |
| νs(B-Hterm) | 2296 | 2291 | 2292 | 2289 | 2287 |
| ν1(B-Hbridge) | 2019 | 2025 | 1993 | 2019 | 2008 |
| ν2(B-Hbridge) | 1838 | 1846 | 1840 | 1840 | 1841 |
| δ(BH) | 1054 | 1060 | 1062 | 1062 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safronov, S.V.; Osipova, E.S.; Nelyubina, Y.V.; Filippov, O.A.; Barakovskaya, I.G.; Belkova, N.V.; Shubina, E.S. Steric and Electronic Effect of Cp-Substituents on the Structure of the Ruthenocene Based Pincer Palladium Borohydrides. Molecules 2020, 25, 2236. https://doi.org/10.3390/molecules25092236
Safronov SV, Osipova ES, Nelyubina YV, Filippov OA, Barakovskaya IG, Belkova NV, Shubina ES. Steric and Electronic Effect of Cp-Substituents on the Structure of the Ruthenocene Based Pincer Palladium Borohydrides. Molecules. 2020; 25(9):2236. https://doi.org/10.3390/molecules25092236
Chicago/Turabian StyleSafronov, Sergey V., Elena S. Osipova, Yulia V. Nelyubina, Oleg A. Filippov, Irina G. Barakovskaya, Natalia V. Belkova, and Elena S. Shubina. 2020. "Steric and Electronic Effect of Cp-Substituents on the Structure of the Ruthenocene Based Pincer Palladium Borohydrides" Molecules 25, no. 9: 2236. https://doi.org/10.3390/molecules25092236
APA StyleSafronov, S. V., Osipova, E. S., Nelyubina, Y. V., Filippov, O. A., Barakovskaya, I. G., Belkova, N. V., & Shubina, E. S. (2020). Steric and Electronic Effect of Cp-Substituents on the Structure of the Ruthenocene Based Pincer Palladium Borohydrides. Molecules, 25(9), 2236. https://doi.org/10.3390/molecules25092236









