Functionalized Carbon Nanostructures Versus Drug Resistance: Promising Scenarios in Cancer Treatment
Abstract
1. Introduction
2. Carbon Nanostructures and Cancer: Toxicity Concerns and Needs for Tailored Functionalization
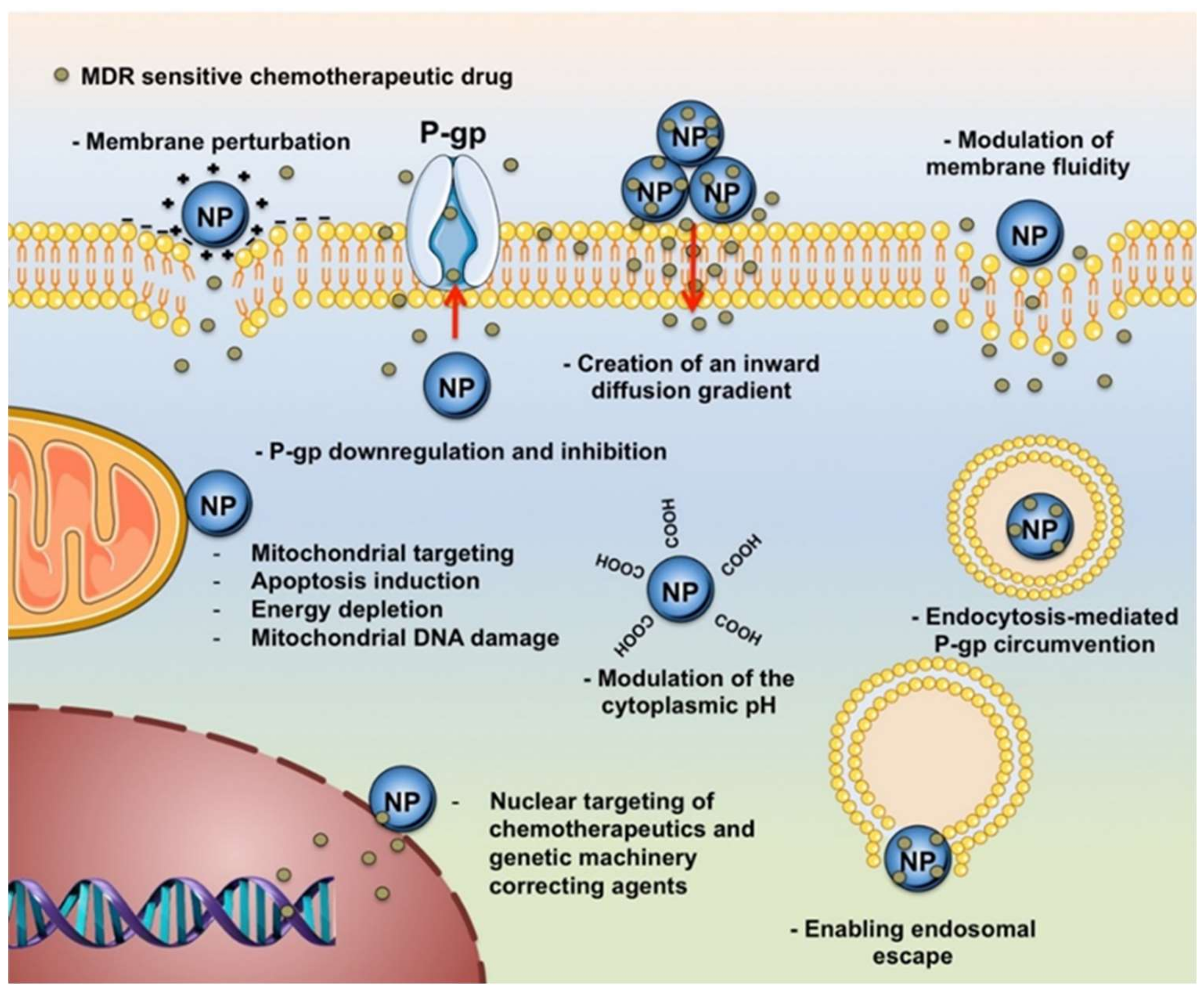
3. Carbon Nanostructures Fighting Multi-Drug Resistance
- a)
- inhibition of drug efflux pumps;
- b)
- increase of intracellular drug concentration and endosomal escape (enhanced uptake);
- c)
- damage of cell membrane and/or intracellular organelles;
- d)
- phototherapy.
3.1. MDR Reversal by Inhibition of Efflux Pumps
3.2. MDR Reversal by Enhanced Cellular Uptake
3.2.1. Pristine and Non-Covalently Functionalized CN
3.2.2. Covalently Functionalized CN
3.3. MDR Reversal by Cell Damage
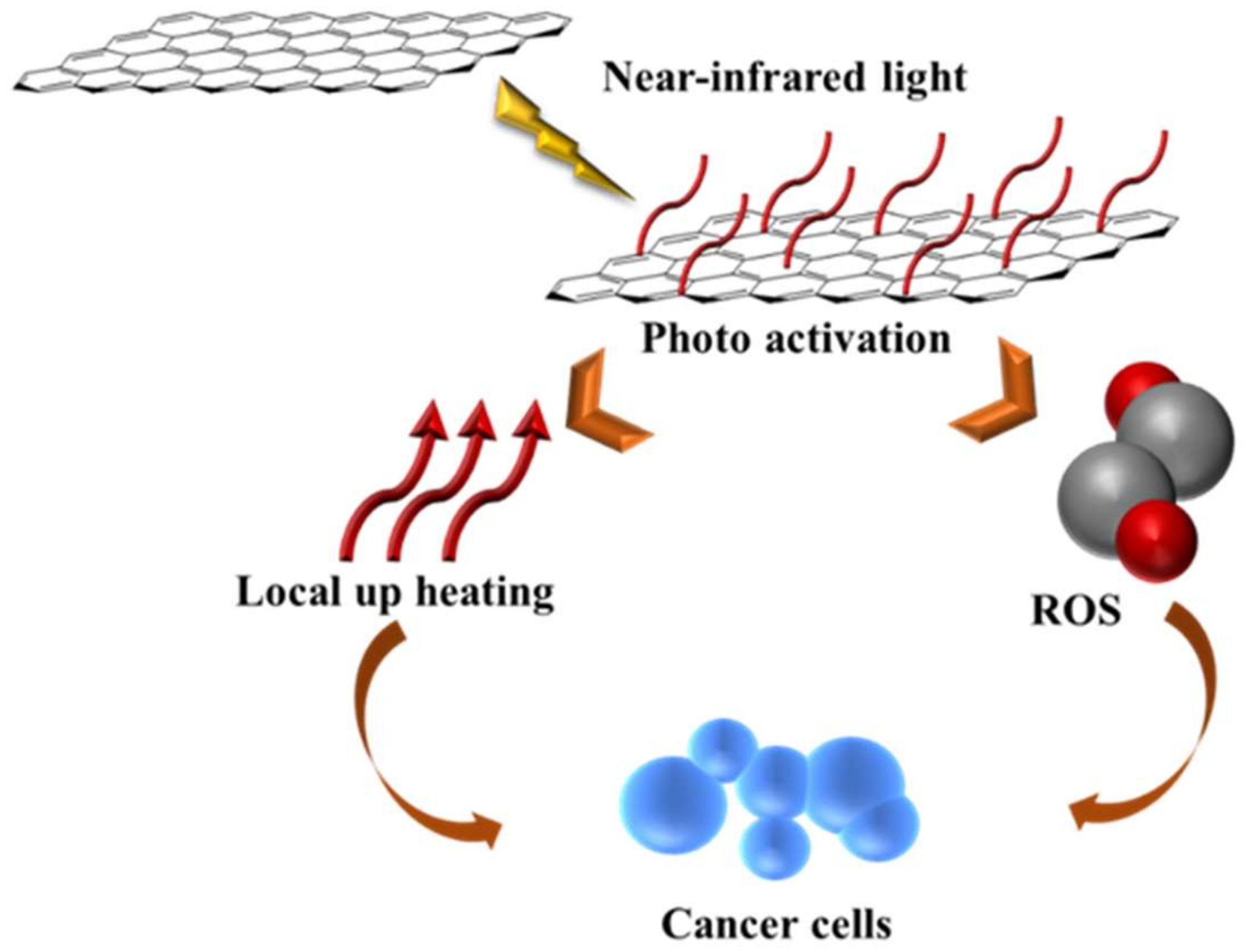
3.4. MDR Reversal by Phototherapy
3.4.1. Pristine and Non-Covalently Coated CN
3.4.2. Covalently Functionalized CN
4. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Frank, D.; Tyagi, C.; Tomar, L.; Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Penny, C.; Pillay, V. Overview of the role of nanotechnological innovations in the detection and treatment of solid tumors. Int. J. Nanomed. 2014, 9, 589–613. [Google Scholar] [CrossRef]
- Iyer, A.K.; Singh, A.; Ganta, S.; Amiji, M.M. Role of integrated cancer nanomedicine in overcoming drug resistance. Adv. Drug Deliver. Rev. 2013, 65, 1784–1802. [Google Scholar] [CrossRef] [PubMed]
- Tsuruo, T.; Naito, M.; Tomida, A.; Fujita, N.; Mashima, T.; Sakamoto, H.; Haga, N. Molecular targeting therapy of cancer: Drug resistance, apoptosis and survival signal. Cancer Sci. 2003, 94, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.S.; Tammam, S.N.; Boushehri, M.A.S.; Lamprecht, A. MDR in cancer: Addressing the underlying cellular alterations with the use of nanocarriers. Pharmacol. Res. 2017, 126, 2–30. [Google Scholar] [CrossRef]
- Fojo, T.; Bates, S. Strategies for reversing drug resistance. Oncogene 2003, 22, 7512–7523. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Peetla, C.; Vijayaraghavalu, S.; Labhasetwar, V. Biophysics of cell membrane lipids in cancer drug resistance: Implications for drug transport and drug delivery with nanoparticles. Adv. Drug Deliver. Rev. 2013, 65, 1686–1698. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Kunz-Schughart, L.A.; Dubrovska, A.; Peitzsch, C.; Ewe, A.; Aigner, A.; Schellenburg, S.; Muders, M.H.; Hampel, S.; Cirillo, G.; Iemma, F.; et al. Nanoparticles for radiooncology: Mission, vision, challenges. Biomaterials 2017, 120, 155–184. [Google Scholar] [CrossRef]
- Bakry, R.; Vallant, R.M.; Najam-Ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal applications of fullerenes. Int. J. Nanomed. 2007, 2, 639–649. [Google Scholar]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of carbon nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.H.; Wang, J.; Li, J.H.; Lin, Y.H. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.K.; Cheng, C.L.; Chang, C.C.; Chao, J.I. Biocompatible and detectable carboxylated nanodiamond on human cell. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Berger, M.L. The World of Graphene. In Nanoengineering: The Skills and Tools Making Technology Invisible; The Royal Society of Chemistry: London, UK, 2020; pp. 1–60. [Google Scholar]
- Malhotra, B.D.; Ali, M.A. (Eds.) Functionalized Carbon Nanomaterials for Biosensors. In Nanomaterials for Biosensors; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 75–103. [Google Scholar]
- Palmer, B.C.; Phelan-Dickenson, S.J.; DeLouise, L.A. Multi-walled carbon nanotube oxidation dependent keratinocyte cytotoxicity and skin inflammation. Part. Fibre Toxicol. 2019, 16. [Google Scholar] [CrossRef]
- Rout, C.S.; Kumar, A.; Fisher, T.S.; Gautam, U.K.; Bando, Y.; Golberg, D. Synthesis of chemically bonded CNT-graphene heterostructure arrays. RSC Adv. 2012, 2, 8250–8253. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing Graphene Quantum Dots and Carbon Dots: Properties, Syntheses, and Biological Applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Terrones, M.; Botello-Mendez, A.R.; Campos-Delgado, J.; Lopez-Urias, F.; Vega-Cantu, Y.I.; Rodriguez-Macias, F.J.; Elias, A.L.; Munoz-Sandoval, E.; Cano-Marquez, A.G.; Charlier, J.C.; et al. Graphene and graphite nanoribbons: Morphology, properties, synthesis, defects and applications. Nano Today 2010, 5, 351–372. [Google Scholar] [CrossRef]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, Properties, Functionalization, and Applications of Carbon Nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef]
- Heiberg-Andersen, H.; Skjeltorp, A.T.; Sattler, K. Carbon nanocones: A variety of non-crystalline graphite. J. Non-Cryst. Solids 2008, 354, 5247–5249. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.Z.; Hong, H.; Cai, W.B.; Liu, Z. Preparation and functionalization of graphene nanocomposites for biomedical applications. Nat. Protoc. 2013, 8, 2392–2403. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, L.; Bianco, A.; Prato, M.; Kostarelos, K. Carbon nanotubes as nanomedicines: From toxicology to pharmacology. Adv. Drug Deliver. Rev. 2006, 58, 1460–1470. [Google Scholar] [CrossRef]
- Bartelmess, J.; Quinn, S.J.; Giordani, S. Carbon nanomaterials: Multi-functional agents for biomedical fluorescence and Raman imaging. Chem. Soc. Rev. 2015, 44, 4672–4698. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.S.; Yoong, S.L.; Jagusiak, A.; Panczyk, T.; Ho, H.K.; Ang, W.H.; Pastorin, G. Carbon nanotubes for delivery of small molecule drugs. Adv. Drug Deliver. Rev. 2013, 65, 1964–2015. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Kostarelos, K.; Prato, M. Opportunities and challenges of carbon-based nanomaterials for cancer therapy. Expert Opin. Drug Del. 2008, 5, 331–342. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zhang, X.Y.; Ma, Y.F.; Huang, Y.; Wang, Y.S.; Chen, Y.S. Superparamagnetic graphene oxide-Fe3O4 nanoparticles hybrid for controlled targeted drug carriers. J. Mater. Chem 2009, 19, 2710–2714. [Google Scholar] [CrossRef]
- Cirillo, G.; Curcio, M.; Spizzirri, U.G.; Vittorio, O.; Tucci, P.; Picci, N.; Iemma, F.; Hampel, S.; Nicoletta, F.P. Carbon nanotubes hybrid hydrogels for electrically tunable release of Curcumin. Eur. Polym. J. 2017, 90, 1–12. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Z.Y.; Zong, S.F.; Zhu, D.; Chen, H.; Zhang, Y.Z.; Wu, L.; Cui, Y.P. pH-sensitive nanocarrier based on gold/silver core-shell nanoparticles decorated multi-walled carbon nanotubes for tracing drug release in living cells. Biosens. Bioelectron. 2016, 75, 446–451. [Google Scholar] [CrossRef]
- Cirillo, G.; Curcio, M.; Vittorio, O.; Spizzirri, U.G.; Nicoletta, F.P.; Picci, N.; Hampel, S.; Lemma, F. Dual Stimuli Responsive Gelatin-CNT Hybrid Films as a Versatile Tool for the Delivery of Anionic Drugs. Macromol. Mater. Eng. 2016, 301, 1537–1547. [Google Scholar] [CrossRef]
- Samadian, H.; Salami, M.S.; Jaymand, M.; Azarnezhad, A.; Najafi, M.; Barabadi, H.; Ahmadi, A. Genotoxicity assessment of carbon-based nanomaterials; Have their unique physicochemical properties made them double-edged swords? Mutat. Res.-Rev. Mutat. Res. 2020, 783. [Google Scholar] [CrossRef] [PubMed]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Van Berlo, D.; Clift, M.; Albrecht, C.; Schins, R. Carbon nanotubes: An insight into the mechanisms of their potential genotoxicity. Swiss Med. Wkly. 2012, 142. [Google Scholar] [CrossRef]
- Sasidharan, A.; Panchakarla, L.S.; Chandran, P.; Menon, D.; Nair, S.; Rao, C.N.R.; Koyakutty, M. Differential nano-bio interactions and toxicity effects of pristine versus functionalized graphene. Nanoscale 2011, 3, 2461–2464. [Google Scholar] [CrossRef] [PubMed]
- Battigelli, A.; Menard-Moyon, C.; Da Ros, T.; Prato, M.; Bianco, A. Endowing carbon nanotubes with biological and biomedical properties by chemical modifications. Adv. Drug Deliver. Rev. 2013, 65, 1899–1920. [Google Scholar] [CrossRef]
- Shim, M.; Kam, N.W.S.; Chen, R.J.; Li, Y.M.; Dai, H.J. Functionalization of carbon nanotubes for biocompatibility and biomolecular recognition. Nano Lett. 2002, 2, 285–288. [Google Scholar] [CrossRef]
- Karousis, N.; Tagmatarchis, N.; Tasis, D. Current Progress on the Chemical Modification of Carbon Nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef]
- Antonucci, A.; Kupis-Rozmyslowicz, J.; Boghossian, A.A. Noncovalent Protein and Peptide Functionalization of Single-Walled Carbon Nanotubes for Biodelivery and Optical Sensing Applications. ACS Appl. Mater. Inter. 2017, 9, 11321–11331. [Google Scholar] [CrossRef]
- Nava, A.C.; Cojoc, M.; Peitzsch, C.; Cirillo, G.; Kurth, I.; Fuessel, S.; Erdmann, K.; Kunhardt, D.; Vittorio, O.; Hampel, S.; et al. Development of novel radiochemotherapy approaches targeting prostate tumor progenitor cells using nanohybrids. Int. J. Cancer 2015, 137, 2492–2503. [Google Scholar] [CrossRef]
- Wu, H.C.; Chang, X.L.; Liu, L.; Zhao, F.; Zhao, Y.L. Chemistry of carbon nanotubes in biomedical applications. J. Mater. Chem. 2010, 20, 1036–1052. [Google Scholar] [CrossRef]
- Wang, X.L.; Shi, G.Q. An introduction to the chemistry of graphene. Phys. Chem. Chem. Phys. 2015, 17, 28484–28504. [Google Scholar] [CrossRef]
- Matson, M.L.; Villa, C.H.; Ananta, J.S.; Law, J.J.; Scheinberg, D.A.; Wilson, L.J. Encapsulation of alpha-Particle-Emitting Ac-225(3+) Ions Within Carbon Nanotubes. J. Nucl. Med. 2015, 56, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulos, D.D.; Bakandritsos, A.; Pykal, M.; Zboril, R.; Otyepka, M. Chemistry, properties, and applications of fluorographene. Appl. Mater. Today 2017, 9, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R. Fluorinated fullerenes. Chem.-Eur. J. 2001, 7, 4074–4083. [Google Scholar] [CrossRef]
- Mohammadi, S.; Kolahdouz, Z.; Mohajerzadeh, S. Hydrogenation-assisted unzipping of carbon nanotubes to realize graphene nano-sheets. J. Mater. Chem. C 2013, 1, 1309–1316. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C. Self-assembly of graphene sheets actuated by surface topological defects: Toward the fabrication of novel nanostructures and drug delivery devices. Appl. Surf. Sci. 2020, 505. [Google Scholar] [CrossRef]
- Schur, D.V.; Zaginaichenko, S.Y.; Veziroğlu, T.N.; Javadov, N.F. The Peculiarities of Hydrogenation of Fullerene Molecules C60 and Their Transformation. In Black Sea Energy Resource Development and Hydrogen Energy Problems; Veziroğlu, A., Tsitskishvili, M., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 191–204. [Google Scholar]
- Cheng, Q.S.; Blais, M.O.; Harris, G.; Jabbarzadeh, E. PLGA-Carbon Nanotube Conjugates for Intercellular Delivery of Caspase-3 into Osteosarcoma Cells. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Chen, S.; Liu, J.W.; Chen, M.L.; Chen, X.W.; Wang, J.H. Unusual emission transformation of graphene quantum dots induced by self-assembled aggregation. Chem. Commun. 2012, 48, 7637–7639. [Google Scholar] [CrossRef]
- Chua, C.K.; Sofer, Z.; Simek, P.; Jankovsky, O.; Klimova, K.; Bakardjieva, S.; Kuckova, S.H.; Pumera, M. Synthesis of Strongly Fluorescent Graphene Quantum Dots by Cage-Opening Buckminsterfullerene. ACS Nano 2015, 9, 2548–2555. [Google Scholar] [CrossRef]
- Pippa, N.; Chronopoulos, D.D.; Stellas, D.; Fernandez-Pacheco, R.; Arenal, R.; Demetzos, C.; Tagmatarchis, N. Design and development of multi-walled carbon nanotube-liposome drug delivery platforms. Int. J. Pharmaceut. 2017, 528, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Afreen, S.; Kokubo, K.; Muthoosamy, K.; Manickam, S. Hydration or hydroxylation: Direct synthesis of fullerenol from pristine fullerene [C-60] via acoustic cavitation in the presence of hydrogen peroxide. RSC Adv. 2017, 7, 31930–31939. [Google Scholar] [CrossRef]
- Beckler, B.; Cowan, A.; Farrar, N.; Murawski, A.; Robinson, A.; Diamanduros, A.; Scarpinato, K.; Sittaramane, V.; Quirino, R.L. Microwave Heating of Antibody-functionalized Carbon Nanotubes as a Feasible Cancer Treatment. Biomed. Phys. Eng. Expr. 2018, 4. [Google Scholar] [CrossRef]
- Campbell, E.; Hasan, M.T.; Pho, C.; Callaghan, K.; Akkaraju, G.R.; Naumov, A.V. Graphene Oxide as a Multifunctional Platform for Intracellular Delivery, Imaging, and Cancer Sensing. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Sabirov, D.S.; Khursan, S.L.; Bulgakov, R.G. 1,3-Dipolar addition reactions to fullerenes: The role of the local curvature of carbon surface. Russ. Chem. B+ 2008, 57, 2520–2525. [Google Scholar] [CrossRef]
- Viswanathan, G.; Chakrapani, N.; Yang, H.C.; Wei, B.Q.; Chung, H.S.; Cho, K.W.; Ryu, C.Y.; Ajayan, P.M. Single-step in situ synthesis of polymer-grafted single-wall nanotube composites. J. Am. Chem. Soc. 2003, 125, 9258–9259. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.S.; Matsuo, Y. Functionalization of [60]fullerene through fullerene cation intermediates. Chem. Commun. 2018, 54, 11244–11259. [Google Scholar] [CrossRef] [PubMed]
- Kooi, S.E.; Schlecht, U.; Burghard, M.; Kern, K. Electrochemical modification of single carbon nanotubes. Angew. Chem. Int. Edit. 2002, 41, 1353–1355. [Google Scholar] [CrossRef]
- Qi, M.; Zhang, Y.; Cao, C.M.; Zhang, M.X.; Liu, S.H.; Liu, G.Z. Decoration of Reduced Graphene Oxide Nanosheets with Aryldiazonium Salts and Gold Nanoparticles toward a Label-Free Amperometric Immunosensor for Detecting Cytokine Tumor Necrosis Factor-alpha in Live Cells. Anal. Chem. 2016, 88, 9614–9621. [Google Scholar] [CrossRef]
- Flavin, K.; Chaur, M.N.; Echegoyen, L.; Giordani, S. Functionalization of Multilayer Fullerenes (Carbon Nano-Onions) using Diazonium Compounds and “Click” Chemistry. Org. Lett. 2010, 12, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Koromilas, N.D.; Lainioti, G.C.; Gialeli, C.; Barbouri, D.; Kouravelou, K.B.; Karamanos, N.K.; Voyiatzis, G.A.; Kallitsis, J.K. Preparation and Toxicological Assessment of Functionalized Carbon Nanotube-Polymer Hybrids. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Vusa, C.S.R.; Venkatesan, M.; Aneesh, K.; Berchmans, S.; Arumugam, P. Tactical tuning of the surface and interfacial properties of graphene: A Versatile and rational electrochemical approach. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Calera, I.J.; Meza-Laguna, V.; Gromovoy, T.Y.; Chavez-Uribe, M.I.; Basiuk, V.A.; Basiuk, E.V. Solvent-free functionalization of fullerene C-60 and pristine multi-walled carbon nanotubes with aromatic amines. Appl. Surf. Sci. 2015, 328, 45–62. [Google Scholar] [CrossRef]
- Homenick, C.M.; Lawson, G.; Adronov, A. Polymer grafting of carbon nanotubes using living free-radical polymerization. Polym. Rev. 2007, 47, 265–290. [Google Scholar] [CrossRef]
- Servant, A.; Bianco, A.; Prato, M.; Kostarelos, K. Graphene for multi-functional synthetic biology: The last ‘zeitgeist’ in nanomedicine. Bioorg. Med. Chem. Lett. 2014, 24, 1638–1649. [Google Scholar] [CrossRef]
- Hasanzadeh, A.; Khataee, A.; Zarei, M.; Zhang, Y.F. Two-electron oxygen reduction on fullerene C-60-carbon nanotubes covalent hybrid as a metal-free electrocatalyst. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Ismaili, H.; Lagugne-Labarthet, F.; Workentin, M.S. Covalently Assembled Gold Nanoparticle-Carbon Nanotube Hybrids via a Photoinitiated Carbene Addition Reaction. Chem. Mater. 2011, 23, 1519–1525. [Google Scholar] [CrossRef]
- Ismaili, H.; Geng, D.S.; Sun, A.X.L.; Kantzas, T.T.; Workentin, M.S. Light-Activated Covalent Formation of Gold Nanoparticle Graphene and Gold Nanoparticle-Glass Composites. Langmuir 2011, 27, 13261–13268. [Google Scholar] [CrossRef]
- Lorbach, A.; Maverick, E.; Carreras, A.; Alemany, P.; Wu, G.; Garcia-Garibay, M.A.; Bazan, G.C. A fullerene-carbene adduct as a crystalline molecular rotor: Remarkable behavior of a spherically-shaped rotator. Phys. Chem. Chem. Phys. 2014, 16, 12980–12986. [Google Scholar] [CrossRef] [PubMed]
- Boncel, S.; Pluta, A.; Skonieczna, M.; Gondela, A.; Maciejewska, B.; Herman, A.P.; Jedrysiak, R.G.; Budniok, S.; Komedera, K.; Blachowski, A.; et al. Hybrids of Iron-Filled Multiwall Carbon Nanotubes and Anticancer Agents as Potential Magnetic Drug Delivery Systems: In Vitro Studies against Human Melanoma, Colon Carcinoma, and Colon Adenocarcinoma. J. Nanomater. 2017. [Google Scholar] [CrossRef]
- Quintana, M.; Spyrou, K.; Grzelczak, M.; Browne, W.R.; Rudolf, P.; Prato, M. Functionalization of Graphene via 1,3-Dipolar Cycloaddition. ACS Nano 2010, 4, 3527–3533. [Google Scholar] [CrossRef] [PubMed]
- Nakahodo, T.; Okada, M.; Morita, H.; Yoshimura, T.; Ishitsuka, M.O.; Tsuchiya, T.; Maeda, Y.; Fujihara, H.; Akasaka, T.; Gao, X.; et al. [2+1] cycloaddition of nitrene onto C(60) revisited: Interconversion between an aziridinofullerene and an azafulleroid. Angew. Chem. Int. Edit. 2008, 47, 1298–1300. [Google Scholar] [CrossRef] [PubMed]
- Samori, C.; Ali-Boucetta, H.; Sainz, R.; Guo, C.; Toma, F.M.; Fabbro, C.; da Ros, T.; Prato, M.; Kostarelos, K.; Bianco, A. Enhanced anticancer activity of multi-walled carbon nanotube-methotrexate conjugates using cleavable linkers. Chem. Commun. 2010, 46, 1494–1496. [Google Scholar] [CrossRef] [PubMed]
- Bekiari, V.; Karakassides, A.; Georgitsopoulou, S.; Kouloumpis, A.; Gournis, D.; Georgakilas, V. Self-assembly of one-side-functionalized graphene nanosheets in bilayered superstructures for drug delivery. J. Mater. Sci. 2018, 53, 11167–11175. [Google Scholar] [CrossRef]
- Pacor, S.; Grillo, A.; Dordevic, L.; Zorzet, S.; Lucafo, M.; Da Ros, T.; Prato, M.; Sava, G. Effects of Two Fullerene Derivatives on Monocytes and Macrophages. Biomed. Res. Int. 2015. [Google Scholar] [CrossRef]
- Irannejad, S.; Amini, M.; Modanlookordi, M.; Shokrzadeh, M.; Irannejad, H. Preparation of Diaminedicarboxyplatinum (II) Functionalized Single-Wall Carbon Nanotube via Bingel Reaction as a Novel Cytotoxic Agent. Iran. J. Pharm. Res. 2016, 15, 753–762. [Google Scholar]
- Stergiou, A.; Pagona, G.; Tagmatarchis, N. Donor-acceptor graphene-based hybrid materials facilitating photo-induced electron-transfer reactions. Beilstein J. Nanotech. 2014, 5, 1580–1589. [Google Scholar] [CrossRef]
- Biglova, Y.N.; Mustafin, A.G. Nucleophilic cyclopropanation of [60]fullerene by the addition-elimination mechanism. RSC Adv. 2019, 9, 22428–22498. [Google Scholar] [CrossRef]
- Ondera, T.J.; Hamme, A.T. A gold nanopopcorn attached single-walled carbon nanotube hybrid for rapid detection and killing of bacteria. J. Mater. Chem. B 2014, 2, 7534–7543. [Google Scholar] [CrossRef] [PubMed]
- Barrejon, M.; Gomez-Escalonilla, M.J.; Fierro, J.L.G.; Prieto, P.; Carrillo, J.R.; Rodriguez, A.M.; Abellan, G.; Lopez-Escalante, M.C.; Gabas, M.; Lopez-Navarrete, J.T.; et al. Modulation of the exfoliated graphene work function through cycloaddition of nitrile imines. Phys. Chem. Chem. Phys. 2016, 18, 29582–29590. [Google Scholar] [CrossRef]
- Sugawara, Y.; Jasinski, N.; Kaupp, M.; Welle, A.; Zydziak, N.; Blasco, E.; Barner-Kowollik, C. Light-driven nitrile imine-mediated tetrazole-ene cycloaddition as a versatile platform for fullerene conjugation. Chem. Commun. 2015, 51, 13000–13003. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.T.; Patil, M.P.; Phan, Q.T.; Le, C.M.Q.; Ahn, B.-H.; Kim, G.-D.; Lim, K.T. Green and direct functionalization of poly (ethylene glycol) grafted polymers onto single walled carbon nanotubes: Effective nanocarrier for doxorubicin delivery. J. Ind. Eng. Chem. 2020, 83, 173–180. [Google Scholar] [CrossRef]
- Yuan, J.C.; Chen, G.H.; Weng, W.G.; Xu, Y.Z. One-step functionalization of graphene with cyclopentadienyl-capped macromolecules via Diels-Alder “click” chemistry. J. Mater. Chem. 2012, 22, 7929–7936. [Google Scholar] [CrossRef]
- Tsuda, M.; Ishida, T.; Nogami, T.; Kurono, S.; Ohashi, M. Isolation and Characterization of Diels-Alder Adducts of C-60 with Anthracene and Cyclopentadiene. J. Chem. Soc. Chem. Comm. 1993, 1296–1298. [Google Scholar] [CrossRef]
- Pastorin, G. Crucial Functionalizations of Carbon Nanotubes for Improved Drug Delivery: A Valuable Option? Pharm. Res.-Dordr. 2009, 26, 746–769. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, K.J.; Gu, Z.N.; Peng, H.Q.; Flor, E.L.; Hauge, R.H.; Smalley, R.E. Controlled oxidative cutting of single-walled carbon nanotubes. J. Am. Chem. Soc. 2005, 127, 1541–1547. [Google Scholar] [CrossRef]
- Savage, T.; Bhattacharya, S.; Sadanadan, B.; Gaillard, J.; Tritt, T.M.; Sun, Y.P.; Wu, Y.; Nayak, S.; Car, R.; Marzari, N.; et al. Photoinduced oxidation of carbon nanotubes. J. Phys.: Condens. Matter 2003, 15, 5915–5921. [Google Scholar] [CrossRef]
- Felten, A.; Bittencourt, C.; Pireaux, J.J. Gold clusters on oxygen plasma functionalized carbon nanotubes: XPS and TEM studies. Nanotechnology 2006, 17, 1954–1959. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Gao, L.; Sun, J. Production of aqueous colloidal dispersions of carbon nanotubes. J. Colloid Interface Sci. 2003, 260, 89–94. [Google Scholar] [CrossRef]
- Rosca, I.D.; Watari, F.; Uo, M.; Akaska, T. Oxidation of multiwalled carbon nanotubes by nitric acid. Carbon 2005, 43, 3124–3131. [Google Scholar] [CrossRef]
- Witzmann, F.A.; Monteiro-Riviere, N.A. Multi-walled carbon nanotube exposure alters protein expression in human keratinocytes. Nanomedicine 2006, 2, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Bussy, C.; Hadad, C.; Prato, M.; Bianco, A.; Kostarelos, K. Intracellular degradation of chemically functionalized carbon nanotubes using a long-term primary microglial culture model. Nanoscale 2016, 8, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Tagmatarchis, N.; Prato, M. Functionalization of carbon nanotubes via 1,3-dipolar cycloadditions. J. Mater. Chem. 2004, 14, 437–439. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Maksimova, N.; Duda, T.A.; Kudashov, A.G.; Shubin, Y.V.; Su, D.S.; Pazhetnov, E.M.; Boronin, A.I.; Bulusheva, L.G. Fluorination of CNx nanotubes. Fuller. Nanotub. Carbon Nanostructures 2004, 12, 99–104. [Google Scholar] [CrossRef]
- Struzzi, C.; Scardamaglia, M.; Hemberg, A.; Petaccia, L.; Colomer, J.F.; Snyders, R.; Bittencourt, C. Plasma fluorination of vertically aligned carbon nanotubes: Functionalization and thermal stability. Beilstein J. Nanotech. 2015, 6, 2263–2271. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.F.; Feng, Y.Y.; Zhao, S.L.; Lu, P.; Yuan, X.Y.; Feng, W. Progress of synthesizing methods and properties of fluorinated carbon nanotubes. Sci. China Technol. Sci. 2010, 53, 1225–1233. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Makharza, S.; Cirillo, G.; Bachmatiuk, A.; Vittorio, O.; Mendes, R.G.; Oswald, S.; Hampel, S.; Rummeli, M.H. Size-dependent nanographene oxide as a platform for efficient carboplatin release. J. Mater. Chem. B 2013, 1, 6107–6114. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.M.; Xia, J.G.; Zhao, Q.H.; Liu, L.W.; Zhang, Z.J. Functional Graphene Oxide as a Nanocarrier for Controlled Loading and Targeted Delivery of Mixed Anticancer Drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Choi, W.I.; Lee, J.H.; Tae, G. Graphene oxide mediated delivery of methylene blue for combined photodynamic and photothermal therapy. Biomaterials 2013, 34, 6239–6248. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Y.; Wang, S.; Li, Y.J.; Wang, M.W.; Shi, P.; Huang, X.Y. Covalent Functionalization of Graphene Oxide with Biocompatible Poly(ethylene glycol) for Delivery of Paclitaxel. ACS Appl. Mater. Inter. 2014, 6, 17268–17276. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Cherian, R.S.; Anju, S.; Paul, W.; Sabareeswaran, A.; Mohanan, P.V. Organ distribution and biological compatibility of surface-functionalized reduced graphene oxide. Nanotechnology 2020, 31. [Google Scholar] [CrossRef]
- Darabdhara, G.; Das, M.R.; Turcheniuk, V.; Turcheniuk, K.; Zaitsev, V.; Boukherroub, R.; Szunerits, S. Reduced graphene oxide nanosheets decorated with AuPd bimetallic nanoparticles: A multifunctional material for photothermal therapy of cancer cells. J. Mater. Chem. B 2015, 3, 8366–8374. [Google Scholar] [CrossRef]
- Zainuddin, M.F.; Raikhan, N.H.N.; Othman, N.H.; Abdullah, W.F.H. Synthesis of reduced Graphene Oxide (rGO) using different treatments of Graphene Oxide (GO). IOP Conf. Ser.-Mat. Sci. 2018. [Google Scholar] [CrossRef]
- Kalluri, A.; Debnath, D.; Dharmadhikari, B.; Patra, P. Graphene Quantum Dots: Synthesis and Applications. In Methods in Enzymology; Kumar, C.V., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 609, pp. 335–354. [Google Scholar]
- Zhao, M.L. Direct Synthesis of Graphene Quantum Dots with Different Fluorescence Properties by Oxidation of Graphene Oxide Using Nitric Acid. Appl. Sci. 2018, 8. [Google Scholar] [CrossRef]
- Pan, D.Y.; Guo, L.; Zhang, J.C.; Xi, C.; Xue, Q.; Huang, H.; Li, J.H.; Zhang, Z.W.; Yu, W.J.; Chen, Z.W.; et al. Cutting sp(2) clusters in graphene sheets into colloidal graphene quantum dots with strong green fluorescence. J. Mater. Chem. 2012, 22, 3314–3318. [Google Scholar] [CrossRef]
- Milane, L.; Ganesh, S.; Shah, S.; Duan, Z.F.; Amiji, M. Multi-modal strategies for overcoming tumor drug resistance: Hypoxia, the Warburg effect, stem cells, and multifunctional nanotechnology. J. Control. Release 2011, 155, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, S.; Ozluer, O.; Gunduz, U. Nanoparticle-based drug delivery in cancer: The role of cell membrane structures. Ther. Deliv. 2016, 7, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.W.; Mumper, R.J. Nanomedicinal strategies to treat multidrug-resistant tumors: Current progress. Nanomedicine 2010, 5, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.S.; Muntimadugu, E.; Rafeeqi, T.A.; Domb, A.J.; Khan, W. Co-delivery of rapamycin- and piperine-loaded polymeric nanoparticles for breast cancer treatment. Drug Deliv. 2016, 23, 2608–2616. [Google Scholar] [CrossRef]
- Alibert-Franco, S.; Pradines, B.; Mahamoud, A.; Davin-Regli, A.; Pages, J.M. Efflux Mechanism, an Attractive Target to Combat Multidrug Resistant Plasmodium falciparum and Pseudomonas aeruginosa. Curr. Med. Chem. 2009, 16, 301–317. [Google Scholar] [CrossRef]
- Gillet, J.P.; Gottesman, M.M. Advances in the Molecular Detection of ABC Transporters Involved in Multidrug Resistance in Cancer. Curr. Pharm. Biotechnol. 2011, 12, 686–692. [Google Scholar] [CrossRef]
- Yu, M.; Ocana, A.; Tannock, I.F. Reversal of ATP-binding cassette drug transporter activity to modulate chemoresistance: Why has it failed to provide clinical benefit? Cancer Metast. Rev. 2013, 32, 211–227. [Google Scholar] [CrossRef]
- Fromm, M.F. Importance of P-glycoprotein at blood-tissue barriers. Trends Pharmacol. Sci. 2004, 25, 423–429. [Google Scholar] [CrossRef]
- Wang, Z.J.; Xu, Y.H.; Meng, X.N.; Watari, F.M.; Liu, H.D.; Chen, X. Suppression of c-Myc is involved in multi-walled carbon nanotubes’ down-regulation of ATP-binding cassette transporters in human colon adenocarcinoma cells. Toxicol. Appl. Pharm. 2015, 282, 42–51. [Google Scholar] [CrossRef]
- Fabbro, C.; Ali-Boucetta, H.; Da Ros, T.; Kostarelos, K.; Bianco, A.; Prato, M. Targeting carbon nanotubes against cancer. Chem. Commun. 2012, 48, 3911–3926. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Förster, C. Multidrug resistance protein P-gp interaction with nanoparticles (fullerenes and carbon nanotube) to assess their drug delivery potential: A theoretical molecular docking study. Int. J. Comput. Biol. Drug Des. 2013, 6, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.P.; Meziani, M.J.; Sun, Y.P.; Cheng, S.H. Poly(ethylene glycol)-conjugated multi-walled carbon nanotubes as an efficient drug carrier for overcoming multidrug resistance. Toxicol. Appl. Pharm. 2011, 250, 184–193. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, G.; Misra, C.; Kumar, R.; Singh, B.; Katare, O.P.; Raza, K. N-desmethyl tamoxifen and quercetin-loaded multiwalled CNTs: A synergistic approach to overcome MDR in cancer cells. Mat. Sci. Eng. C 2018, 89, 274–282. [Google Scholar] [CrossRef]
- Luo, C.; Li, Y.F.; Guo, L.J.; Zhang, F.W.; Liu, H.; Zhang, J.L.; Zheng, J.; Zhang, J.Y.; Guo, S.W. Graphene Quantum Dots Downregulate Multiple Multidrug-Resistant Genes via Interacting with Their C-Rich Promoters. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.Y.; Zhang, H.X.; Pan, Y.B.; Ren, J.; Ye, M.M.; Xia, F.F.; Huang, R.; Lin, Z.H.; Jiang, S.; Zhang, Y.; et al. Single-walled carbon nanotube: One specific inhibitor of cancer stem cells in osteosarcoma upon downregulation of the TGF beta 1 signaling. Biomaterials 2017, 149, 29–40. [Google Scholar] [CrossRef]
- Da Silva, C.G.; Peters, G.J.; Ossendorp, F.; Cruz, L.J. The potential of multi-compound nanoparticles to bypass drug resistance in cancer. Cancer Chemother. Pharmacol. 2017, 80, 881–894. [Google Scholar] [CrossRef]
- Salvioni, L.; Rizzuto, M.A.; Bertolini, J.A.; Pandolfi, L.; Colombo, M.; Prosperi, D. Thirty Years of Cancer Nanomedicine: Success, Frustration, and Hope. Cancers 2019, 11. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Thakur, M.; Gurung, R.B.; Srivastava, R. Graphene Quantum Dots for Cell Proliferation, Nucleus Imaging, and Photoluminescent Sensing Applications. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Mu, Q.X.; Broughton, D.L.; Yan, B. Endosomal Leakage and Nuclear Translocation of Multiwalled Carbon Nanotubes: Developing a Model for Cell Uptake. Nano Lett. 2009, 9, 4370–4375. [Google Scholar] [CrossRef]
- Shi, X.H.; von dem Bussche, A.; Hurt, R.H.; Kane, A.B.; Gao, H.J. Cell entry of one-dimensional nanomaterials occurs by tip recognition and rotation. Nat. Nanotechnol. 2011, 6, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Kam, N.W.S.; Jessop, T.C.; Wender, P.A.; Dai, H.J. Nanotube molecular transporters: Internalization of carbon nanotube-protein conjugates into mammalian cells. J. Am. Chem Soc. 2004, 126, 6850–6851. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.M.; Bourgognon, M.; Wang, J.T.W.; Al-Jamal, K.T. Functionalised carbon nanotubes: From intracellular uptake and cell-related toxicity to systemic brain delivery. J. Control. Release 2016, 241, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zong, C.; Shen, H.; Liu, M.; Chen, B.A.; Ren, B.; Zhang, Z.J. Mechanism of Cellular Uptake of Graphene Oxide Studied by Surface-Enhanced Raman Spectroscopy. Small 2012, 8, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Yamanaka, T.; Takamura-Enya, T. Synthesis of novel fluorescently labeled water-soluble fullerenes and their application to its cellar uptake and distribution properties. J. Nanopart. Res. 2017, 19. [Google Scholar] [CrossRef]
- Mahajan, S.; Patharkar, A.; Kuche, K.; Maheshwari, R.; Deb, P.K.; Kalia, K.; Tekade, R.K. Functionalized carbon nanotubes as emerging delivery system for the treatment of cancer. Int. J. Pharmaceut. 2018, 548, 540–558. [Google Scholar] [CrossRef]
- Gong, P.W.; Zhang, L.; Yuan, X.A.; Liu, X.C.; Diao, X.L.; Zhao, Q.; Tian, Z.Z.; Sun, J.; Liu, Z.; You, J.M. Multifunctional fluorescent PEGylated fluorinated graphene for targeted drug delivery: An experiment and DFT study. Dyes Pigments 2019, 162, 573–582. [Google Scholar] [CrossRef]
- Mahmood, M.; Xu, Y.; Dantuluri, V.; Mustafa, T.; Zhang, Y.; Karmakar, A.; Casciano, D.; Ali, S.; Biris, A. Carbon nanotubes enhance the internalization of drugs by cancer cells and decrease their chemoresistance to cytostatics. Nanotechnology 2013, 24. [Google Scholar] [CrossRef]
- Wu, P.P.; Li, S.; Zhang, H.J. Design real-time reversal of tumor multidrug resistance cleverly with shortened carbon nanotubes. Drug Des. Dev. Ther. 2014, 8, 2431–2438. [Google Scholar] [CrossRef]
- Alizadeh, D.; White, E.E.; Sanchez, T.C.; Liu, S.N.; Zhang, L.Y.; Badie, B.; Berlin, J.M. lmmunostimulatory CpG on Carbon Nanotubes Selectively Inhibits Migration of Brain Tumor Cells. Bioconjugate Chem. 2018, 29, 1659–1668. [Google Scholar] [CrossRef]
- Lin, K.C.; Lin, M.W.; Hsu, M.N.; Guan, Y.C.; Chao, Y.C.; Tuan, H.Y.; Chiang, C.S.; Hu, Y.C. Graphene oxide sensitizes cancer cells to chemotherapeutics by inducing early autophagy events, promoting nuclear trafficking and necrosis. Theranostics 2018, 8, 2477–2487. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Gao, X.N.; Yu, Z.Z.; Liu, B.; Pan, W.; Li, N.; Tang, B. Reversing Multidrug Resistance by Multiplexed Gene Silencing for Enhanced Breast Cancer Chemotherapy. ACS Appl. Mater. Inter. 2018, 10, 15461–15466. [Google Scholar] [CrossRef] [PubMed]
- Guven, A.; Rusakova, I.A.; Lewis, M.T.; Wilson, L.J. Cisplatin@US-tube carbon nanocapsules for enhanced chemotherapeutic delivery. Biomaterials 2012, 33, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Guven, A.; Villares, G.J.; Hilsenbeck, S.G.; Lewis, A.; Landua, J.D.; Dobrolecki, L.E.; Wilson, L.J.; Lewis, M.T. Carbon nanotube capsules enhance the in vivo efficacy of cisplatin. Acta Biomater. 2017, 58, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Muzi, L.; Menard-Moyon, C.; Russier, J.; Li, J.; Chin, C.F.; Ang, W.H.; Pastorin, G.; Risuleo, G.; Bianco, A. Diameter-dependent release of a cisplatin pro-drug from small and large functionalized carbon nanotubes. Nanoscale 2015, 7, 5383–5394. [Google Scholar] [CrossRef]
- Sui, X.; Luo, C.; Wang, C.; Zhang, F.W.; Zhang, J.Y.; Guo, S.W. Graphene quantum dots enhance anticancer activity of cisplatin via increasing its cellular and nuclear uptake. Nanomedicine 2016, 12, 1997–2006. [Google Scholar] [CrossRef]
- Wei, Z.; Yin, X.T.; Cai, Y.; Xu, W.G.; Song, C.H.; Wang, Y.F.; Zhang, J.W.; Kang, A.; Wang, Z.Y.; Han, W. Antitumor effect of a Pt-loaded nanocomposite based on graphene quantum dots combats hypoxia-induced chemoresistance of oral squamous cell carcinoma. Int. J. Nanomed. 2018, 13, 1505–1524. [Google Scholar] [CrossRef]
- Wang, C.; Wu, C.Y.; Zhou, X.J.; Han, T.; Xin, X.Z.; Wu, J.Y.; Zhang, J.Y.; Guo, S.W. Enhancing Cell Nucleus Accumulation and DNA Cleavage Activity of Anti-Cancer Drug via Graphene Quantum Dots. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.S.; Yang, X.Y.; Liu, Y.Y.; Yang, J.R.; Yang, R.; Zhang, N. Graphene oxide used as a carrier for adriamycin can reverse drug resistance in breast cancer cells. Nanotechnology 2012, 23. [Google Scholar] [CrossRef]
- Jin, R.; Ji, X.J.; Yang, Y.X.; Wang, H.F.; Cao, A.N. Self-Assembled Graphene-Dextran Nanohybrid for Killing Drug-Resistant Cancer Cells. ACS Appl. Mater. Inter. 2013, 5, 7181–7189. [Google Scholar] [CrossRef]
- Zhang, Q.; Chi, H.R.; Tang, M.Z.; Chen, J.B.; Li, G.L.; Liu, Y.S.; Liu, B. Mixed surfactant modified graphene oxide nanocarriers for DOX delivery to cisplatin-resistant human ovarian carcinoma cells. RSC Adv. 2016, 6, 87258–87269. [Google Scholar] [CrossRef]
- Wu, C.H.; Cao, C.; Kim, J.H.; Hsu, C.H.; Wanebo, H.J.; Bowen, W.D.; Xu, J.; Marshall, J. Trojan-Horse Nanotube On-Command Intracellular Drug Delivery. Nano Lett. 2012, 12, 5475–5480. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yao, H.J.; Sun, L.; Liu, Y.; Jiang, S.; Pu, Y.Z.; Li, J.C.; Zhang, Y.G. Monodistearoylphosphatidylethanolamine-hyaluronic acid functionalization of single-walled carbon nanotubes for targeting intracellular drug delivery to overcome multidrug resistance of cancer cells. Carbon 2016, 96, 362–376. [Google Scholar] [CrossRef]
- Zhi, F.; Dong, H.F.; Jia, X.F.; Guo, W.J.; Lu, H.T.; Yang, Y.L.; Ju, H.X.; Zhang, X.J.; Hu, Y.Q. Functionalized Graphene Oxide Mediated Adriamycin Delivery and miR-21 Gene Silencing to Overcome Tumor Multidrug Resistance In Vitro. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Lu, C.H.; Zhu, C.L.; Li, J.; Liu, J.J.; Chen, X.; Yang, H.H. Using graphene to protect DNA from cleavage during cellular delivery. Chem. Commun. 2010, 46, 3116–3118. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Hartman, K.B.; Boudjemaa, S.; Ananta, J.S.; Morgant, G.; Szwarc, H.; Wilson, L.J.; Moussa, F. In Vivo Behavior of Large Doses of Ultrashort and Full-Length Single-Walled Carbon Nanotubes after Oral and Intraperitoneal Administration to Swiss Mice. ACS Nano 2010, 4, 1481–1492. [Google Scholar] [CrossRef]
- Kim, S.W.; Lee, Y.K.; Kim, S.H.; Park, J.Y.; Lee, D.U.; Choi, J.; Hong, J.H.; Kim, S.; Khang, D. Covalent, Non-Covalent, Encapsulated Nanodrug Regulate the Fate of Intra- and Extracellular Trafficking: Impact on Cancer and Normal Cells. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Eassa, S.; Parnell, C.; Nima, Z.; Ghosh, A.; Biris, A.S.; Khodakovskaya, M.V. Carbon nanotubes as carriers of Panax ginseng metabolites and enhancers of ginsenosides Rb1 and Rg1 anti-cancer activity. Nanotechnology 2017, 28. [Google Scholar] [CrossRef]
- Wang, N.; Feng, Y.X.; Zeng, L.L.; Zhao, Z.N.; Chen, T.F. Functionalized Multiwalled Carbon Nanotubes as Carriers of Ruthenium Complexes to Antagonize Cancer Multidrug Resistance and Radioresistance. ACS Appl. Mater. Inter. 2015, 7, 14933–14945. [Google Scholar] [CrossRef]
- Zakaria, A.; Picaud, F.; Rattier, T.; Pudlo, M.; Saviot, L.; Chassagnon, R.; Lherminier, J.; Gharbi, T.; Micheau, O.; Herlem, G. Nanovectorization of TRAIL with Single Wall Carbon Nanotubes Enhances Tumor Cell Killing. Nano Lett. 2015, 15, 891–895. [Google Scholar] [CrossRef]
- Jiang, T.Y.; Sun, W.J.; Zhu, Q.W.; Burns, N.A.; Khan, S.A.; Mo, R.; Gu, Z. Furin-Mediated Sequential Delivery of Anticancer Cytokine and Small-Molecule Drug Shuttled by Graphene. Adv. Mater. 2015, 27, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.B.; Ma, Y.Y.; Chen, X.Y.; Zhao, Y.Y.; Mou, X.Z. Ceramide-Graphene Oxide Nanoparticles Enhance Cytotoxicity and Decrease HCC Xenograft Development: A Novel Approach for Targeted Cancer Therapy. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Al Faraj, A.; Shaik, A.S.; Ratemi, E.; Halwani, R. Combination of drug-conjugated SWCNT nanocarriers for efficient therapy of cancer stem cells in a breast cancer animal model. J. Control. Release 2016, 225, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.J.; Weng, Z.Y.; Wang, C.Y.; Zhu, M.J.; Lu, Y.S.; Ding, L.L.; Wang, Y.K.; Cheng, X.H.; Lin, Q.; Wu, K.J. Increased chemosensitivity and radiosensitivity of human breast cancer cell lines treated with novel functionalized single-walled carbon nanotubes. Oncol. Lett. 2017, 13, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.A.; Cai, X.L.; Li, H.; Lin, Y.H.; Du, D. Hyaluronic Acid-Modified Multifunctional Q-Graphene for Targeted Killing of Drug-Resistant Lung Cancer Cells. ACS Appl. Mater. Inter. 2016, 8, 4048–4055. [Google Scholar] [CrossRef]
- Nigam, P.; Waghmode, S.; Louis, M.; Wangnoo, S.; Chavan, P.; Sarkar, D. Graphene quantum dots conjugated albumin nanoparticles for targeted drug delivery and imaging of pancreatic cancer. J. Mater. Chem. B 2014, 2, 3190–3195. [Google Scholar] [CrossRef]
- Vittorio, O.; Le Grand, M.; Makharza, S.A.; Curcio, M.; Tucci, P.; Iemma, F.; Nicoletta, F.P.; Hampel, S.; Cirillo, G. Doxorubicin synergism and resistance reversal in human neuroblastoma BE(2)C cell lines: An in vitro study with dextran-catechin nanohybrids. Eur. J. Pharm. Biopharm. 2018, 122, 176–185. [Google Scholar] [CrossRef]
- Li, R.B.; Wu, R.; Zhao, L.; Wu, M.H.; Yang, L.; Zou, H.F. P-Glycoprotein Antibody Functionalized Carbon Nanotube Overcomes the Multidrug Resistance of Human Leukemia Cells. ACS Nano 2010, 4, 1399–1408. [Google Scholar] [CrossRef]
- Zhang, H.J.; Xiong, J.; Guo, L.T.; Patel, N.; Guang, X.N. Integrated traditional Chinese and western medicine modulator for overcoming the multidrug resistance with carbon nanotubes. RSC Adv. 2015, 5, 71287–71296. [Google Scholar] [CrossRef]
- Nowacki, M.; Wisniewski, M.; Werengowska-Ciecwierz, K.; Roszek, K.; Czarnecka, J.; Lakomska, I.; Kloskowski, T.; Tyloch, D.; Debski, R.; Pietkun, K.; et al. Nanovehicles as a novel target strategy for hyperthermic intraperitoneal chemotherapy: A multidisciplinary study of peritoneal carcinomatosis. Oncotarget 2015, 6, 22776–22798. [Google Scholar] [CrossRef]
- Zhang, G.L.; Du, R.H.; Qian, J.C.; Zheng, X.J.; Tian, X.H.; Cai, D.Q.; He, J.C.; Wu, Y.Q.; Huang, W.; Wang, Y.Y.; et al. A tailored nanosheet decorated with a metallized dendrimer for angiography and magnetic resonance imaging-guided combined chemotherapy. Nanoscale 2018, 10, 488–498. [Google Scholar] [CrossRef]
- Gu, Y.M.; Guo, Y.Z.; Wang, C.Y.; Xu, J.K.; Wu, J.P.; Kirk, T.B.; Ma, D.; Xue, W. A polyamidoamne dendrimer functionalized graphene oxide for DOX and MMP-9 shRNA plasmid co-delivery. Mat. Sci. Eng. C 2017, 70, 572–585. [Google Scholar] [CrossRef]
- Cao, X.F.; Feng, F.L.; Wang, Y.S.; Yang, X.Y.; Duan, H.Q.; Chen, Y.S. Folic acid-conjugated graphene oxide as a transporter of chemotherapeutic drug and siRNA for reversal of cancer drug resistance. J. Nanopart. Res. 2013, 15. [Google Scholar] [CrossRef]
- Liang, X.J.; Meng, H.; Wang, Y.Z.; He, H.Y.; Meng, J.; Lu, J.; Wang, P.C.; Zhao, Y.L.; Gao, X.Y.; Sun, B.Y.; et al. Metallofullerene nanoparticles circumvent tumor resistance to cisplatin by reactivating endocytosis. Proc. Natl. Acad. Sci. USA 2010, 107, 7449–7454. [Google Scholar] [CrossRef]
- Tredan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug resistance and the solid tumor microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef]
- Rundqvist, H.; Johnson, R.S. Tumour oxygenation: Implications for breast cancer prognosis. J. Intern. Med. 2013, 274, 105–112. [Google Scholar] [CrossRef]
- Vittorio, O.; Cojoc, M.; Curcio, M.; Spizzirri, U.G.; Hampel, S.; Nicoletta, F.P.; Iemma, F.; Dubrovska, A.; Kavallaris, M.; Cirillo, G. Polyphenol Conjugates by Immobilized Laccase: The Green Synthesis of Dextran-Catechin. Macromol. Chem. Phys. 2016, 217, 1488–1492. [Google Scholar] [CrossRef]
- Septiadi, D.; Crippa, F.; Moore, T.L.; Rothen-Rutishauser, B.; Petri-Fink, A. Nanoparticle-Cell Interaction: A Cell Mechanics Perspective. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, L.; Valiente, R.; Gonzalez, J.; Villegas, J.C.; Fanarraga, M.L. Multiwalled Carbon Nanotubes Display Microtubule Biomimetic Properties in Vivo, Enhancing Microtubule Assembly and Stabilization. ACS Nano 2012, 6, 6614–6625. [Google Scholar] [CrossRef]
- Madannejad, R.; Shoaie, N.; Jahanpeyma, F.; Darvishi, M.H.; Azimzadeh, M.; Javadi, H. Toxicity of carbon-based nanomaterials: Reviewing recent reports in medical and biological systems. Chem.-Biol. Interact. 2019, 307, 206–222. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, X.X.; Sun, L.; Wei, Y.Q.; Wei, X.W. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Fu, Y.J.; Wei, T.T.; Le Guyader, L.; Gao, G.; Liu, R.S.; Chang, Y.Z.; Chen, C.Y. The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways. Biomaterials 2012, 33, 402–411. [Google Scholar] [CrossRef]
- Gao, X.; Schottker, B. Reduction-oxidation pathways involved in cancer development: A systematic review of literature reviews. Oncotarget 2017, 8, 51888–51906. [Google Scholar] [CrossRef]
- Garcia-Hevia, L.; Villegas, J.C.; Fernandez, F.; Casafont, I.; Gonzalez, J.; Valiente, R.; Fanarraga, M.L. Multiwalled Carbon Nanotubes Inhibit Tumor Progression in a Mouse Model. Adv. Healthc. Mater. 2016, 5, 1080–1087. [Google Scholar] [CrossRef]
- González-Lavado, E.; Valdivia, L.; García-Castaño, A.; González, F.; Pesquera, C.; Valiente, R.; Fanarraga, M.L. Multi-walled carbon nanotubes complement the anti-tumoral effect of 5-Fluorouracil. Oncotarget 2019, 10, 2022–2029. [Google Scholar]
- Ling, B.P.; Chen, H.T.; Liang, D.Y.; Lin, W.; Qi, X.Y.; Liu, H.P.; Deng, X. Acidic pH and High-H2O2 Dual Tumor Microenvironment-Responsive Nanocatalytic Graphene Oxide for Cancer Selective Therapy and Recognition. ACS Appl. Mater. Inter. 2019, 11, 11157–11166. [Google Scholar] [CrossRef]
- Zhang, X.F.; Huang, F.H.; Zhang, G.L.; Bai, D.P.; Massimo, D.F.; Huang, Y.F.; Gurunathan, S. Novel biomolecule lycopene-reduced graphene oxide-silver nanoparticle enhances apoptotic potential of trichostatin A in human ovarian cancer cells (SKOV3). Int. J. Nanomed. 2017, 12, 7551–7575. [Google Scholar] [CrossRef]
- Yang, C.; Peng, S.; Sun, Y.M.; Miao, H.T.; Lyu, M.; Ma, S.J.; Luo, Y.; Xiong, R.; Xie, C.H.; Quan, H. Development of a hypoxic nanocomposite containing high-Z element as 5-fluorouracil carrier activated self-amplified chemoradiotherapy co-enhancement. Roy. Soc. Open Sci. 2019, 6. [Google Scholar] [CrossRef]
- Denkova, A.G.; de Kruijff, R.M.; Serra-Crespo, P. Nanocarrier-Mediated Photochemotherapy and Photoradiotherapy. Adv. Healthc. Mater. 2018, 7. [Google Scholar] [CrossRef]
- Shanmugam, V.; Selvakumar, S.; Yeh, C.S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem. Soc. Rev. 2014, 43, 6254–6287. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Dougherty, T.J. Basic principles of photodynamic therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.T.; Wu, F.; Yuan, P.; Chi, C.; Zhou, N.L. Magnetic and fluorescent carbon nanotubes for dual modal imaging and photothermal and chemo-therapy of cancer cells in living mice. Carbon 2017, 123, 70–83. [Google Scholar] [CrossRef]
- Chen, H.L.; Liu, Z.M.; Li, S.Y.; Su, C.K.; Qiu, X.J.; Zhong, H.Q.; Guo, Z.Y. Fabrication of Graphene and AuNP Core Polyaniline Shell Nanocomposites as Multifunctional Theranostic Platforms for SERS Real-time Monitoring and Chemo-photothermal Therapy. Theranostics 2016, 6, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Dong, J.; Zhang, T.; Peng, Q. Graphene-based nanomaterials and their potentials in advanced drug delivery and cancer therapy. J. Control. Release 2018, 286, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Fortner, J.D.; Lyon, D.Y.; Sayes, C.M.; Boyd, A.M.; Falkner, J.C.; Hotze, E.M.; Alemany, L.B.; Tao, Y.J.; Guo, W.; Ausman, K.D.; et al. C-60 in water: Nanocrystal formation and microbial response. Environ. Sci. Technol. 2005, 39, 4307–4316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, W.J.; Man, N.; Zheng, F.; Shen, Y.Y.; Sun, K.J.; Li, Y.; Wen, L.P. Autophagy-mediated chemosensitization in cancer cells by fullerene C60 nanocrystal. Autophagy 2009, 5, 1107–1117. [Google Scholar] [CrossRef]
- Wei, P.F.; Zhang, L.; Lu, Y.; Man, N.; Wen, L.P. C60(Nd) nanoparticles enhance chemotherapeutic susceptibility of cancer cells by modulation of autophagy. Nanotechnology 2010, 21. [Google Scholar] [CrossRef]
- Mocan, T.; Matea, C.T.; Cojocaru, I.; Ilie, I.; Tabaran, F.A.; Zaharie, F.; Iancu, C.; Bartos, D.; Mocan, L. Photothermal Treatment of Human Pancreatic Cancer Using PEGylated Multi-Walled Carbon Nanotubes Induces Apoptosis by Triggering Mitochondrial Membrane Depolarization Mechanism. J. Cancer 2014, 5, 679–688. [Google Scholar] [CrossRef]
- Burke, A.R.; Singh, R.N.; Carroll, D.L.; Wood, J.C.S.; D’Agostino, R.B.; Ajayan, P.M.; Torti, F.M.; Torti, S.V. The resistance of breast cancer stem cells to conventional hyperthermia and their sensitivity to nanoparticle-mediated photothermal therapy. Biomaterials 2012, 33, 2961–2970. [Google Scholar] [CrossRef]
- Suo, X.B.; Eldridge, B.N.; Zhang, H.; Mao, C.Q.; Min, Y.Z.; Sun, Y.; Singh, R.; Ming, X. P-Glycoprotein-Targeted Photothermal Therapy of Drug-Resistant Cancer Cells Using Antibody-Conjugated Carbon Nanotubes. ACS Appl. Mater. Inter. 2018, 10, 33464–33473. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, R.; Zhang, F.R.; Yin, Y.J.; Mei, L.X.; Song, F.J.; Tao, M.T.; Yue, W.Q.; Zhong, W.Y. Overcoming multidrug resistance by a combination of chemotherapy and photothermal therapy mediated by carbon nanohorns. J. Mater. Chem. B 2016, 4, 6043–6051. [Google Scholar] [CrossRef] [PubMed]
- Bhirde, A.A.; Chikkaveeraiah, B.V.; Srivatsan, A.; Niu, G.; Jin, A.J.; Kapoor, A.; Wang, Z.; Patel, S.; Patel, V.; Gorbach, A.M.; et al. Targeted Therapeutic Nanotubes Influence the Viscoelasticity of Cancer Cells to Overcome Drug Resistance. ACS Nano 2014, 8, 4177–4189. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.P.; Xu, C.; Li, M.; Zhang, H.L.; Wang, D.C.; Xia, M.; Meng, G.; Kang, B.; Chen, H.Y.; Wei, J.W. Photoacoustic “nanobombs” fight against undesirable vesicular compartmentalization of anticancer drugs. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Chiou, S.H.; Chou, C.P.; Chen, Y.C.; Huang, Y.J.; Peng, C.A. Photothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with CD133 monoclonal antibody. Nanomedicine 2011, 7, 69–79. [Google Scholar] [CrossRef]
- Zhou, F.F.; Wu, S.; Song, S.; Chen, W.R.; Resasco, D.E.; Xing, D. Antitumor immunologically modified carbon nanotubes for photothermal therapy. Biomaterials 2012, 33, 3235–3242. [Google Scholar] [CrossRef]
- Thapa, R.K.; Byeon, J.H.; Choi, H.G.; Yong, C.S.; Kim, J.O. PEGylated lipid bilayer-wrapped nanographene oxides for synergistic co-delivery of doxorubicin and rapamycin to prevent drug resistance in cancers. Nanotechnology 2017, 28. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, H.T.; Pham, T.T.; Choi, J.Y.; Choi, H.G.; Yong, C.S.; Kim, J.O. Development of a Graphene Oxide Nanocarrier for Dual-Drug Chemo-phototherapy to Overcome Drug Resistance in Cancer. ACS Appl. Mater. Inter. 2015, 7, 28647–28655. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.Q.; Liu, B.; Wu, H.M.; Kang, Y.J.; Li, M.; Zeng, X.; He, N.Y.; Zhang, G. The effects of multifunctional MiR-122-loaded graphene-gold composites on drug-resistant liver cancer. J. Nanobiotechnol. 2015, 13. [Google Scholar] [CrossRef]
- Hou, L.; Feng, Q.H.; Wang, Y.T.; Yang, X.M.; Ren, J.X.; Shi, Y.Y.; Shan, X.N.; Yuan, Y.J.; Wang, Y.C.; Zhang, Z.Z. Multifunctional hyaluronic acid modified graphene oxide loaded with mitoxantrone for overcoming drug resistance in cancer. Nanotechnology 2016, 27. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, Y.; Li, Y.; Wu, J.H.; Li, F.Y.; Ling, D.S.; Gao, J.Q. Reactive oxygen species and near-infrared light dual-responsive indocyanine green-loaded nanohybrids for overcoming tumour multidrug resistance. Eur. J. Pharm. Sci. 2019, 134, 185–193. [Google Scholar] [CrossRef]
- Wang, M.; Wu, J.H.; Li, Y.; Li, F.Y.; Hu, X.; Wang, G.; Han, M.; Ling, D.S.; Gao, J.Q. A tumor targeted near-infrared light-controlled nanocomposite to combat with multidrug resistance of cancer. J. Control. Release 2018, 288, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.Z.; Li, K.Y.; Shi, X.Z.; Gao, M.; Liu, J.; Liu, Z. Smart pH-Responsive Nanocarriers Based on Nano-Graphene Oxide for Combined Chemo- and Photothermal Therapy Overcoming Drug Resistance. Adv. Healthc. Mater. 2014, 3, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.P.; Yang, Z.Y.; Li, H.; Hao, Y.H.; Liu, C.; Zhu, L.; Liu, J.; Lu, B.H.; Li, R. Multifunctional Nanographene Oxide for Targeted Gene-Mediated Thermochemotherapy of Drug-resistant Tumour. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, C.R.; Gao, L.Q.; Yu, X.H.; Lai, J.H.; Lu, D.H.; Bao, R.; Wang, Y.P.; Jia, B.; Wang, F.; et al. Chemotherapy-Induced Macrophage Infiltration into Tumors Enhances Nanographene-Based Photodynamic Therapy. Cancer Res. 2017, 77, 6021–6032. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, A.; Sivaram, A.J.; Retnakumari, A.P.; Chandran, P.; Malarvizhi, G.L.; Nair, S.; Koyakutty, M. Radiofrequency Ablation of Drug-Resistant Cancer Cells Using Molecularly Targeted Carboxyl-Functionalized Biodegradable Graphene. Adv. Healthc. Mater. 2015, 4, 679–684. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, P.F.; Shu, Z.; Wu, M.Y.; Wang, L.Z.; Zhang, S.J.; Zheng, Y.Y.; Chen, H.R.; Wang, J.; Li, Y.P.; et al. Multifunctional Graphene Oxide-based Triple Stimuli-Responsive Nanotheranostics. Adv. Funct. Mater. 2014, 24, 4386–4396. [Google Scholar] [CrossRef]
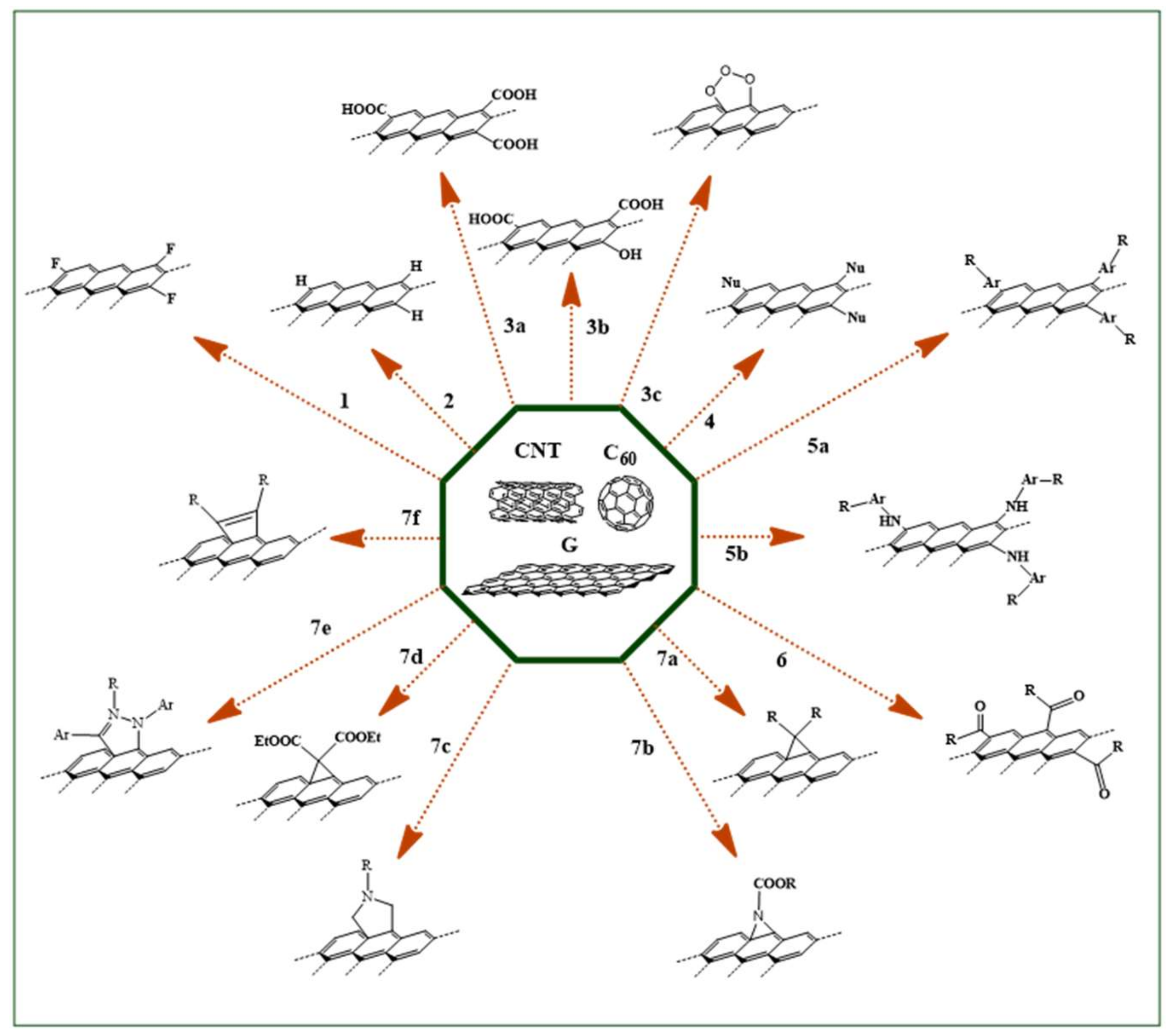

| Reaction | Ref | ||||
|---|---|---|---|---|---|
| N. | Type | Derivatizing Agents | CNT | G | C60 |
| 1 | Halogenation | F2 | [45] | [46] | [47] |
| 2 | Hydrogenation | H2 | [48] | [49] | [50] |
| 3 | Oxidation | a) HNO3/H2SO4 | [51] | [52] | [53] |
| b) H2O2 | [54] | [55] | [56] | ||
| c) O3 | [57] | [58] | [59] | ||
| 4 | Nucleophilic Addition | Nu− | [60] | [61] | [62] |
| 5 | Radical Coupling | a) R-Ar-N2+ | [63] | [64] | [65] |
| b) R-Ar-NH2 | [66] | [67] | [68] | ||
| 6 | Electrophilic Addition | RCOX | [69] | [70] | [71] |
| 7 | Cycloaddition | a) R2C: | [72] | [73] | [74] |
| b) N3-COOR | [75] | [76] | [77] | ||
| c) R-NHCH2COOH/(CH2O)n | [78] | [79] | [80] | ||
| d) EtOOCCH2COOEt | [81] | [82] | [83] | ||
| e) R-C=N-NH-Ar | [84] | [85] | [86] | ||
| f) -C=C(R)-C(R)=C- | [87] | [88] | [89] | ||
| Carrier | Delivery Properties | Cancer Model | Ref | |||||
|---|---|---|---|---|---|---|---|---|
| CN | Derivatizing Agent | Bioactive Agent | DL | Responsivity | Tissue | In Vitro | In Vivo | |
| oxMWCNT | PEG-NH2 Condensation | --- | --- | --- | Cervix | HeLa | --- | [127] |
| Liver | HepG2 | |||||||
| HepG2/R | ||||||||
| Blood | K562 | |||||||
| K562R | ||||||||
| oxSWCNT | --- | N-TAM-TEG Condensation | pH | Breast | MDA-MB-231/R | --- | [128] | |
| Q π-π Stacking | ||||||||
| MWCNT | TCM Coating | --- | --- | --- | Colon | Caco-2 | --- | [124] |
| GQD | --- | DOX π-π Stacking | --- | --- | Breast | MCF-7 | --- | [129] |
| MCF-7/ADR | ||||||||
| Liver | SMMC-7721 | |||||||
| Colon | Caco-2 | |||||||
| Blood | HL-60 | |||||||
| SWCNT/oxSWCNT/MWCNT | --- | --- | --- | --- | Bone | MNNG/HOS | MNNG/HOS | [130] |
| Carrier | Delivery Properties | Cancer Model | Ref | |||||
|---|---|---|---|---|---|---|---|---|
| CN | Derivatizing Agent | Bioactive Agent | DL | Responsivity | Tissue | In Vitro | In Vivo | |
| SWCNT | TCM π-π Staking | ETP* | 11-88§ | --- | Pancreas | PANC-1 | --- | [142] |
| oxMWCNT | --- | VER π-π Stacking DOXπ-π Stacking | 149 164 | --- | Blood | K562/A02/R | --- | [143] |
| SWCNT | --- | CpG | --- | --- | Brain | K-Luc | --- | [144] |
| GL261 | ||||||||
| Ovary | OVCAR8 | |||||||
| Cervix | HeLa | |||||||
| GO | --- | CDDP π-π Staking | 400 | --- | Ovary | SCOV-3 | --- | [145] |
| Cervix | HeLa | |||||||
| Prostate | Tramp-C1 | |||||||
| Lung | A549 | |||||||
| Colon | CT26 | |||||||
| GO | ASO Hybridization | DOX π-π Staking | 35.25 | --- | Breast | MCF-7/ADR | MCF-7/ADR | [146] |
| usSWCNT | --- | CDDP Filling | 6.4 | --- | Breast | MCF-7 | --- | [147] |
| PF108 π-π Staking | MDA-MB-231 | |||||||
| usSWCNT | --- | CDDP Filling | 6.4 | --- | Breast | --- | MCF-7 | [148] |
| PF108 π-π Staking | MDA-MB-231 | |||||||
| oxMWCNT | --- | Pt(IV) Filling | 37 | --- | Cervix | HeLa | --- | [149] |
| GQD | --- | CDDP π-π Staking | 0-50 | --- | Liver | SMMC-7721 | --- | [150] |
| Cervix | HeLa | |||||||
| Lung | A549 | |||||||
| Breast | MCF-7 | |||||||
| Stomach | MGC-803 | |||||||
| GQD | --- | CDDP Condensation | --- | pH | Os | HSC3 | HSC3 | [151] |
| PEG-NH2 Condensation | SCC4 | --- | ||||||
| CAL-27 | --- | |||||||
| GQD | ---- | DOX π-π Staking | 10 | pH | Breast | MCF-7 | --- | [152] |
| MCF-7/ADR | ||||||||
| Stomach | MGC-803 | |||||||
| GO | --- | DOX π-π Staking | 47 | pH | Breast | MCF-7 | --- | [153] |
| MCF-7/ADR | ||||||||
| GO | HDex π-π Staking | DOX π-π Staking | 350 | pH | Breast | MCF-7/ADR | --- | [154] |
| GO | HEC/PAC π-π Staking | DOX π-π Staking | 49 | pH | Ovary | SCOV-3 | --- | [155] |
| SCOV-3/DDP | ||||||||
| SWCNT | DISPE-PEG π-π Staking | PTX/C6/QD π-π Stacking | 14.3 | Magnetic | Pancreas | PANC-1 | --- | [156] |
| MIA PaCa-2 | ||||||||
| L3.6 | ||||||||
| oxSWCNT | DISPE-HA π-π Staking | ERU π-π Stacking | 45 | pH | Lung | A549 | --- | [157] |
| A549/TXR | ||||||||
| GO | PEI/PSS π-π Staking | DOX π-π Staking Anti-miR-21 | --- | --- | Breast | MCF-7 | --- | [158] |
| MCF-7/ADR | ||||||||
| Carrier | Delivery Properties | Cancer Model | Ref | |||||
|---|---|---|---|---|---|---|---|---|
| CN | Derivatizing Agent | Bioactive Agent | DL | Responsivity | Tissue | In Vitro | In Vivo | |
| oxMWCNT | --- | DOX Condensation | 112 | pH | Lung | A549 | --- | [161] |
| PEG π-π Stacking | DOX π-π Stacking | 31.4 | Breast | MDA-MB-231 | ||||
| oxMWCNT | RB1 Condensation | --- | 25 | --- | Breast | MCF-7 | --- | [162] |
| RG1 Condensation | --- | Pancreas | PANC-1 | |||||
| oxMWCNT | PEG-NH2 Condensation | RuPOP π-π Stacking | 9.8 | pH X-ray | Liver | HepG2 | --- | [163] |
| R-HepG2 | ||||||||
| oxSWCNT | PSE- PEG-NH2 Condensation | TRAIL Condensation | 61 | --- | Liver | HepG2 | --- | [164] |
| Colon | HCT116 | |||||||
| Lung | H1703 | |||||||
| GO | NH2-PEG-N3 Condensation | DOX π-π Stacking TRAIL Condensation | 78 8 | pH | Lung | A549 | A549 | [165] |
| Colon | LoVo | --- | ||||||
| GO | H2N-PEG-PEI Condensation | CER Ionic SRB* | --- | --- | Liver | HepG2 | --- | [166] |
| HuH7 | HuH7 | |||||||
| HuH7-SR | ||||||||
| HepG2 | --- | |||||||
| oxSWCNT | PEG-HBA/PEG-CD44 Ab Condensation | PTX Condensation | 180 | pH | Breast | MDA-MB-231 | MDA-MB-231 | [167] |
| SAL Condensation | 170 | |||||||
| oxSWCNT | CS-FA Condensation | O2 Complexation | --- | --- | Breast | MDA-MB-231 | --- | [168] |
| 5-FU* ERU* PRU* PTX* CBPT* | 3.3§ 20§ 125§ 21§ 10§ | |||||||
| ZR-75-1 | ||||||||
| GQD | HA-PEG-NH2 Condensation | DOX π-π Stacking | 30 | pH | Lung | A549 | --- | [169] |
| GQD | HA-HSA NPs Condensation | GEM π-π Stacking | 16 | --- | Pancreas | Panc-1 | --- | [170] |
| rGO | DEX-CT Redox coupling | DOX π-π Stacking | 20 | pH | Neural Crest | BE(2)C | --- | [171] |
| BE(2)C/ ADR | ||||||||
| oxSWCNT | P-gp Ab Condensation | DOX π-π Stacking | 20 | NIR | Blood | K562 | --- | [172] |
| K562R | ||||||||
| oxMWCNT | P-gp Ab Condensation | DOX π-π Stacking GA π-π Stacking | 39.4 30.3 | pH | Blood | K562/A02/R | K562/A02/R | [173] |
| oxSWCNT | CD133 Ab Condensation | CDDP π-π Stacking/Condensation | 66 | --- | Skin | B16-F10 | B16-F10 | [174] |
| Pt(IV) π-π Stacking/Condensation | 66 | |||||||
| GO | FA-PAMAM-DTPA Condensation | DOXnπ-π Stacking COLCnπ-π Stacking | 154 154 | pH | Liver | HepG2 | HepG2 | [175] |
| GO | PAMAM Condensation | DOX π-π Stacking sRNA Hybridization | 28.6 5 | pH | Breast | MCF-7 | --- | [176] |
| GO | FA-CO Condensation | DOX π-π Stacking sRNA Hybridization | 56 | pH | Breast | MCF-7 | --- | [177] |
| MCF-7/ ADR | ||||||||
| Lung | A549 | |||||||
| C60 | Br-C- (COOEt)2 Bingel | CDDP* | Prostate | PC-3 | PC-3R | [178] | ||
| Carrier | Delivery Properties | Cancer Model | Ref | |||||
|---|---|---|---|---|---|---|---|---|
| CN | Derivatizing Agent | Bioactive Agent | DL | Responsivity | Tissue | In Vitro | In Vivo | |
| MWCNT | TCM π-π Stacking | --- | --- | --- | Skin | B16-F10 | B16-F10 | [188] |
| MWCNT | 5-FU π-π Stacking | 3 | --- | Skin | --- | B16-F10 | [189] | |
| Cervix | HeLa | --- | ||||||
| GO | NH3 Oxidation | --- | --- | pH | Cervix | HeLa | HeLa | [190] |
| rGO | Ag NPs Redox Coupling | TSA π-π Stacking | 100 | --- | Ovary | SKOV-3 | --- | [191] |
| GO | PEG Condensation FePt MNPs π-π Stacking | MI π-π Stacking 5-FU π-π Stacking | 12.3 9.5 | O2 | Lung | A549 | --- | [192] |
| H1975 | ||||||||
| Carrier | Delivery Properties | Cancer Model | Ref | |||||
|---|---|---|---|---|---|---|---|---|
| CN | Derivatizing Agent | Bioactive Agent | DL | Responsivity | Tissue | In Vitro | In Vivo | |
| nC60 | --- | DOX* | --- | --- | Cervix | HeLa | --- | [200] |
| Breast | MCF-7/ADR | |||||||
| nC60 | Nd Encapsulation | --- | --- | --- | Cervix | HeLa | --- | [201] |
| H1975 | ||||||||
| MWCNT | PEG π-π Stacking | --- | --- | NIR | Pancreas | PANC1 | --- | [202] |
| oxMWCNT | H2NC2H4NH2 Amidation DISPE-PEG π-π Stacking | PTX* | --- | NIR | Breast | HMLER | --- | [203] |
| SAL* | HMLER CSC | HMLER CSC | ||||||
| 17DMAG* | ||||||||
| oxSWCNT | DISPE-PEG/P-gp Ab π-π Stacking | --- | --- | --- | Fibroblast | 3T3-MDR1 | --- | [204] |
| Ovary | NCI/ADR | |||||||
| oxCNH | PEG/P-gp Ab π-π Stacking | ETP Filling | --- | NIR | Lung | A549 | [205] | |
| A549R | A549R | |||||||
| SWCNT | CA-HA π-π Stacking | DOX π-π Stacking | 300 | --- | Ovary | OVCAR8 | --- | [206] |
| OVCAR8/ADR | OVCAR8/ADR | |||||||
| oxSWCNT | CS-FA π-π Stacking | DOX π-π Stacking | 33.3 | NIR | Lung | A549 | A549 | [207] |
| SWCNT | CS-CD133 Ab π-π Stacking | --- | --- | NIR | Brain | GMB-CD133+ | GMB-CD133+ | [208] |
| GMB-CD133- | GMB-CD133- | |||||||
| SWCNT | GCS π-π Stacking | --- | --- | NIR | Breast | EMT6 | EMT6 | [209] |
| GO | PEGylated Liposome Encapsulation | DOX π-π Stacking RAPA π-π Stacking | 10 10 | pH | Breast | MCF-7 | --- | [210] |
| MDA-MB-231 | ||||||||
| BT4T4 | ||||||||
| GO | PF 68 | DOX π-π Stacking IRI π-π Stacking | 7 7 | pH | Breast | MCF-7 | --- | [211] |
| MDA-MB-231 | ||||||||
| Head/ Neck | SCC-7 | |||||||
| Carrier | Delivery Properties | Cancer Model | Ref | |||||
|---|---|---|---|---|---|---|---|---|
| CN | Derivatizing Agent | Bioactive Agent | DL | Responsivity | Tissue | In Vitro | In Vivo | |
| GO | P-gp Ab Condensation FA-Au NPs π-π Stacking | MiR-122 Hybridization | --- | --- | Liver | Hep-G2/ADR | Hep-G2/ADR | [212] |
| GO | HA Condensation PF 68 π-π Stacking | MIT π-π Stacking | 3 | pH | Breast | MCF-7 | MCF-7 | [213] |
| MCF-7/ADR | MCF-7/ADR | |||||||
| GO | PF 68-PAMAM Diselenide | ICG π-π Stacking | 52.1 | ROS | Breast | MCF-7 | --- | [214] |
| MCF-7/ADR | ||||||||
| GO | PF 68-PAMAM Diselenide | ICG π-π Stacking | 52.1 | ROS | Breast | MCF-7 | --- | [215] |
| MCF-7/ADR | MCF-7/ADR | |||||||
| GO | PEG-PAH Condensation | DOX π-π Stacking | 50 | pH | Breast | MCF-7 | --- | [216] |
| MCF-7/ADR | ||||||||
| GO | FA-PEG-PEI Condensation | DOX π-π Stacking sRNA Hybridization | --- | pH | Breast | MCF-7 | --- | [217] |
| MCF-7/ADR | ||||||||
| GO | PEG-NH2 Condensation HPPH π-π Stacking | CTX π-π Stacking | 1 | NIR | Breast | 4T1 | 4T1 | [218] |
| DOX π-π Stacking | ||||||||
| DTX π-π Stacking | ||||||||
| 5-FU π-π Stacking | ||||||||
| GO | TRF Condensation | --- | --- | --- | Blood | K562 | --- | [219] |
| K562R | ||||||||
| GO | Fe3O4/MnOx Redox Coupling | DOX π-π Stacking | 38 | pH Redox Magnetic | Breast | MDA-MB-231 | --- | [220] |
| MCF-7/ADR | ||||||||
| CNs | Deriv | Ref | Total Studies | Drug | Cancer Model | Direct MDR Reversal | Enhanced Drug Efficiency | Reduced Side Effects |
|---|---|---|---|---|---|---|---|---|
| Studies (%) | Success (%) | |||||||
| F | --- | [178] [200] [201] | 4 | None (25) DOX (50) CDDP (25) | Cervix (50) Prostate (25) Breast (25) | 75 * 25 # | 75 * 25 # | 0 * 0 # |
| CNT | --- | [124] [128] [142] [144] [147] [148] [188] [189] | 11 | None (46) 5-FU (18) CDDP (18) TAM (9) ETP (9) | Breast (28) Cervix (18) Skin (18) Brain (9) Ovary (9) Colon (9) Pancreas (9) | 55 * 18 # | 36 * 18 # | 0 * 9 # |
| CNT | Ox | [130] [143] [149] [161] [162] [164] [172] [173] [174] | 16 | None (6) DOX (30) TRAIL (19) RB1 (13) RG1 (13) Pt(IV) (13) CDDP (6) | Breast (18.5) Blood (18.5) Lung (13) Pancreas (13) Skin (13) Cervix (6) Bone (6) Liver (6) Colon (6) | 50 * 25 # | 94 * 19 # | 31 * 0 # |
| GO | --- | [129] [145] [146] [150] [151] [152] [153] [190] [191] [212] [220] | 23 | None (22) CDDP (48) DOX (26) TSA (4) | Breast (26) Cervix (13) Liver (13) Ovary (9) Lung (9) Stomach (9) Colon (9) Blood (4) Prostate (4) Os (4) | 12 * 4 # | 18 * 2 # | 10 * 1 # |
| CNT | PEG | [127] [161] [163] [167] [202] | 9 | None (45) DOX (22) PTX (11) SAL (11) RuPOP (11) | Breast (34) Liver (23) Cervix (11) Blood (11) Pancreas (11) Lung (11) | 56 * 0 # | 56 * 22 # | 33 * 11 # |
| CNH | PEG | [205] | 1 | ETP (100) | Lung (100) | 100 * 100 # | 100 * 100 # | 0 * 0 # |
| GO | PEG | [151] [165] [169] [170] [192] [210] [216] [218] | 12 | DOX (50) 5-FU (17) CDDP (8) GEM (8) CTX (8) DTX (8) | Breast (50) Lung (25) Os (8) Colon (8) Pancreas (8 | 75 * 42 # | 92 * 42 # | 42 * 0 # |
| CNT | Surf | [147] [148] [156] [157] [203] [204] | 9 | None (22) CDDP (22) PTX (22) SAL (11) 17DMAG (11) ERU (11) | Breast (56) Fibroblast (11) Ovary (11) Pancreas (11) Lung (11) | 78 * 33 # | 67 * 44 # | 56 * 0 # |
| GO | Surf | [211] [213] | 3 | DOX (67) MIT (33) | Breast (67) Head and Neck (33) | 33 * 33 # | 100 * 33 # | 0 * 0 # |
| GO | PEI | [158] [166] [217] | 3 | DOX (67) SRB (33) | Breast (67) Liver (33 | 100 * 33 # | 100 * 33 # | 0 * 0 # |
| GO | Dend | [175] [176] [214] [215] | 4 | DOX (50) ICG (50) | Breast (75) Liver (25) | 100 * 50 # | 100 * 50 # | 25 * 25 # |
| CNT | PS | [168] [206] [207] [208] [209] | 9 | None (22) DOX (22) 5-FU (11) ERU (11) PRU (11) PTX (11) CBPT (11) | Breast (67) Brain (11) Ovary (11) Lung (11) | 33 * 33 # | 78 * 22 # | 11 * 0 # |
| GO | PS | [154] [155] [171] [177] | 5 | DOX (100) | Breast (40) Ovary (20) Lung (20) Neural Crest (20) | 80 * 0 # | 100 * 0 # | 20 * 0 # |
| GO | PR | [219] | 1 | None (100) | Blood (100) | 100 * 100 # | 0 * 0 # | 100 * 0 # |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curcio, M.; Farfalla, A.; Saletta, F.; Valli, E.; Pantuso, E.; Nicoletta, F.P.; Iemma, F.; Vittorio, O.; Cirillo, G. Functionalized Carbon Nanostructures Versus Drug Resistance: Promising Scenarios in Cancer Treatment. Molecules 2020, 25, 2102. https://doi.org/10.3390/molecules25092102
Curcio M, Farfalla A, Saletta F, Valli E, Pantuso E, Nicoletta FP, Iemma F, Vittorio O, Cirillo G. Functionalized Carbon Nanostructures Versus Drug Resistance: Promising Scenarios in Cancer Treatment. Molecules. 2020; 25(9):2102. https://doi.org/10.3390/molecules25092102
Chicago/Turabian StyleCurcio, Manuela, Annafranca Farfalla, Federica Saletta, Emanuele Valli, Elvira Pantuso, Fiore Pasquale Nicoletta, Francesca Iemma, Orazio Vittorio, and Giuseppe Cirillo. 2020. "Functionalized Carbon Nanostructures Versus Drug Resistance: Promising Scenarios in Cancer Treatment" Molecules 25, no. 9: 2102. https://doi.org/10.3390/molecules25092102
APA StyleCurcio, M., Farfalla, A., Saletta, F., Valli, E., Pantuso, E., Nicoletta, F. P., Iemma, F., Vittorio, O., & Cirillo, G. (2020). Functionalized Carbon Nanostructures Versus Drug Resistance: Promising Scenarios in Cancer Treatment. Molecules, 25(9), 2102. https://doi.org/10.3390/molecules25092102









