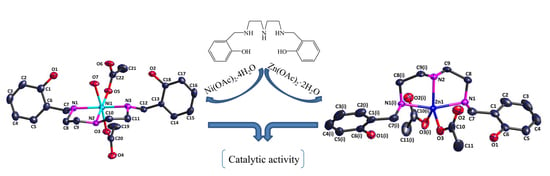
Synthesis and Catalytic Application of Two Mononuclear Complexes Bearing Diethylenetriamine Derivative Ligand
Abstract
1. Introduction
2. Results and Discussion
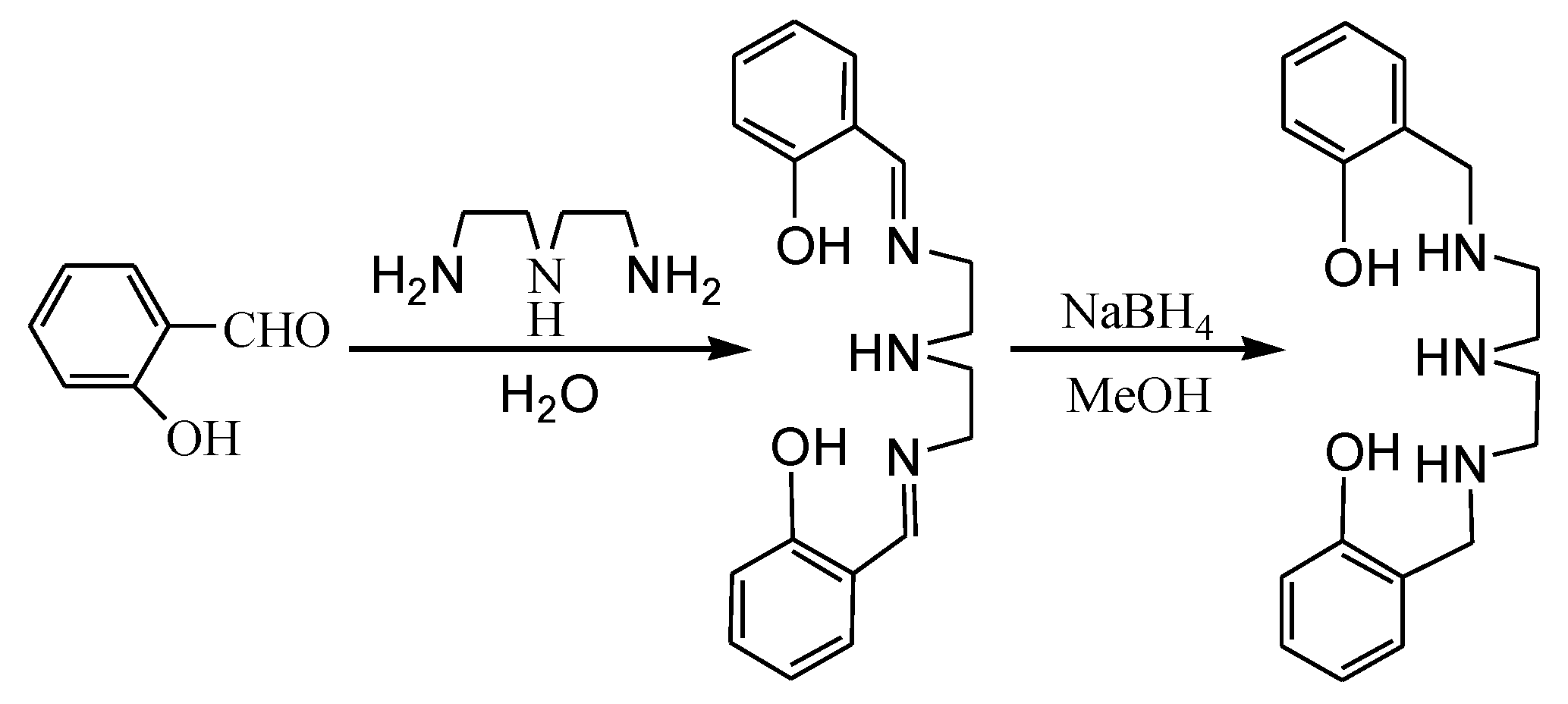
2.1. Synthesis and IR Spectra
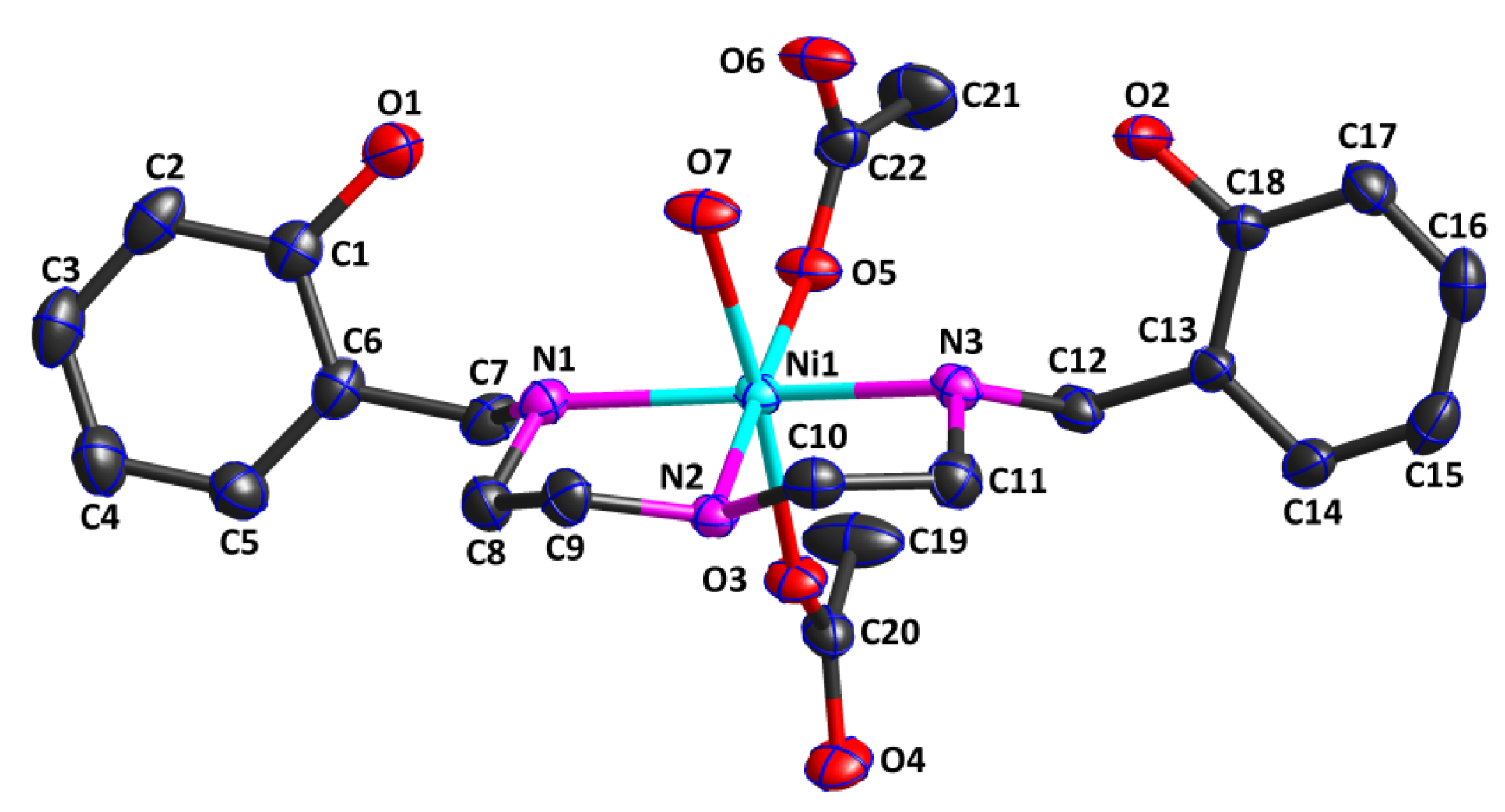
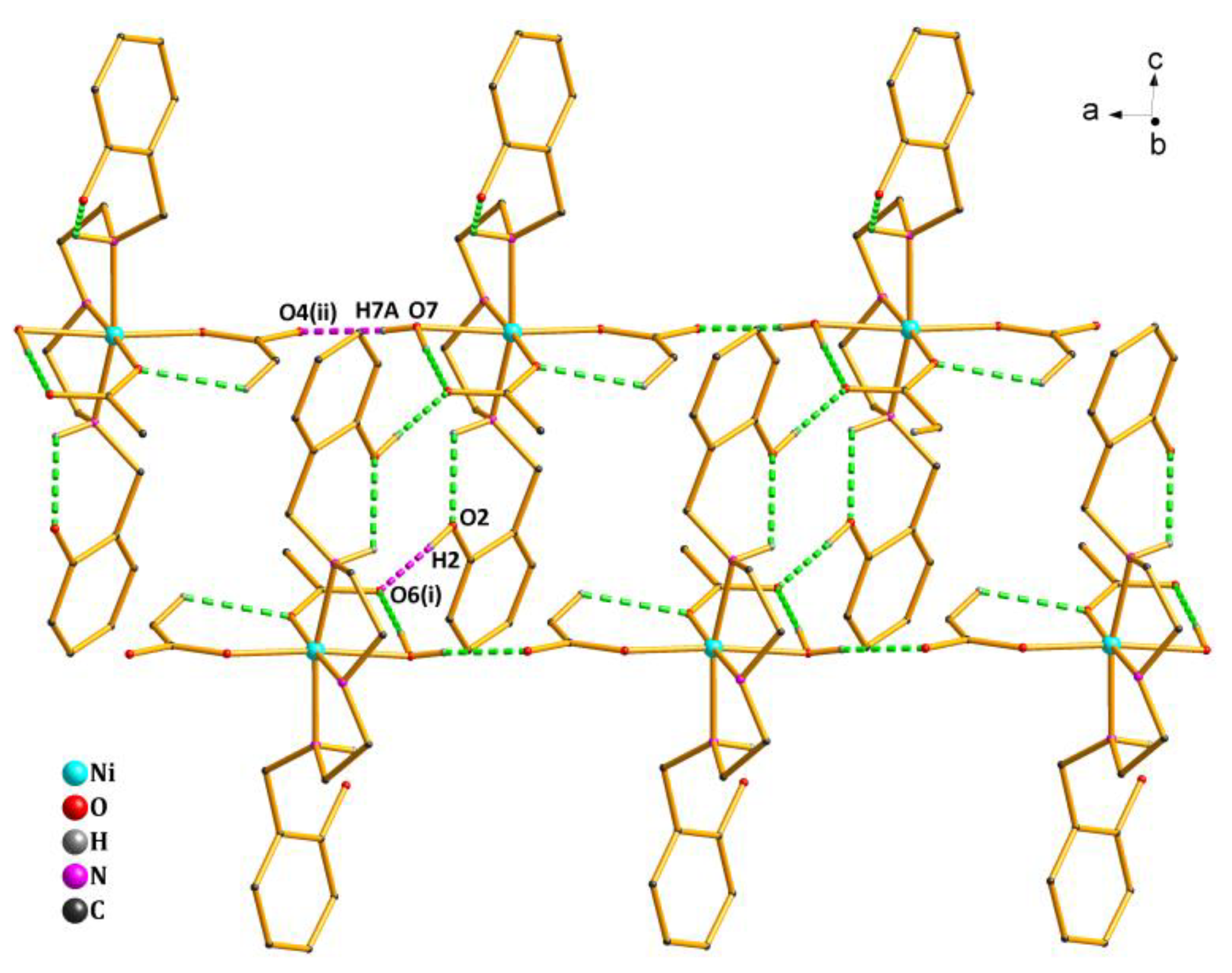
2.2. Structure Description of Complex 1
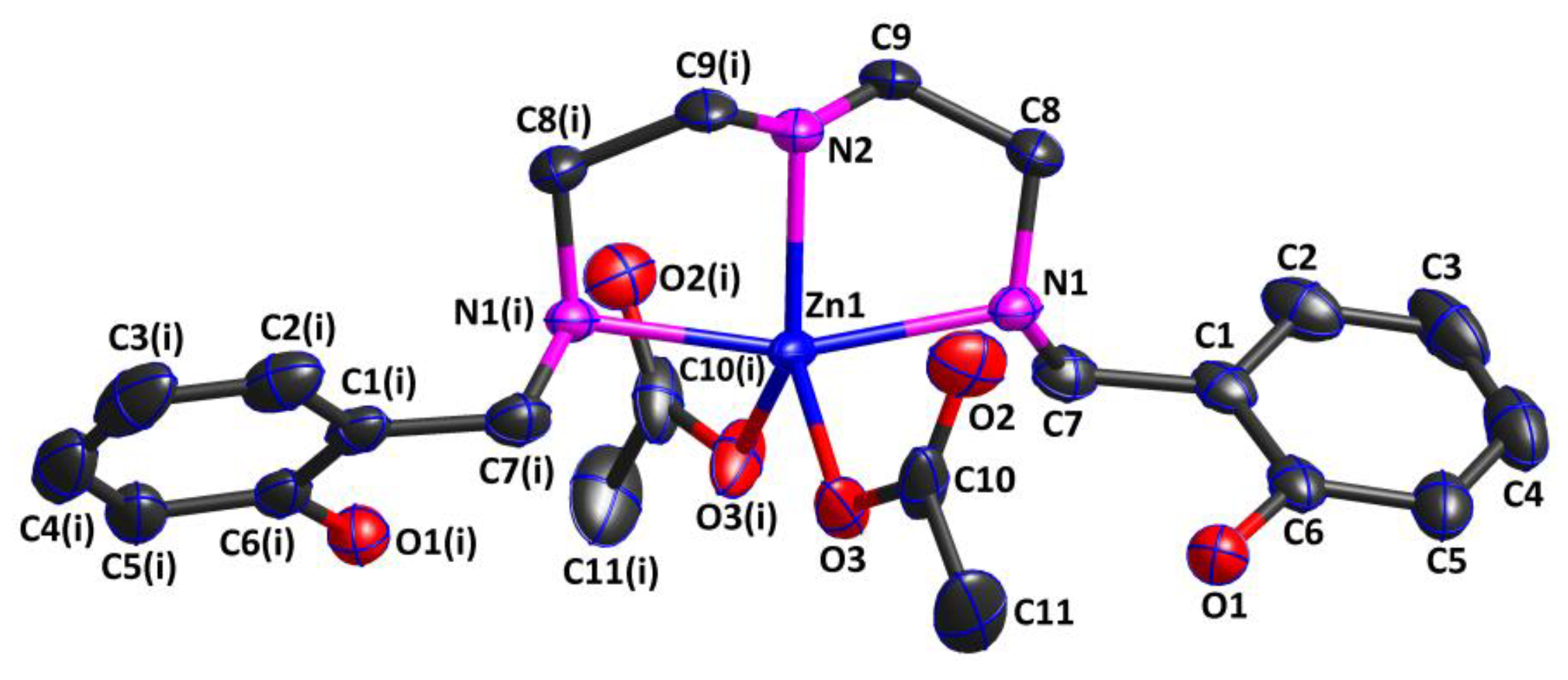
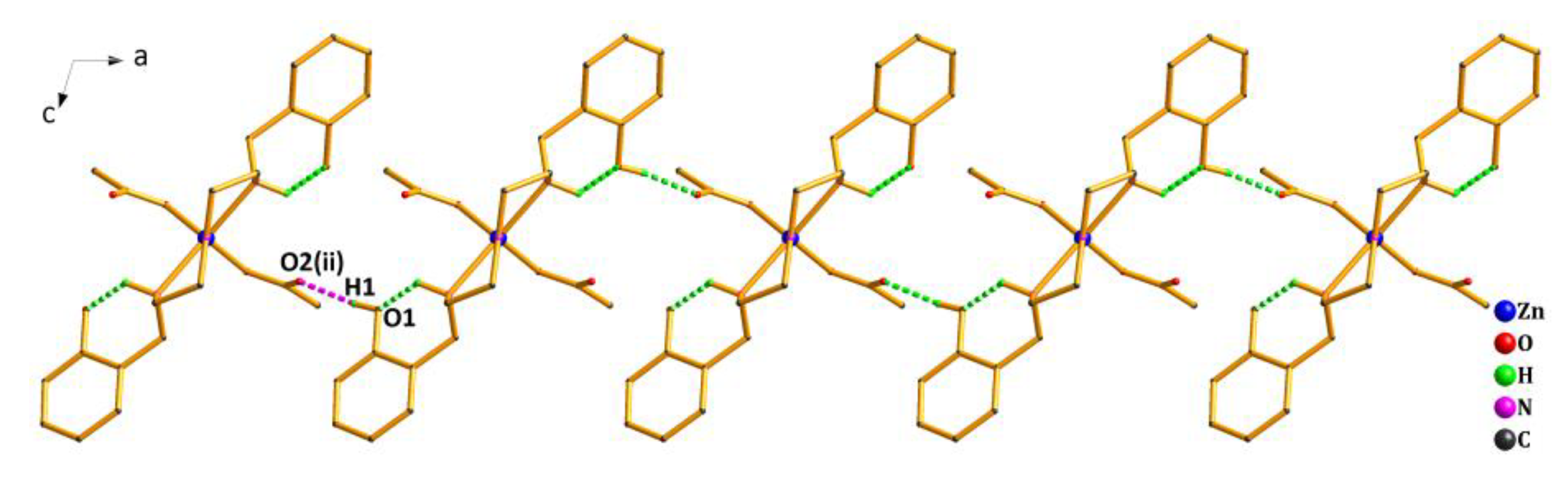
2.3. Structure Description of Complex 2
2.4. PXRD of Complexes 1 and 2
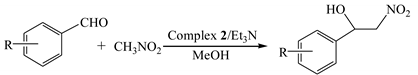
2.5. Catalytic Application on the Henry Reaction
2.5.1. Optimization of Catalytic Reaction Conditions
2.5.2. Expansion of the Substrate Scope on the Henry Reaction
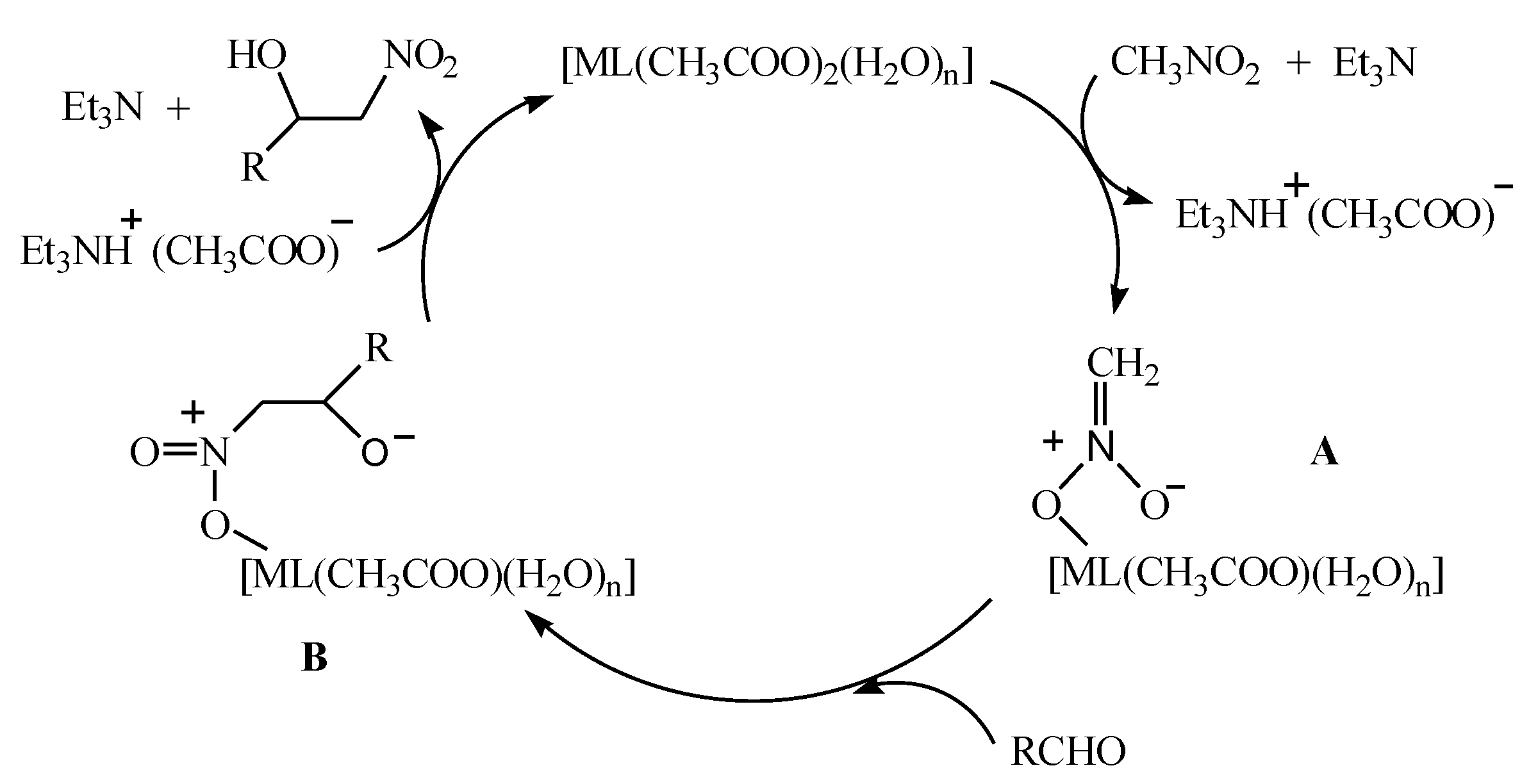
2.5.3. Mechanistic Investigation of the Henry Reaction
3. Materials and Methods
3.1. General
3.2. Synthesis
3.2.1. Synthesis of the Ligand
3.2.2. Synthesis of [NiL(CH3COO)2(H2O)] (1)
3.2.3. Synthesis of [ZnL(CH3COO)2] (2)
3.3. Single-Crystal X-ray Diffraction
3.4. General Procedure for the Henry Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rodríguez, J.M.; Pujol, M.D. Straightforward synthesis of nitroolefins by microwave- or ultrasound-assisted Henry reaction. Tetrahedron Lett. 2011, 52, 2629–2632. [Google Scholar] [CrossRef]
- Tseberlidis, G.; Dell’Acqua, M.; Valcarenghi, D.; Gallo, E. Silver comes into play: Henry reaction and domino cycloisomerisation sequence catalysed by [Ag(i)(Pc-L)] complexes. RSC Adv. 2016, 6, 97404–97419. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Z.-H.; Chen, H.-B. Efficient synthesis of chiral benzofuryl β-amino alcohols via a catalytic asymmetric Henry reaction. Org. Biomol. Chem. 2017, 15, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Gorczynski, M.J.; Huang, J.; Lee, H.; King, S.B. Evaluation of nitroalkenes as nitric oxide donors. Bioorg. Med. Chem. Lett. 2007, 17, 2013–2017. [Google Scholar] [CrossRef]
- Tarek, E.; Lyle, C. Chromate oxidation of α-nitro alcohols to α-nitro ketones: Significant improvements to a classic method. Molecules 2005, 10, 1458–1461. [Google Scholar] [CrossRef]
- Luzzio, F.A. The Henry reaction: Recent examples. Tetrahedron 2001, 57, 915–945. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Wu, X.-Y.; Han, J.-W. Chiral iminophosphorane catalyzed asymmetric Henry reaction of α,β-alkynyl ketoesters. Chin. Chem. Lett. 2019, 30, 1519–1522. [Google Scholar] [CrossRef]
- Kumpulainen, T.; Qian, J.; Brouwer, A.-M. Spectroscopic study of a cinchona alkaloid-catalyzed Henry reaction. ACS Omega 2018, 3, 1871–1880. [Google Scholar] [CrossRef]
- Shinde, P.S.; Shinde, S.S.; Dake, S.A.; Sonekar, V.S.; Deshmukh, S.U.; Thorat, V.V.; Andurkar, N.M.; Pawar, R.P. CsF/[bmim][BF4]: An efficient and reusable system for Henry reaction. Arab. J. Chem. 2014, 7, 1013–1016. [Google Scholar] [CrossRef]
- Yadav, L.D.S.; Rai, A. The first ionic liquid-promoted three-component coupling strategy for an expeditious synthesis of β-nitrocarbonitriles/thiocyanates. Tetrahedron Lett. 2009, 50, 640–643. [Google Scholar] [CrossRef]
- Markad, D.; Mandal, S.K. Synthesis and structural characterization of a novel dinuclear Cu(II) complex: An efficient and recyclable bifunctional heterogeneous catalyst for the diastereoselective Henry reaction. Dalton Trans. 2018, 47, 5928–5932. [Google Scholar] [CrossRef] [PubMed]
- Drabina, P.; Horáková, E.; Růžičková, Z.; Sedlák, M. Henry reaction catalyzed by new series of imidazolidine-4-one Cu-complexes. Tetrahedron Asymmetry 2015, 26, 141–147. [Google Scholar] [CrossRef]
- Das, A.; Choudhary, M.K.; Kureshy, R.I.; Jana, K.; Verma, S.; Khan, N.H.; Abdi, S.H.R.; Bajaj, H.C.; Ganguly, B. Asymmetric Henry reaction of trifluoromethyl ketone and aldehyde using Cu(II)-complex: Computational study offers the origin of enantioselectivity with varied size of catalysts. Tetrahedron 2015, 71, 5229–5237. [Google Scholar] [CrossRef]
- Wei, Y.-L.; Yang, K.-F.; Li, F.; Zheng, Z.-J. Probing the evolution of an Ar-BINMOL-derived salen–Co(III) complex for asymmetric Henry reactions of aromatic aldehydes: Salan–Cu(II) versus salen–Co(III) catalysis. RSC Adv. 2015, 46, 37859–37867. [Google Scholar] [CrossRef]
- Ouyang, G.-H.; He, Y.-M.; Fan, Q.-H. Podand-based dimeric chromium(III)–salen complex for asymmetric Henry Reaction: Cooperative catalysis promoted by complexation of alkali metal ions. Chemistry 2015, 46, 16454–16457. [Google Scholar] [CrossRef]
- Wang, J.-K.; Lu, Z.-J.; Wang, L.-Y.; Bai, J.-F. The Henry reaction efficiently catalyzed by a Cd-proline complex. Lett. Org. Chem. 2008, 5, 286–289. [Google Scholar] [CrossRef]
- Gogoi, N.; Boruwa, J.; Barua, N.C. A total synthesis of (-)-bestatin using shibasaki′s asymmetric Henry reaction. Tetrahedron Lett. 2006, 46, 7581–7582. [Google Scholar] [CrossRef]
- Burks, D.B.; Davis, S.; Lamb, R.W.; Liu, X.; Rodrigues, R.R.; Liyanage, N.P.; Sun, Y.; Webster, C.E.; Delcamp, J.H.; Papish, E.T. Nickel(II) pincer complexes demonstrate that the remote substituent controls catalytic carbon dioxide reduction. Chem. Commun. 2018, 54, 3819–3822. [Google Scholar] [CrossRef]
- Asano, E.; Hatayama, Y.; Kurisu, N.; Ohtani, A.; Hashimoto, T.; Kurihara, Y.; Ueda, K.; Ishihara, S.; Nagao, H.; Yamaguchi, Y. Acetylacetonato-based pincer-type nickel(II) complexes: Synthesis and catalysis in cross-couplings of aryl chlorides with aryl Grignard reagents. Dalton Trans. 2018, 47, 8003–8012. [Google Scholar] [CrossRef]
- Mantri, S.; Routaray, A.; Nath, N.; Sutar, A.K.; Maharana, T. Synthesis, characterization and catalytic activity of zinc complex for ring-opening polymerization of lactide. Polym. Int. 2017, 66, 313–319. [Google Scholar] [CrossRef]
- Balinge, K.R.; Khiratkar, A.G.; Muskawar, P.N.; Thenmozhi, K.; Bhagat, P.R. Facile access to polymer supported zinc-salen complex: Highly efficient heterogeneous catalyst for synthesizing hydantoins, thiohydantoins and Schiff bases in aqueous medium. Res. Chem. Intermed. 2017, 44, 2075–2097. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Mac Leod, T.C.; Mahmudov, K.T.; da Silva, M.F.C.G.; Pombeiro, A.J. Zinc(II) ortho-hydroxyphenylhydrazo-β-diketonate complexes and their catalytic ability towards diastereoselective nitroaldol (Henry) reaction. Dalton Trans. 2011, 40, 5352–5361. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhang, Z.J.; Wang, L.; Zhang, J.C. Synthesis and catalytic activity of nickel(Ⅱ)and cobalt(Ⅱ) complexes involving chiral leucinol. Chin. J. Struct. Chem. 2015, 34, 1851–1856. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.-A.; Xu, Y.-G.; Wang, Z.-Y. Recent progress in copper catalyzed asymmetric Henry reaction. Chin. Chem. Lett. 2018, 29, 873–883. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.-J.; Zhang, L.-L.; Jiang, K.; Zhang, L. Two coordination compounds bearing bis(2-dimethylaminoethyl) ether: Syntheses, crystal structures and catalytic application to the Henry reaction. Chem. Res. Chin. Univ. 2018, 34, 358–362. [Google Scholar] [CrossRef]
- Luo, M.; Tang, H.-M.; Li, Q.-R.; Sun, J.; Yang, S.-Z.; Li, X.-L. The synthesis of N-Zn, N-Cu complexes involving 2-amino pyridine and ethylenediamine ligands and application to the Henry reaction. J. Chem. Sci. 2009, 121, 435–440. [Google Scholar] [CrossRef][Green Version]
- Venkateswarlu, K.; Ganji, N.; Daravath, S.; Kanneboina, K.; Rangan, K. Crystal structure, DNA interactions, antioxidant and antitumor activity of thermally stable Cu(II), Ni(II) and Co(III) complexes of an N,O donor Schiff base ligand. Polyhedron 2019, 171, 86–97. [Google Scholar] [CrossRef]
- Guan, L.; Wang, Y.; Lu, S.; Zhang, Z.-J.; Fan, W.-T.; Gao, W. Synthesis, crystal structure and properties of nickel complex containing pyridine [Ni(py)3(H2O)3](1,5-nds). Chin. J. Inorg. Chem. 2013, 29, 2079–2084. [Google Scholar] [CrossRef]
- Xie, Y.-S.; Xue, Y.; Kou, F.-P.; Lin, R.-S.; Liu, Q.-L. Solution study and crystal structure of a pentacoordinate Zinc(II) complex with N,N″-bis-(2-hydroxybenzyl)-diethylenetriamine. J. Coord. Chem. 2001, 53, 91–97. [Google Scholar] [CrossRef]
- Munzeiwa, W.A.; Nyamori, V.O.; Omondi, B. N,O-Amino-phenolate Mg(II) and Zn(II) Schiff base complexes: Synthesis and application in ring-opening polymerization of ε-caprolactone and lactides. Inorg. Chim. Acta 2019, 487, 264–274. [Google Scholar] [CrossRef]
- Qian, B.-B.; Chang, Z.; Bu, X.-H. Zinc-coordination polymers based on a donor-acceptor mix-ligand system: Syntheses, crystal structures and photophysical properties. Chem. Res. Chin. Univ. 2020, 36, 74–80. [Google Scholar] [CrossRef]
- Ginotra, S.K.; Singh, V.K. Enantioselective Henry reaction catalyzed by a C2-symmetric bis(oxazoline)–Cu(OAc)2·H2O complex. Org. Biomol. Chem. 2007, 5, 3932–3937. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Gou, S.-H.; Li, L. Asymmetric Henry reactions of aldehydes with various nitroalkanes catalyzed by copper(II) complexes of novel chiral N-monoalkyl cyclohexane-1,2-diamines. Appl. Organometal. Chem. 2014, 28, 186–193. [Google Scholar] [CrossRef]
- Guo, J.; Mao, J.C. Asymmetric Henry reaction catalyzed by bifunctional copper-based catalysts. Chirality 2009, 21, 619–627. [Google Scholar] [CrossRef]
- Sanjeevakumar, N.; Periasamy, M. Highly enantioselective Henry reaction catalyzed by a new chiral C2-symmetric N,N’-bis(isobornyl)ethylenediamine-copper complex. Tetrahedron Asymmetry 2009, 20, 1842–1847. [Google Scholar] [CrossRef]
- Aydin, A.E. Synthesis of novel thiophene-based chiral ligands and their application in asymmetric Henry reaction. Appl. Organometal. Chem. 2013, 27, 283–289. [Google Scholar] [CrossRef]
- Shen, T.h.; Qin, Q.; Ni, H.; Xia, T.; Zhou, X.-C.; Cui, F.; Li, J.-Q.; Ran, D.-Q.; Song, Q.-B. Asymmetric Henry reaction catalyzed by Cu(I)-based chiral amino alcohol complex. Turk. J. Chem. 2013, 37, 966–977. [Google Scholar] [CrossRef]
- Zhang, P.; He, J.; Wan, N.-N.; Zhang, W.-D.; Hui, Y.-H.; Xie, Z.-F. Bis-thiocarbohydrazones schiff base Mn(II) complex as a catalyst for Henry reaction. Chin. J. Org. Chem. 2012, 32, 254–258. [Google Scholar] [CrossRef]
- Evans, D.A.; Seidel, D.; Rueping, M.; Lam, H.W.; Shaw, J.T.; Downey, C.W. A new copper acetate-bis(oxazoline)-catalyzed, enantioselective Henry reaction. J. Am. Chem. Soc. 2003, 125, 12692–12693. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]


| Entry | Catalyst Loading b (mol%) | Catalyst | Et3N Loading c (mol%) | Solvent | Yield (%) |
|---|---|---|---|---|---|
| 1 | 6 | 1 + Et3N | 20 | MeOH | 39 |
| 2 | 8 | 1 + Et3N | 20 | MeOH | 68 |
| 3 | 10 | 1 + Et3N | 20 | MeOH | 81 |
| 4 | 12 | 1 + Et3N | 20 | MeOH | 81 |
| 5 | 14 | 1 + Et3N | 20 | MeOH | 82 |
| 6 | 10 | 1 + Et3N | 20 | MeOH | 78 |
| 7 | 10 | 1 | 20 | MeOH | 35 |
| 8 | 10 | 2 | 20 | MeOH | 33 |
| 9 | 10 | L | 20 | MeOH | 0 |
| 10 | 10 | Et3N | 20 | MeOH | 35 |
| 11 | 10 | Ni(OAc)2 × 4H2O | 0 | MeOH | 19 |
| 12 | 10 | Zn(OAc)2 × 2H2O | 0 | MeOH | 15 |
| 13 | 10 | 1 + Et3N | 10 | MeOH | 56 |
| 14 | 10 | 1 + Et3N | 15 | MeOH | 73 |
| 15 | 10 | 1 + Et3N | 25 | MeOH | 81 |
| 16 | 10 | 1 + Et3N | 30 | MeOH | 81 |
| 17 | 10 | 1 + Et3N | 20 | iPrOH | 79 |
| 18 | 10 | 1 + Et3N | 20 | EtOH | 76 |
| 19 | 10 | 1 + Et3N | 20 | THF | 71 |
| 20 | 10 | 1 + Et3N | 20 | CH2Cl2 | 62 |
| 21 | 10 | 1 + Et3N | 20 | Toluene | 66 |
| Entry | Compound | R | Yield (%) |
|---|---|---|---|
| 1 | 3 | H | 81 |
| 2 | 4 | p-Me | 72 |
| 3 | 5 | o-Me | 77 |
| 4 | 6 | p-Cl | 90 |
| 5 | 7 | o-Cl | 87 |
| 6 | 8 | m-Cl | 83 |
| 7 | 9 | p-Br | 85 |
| 8 | 10 | m-Br | 82 |
| 9 | 11 | p-MeO | 66 |
| 10 | 12 | o-MeO | 58 |
| 11 | 13 | p-NO2 | 93 |
| 12 | 14 | m-NO2 | 88 |
| Complex | 1 | 2 |
|---|---|---|
| Formula | C22H33N3NiO7 | C22H31N3O6Zn |
| Fw | 510.22 | 498.87 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/n | C2/c |
| a (Å) | 8.687 (5) | 16.798 (4) |
| b (Å) | 15.248 (8) | 6.5119 (17) |
| c (Å) | 20.858 (11) | 22.933 (6) |
| α (◦) | 90 | 90 |
| β (◦) | 93.384 (10) | 106.792 (9) |
| γ (◦) | 90 | 90 |
| Volume (Å3) | 2758 (3) | 2401.6 (11) |
| Z | 4 | 4 |
| Dcalcd/(g·cm−3) | 1.229 | 1.38 |
| θ range (◦) | 1.7–27.3 | 2.7–27.4 |
| μ (mm−1) | 0.744 | 1.064 |
| F (000) | 1080 | 1048 |
| Crystal size (mm) | 0.12 × 0.13 × 0.14 | 0.22 × 0.24 × 0.26 |
| Reflections collected | 15,493 | 10,092 |
| Unique reflections (Rint) | 5971 (0.050) | 2740 (0.042) |
| Goodness-of-fit on F2 | 1.014 | 1.031 |
| Final R indices [I > 2σ(I)] | R1 = 0.0509, wR2 = 0.1195 | R1 = 0.0376, wR2 = 0.0890 |
| R indices (all data) | R1 = 0.0936,wR2 = 0.1351 | R1 = 0.0500, wR2 = 0.0945 |
| Largest diff. peak and hole (e Å−3) | 0.55 and −0.38 | 0.29 and −0.35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Yang, Z.; Guo, H.; Zhao, Y.-C. Synthesis and Catalytic Application of Two Mononuclear Complexes Bearing Diethylenetriamine Derivative Ligand. Molecules 2020, 25, 2101. https://doi.org/10.3390/molecules25092101
Liu C, Yang Z, Guo H, Zhao Y-C. Synthesis and Catalytic Application of Two Mononuclear Complexes Bearing Diethylenetriamine Derivative Ligand. Molecules. 2020; 25(9):2101. https://doi.org/10.3390/molecules25092101
Chicago/Turabian StyleLiu, Chao, Zhao Yang, Hao Guo, and Yu-Cai Zhao. 2020. "Synthesis and Catalytic Application of Two Mononuclear Complexes Bearing Diethylenetriamine Derivative Ligand" Molecules 25, no. 9: 2101. https://doi.org/10.3390/molecules25092101
APA StyleLiu, C., Yang, Z., Guo, H., & Zhao, Y.-C. (2020). Synthesis and Catalytic Application of Two Mononuclear Complexes Bearing Diethylenetriamine Derivative Ligand. Molecules, 25(9), 2101. https://doi.org/10.3390/molecules25092101