Crosslinked Enzyme Aggregates (CLEAs) of Laccases from Pleurotus citrinopileatus Induced in Olive Oil Mill Wastewater (OOMW)
Abstract
1. Introduction
2. Results and Discussion
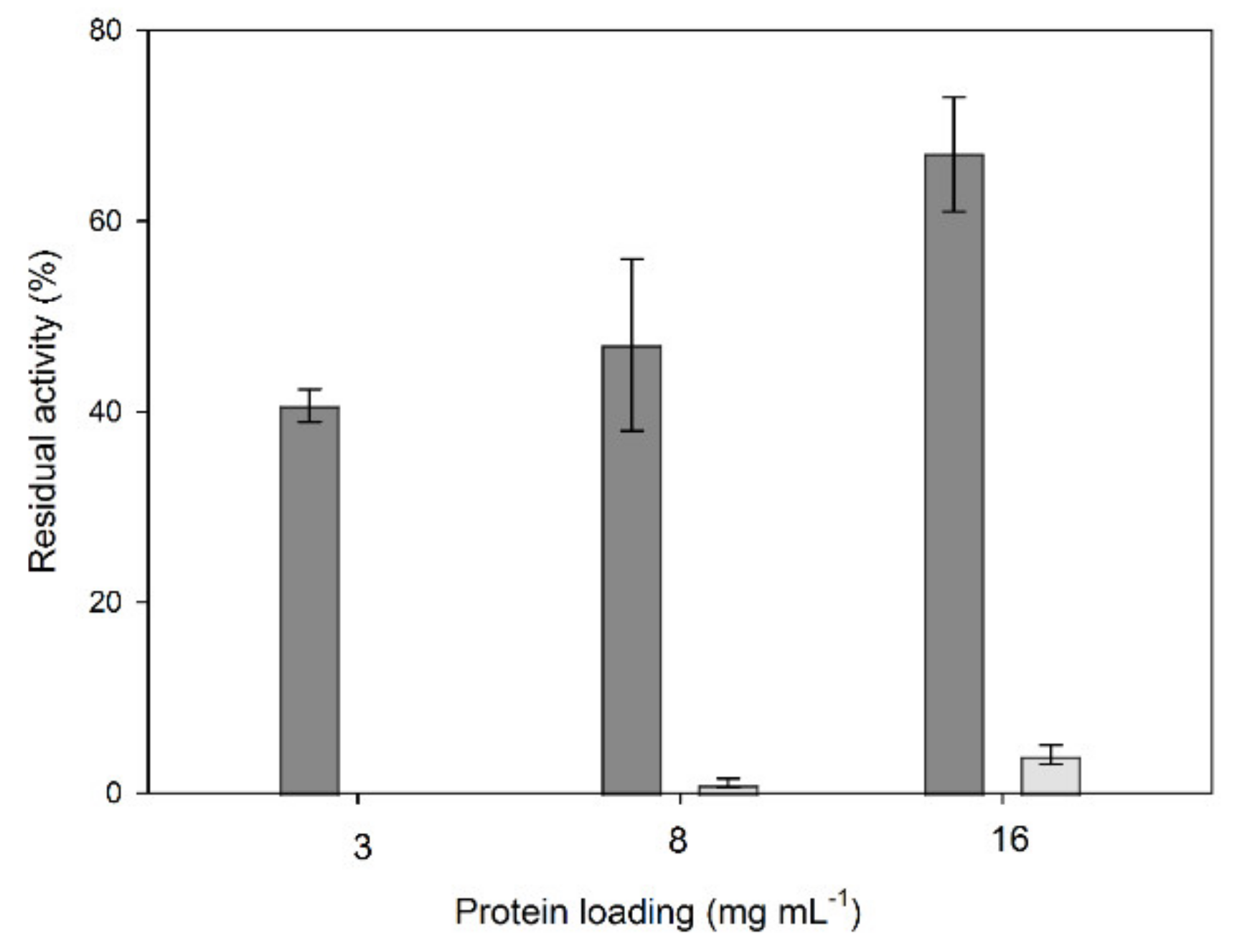
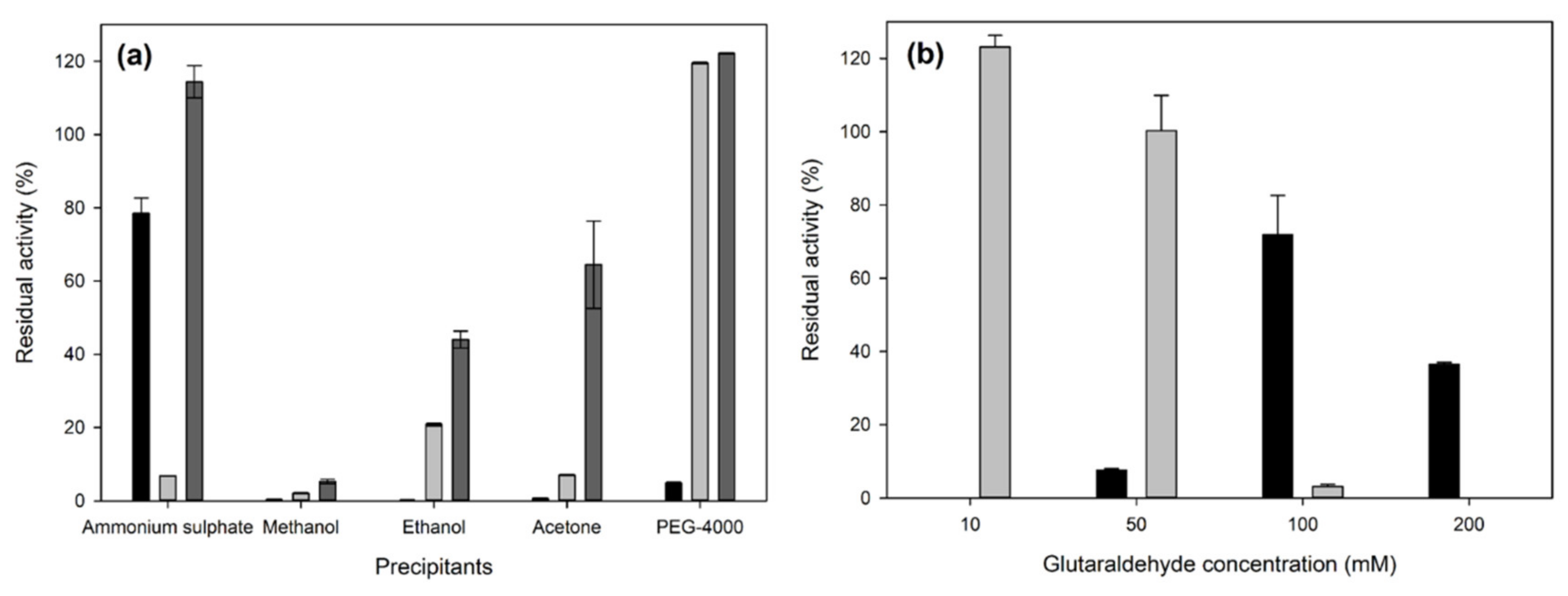
2.1. Immobilization of Crude Laccase Extract from OOMW-Grown P. citrinopileatus in CLEAs
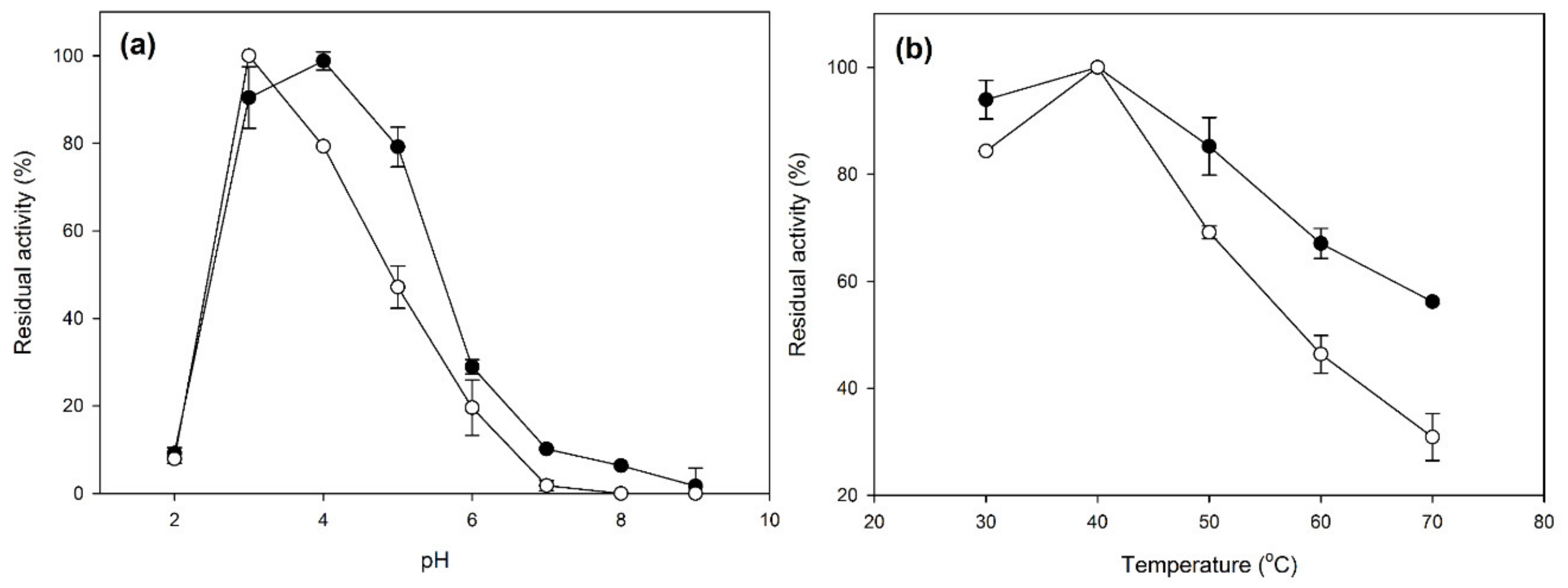
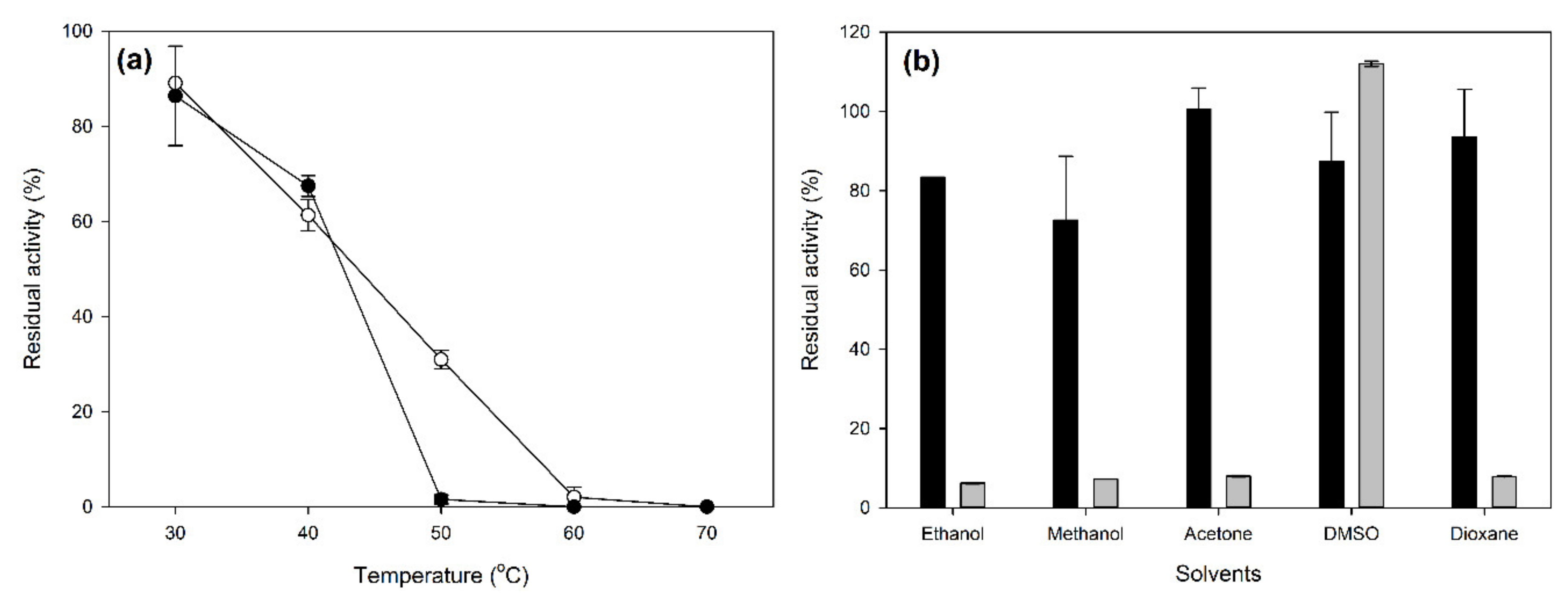
2.2. Characterization of Laccase CLEAs
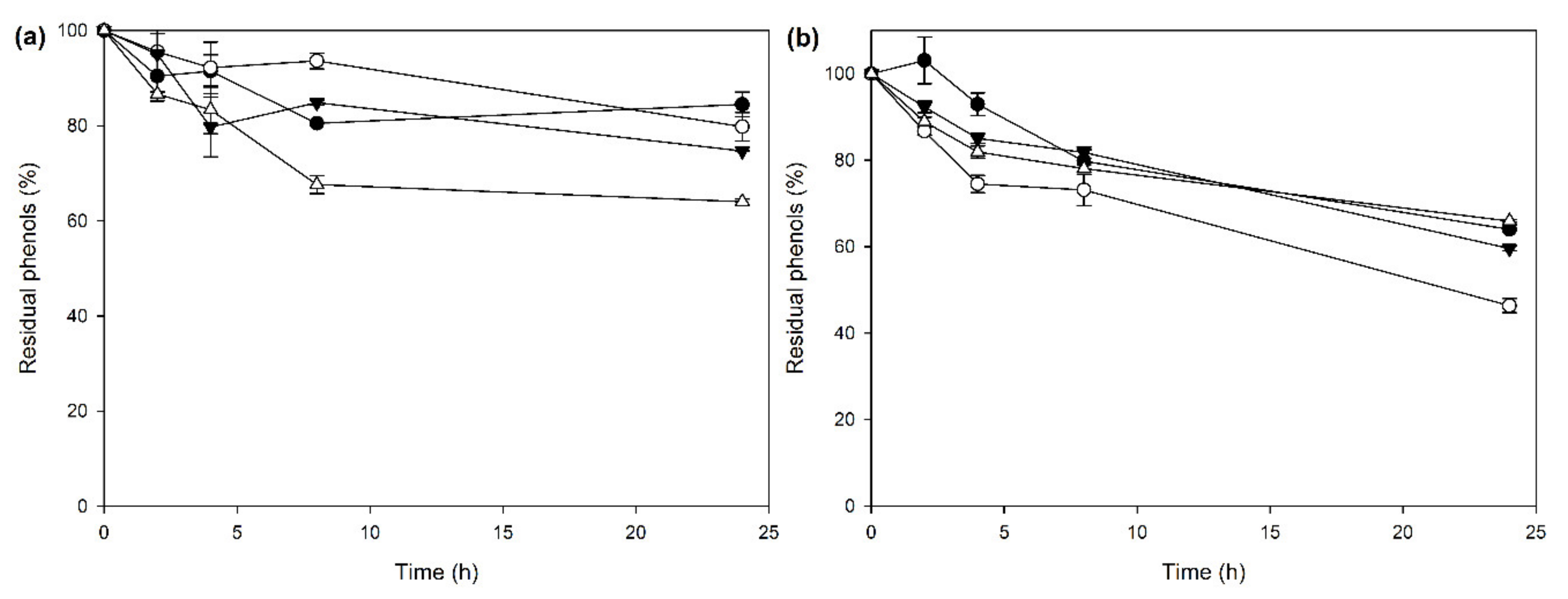
2.3. CLEA Application on OOMW Phenols Removal
3. Materials and Methods
3.1. Olive Mill Wastewater
3.2. Microorganisms and Culture Procedures
3.3. Enzyme Activities
3.4. Immobilization of Crude Laccase Extract
3.5. Characterization of Laccase-CLEAs
3.6. Application of Laccase-CLEAs on OOMW Phenol Removal
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zerva, A.; Zervakis, G.I.; Christakopoulos, P.; Topakas, E. Degradation of olive mill wastewater by the induced extracellular ligninolytic enzymes of two wood-rot fungi. J. Environ. Manag. 2017, 203, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Falade, A.O.; Mabinya, L.V.; Okoh, A.I.; Nwodo, U.U. Ligninolytic enzymes: Versatile biocatalysts for the elimination of endocrine-disrupting chemicals in wastewater. Microbiol. Open 2018, 7, e00722. [Google Scholar] [CrossRef]
- Singh, R.L.; Singh, P.K.; Singh, R.P. Enzymatic decolorization and degradation of azo dyes—A review. Int. Biodeterior. Biodegrad. 2015, 104, 21–31. [Google Scholar] [CrossRef]
- Wang, X.; Yao, B.; Su, X. Linking Enzymatic Oxidative Degradation of Lignin to Organics Detoxification. Int. J. Mol. Sci. 2018, 19, 3373. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Ashraf, S.S.; Barceló, D.; Iqbal, H.M.N. Biocatalytic degradation/redefining “removal” fate of pharmaceutically active compounds and antibiotics in the aquatic environment. Sci. Total Environ. 2019, 691, 1190–1211. [Google Scholar] [CrossRef] [PubMed]
- Babić, S.; Malev, O.; Pflieger, M.; Lebedev, A.T.; Mazur, D.M.; Kužić, A.; Čož-Rakovac, R.; Trebše, P. Toxicity evaluation of olive oil mill wastewater and its polar fraction using multiple whole-organism bioassays. Sci. Total Environ. 2019, 686, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Ntougias, S.; Baldrian, P.; Ehaliotis, C.; Nerud, F.; Merhautová, V.; Zervakis, G.I. Olive mill wastewater biodegradation potential of white-rot fungi–Mode of action of fungal culture extracts and effects of ligninolytic enzymes. Bioresour. Technol. 2015, 189, 121–130. [Google Scholar] [CrossRef]
- Ntougias, S.; Baldrian, P.; Ehaliotis, C.; Nerud, F.; Antoniou, T.; Merhautová, V.; Zervakis, G.I. Biodegradation and detoxification of olive mill wastewater by selected strains of the mushroom genera Ganoderma and Pleurotus. Chemosphere 2012, 88, 620–626. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Zervakis, G.I. Comparative Examination of the Olive Mill Wastewater Biodegradation Process by Various Wood-Rot Macrofungi. BioMed Res. Int. 2014, 2014, 14. [Google Scholar] [CrossRef]
- Feldman, D.; Kowbel, D.J.; Glass, N.L.; Yarden, O.; Hadar, Y. Detoxification of 5-hydroxymethylfurfural by the Pleurotus ostreatus lignolytic enzymes aryl alcohol oxidase and dehydrogenase. Biotechnol. Biofuels 2015, 8, 63. [Google Scholar] [CrossRef]
- Rivera-Hoyos, C.M.; Morales-Álvarez, E.D.; Abelló-Esparza, J.; Buitrago-Pérez, D.F.; Martínez-Aldana, N.; Salcedo-Reyes, J.C.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M. Detoxification of pulping black liquor with Pleurotus ostreatus or recombinant Pichia pastoris followed by CuO/TiO2/visible photocatalysis. Sci. Rep. 2018, 8, 3503. [Google Scholar] [CrossRef] [PubMed]
- Castanera, R.; Perez, G.; Omarini, A.; Alfaro, M.; Pisabarro, A.G.; Faraco, V.; Amore, A.; Ramirez, L. Transcriptional and enzymatic profiling of Pleurotus ostreatus laccase genes in submerged and solid-state fermentation cultures. Appl. Environ. Microbiol. 2012, 78, 4037–4045. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Kachrimanidou, V.; Papanikolaou, S.; Philippoussis, A.; Diamantopoulou, P. Upgrading Grape Pomace through Pleurotus spp. Cultivation for the Production of Enzymes and Fruiting Bodies. Microorganisms 2019, 7, 207. [Google Scholar] [CrossRef] [PubMed]
- De Salas, F.; Aza, P.; Gilabert, J.F.; Santiago, G.; Kilic, S.; Sener, M.E.; Vind, J.; Guallar, V.; Martínez, A.T.; Camarero, S. Engineering of a fungal laccase to develop a robust, versatile and highly-expressed biocatalyst for sustainable chemistry. Green Chem. 2019, 21, 5374–5385. [Google Scholar] [CrossRef]
- García-Ruiz, E.; Maté, D.; Ballesteros, A.; Martinez, A.T.; Alcalde, M. Evolving thermostability in mutant libraries of ligninolytic oxidoreductases expressed in yeast. Microb. Cell Fact. 2010, 9, 17. [Google Scholar] [CrossRef]
- García-Ruiz, E.; Gonzalez-Perez, D.; Ruiz-Duenas, F.J.; Martinez, A.T.; Alcalde, M. Directed evolution of a temperature-, peroxide- and alkaline pH-tolerant versatile peroxidase. Biochem. J. 2012, 441, 487–498. [Google Scholar] [CrossRef]
- Voběrková, S.; Solčány, V.; Vršanská, M.; Adam, V. Immobilization of ligninolytic enzymes from white-rot fungi in cross-linked aggregates. Chemosphere 2018, 202, 694–707. [Google Scholar] [CrossRef]
- Ba, S.; Jones, J.P.; Cabana, H. Hybrid bioreactor (HBR) of hollow fiber microfilter membrane and cross-linked laccase aggregates eliminate aromatic pharmaceuticals in wastewaters. J. Hazard. Mater. 2014, 280, 662–670. [Google Scholar] [CrossRef]
- Ba, S.; Haroune, L.; Cruz-Morató, C.; Jacquet, C.; Touahar, I.E.; Bellenger, J.-P.; Legault, C.Y.; Jones, J.P.; Cabana, H. Synthesis and characterization of combined cross-linked laccase and tyrosinase aggregates transforming acetaminophen as a model phenolic compound in wastewaters. Sci. Total Environ. 2014, 487, 748–755. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-linked enzyme aggregates (CLEAs): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef]
- Zerva, A.; Antonopoulou, I.; Enman, J.; Iancu, L.; Rova, U.; Christakopoulos, P. Cross-Linked Enzyme Aggregates of Feruloyl Esterase Preparations from Thermothelomyces thermophila and Talaromyces wortmannii. Catalysts 2018, 8, 208. [Google Scholar] [CrossRef]
- Han, J.Y.; Kim, H.K. Transesterification using the cross-linked enzyme aggregate of Photobacterium lipolyticum lipase M37. J. Microbiol. Biotechnol. 2011, 21, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, W.; Yang, Z.; Yang, X.; Wang, N.; Yu, X. Novel Magnetic Cross-Linked Cellulase Aggregates with a Potential Application in Lignocellulosic Biomass Bioconversion. Molecules 2017, 22, 269. [Google Scholar] [CrossRef] [PubMed]
- Hero, J.S.; Romero, C.M.; Pisa, J.H.; Perotti, N.I.; Olivaro, C.; Martinez, M.A. Designing cross-linked xylanase aggregates for bioconversion of agroindustrial waste biomass towards potential production of nutraceuticals. Int. J. Biol. Macromol. 2018, 111, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Pandharbale, A.; Ladole, M.; Nadar, S.; Mulla, M.; Japhalekar, K.; Pattankude, K.; Arage, D. Carrier free co-immobilization of alpha amylase, glucoamylase and pullulanase as combined cross-linked enzyme aggregates (combi-CLEAs): A tri-enzyme biocatalyst with one pot starch hydrolytic activity. Bioresour. Technol. 2013, 147, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Pletschke, B.I. Magnetic cross-linked enzyme aggregates (CLEAs): A novel concept towards carrier free immobilization of lignocellulolytic enzymes. Enzyme. Microb. Technol. 2014, 61–62, 17–27. [Google Scholar] [CrossRef]
- Talekar, S.; Shah, V.; Patil, S.; Nimbalkar, M. Porous cross linked enzyme aggregates (p-CLEAs) of Saccharomyces cerevisiae invertase. Catal. Sci. Technol. 2012, 2, 1575–1579. [Google Scholar] [CrossRef]
- Sheldon, R.A. CLEAs, Combi-CLEAs and ‘Smart’ Magnetic CLEAs: Biocatalysis in a Bio-Based Economy. Catalysts 2019, 9, 261. [Google Scholar] [CrossRef]
- Vinoth Kumar, V.; Prem Kumar, M.P.; Thiruvenkadaravi, K.V.; Baskaralingam, P.; Senthil Kumar, P.; Sivanesan, S. Preparation and characterization of porous cross linked laccase aggregates for the decolorization of triphenyl methane and reactive dyes. Bioresour. Technol. 2012, 119, 28–34. [Google Scholar] [CrossRef]
- Touahar, I.E.; Haroune, L.; Ba, S.; Bellenger, J.P.; Cabana, H. Characterization of combined cross-linked enzyme aggregates from laccase, versatile peroxidase and glucose oxidase, and their utilization for the elimination of pharmaceuticals. Sci. Total Environ. 2014, 481, 90–99. [Google Scholar] [CrossRef]
- Vrsanska, M.; Voberkova, S.; Jimenez Jimenez, A.M.; Strmiska, V.; Adam, V. Preparation and Optimisation of Cross-Linked Enzyme Aggregates Using Native Isolate White Rot Fungi Trametes versicolor and Fomes fomentarius for the Decolourisation of Synthetic Dyes. Int. J. Environ. Res. Public Health 2017, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.V.; Sivanesan, S.; Cabana, H. Magnetic cross-linked laccase aggregates—Bioremediation tool for decolorization of distinct classes of recalcitrant dyes. Sci. Total Environ. 2014, 487, 830–839. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Lin, Y.; Ng, T.B.; Ye, X.; Lin, J. Immobilized Cerrena sp. laccase: Preparation, thermal inactivation, and operational stability in malachite green decolorization. Sci. Rep. 2017, 7, 16429. [Google Scholar] [CrossRef] [PubMed]
- Zerva, A.; Papaspyridi, L.-M.; Christakopoulos, P.; Topakas, E. Valorization of Olive Mill Wastewater for the Production of β-glucans from Selected Basidiomycetes. Waste Biomass. Valorization 2017, 8, 1721–1731. [Google Scholar] [CrossRef]
- López-Serrano, P.; Cao, L.; van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates with enhanced activity: Application to lipases. Biotechnol. Lett. 2002, 24, 1379–1383. [Google Scholar] [CrossRef]
- Cabana, H.; Jones, J.P.; Agathos, S.N. Preparation and characterization of cross-linked laccase aggregates and their application to the elimination of endocrine disrupting chemicals. J. Biotechnol. 2007, 132, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lin, Y.; Yang, X.; Ng, T.B.; Ye, X.; Lin, J. Degradation of tetracycline by immobilized laccase and the proposed transformation pathway. J. Hazard. Mater. 2017, 322, 525–531. [Google Scholar] [CrossRef]
- Cabana, H.; Jones, J.P.; Agathos, S.N. Utilization of cross-linked laccase aggregates in a perfusion basket reactor for the continuous elimination of endocrine-disrupting chemicals. Biotechnol. Bioeng. 2009, 102, 1582–1592. [Google Scholar] [CrossRef]
- Hassani, T.; Ba, S.; Cabana, H. Formation of enzyme polymer engineered structure for laccase and cross-linked laccase aggregates stabilization. Bioresour. Technol. 2013, 128, 640–645. [Google Scholar] [CrossRef]
- Arca-Ramos, A.; Kumar, V.V.; Eibes, G.; Moreira, M.T.; Cabana, H. Recyclable cross-linked laccase aggregates coupled to magnetic silica microbeads for elimination of pharmaceuticals from municipal wastewater. Environ. Sci. Pollut. Res. 2016, 23, 8929–8939. [Google Scholar] [CrossRef]
- Justino, C.; Marques, A.G.; Duarte, K.R.; Duarte, A.C.; Pereira, R.; Rocha-Santos, T.; Freitas, A.C. Degradation of phenols in olive oil mill wastewater by biological, enzymatic, and photo-Fenton oxidation. Environ. Sci. Pollut. Res. 2010, 17, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Nugroho Prasetyo, E.; Rodríguez, R.D.; Lukesch, B.; Weiss, S.; Murkovic, M.; Katsoyannos, E.; Sygmund, C.; Ludwig, R.; Nyanhongo, G.S.; Guebitz, G.M. Laccase–cellobiose dehydrogenase-catalyzed detoxification of phenolic-rich olive processing residues. Int. J. Environ. Sci. Technol. 2015, 12, 1343–1352. [Google Scholar] [CrossRef][Green Version]
- Ngo, T.T.; Lenhoff, H.M. A sensitive and versatile chromogenic assay for peroxidase and peroxidase-coupled reactions. Anal. Biochem. 1980, 105, 389–397. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Schoevaart, R.; Wolbers, M.W.; Golubovic, M.; Ottens, M.; Kieboom, A.P.; van Rantwijk, F.; van der Wielen, L.A.; Sheldon, R.A. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerva, A.; Pentari, C.; Topakas, E. Crosslinked Enzyme Aggregates (CLEAs) of Laccases from Pleurotus citrinopileatus Induced in Olive Oil Mill Wastewater (OOMW). Molecules 2020, 25, 2221. https://doi.org/10.3390/molecules25092221
Zerva A, Pentari C, Topakas E. Crosslinked Enzyme Aggregates (CLEAs) of Laccases from Pleurotus citrinopileatus Induced in Olive Oil Mill Wastewater (OOMW). Molecules. 2020; 25(9):2221. https://doi.org/10.3390/molecules25092221
Chicago/Turabian StyleZerva, Anastasia, Christina Pentari, and Evangelos Topakas. 2020. "Crosslinked Enzyme Aggregates (CLEAs) of Laccases from Pleurotus citrinopileatus Induced in Olive Oil Mill Wastewater (OOMW)" Molecules 25, no. 9: 2221. https://doi.org/10.3390/molecules25092221
APA StyleZerva, A., Pentari, C., & Topakas, E. (2020). Crosslinked Enzyme Aggregates (CLEAs) of Laccases from Pleurotus citrinopileatus Induced in Olive Oil Mill Wastewater (OOMW). Molecules, 25(9), 2221. https://doi.org/10.3390/molecules25092221






