Bioanalytical HPLC Applications of In-Tube Solid Phase Microextraction: A Two-Decade Overview
Abstract
1. Introduction
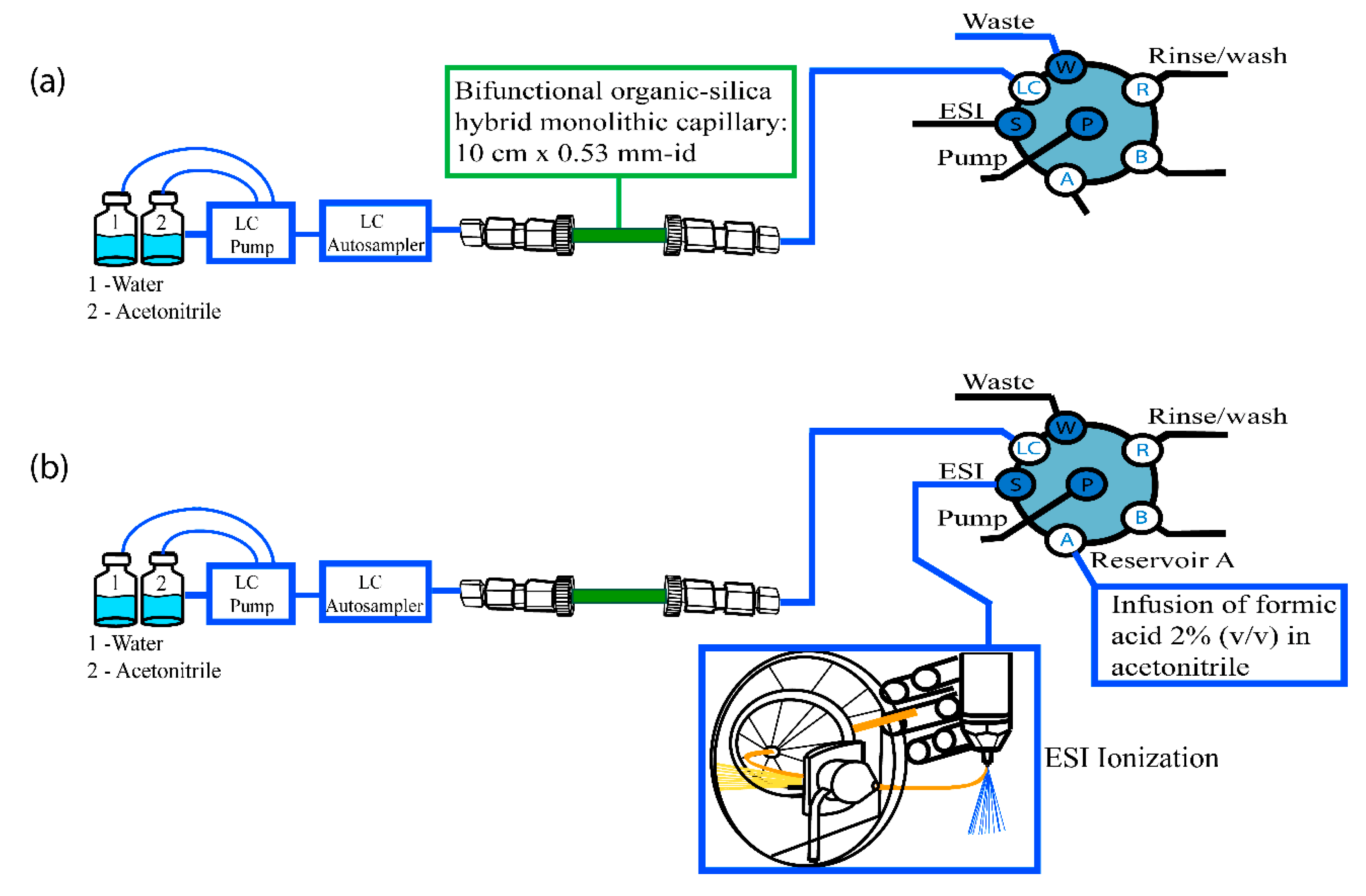
2. Instrumental Configurations
3. Materials and Coatings
3.1. Conventional Capillary Columns Coating
3.2. Monolithic Capillary Columns
3.3. Restricted Access Materials (RAMs)
3.4. Immunosorbents
3.5. Molecularly Imprinted Polymers (MIPs)
3.6. Carbon-Based Materials
3.7. Metal–Organic Frameworks
3.8. Ionic Liquids and Deep Eutectic Solvents
3.9. Other Materials
4. Applications
4.1. Plasma and Serum
4.2. Urine
4.3. Saliva
4.4. Miscellaneous
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Honore Hansen, S.; Pedersen-Bjergaard, S. Bioanalysis of Pharmaceuticals. Sample Preparation, Separation Techniques, and Mass Spectrometry; John Wiley & Sons, Ltd.: Chichester, UK, 2015; ISBN 9781118716816. [Google Scholar]
- Clark, K.D.; Zhang, C.; Anderson, J.L. Sample Preparation for Bioanalytical and Pharmaceutical Analysis. Anal. Chem. 2016, 88, 11262–11270. [Google Scholar] [CrossRef] [PubMed]
- Jannetto, P.J.; Laleli-Sahin, E.; Wong, S.H. Drug Monitoring and Clinical Chemistry; Elsevier Science Publishing Co Inc.: Amsterdam, The Netherlands, 2004; ISBN 9780444509727. [Google Scholar]
- Vuckovic, D.; Zhang, X.; Cudjoe, E.; Pawliszyn, J. Solid-phase microextraction in bioanalysis: New devices and directions. J. Chromatogr. A 2010, 1217, 4041–4060. [Google Scholar] [CrossRef]
- Kataoka, H. Sample preparation for liquid chromatography. In Liquid Chromatography: Applications, 2nd ed.; Elsevier Science Publishing Co Inc.: New York, NY, USA, 2017; ISBN 9780128053928. [Google Scholar]
- Smith, R.M. Before the injection--modern methods of sample preparation for separation techniques. J. Chromatogr. A 2003, 1000, 3–27. [Google Scholar] [CrossRef]
- Kataoka, H.; Saito, K. Recent advances in SPME techniques in biomedical analysis. J. Pharm. Biomed. Anal. 2011, 54, 926–950. [Google Scholar] [CrossRef] [PubMed]
- Navitha Reddy, G.; Dilip Zagade, A.; Sengupta, P. Current direction and advances in analytical sample extraction techniques for drugs with special emphasis on bioanalysis. Bioanalysis 2019, 11, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Pawliszyn, J. Handbook of Solid Phase Microextraction; Elsevier Inc.: New York, NY, USA, 2012; ISBN 9780124160170. [Google Scholar]
- Piri-Moghadam, H.; Alam, M.N.; Pawliszyn, J. Review of geometries and coating materials in solid phase microextraction: Opportunities, limitations, and future perspectives. Anal. Chim. Acta 2017, 984, 42–65. [Google Scholar] [CrossRef]
- Kataoka, H.; Ishizaki, A.; Nonaka, Y.; Saito, K. Developments and applications of capillary microextraction techniques: A review. Anal. Chim. Acta 2009, 655, 8–29. [Google Scholar] [CrossRef]
- Kataoka, H. New trends in sample preparation for clinical and pharmaceutical analysis. Trac Trends Anal. Chem. 2003, 22, 232–244. [Google Scholar] [CrossRef]
- Kataoka, H. Recent developments and applications of microextraction techniques in drug analysis. Anal. Bioanal. Chem. 2010, 396, 339–364. [Google Scholar] [CrossRef]
- Eisert, R.; Pawliszyn, J. Automated In-Tube Solid-Phase Microextraction Coupled to High-Performance Liquid Chromatography. Anal. Chem. 1997, 69, 3140–3147. [Google Scholar] [CrossRef]
- Filipiak, W.; Bojko, B. SPME in clinical, pharmaceutical, and biotechnological research—How far are we from daily practice? Trac Trends Anal. Chem. 2019, 115, 203–213. [Google Scholar] [CrossRef]
- Sajid, M.; Khaled Nazal, M.; Rutkowska, M.; Szczepańska, N.; Namieśnik, J.; Płotka-Wasylka, J. Solid Phase Microextraction: Apparatus, Sorbent Materials, and Application. Crit. Rev. Anal. Chem. 2019, 49, 271–288. [Google Scholar] [CrossRef]
- Godage, N.H.; Gionfriddo, E. A critical outlook on recent developments and applications of matrix compatible coatings for solid phase microextraction. Trac Trends Anal. Chem. 2019, 111, 220–228. [Google Scholar] [CrossRef]
- Roszkowska, A.; Miękus, N.; Bączek, T. Application of solid-phase microextraction in current biomedical research. J. Sep. Sci. 2019, 42, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Lashgari, M.; Yamini, Y. An overview of the most common lab-made coating materials in solid phase microextraction. Talanta 2019, 191, 283–306. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Huang, J.; Yang, Q.; Ni, C.; Xie, X.; Shi, Y.; Sun, J.; Zhu, F.; Ouyang, G. Fabrications of novel solid phase microextraction fiber coatings based on new materials for high enrichment capability. Trac Trends Anal. Chem. 2018, 108, 135–153. [Google Scholar] [CrossRef]
- Hussain, D.; Raza Naqvi, S.T.; Ashiq, M.N.; Najam-ul-Haq, M. Analytical sample preparation by electrospun solid phase microextraction sorbents. Talanta 2020, 208, 120413. [Google Scholar] [CrossRef]
- Costa Queiroz, M.E.; Donizeti de Souza, I.; Marchioni, C. Current advances and applications of in-tube solid-phase microextraction. Trac Trends Anal. Chem. 2019, 111, 261–278. [Google Scholar] [CrossRef]
- Bagheri, H.; Piri-Moghadam, H. Recent advances in capillary microextraction. Trac Trends Anal. Chem. 2015, 73, 64–80. [Google Scholar] [CrossRef]
- Moliner-Martinez, Y.; Herráez-Hernández, R.; Verdú-Andrés, J.; Molins-Legua, C.; Campíns-Falcó, P. Recent advances of in-tube solid-phase microextraction. Trac Trends Anal. Chem. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Kataoka, H.; Ishizaki, A.; Saito, K. Recent progress in solid-phase microextraction and its pharmaceutical and biomedical applications. Anal. Methods 2016, 8, 5773–5788. [Google Scholar] [CrossRef]
- Queiroz, M.E.C.; Melo, L.P. Selective capillary coating materials for in-tube solid-phase microextraction coupled to liquid chromatography to determine drugs and biomarkers in biological samples: A review. Anal. Chim. Acta 2014, 826, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Garcés, N.; Gionfriddo, E.; Gómez-Ríos, G.A.; Alam, M.N.; Boyacı, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in Solid Phase Microextraction and Perspective on Future Directions. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, G.; Ye, N.; Kou, X.; Zhu, F.; Shen, J.; Ouyang, G. Solid-phase microextraction: An appealing alternative for the determination of endogenous substances—A review. Anal. Chim. Acta 2019, 1077, 67–86. [Google Scholar] [CrossRef]
- Jalili, V.; Barkhordari, A.; Ghiasvand, A. A comprehensive look at solid-phase microextraction technique: A review of reviews. Microchem. J. 2020, 152, 104319. [Google Scholar] [CrossRef]
- Inukai, T.; Kaji, S.; Kataoka, H. Analysis of nicotine and cotinine in hair by on-line in-tube solid-phase microextraction coupled with liquid chromatography-tandem mass spectrometry as biomarkers of exposure to tobacco smoke. J. Pharm. Biomed. Anal. 2018, 156, 272–277. [Google Scholar] [CrossRef]
- Saito, A.; Hamano, M.; Kataoka, H. Simultaneous analysis of multiple urinary biomarkers for the evaluation of oxidative stress by automated online in-tube solid-phase microextraction coupled with negative/positive ion-switching mode liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2018, 41, 2743–2749. [Google Scholar] [CrossRef]
- Caris, J.A.; Silva, B.J.G.; Moisés, E.C.D.; Lanchote, V.L.; Queiroz, M.E.C. Automated analysis of lidocaine and its metabolite in plasma by in-tube solid-phase microextraction coupled with LC-UV for pharmacokinetic study. J. Sep. Sci. 2012, 35, 734–741. [Google Scholar] [CrossRef]
- Melo, L.P.; Queiroz, R.H.C.; Queiroz, M.E.C. Automated determination of rifampicin in plasma samples by in-tube solid-phase microextraction coupled with liquid chromatography. J. Chromatogr. B 2011, 879, 2454–2458. [Google Scholar] [CrossRef]
- Silva, B.J.G.; Lanças, F.M.; Queiroz, M.E.C. In-tube solid-phase microextraction coupled to liquid chromatography (in-tube SPME/LC) analysis of nontricyclic antidepressants in human plasma. J. Chromatogr. B 2008, 862, 181–188. [Google Scholar] [CrossRef]
- Kataoka, H.; Inoue, T.; Saito, K.; Kato, H.; Masuda, K. Analysis of heterocyclic amines in hair by on-line in-tube solid-phase microextraction coupled with liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2013, 786, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Rodríguez, H.D.; García-Robles, A.A.; Sáenz-González, P.; Verdú-Andrés, J.; Campíns-Falcó, P. On-line in-tube solid phase microextraction coupled to capillary liquid chromatography-diode array detection for the analysis of caffeine and its metabolites in small amounts of biological samples. J. Pharm. Biomed. Anal. 2020, 178, 112914. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lord, H.; Pawliszyn, J. A new strategy to eliminate sample mixing during in-tube solid phase microextraction. J. Chromatogr. A 2013, 1318, 12–21. [Google Scholar] [CrossRef]
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Automated in-tube solid-phase microextraction–liquid chromatography–electrospray ionization mass spectrometry for the determination of ranitidine. J. Chromatogr. B Biomed. Sci. Appl. 1999, 731, 353–359. [Google Scholar] [CrossRef]
- Kataoka, H.; Narimatsu, S.; Lord, H.L.; Pawliszyn, J. Automated In-Tube Solid-Phase Microextraction Coupled with Liquid Chromatography/Electrospray Ionization Mass Spectrometry for the Determination of β-Blockers and Metabolites in Urine and Serum Samples. Anal. Chem. 1999, 71, 4237–4244. [Google Scholar]
- Farka, Z.; Juřík, T.; Kovář, D.; Trnková, L.; Skládal, P. Nanoparticle-Based Immunochemical Biosensors and Assays: Recent Advances and Challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef]
- Saito, Y.; Kawazoe, M.; Jinno, K.; Hayashida, M. Direct coupling of microcolumn liquid chromatography with in-tube solid-phase microextraction for the analysis of antidepressant drugs. Analyst 2000, 125, 807–809. [Google Scholar] [CrossRef]
- Jornet-Martínez, N.; Ortega-Sierra, A.; Verdú-Andrés, J.; Herráez-Hernández, R.; Campíns-Falcó, P. Analysis of Contact Traces of Cannabis by In-Tube Solid-Phase Microextraction Coupled to Nanoliquid Chromatography. Molecules 2018, 23, 2359. [Google Scholar] [CrossRef]
- Jinno, K.; Kawazoe, M.; Saito, Y.; Takeichi, T.; Hayashida, M. Sample preparation with fiber-in-tube solid-phase microextraction for capillary electrophoretic separation of tricyclic antidepressant drugs in human urine. Electrophoresis 2001, 22, 3785–3790. [Google Scholar] [CrossRef]
- Hakobyan, L.; Pla Tolos, J.; Moliner-Martinez, Y.; Molins-Legua, C.; Ramos, J.R.; Gordon, M.; Ramirez-Galleymore, P.; Campins-Falco, P. Determination of meropenem in endotracheal tubes by in-tube solid phase microextraction coupled to capillary liquid chromatography with diode array detection. J. Pharm. Biomed. Anal. 2018, 151, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, T.; Saeki, K.; Yanagisawa, I.; Uchigasaki, S.; Hasegawa, C.; Seno, H.; Suzuki, O.; Sato, K. Automated on-line in-tube solid-phase microextraction coupled with HPLC/MS/MS for the determination of butyrophenone derivatives in human plasma. Anal. Bioanal. Chem. 2009, 394, 1161–1170. [Google Scholar] [CrossRef]
- Yasuhara, R.; Ehara, K.; Saito, K.; Kataoka, H. Automated analysis of salivary stress-related steroid hormones by online in-tube solid-phase microextraction coupled with liquid chromatography-tandem mass spectrometry. Anal. Methods 2012, 4, 3625–3630. [Google Scholar] [CrossRef]
- Kataoka, H.; Inoue, R.; Yagi, K.; Saito, K. Determination of nicotine, cotinine, and related alkaloids in human urine and saliva by automated in-tube solid-phase microextraction coupled with liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2009, 49, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Mizuno, K.; Oda, E.; Saito, A. Determination of the oxidative stress biomarker urinary 8-hydroxy-2’-deoxyguanosine by automated on-line in-tube solid-phase microextraction coupled with liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2016, 1019, 140–146. [Google Scholar] [CrossRef]
- Mizuno, K.; Kataoka, H. Analysis of urinary 8-isoprostane as an oxidative stress biomarker by stable isotope dilution using automated online in-tube solid-phase microextraction coupled with liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2015, 112, 36–42. [Google Scholar] [CrossRef]
- Mitani, K.; Narimatsu, S.; Izushi, F.; Kataoka, H. Simple and rapid analysis of endocrine disruptors in liquid medicines and intravenous injection solutions by automated in-tube solid-phase microextraction/high performance liquid chromatography. J. Pharm. Biomed. Anal. 2003, 32, 469–478. [Google Scholar] [CrossRef]
- Ishizaki, A.; Uemura, A.; Kataoka, H. A sensitive method to determine melatonin in saliva by automated online in-tube solid-phase microextraction coupled with stable isotope-dilution liquid chromatography-tandem mass spectrometry. Anal. Methods 2017, 9, 3134–3140. [Google Scholar] [CrossRef]
- Kataoka, H.; Matsuura, E.; Mitani, K. Determination of cortisol in human saliva by automated in-tube solid-phase microextraction coupled with liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2007, 44, 160–165. [Google Scholar] [CrossRef]
- Kataoka, H.; Inoue, T.; Ikekita, N.; Saito, K. Development of exposure assessment method based on the analysis of urinary heterocyclic amines as biomarkers by on-line in-tube solid-phase microextraction coupled with liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 2171–2178. [Google Scholar] [CrossRef]
- Kataoka, H.; Ehara, K.; Yasuhara, R.; Saito, K. Simultaneous determination of testosterone, cortisol, and dehydroepiandrosterone in saliva by stable isotope dilution on-line in-tube solid-phase microextraction coupled with liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yagi, K.; Ishizaki, A.; Kataoka, H. Determination of anabolic steroids in human urine by automated in-tube solid-phase microextraction coupled with liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2010, 52, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Bao, M.; Barreiro, R.; Miranda, J.; Cepeda, A.; Regal, P. Recent Advances and Uses of Monolithic Columns for the Analysis of Residues and Contaminants in Food. Chromatography 2015, 2, 79–95. [Google Scholar] [CrossRef]
- Jandera, P. Advances in the development of organic polymer monolithic columns and their applications in food analysis—A review. J. Chromatogr. A 2013, 1313, 37–53. [Google Scholar] [CrossRef]
- Yao, W.; Fan, Z.; Zhang, S. Poly(N-vinylcarbazole-co-divinylbenzene) monolith microextraction coupled to liquid chromatography–high resolution Orbitrap mass spectrometry to analyse benzodiazepines in beer and urine. J. Chromatogr. A 2016, 1465, 55–62. [Google Scholar] [CrossRef]
- Fan, Y.; Feng, Y.-Q.; Da, S.-L.; Shi, Z.-G. Poly (methacrylic acid–ethylene glycol dimethacrylate) monolithic capillary for in-tube solid phase microextraction coupled to high performance liquid chromatography and its application to determination of basic drugs in human serum. Anal. Chim. Acta 2004, 523, 251–258. [Google Scholar] [CrossRef]
- Fan, Y.; Feng, Y.-Q.; Zhang, J.-T.; Da, S.-L.; Zhang, M. Poly(methacrylic acid-ethylene glycol dimethacrylate) monolith in-tube solid phase microextraction coupled to high performance liquid chromatography and analysis of amphetamines in urine samples. J. Chromatogr. A 2005, 1074, 9–16. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, M.; Fan, Y.; Wen, Y.; Xiang, B.; Feng, Y. Biocompatible in-tube solid-phase microextraction coupled to HPLC for the determination of angiotensin II receptor antagonists in human plasma and urine. J. Chromatogr. B 2005, 828, 62–69. [Google Scholar] [CrossRef]
- Lin, B.; Zheng, M.-M.; Ng, S.-C.; Feng, Y.-Q. Development of in-tube solid-phase microextraction coupled to pressure-assisted CEC and its application to the analysis of propranolol enantiomers in human urine. Electrophoresis 2007, 28, 2771–2780. [Google Scholar] [CrossRef]
- Nie, J.; Zhao, Q.; Huang, J.; Xiang, B.; Feng, Y.-Q. Determination of telmisartan in rat tissues by in-tube solid-phase microextraction coupled to high performance liquid chromatography. J. Sep. Sci. 2006, 29, 650–655. [Google Scholar] [CrossRef]
- Huang, J.-F.; Lin, B.; Yu, Q.-W.; Feng, Y.-Q. Determination of fluoroquinolones in eggs using in-tube solid-phase microextraction coupled to high-performance liquid chromatography. Anal. Bioanal. Chem. 2006, 384, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Fan, Y.; Zhang, M.; Feng, Y.-Q. Determination of camptothecin and 10-hydroxycamptothecin in human plasma using polymer monolithic in-tube solid phase microextraction combined with high-performance liquid chromatography. Anal. Bioanal. Chem. 2005, 382, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhang, M.; Zhao, Q.; Feng, Y.-Q. Monitoring of Five Sulfonamide Antibacterial Residues in Milk by In-Tube Solid-Phase Microextraction Coupled to High-Performance Liquid Chromatography. J. Agric. Food Chem. 2005, 53, 8468–8473. [Google Scholar] [CrossRef]
- Fan, Y.; Feng, Y.-Q.; Da, S.-L.; Gao, X.-P. In-tube solid-phase microextraction with poly(methacrylic acid-ethylene glycol dimethacrylate) monolithic capillary for direct high-performance liquid chromatographic determination of ketamine in urine samples. Analyst 2004, 129, 1065–1069. [Google Scholar] [CrossRef]
- Zheng, M.-M.; Ruan, G.-D.; Feng, Y.-Q. Evaluating polymer monolith in-tube solid-phase microextraction coupled to liquid chromatography/quadrupole time-of-flight mass spectrometry for reliable quantification and confirmation of quinolone antibacterials in edible animal food. J. Chromatogr. A 2009, 1216, 7510–7519. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wang, Y.; Feng, Y. Simultaneous residue monitoring of four tetracycline antibiotics in fish muscle by in-tube solid-phase microextraction coupled with high-performance liquid chromatography. Talanta 2006, 70, 153–159. [Google Scholar] [CrossRef]
- Wei, F.; Fan, Y.; Zhang, M.; Feng, Y.-Q. Poly(methacrylic acid-ethylene glycol dimethacrylate) monolith in-tube solid-phase microextraction applied to simultaneous analysis of some amphetamine derivatives in urine by capillary zone electrophoresis. Electrophoresis 2005, 26, 3141–3150. [Google Scholar] [CrossRef]
- Lin, Z.; Lin, Y.; Sun, X.; Yang, H.; Zhang, L.; Chen, G. One-pot preparation of a molecularly imprinted hybrid monolithic capillary column for selective recognition and capture of lysozyme. J. Chromatogr. A 2013, 1284, 8–16. [Google Scholar] [CrossRef]
- Zhang, S.-W.; Xing, J.; Cai, L.-S.; Wu, C.-Y. Molecularly imprinted monolith in-tube solid-phase microextraction coupled with HPLC/UV detection for determination of 8-hydroxy-2′-deoxyguanosine in urine. Anal. Bioanal. Chem. 2009, 395, 479–487. [Google Scholar] [CrossRef]
- Zhang, S.W.; Zou, C.J.; Luo, N.; Weng, Q.F.; Cai, L.S.; Wu, C.Y.; Xing, J. Determination of urinary 8-hydroxy-2′-deoxyguanosine by capillary electrophoresis with molecularly imprinted monolith in-tube solid phase microextraction. Chin. Chem. Lett. 2010, 21, 85–88. [Google Scholar] [CrossRef]
- Lin, C.-L.; Lirio, S.; Chen, Y.-T.; Lin, C.-H.; Huang, H.-Y. A Novel Hybrid Metal-Organic Framework-Polymeric Monolith for Solid-Phase Microextraction. Chem. A Eur. J. 2014, 20, 3317–3321. [Google Scholar] [CrossRef]
- Lirio, S.; Liu, W.-L.; Lin, C.-L.; Lin, C.-H.; Huang, H.-Y. Aluminum based metal-organic framework-polymer monolith in solid-phase microextraction of penicillins in river water and milk samples. J. Chromatogr. A 2016, 1428, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Liao, Y.; Huang, X.; Ye, Z.; Yuan, D. Metal-organic framework-monolith composite-based in-tube solid phase microextraction on-line coupled to high-performance liquid chromatography-fluorescence detection for the highly sensitive monitoring of fluoroquinolones in water and food samples. Talanta 2019, 199, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, G.; Hu, Y. In-tube solid-phase microextraction based on NH 2 -MIL-53(Al)-polymer monolithic column for online coupling with high-performance liquid chromatography for directly sensitive analysis of estrogens in human urine. Talanta 2017, 165, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, W.; Chen, Z. Solid phase microextraction with poly(deep eutectic solvent) monolithic column online coupled to HPLC for determination of non-steroidal anti-inflammatory drugs. Anal. Chim. Acta 2018, 1018, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.-W.; Wang, X.; Ma, Q.; Yuan, B.-F.; He, H.-B.; Feng, Y.-Q. Automated analysis of non-steroidal anti-inflammatory drugs in human plasma and water samples by in-tube solid-phase microextraction coupled to liquid chromatography-mass spectrometry based on a poly(4-vinylpyridine-co-ethylene dimethacrylate) monolith. Anal. Methods 2012, 4, 1538–1545. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, M.; Shi, Z.-G.; Feng, Y.-Q. Preparation of a poly( N -isopropylacrylamide- co -ethylene dimethacrylate) monolithic capillary and its application for in-tube solid-phase microextrac-tion coupled to high-performance liquid chromatography. J. Sep. Sci. 2009, 32, 2592–2600. [Google Scholar] [CrossRef]
- Wang, R.; Chen, Z. Boronate affinity monolithic column incorporated with graphene oxide for the in-tube solid-phase microextraction of glycoproteins. J. Sep. Sci. 2018, 41, 2767–2773. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.-M.; Wang, S.-T.; Hu, W.-K.; Feng, Y.-Q. In-tube solid-phase microextraction based on hybrid silica monolith coupled to liquid chromatography–mass spectrometry for automated analysis of ten antidepressants in human urine and plasma. J. Chromatogr. A 2010, 1217, 7493–7501. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Q.; Jiang, N.; Li, W.; Chen, L.; Lin, X.; Xie, Z.; You, L.; Zhang, Q. Urea-formaldehyde monolithic column for hydrophilic in-tube solid-phase microextraction of aminoglycosides. J. Chromatogr. A 2017, 1485, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Mullett, W.M.; Levsen, K.; Lubda, D.; Pawliszyn, J. Bio-compatible in-tube solid-phase microextraction capillary for the direct extraction and high-performance liquid chromatographic determination of drugs in human serum. J. Chromatogr. A 2002, 963, 325–334. [Google Scholar] [CrossRef]
- Chaves, A.R.; Silva, B.J.G.; Lanças, F.M.; Queiroz, M.E.C. Biocompatible in-tube solid phase microextraction coupled with liquid chromatography-fluorescence detection for determination of interferon α in plasma samples. J. Chromatogr. A 2011, 1218, 3376–3381. [Google Scholar] [CrossRef] [PubMed]
- Chaves, A.R.; Queiroz, M.E.C. Immunoaffinity in-tube solid phase microextraction coupled with liquid chromatography with fluorescence detection for determination of interferon α in plasma samples. J. Chromatogr. B 2013, 928, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.E.C.; Oliveira, E.B.; Breton, F.; Pawliszyn, J. Immunoaffinity in-tube solid phase microextraction coupled with liquid chromatography–mass spectrometry for analysis of fluoxetine in serum samples. J. Chromatogr. A 2007, 1174, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Cheng, S.; Wang, X.; Wang, D.; Xu, L. Novel polystyrene/antibody nanoparticle-coated capillary for immunoaffinity in-tube solid-phase microextraction. Anal. Bioanal. Chem. 2015, 407, 2771–2775. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.-L.; Ma, Y.-C.; Xu, B.; Wang, X.-H.; Dong, L.-Y.; Wang, D.-M.; Liu, K.; Xu, L. Poly(glycidyl methacrylate) nanoparticle-coated capillary with oriented antibody immobilization for immunoaffinity in-tube solid phase microextraction: Preparation and characterization. J. Chromatogr. A 2017, 1509, 1–8. [Google Scholar] [CrossRef]
- Bitas, D.; Samanidou, V. Molecularly Imprinted Polymers as Extracting Media for the Chromatographic Determination of Antibiotics in Milk. Molecules 2018, 23, 316. [Google Scholar] [CrossRef]
- Tamayo, F.G.; Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymers for solid-phase extraction and solid-phase microextraction: Recent developments and future trends. J. Chromatogr. A 2007, 1152, 32–40. [Google Scholar] [CrossRef]
- Mullett, W.M.; Martin, P.; Pawliszyn, J. In-Tube Molecularly Imprinted Polymer Solid-Phase Microextraction for the Selective Determination of Propranolol. Anal. Chem. 2001, 73, 2383–2389. [Google Scholar] [CrossRef]
- Chaves, A.R.; Costa Queiroz, M.E. In-tube solid-phase microextraction with molecularly imprinted polymer to determine interferon alpha 2a in plasma sample by high performance liquid chromatography. J. Chromatogr. A 2013, 1318, 43–48. [Google Scholar] [CrossRef]
- Asiabi, H.; Yamini, Y.; Seidi, S.; Ghahramanifard, F. Preparation and evaluation of a novel molecularly imprinted polymer coating for selective extraction of indomethacin from biological samples by electrochemically controlled in-tube solid phase microextraction. Anal. Chim. Acta 2016, 913, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Song, C.; Li, G. Fiber-in-tube solid-phase microextraction with molecularly imprinted coating for sensitive analysis of antibiotic drugs by high performance liquid chromatography. J. Chromatogr. A 2012, 1263, 21–27. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Stalikas, C. Recent Advances in Carbon Dots. C 2019, 5, 41. [Google Scholar] [CrossRef]
- De Toffoli, A.L.; Maciel, E.V.S.; Fumes, B.H.; Lanças, F.M. The role of graphene-based sorbents in modern sample preparation techniques. J. Sep. Sci. 2018, 41, 288–302. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Stalikas, C. Carbon-Based Nanomaterials Functionalized with Ionic Liquids for Microextraction in Sample Preparation. Separations 2017, 4, 14. [Google Scholar] [CrossRef]
- Feng, J.; Sun, M.; Bu, Y.; Luo, C. Development of a cheap and accessible carbon fibers-in-poly(ether ether ketone) tube with high stability for online in-tube solid-phase microextraction. Talanta 2016, 148, 313–320. [Google Scholar] [CrossRef]
- Feng, J.; Wang, X.; Tian, Y.; Bu, Y.; Luo, C.; Sun, M. Electrophoretic deposition of graphene oxide onto carbon fibers for in-tube solid-phase microextraction. J. Chromatogr. A 2017, 1517, 209–214. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H. Development of a novel graphene/polyaniline electrodeposited coating for on-line in-tube solid phase microextraction of aldehydes in human exhaled breath condensate. J. Chromatogr. A 2015, 1395, 23–31. [Google Scholar] [CrossRef]
- Shamsayei, M.; Yamini, Y.; Asiabi, H. Polythiophene/graphene oxide nanostructured electrodeposited coating for on-line electrochemically controlled in-tube solid-phase microextraction. J. Chromatogr. A 2016, 1475, 8–17. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, S.; Yan, Y.; Wang, L.; Guo, G.; Wang, X. GO-META-TiO2 composite monolithic columns for in-tube solid-phase microextraction of phosphopeptides. Talanta 2019, 192, 360–367. [Google Scholar] [CrossRef]
- Shuo, Z.; Hao-Tian, W.; Ke, L.; Jing, Z.; Xia-Yan, W.; Guang-Sheng, G. Fast Determination of Residual Sulfonamides in Milk by In-Tube Solid-Phase Microextraction Coupled with Capillary Electrophoresis-Laser Induced Fluorescence. Chin. J. Anal. Chem. 2018, 46, e1810–e1816. [Google Scholar] [CrossRef]
- González-Fuenzalida, R.A.; López-García, E.; Moliner-Martínez, Y.; Campíns-Falcó, P. Adsorbent phases with nanomaterials for in-tube solid-phase microextraction coupled on-line to liquid nanochromatography. J. Chromatogr. A 2016, 1432, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Argente-García, A.; Moliner-Martínez, Y.; López-García, E.; Campíns-Falcó, P.; Herráez-Hernández, R. Application of Carbon Nanotubes Modified Coatings for the Determination of Amphetamines by In-Tube Solid-Phase Microextraction and Capillary Liquid Chromatography. Separations 2016, 3, 7. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Ji, Y.-S.; Zhang, H.-X.; Liu, M.-C. Highly sensitive analysis of substituted aniline compounds in water samples by using oxidized multiwalled carbon nanotubes as an in-tube solid-phase microextraction medium. J. Chromatogr. A 2008, 1212, 10–15. [Google Scholar] [CrossRef]
- Manousi, N.; Zachariadis, G.; Deliyanni, E.; Samanidou, V. Applications of Metal-Organic Frameworks in Food Sample Preparation. Molecules 2018, 23, 2896. [Google Scholar] [CrossRef]
- Manousi, N.; Giannakoudakis, D.A.; Rosenberg, E.; Zachariadis, G.A. Extraction of Metal Ions with Metal–Organic Frameworks. Molecules 2019, 24, 4605. [Google Scholar] [CrossRef]
- Vardali, S.C.; Manousi, N.; Barczak, M.; Giannakoudakis, D.A. Novel Approaches Utilizing Metal-Organic Framework Composites for the Extraction of Organic Compounds and Metal Traces from Fish and Seafood. Molecules 2020, 25, 513. [Google Scholar] [CrossRef]
- Wu, S.; Cai, C.; Cheng, J.; Cheng, M.; Zhou, H.; Deng, J. Polydopamine/dialdehyde starch/chitosan composite coating for in-tube solid-phase microextraction and in-situ derivation to analysis of two liver cancer biomarkers in human blood. Anal. Chim. Acta 2016, 935, 113–120. [Google Scholar] [CrossRef]
- Shamsayei, M.; Yamini, Y.; Asiabi, H.; Safari, M. On-line packed magnetic in-tube solid phase microextraction of acidic drugs such as naproxen and indomethacin by using Fe3O4@SiO2@layered double hydroxide nanoparticles with high anion exchange capacity. Microchim. Acta 2018, 185, 192. [Google Scholar] [CrossRef]
- Han, D.; Row, K.H. Recent Applications of Ionic Liquids in Separation Technology. Molecules 2010, 15, 2405–2426. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Feng, J.; Bu, Y.; Luo, C. Ionic liquid coated copper wires and tubes for fiber-in-tube solid-phase microextraction. J. Chromatogr. A 2016, 1458, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y. In-tube solid phase microextraction using a ?-cyclodextrin coated capillary coupled to high performance liquid chromatography for determination of non-steroidal anti-inflammatory drugs in urine samples. Talanta 2005, 65, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Wang, L.; Pu, Q.; Du, W.; Guo, G. Plasma-assisted alignment in the fabrication of microchannel-array-based in-tube solid-phase microextraction microchips packed with TiO 2 nanoparticles for phosphopeptide analysis. Anal. Chim. Acta 2018, 1018, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Shamsayei, M.; Yamini, Y.; Asiabi, H. Electrochemically controlled fiber-in-tube solid-phase microextraction method for the determination of trace amounts of antipsychotic drugs in biological samples. J. Sep. Sci. 2018, 41, 3598–3606. [Google Scholar] [CrossRef]
- Asiabi, H.; Yamini, Y.; Shamsayei, M. Development of electrochemically controlled packed-in-tube solid phase microextraction method for sensitive analysis of acidic drugs in biological samples. Talanta 2018, 185, 80–88. [Google Scholar] [CrossRef]
- Walles, M.; Mullett, W.; Levsen, K.; Borlak, J.; Wünsch, G.; Pawliszyn, J. Verapamil drug metabolism studies by automated in-tube solid phase microextraction. J. Pharm. Biomed. Anal. 2002, 30, 307–319. [Google Scholar] [CrossRef]
- Ahmadi, S.H.; Manbohi, A.; Heydar, K.T. Electrochemically controlled in-tube solid phase microextraction of naproxen from urine samples using an experimental design. Analyst 2015, 140, 497–505. [Google Scholar] [CrossRef]
- Piri-Moghadam, H.; Lendor, S.; Pawliszyn, J. Development of a Biocompatible In-Tube Solid-Phase Microextraction Device: A Sensitive Approach for Direct Analysis of Single Drops of Complex Matrixes. Anal. Chem. 2016, 88, 12188–12195. [Google Scholar] [CrossRef]
- Wu, J. Determination of stimulants in human urine and hair samples by polypyrrole coated capillary in-tube solid phase microextraction coupled with liquid chromatography-electrospray mass spectrometry. Talanta 2001, 54, 655–672. [Google Scholar] [CrossRef]
- Silva, B.J.G.; Lanças, F.M.; Queiroz, M.E.C. Determination of fluoxetine and norfluoxetine enantiomers in human plasma by polypyrrole-coated capillary in-tube solid-phase microextraction coupled with liquid chromatography-fluorescence detection. J. Chromatogr. A 2009, 1216, 8590–8597. [Google Scholar] [CrossRef]
- Wu, J.; Lord, H.L.; Pawliszyn, J.; Kataoka, H. Polypyrrole-coated capillary in-tube solid phase microextraction coupled with liquid chromatography-electrospray ionization mass spectrometry for the determination of ?-blockers in urine and serum samples. J. Microcolumn Sep. 2000, 12, 255–266. [Google Scholar] [CrossRef]
- Mullett, W.M.; Levsen, K.; Borlak, J.; Wu, J.; Pawliszyn, J. Automated In-Tube Solid-Phase Microextraction Coupled with HPLC for the Determination of N -Nitrosamines in Cell Cultures. Anal. Chem. 2002, 74, 1695–1701. [Google Scholar] [CrossRef]
- Asiabi, H.; Yamini, Y.; Seidi, S.; Safari, M.; Shamsayei, M. Evaluation of in-tube solid-phase microextraction method for co-extraction of acidic, basic, and neutral drugs. Rsc Adv. 2016, 6, 14049–14058. [Google Scholar] [CrossRef]
- Wang, S.; Xu, H. Inorganic-organic hybrid coating material for the online in-tube solid-phase microextraction of monohydroxy polycyclic aromatic hydrocarbons in urine. J. Sep. Sci. 2016, 39, 4610–4620. [Google Scholar] [CrossRef]
- Alhooshani, K.; Kim, T.-Y.; Kabir, A.; Malik, A. Sol–gel approach to in situ creation of high pH-resistant surface-bonded organic–inorganic hybrid zirconia coating for capillary microextraction (in-tube SPME). J. Chromatogr. A 2005, 1062, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Moliner-Martínez, Y.; Prima-Garcia, H.; Ribera, A.; Coronado, E.; Campíns-Falcó, P. Magnetic In-Tube Solid Phase Microextraction. Anal. Chem. 2012, 84, 7233–7240. [Google Scholar] [CrossRef] [PubMed]
- Bigham, S.; Medlar, J.; Kabir, A.; Shende, C.; Alli, A.; Malik, A. Sol–Gel Capillary Microextraction. Anal. Chem. 2002, 74, 752–761. [Google Scholar] [CrossRef]
- Whelpton, R. PHARMACEUTICAL ANALYSIS | Sample Preparation. In Encyclopedia of Analytical Science; Elsevier Ltd.: Amsterdam, The Netherlands, 2005; pp. 107–116. ISBN 9780123693976. [Google Scholar]
- Marchioni, C.; Vieira, T.M.; Miller Crotti, A.E.; Crippa, J.A.; Costa Queiroz, M.E. In-tube solid-phase microextraction with a dummy molecularly imprinted monolithic capillary coupled to ultra-performance liquid chromatography-tandem mass spectrometry to determine cannabinoids in plasma samples. Anal. Chim. Acta 2020, 1099, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Beloti, L.; Miranda, L.; Queiroz, M. Butyl Methacrylate-Co-Ethylene Glycol Dimethacrylate Monolith for Online in-Tube SPME-UHPLC-MS/MS to Determine Chlopromazine, Clozapine, Quetiapine, Olanzapine, and Their Metabolites in Plasma Samples. Molecules 2019, 24, 310. [Google Scholar] [CrossRef] [PubMed]
- Souza, I.D.; Hantao, L.W.; Queiroz, M.E.C. Polymeric ionic liquid open tubular capillary column for on-line in-tube SPME coupled with UHPLC-MS/MS to determine endocannabinoids in plasma samples. Anal. Chim. Acta 2019, 1045, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.F.C.; Gonçalves, R.R.C.; Queiroz, M.E. A Dual Ligand Sol–Gel Organic-Silica Hybrid Monolithic Capillary for In-Tube SPME-MS/MS to Determine Amino Acids in Plasma Samples. Molecules 2019, 24, 1658. [Google Scholar] [CrossRef] [PubMed]
- Asiabi, H.; Yamini, Y.; Shamsayei, M.; Mehraban, J.A. A nanocomposite prepared from a polypyrrole deep eutectic solvent and coated onto the inner surface of a steel capillary for electrochemically controlled microextraction of acidic drugs such as losartan. Microchim. Acta 2018, 185, 169. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, W.; Liao, X.; Zhang, W.; Chen, Z. An etched polyether ether ketone tube covered with immobilized graphene oxide for online solid phase microextraction of quaternary alkaloids prior to their quantitation by HPLC-MS/MS. Microchim. Acta 2017, 184, 2715–2721. [Google Scholar] [CrossRef]
- Alidoust, M.; Seidi, S.; Rouhollahi, A.; Shanehsaz, M. In-tube electrochemically controlled solid phase microextraction of amitriptyline, imipramine and chlorpromazine from human plasma by using an indole-thiophene copolymer nanocomposite. Microchim. Acta 2017, 184, 2473–2481. [Google Scholar] [CrossRef]
- Ling, X.; Zhang, W.; Chen, Z. Electrochemically modified carbon fiber bundles as selective sorbent for online solid-phase microextraction of sulfonamides. Microchim. Acta 2016, 183, 813–820. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Z. Mussel inspired polydopamine functionalized poly(ether ether ketone) tube for online solid-phase microextraction–high performance liquid chromatography and its application in analysis of protoberberine alkaloids in rat plasma. J. Chromatogr. A 2013, 1278, 29–36. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, J.; Tan, X.; Sun, L.; Yu, R.; Yang, H.; Chen, G. Preparation of boronate-functionalized molecularly imprinted monolithic column with polydopamine coating for glycoprotein recognition and enrichment. J. Chromatogr. A 2013, 1319, 141–147. [Google Scholar] [CrossRef]
- Yuan, H.; Mester, Z.; Lord, H.; Pawliszyn, J. Automated In-Tube Solid-Phase Microextraction Coupled with Liquid Chromatography-Electrospray Ionization Mass Spectrometry for the Determination of Selected Benzodiazepines. J. Anal. Toxicol. 2000, 24, 718–725. [Google Scholar] [CrossRef]
- Kataoka, H.; Lord, H.L.; Yamamoto, S.; Narimatsu, S.; Pawliszyn, J. Development of automated in-tube SPME/LC/MS method for drug analysis. J. Microcolumn Sep. 2000, 12, 493–500. [Google Scholar] [CrossRef]
- Manbohi, A.; Ahmadi, S.H.; Jabbari, V. On-line microextraction of moxifloxacin using Fe3O4 nanoparticle-packed in-tube SPME. Rsc Adv. 2015, 5, 57930–57936. [Google Scholar] [CrossRef]
- Manbohi, A.; Ahmadi, S.H. In-tube magnetic solid phase microextraction of some fluoroquinolones based on the use of sodium dodecyl sulfate coated Fe3O4 nanoparticles packed tube. Anal. Chim. Acta 2015, 885, 114–121. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, Z.; Ren, L.; Liu, Y.; Dou, P.; Qian, K.; Chen, H.-Y. On-line coupling of in-tube boronate affinity solid phase microextraction with high performance liquid chromatography–electrospray ionization tandem mass spectrometry for the determination of cis-diol biomolecules. Talanta 2010, 82, 270–276. [Google Scholar] [CrossRef]
- Chen, D.; Xu, H. Simultaneous HPLC-MS determination of 8-hydroxy-2′-deoxyguanosine, 3-hydroxyphenanthrene and 1-hydroxypyrene after online in-tube solid phase microextraction using a graphene oxide/poly(3,4-ethylenedioxythiophene)/polypyrrole composite. Microchim. Acta 2019, 186, 300. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, E.; Kataoka, H. Automated Analysis of Oxytocin by On-Line in-Tube Solid-Phase Microextraction Coupled with Liquid Chromatography-Tandem Mass Spectrometry. Chromatography 2015, 2, 382–391. [Google Scholar] [CrossRef]
- Wang, S.; Hu, S.; Xu, H. Analysis of aldehydes in human exhaled breath condensates by in-tube SPME-HPLC. Anal. Chim. Acta 2015, 900, 67–75. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ishizaki, A.; Kataoka, H. Biomonitoring method for the determination of polycyclic aromatic hydrocarbons in hair by online in-tube solid-phase microextraction coupled with high performance liquid chromatography and fluorescence detection. J. Chromatogr. B 2015, 1000, 187–191. [Google Scholar] [CrossRef]
- Souza-Silva, É.A.; Reyes-Garcés, N.; Gómez-Ríos, G.A.; Boyacı, E.; Bojko, B.; Pawliszyn, J. A critical review of the state of the art of solid-phase microextraction of complex matrices III. Bioanalytical and clinical applications. Trac Trends Anal. Chem. 2015, 71, 249–264. [Google Scholar] [CrossRef]
- Nováková, L.; Vlčková, H. A review of current trends and advances in modern bio-analytical methods: Chromatography and sample preparation. Anal. Chim. Acta 2009, 656, 8–35. [Google Scholar] [CrossRef]
- Kataoka, H. Automated sample preparation using in-tube solid-phase microextraction and its application—A review. Anal. Bioanal. Chem. 2002, 373, 31–45. [Google Scholar] [CrossRef]
- US Food and Drug Administration Guidance for Industry: Bioanalytical Method Validation (5.24.18). Available online: https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs (accessed on 29 April 2020).
- Cruz-Vera, M.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Sorptive microextraction for liquid-chromatographic determination of drugs in urine. Trac Trends Anal. Chem. 2009, 28, 1164–1173. [Google Scholar] [CrossRef]
- Rezaei, F.; Yamini, Y.; Moradi, M.; Ebrahimpour, B. Solid phase extraction as a cleanup step before microextraction of diclofenac and mefenamic acid using nanostructured solvent. Talanta 2013, 105, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Pebdani, A.A.; Shabani, A.M.H.; Dadfarnia, S.; Khodadoust, S. Solid phase microextraction of diclofenac using molecularly imprinted polymer sorbent in hollow fiber combined with fiber optic-linear array spectrophotometry. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2015, 147, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H. Pharmaceutical Analysis | Sample Preparation. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier Ltd.: New York, NY, USA, 2018; ISBN 9780081019832. [Google Scholar]
- Bellagambi, F.G.; Lomonaco, T.; Salvo, P.; Vivaldi, F.; Hangouët, M.; Ghimenti, S.; Biagini, D.; Di Francesco, F.; Fuoco, R.; Errachid, A. Saliva sampling: Methods and devices. An overview. Trac Trends Anal. Chem. 2020, 124, 115781. [Google Scholar] [CrossRef]
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, G.F.E. Diagnostic potential of breath analysis—Focus on volatile organic compounds. Clin. Chim. Acta 2004, 347, 25–39. [Google Scholar] [CrossRef] [PubMed]





| Analyte | Sample | SPME Material | SPME Mode | Detection | LOD/LOQ | Year | Ref |
|---|---|---|---|---|---|---|---|
| Cannabidiol, Δ9-tetrahydrocannabinol | Human plasma | Dummy molecularly imprinted monolithic capillary | In-valve | MS/MS | NM 1/10 ng mL−1 | 2020 | [134] |
| Chlopromazine, clozapine, quetiapine, olanzapine, and their metabolites | Human plasma | Butyl methacrylate-co-ethylene glycol dimethacrylate monolith | In-valve | MS/MS | NM/10 ng mL−1 | 2019 | [135] |
| Anandamide, 2-arachidonoyl glycerol | Human plasma | Polymeric ionic liquid open tubular capillary column | In-valve | MS/MS | NM/0.05, 0.10 ng mL−1 | 2019 | [136] |
| Amino acids, neurotransmitters | Human plasma | Dual ligand sol–gel organic-silica hybrid monolithic capillary | In-valve | MS/MS | NM/6–360 nmol mL−1 | 2019 | [137] |
| Ketoprofen, flurbiprofen, diclofenac | Human plasma | Poly(deep eutectic solvent) monolithic column | In-valve | UV | 0.05–0.5/0.2–2 ng mL−1 | 2018 | [79] |
| Losartan | Human plasma, urine | Polypyrrole-deep eutectic solvent coated capillary | In-valve | UV | 0.2, 0.5 μg L−1/NM | 2018 | [138] |
| Jatorrhizine, palmatine, berberine | Rat plasma | immobilized Graphene oxide on PEEK tube | In-valve | MS/MS | 0.1–0.3 pg mL−1 | 2017 | [139] |
| Amitriptyline, imipramine, chlorpromazine | Human plasma | indole-thiophene copolymer nanocomposite | In-valve | UV | 40 ng mL−1/80 ng mL−1 | 2017 | [140] |
| Amitriptyline, doxepin | Human plasma, urine | Polythiophene/graphene oxide (PTh/GO) nanostructured coating | In-valve | UV | 0.3, 0.5/2.3, 2.9 ng mL−1 | 2016 | [103] |
| Sulfadiazine, sulfadimidine, sulfamethoxazole | Rat plasma | Poly(3,4-ethylenedioxythiophene) | In-valve | UV | 0.002–0.05/0.01–0.25 ng mL−1 | 2016 | [141] |
| Berberine, palmatine, jatrorrhizine | Rat plasma | Poly(acrylamide–ethylene glycol dimethacrylate)monolith | In-valve | UV | 0.01/0.03 ng mL−1 | 2013 | [142] |
| Glycoproteins | Rat plasma | Poly(vinylphenylboronic acid–ethylene glycol dimethacrylate) monolithic material | In-valve | UV | 0.01 μg mL−1/NM | 2018 | [82] |
| Interferon alpha 2a | Human plasma | Molecularly imprinted polymer | Draw-inject | FLD | NM/8 ng mL−1 | 2013 | [94] |
| Interferon alpha 2a | Human plasma | Monoclonal anti-interferon 2a antibody | Draw-inject | FLD | NM/0.006 MIU mL−1 | 2013 | [87] |
| Ketoprofen, fenbufen, ibuprofen | Human plasma | Poly(4-vinylpyridine-co-ethylene dimethacrylate) monolith | In-valve | UV | 2.01–4.77/6.70–15.9 ng mL−1 | 2012 | [80] |
| Lidocaine and its metabolite | Human plasma | 14% cyanopropylphenyl methylpolysiloxane | Draw-inject | UV | 15, 20/50 ng mL−1 | 2012 | [33] |
| Rifampicin | Human plasma | Polyethylene glycol | Draw-inject | UV | MN/0.1 μg mL−1 | 2011 | [34] |
| Interferon alpha 2a | Human plasma | Restricted access material (protein-coated silica) | Draw-inject | FLD | NM/0.06 MIU mL−1 | 2011 | [86] |
| Antidepressants | Human plasma, urine | Hybrid organic–inorganic silica monolith with cyanoethyl functional groups | In-valve | MS | 0.06–2.84/0.19–9.45 ng mL−1 | 2010 | [83] |
| Fluoxetine, norfluoxetine | Human plasma | Polypyrrole-coated capillary | Draw-inject | RF | NM/10–15 ng mL−1 | 2009 | [125] |
| Moperone, floropipamide, haloperidol, spiroperidol, bromperidol, pimozide | Human plasma | DB-17 | Draw-inject | MS/MS | 0.03–0.2/0.1–0.5 ng mL−1 | 2009 | [46] |
| Mirtazapine, citalopram, paroxetine, duloxetine, fluoxetine, sertraline | Human plasma | OV-1701 | Draw-inject | UV | 5–20/20–50 ng mL−1 | 2008 | [35] |
| Candesartan, losartan, irbesartan, valsartan, telmisartan | Human plasma, urine | Poly(MAA-EGDMA) monolithic capillary | In-valve | FLD | 0.1–15.3/0.4–51 ng mL−1 | 2005 | [62] |
| Camptothecin, 10-hydroxycamptothecin | Human plasma | Poly(MAA-EGDMA) monolithic capillary column | In-valve | UV | 1.79–2.62/5.96–8.73 ng mL−1 | 2005 | [66] |
| Verapamil metabolites | Human plasma, urine | Polypyrrole-coated capillary | Draw-inject | UV, MS | 52–83 ng mL−1 (UV), 5–8 ng mL−1 (MS)/NM | 2002 | [121] |
| Indomethacin | Human plasma, urine, blood | Nanostructured copolymer coating consisting of polypyrrole doped with ethylene glycol dimethacrylate | In-valve | UV | 0.6–2.0 μg L−1/NM | 2016 | [95] |
| Theobromine, theophylline, caffeine | Human serum | Poly(methacrylic acid–ethylene glycol dimethacrylate) monolithic | In-valve | UV | 6.5–12.0/21.5–39.6 ng mL−1 | 2004 | [60] |
| Oxazepam, temazepam, nordazepam, diazepam | Human serum | Restricted access material (RAM), alkyl-diol-silica (ADS), | Draw-inject | UV | 22–29/74–98 ng mL−1 | 2002 | [85] |
| Fluoxetine | Human serum | Immunoaffinity-based (BSA-fluoxetine conjugate) | Draw-inject | MS | NM/5 ng mL−1 | 2007 | [88] |
| Glycoprotein | Human serum | Boronate-functionalized molecularly imprinted monolithic column | In-valve | UV | NM | 2013 | [143] |
| Theobromine, paraxanthine, theophylline, caffeine | Human serum | ZB-FFAP (100% nitroterephthalic modified polyethylene glycol). | In-valve | UV | 0.1–0.5/0.4–1.5 μg mL−1 | 2020 | [37] |
| Benzodiazepines | Human serum, urine | Supelco-Q plot capillary column | Draw-inject | MS | 0.02–2/0.5–2 ng mL−1 | 2000 | [144] |
| Beta-blockers | Human serum, urine | Omegawax 250 capillary | Draw-inject | MS | 0.1–1.2 ng mL−1/NM | 1999 | [40] |
| Stimulants, beta-blockers | Human serum, urine | Omegawax 250 capillary | Draw-inject | MS | 0.1–1.2 ng mL−1/NM | 2000 | [145] |
| Propranolol | Human serum | Molecularly imprinted polymer | Draw-inject | UV | 0.32 μg mL−1 | 2001 | [93] |
| 17β-Estradiol, estrone, ethinyl estradiol, progesterone, estriol | Human urine | NH2-MIL-53(Al)-polymer monolithic column | In-valve | UV-FLD | 0.002–0.04 μg L−1/NM | 2017 | [78] |
| Naproxen | Human urine | Polypyrrole (PPy)-coated | In-valve | UV | 0.07 μg L−1 | 2015 | [122] |
| Moxifloxacin | Human urine | Fe3O4 nanoparticles-packed | In-valve | UV | 0.03 μg L−1/NM | 2015 | [146] |
| Ciprofloxacin, enrofloxacin, ofloxacin | Human urine | Sodium dodecyl sulfate coated Fe3O4 nanoparticles | In-valve | UV | 0.01–0.05 μg L−1 | 2015 | [147] |
| Dopamine, 5-hydroxytryptamine | Human urine | Boronate affinity solid phase microextraction | In-valve | MS/MS | 1.2/4.0 ng mL−1 | 2010 | [148] |
| Nicotine, cotinine, nornicotine, anabasine, anatabine | Human urine, saliva | CP-Pora PLOT amine capillary column | Draw-inject | MS | 0.015–0.040 ng mL−1/NM | 2009 | [48] |
| Ketoprofen, fenbufen, ibuprofen | Human urine | Beta-cyclodextrin coated capillary column | Draw-inject | UV | 18–38 ng mL−1/NM | 2005 | [117] |
| Ketamine | Human urine | Poly(methacrylic acid- ethylene glycol dimethacrylate) monolithic capillary | In-valve | UV | 6.4 ng mL−1/NM | 2004 | [68] |
| Stimulants | Human urine, hair | Polypyrrole coated capillary column | Draw-inject | MS | 8–56 ng L−1/NM | 2001 | [124] |
| Diclofenac, mefenamic acid | Human urine, plasma | Nanostructured polypyrrole | In-valve | UV | 0.08–1.6 μg L−1/NM | 2018 | [120] |
| Urinary biomarkers (8-isoprostane, 8-hydroxy-2′-deoxyguanosine, 3-nitro-L-tyrosine) | Human urine | Carboxen 1006 PLOT capillary column | Draw-inject | MS/MS | 3.4–21.5 pg mL−1/0.02 ng mL−1 | 2018 | [32] |
| Heterocyclic amines | Human urine | Supel-Q PLOT capillary column | Draw-inject | MS/MS | NM/1.7–4.1 pg mL−1 | 2014 | [54] |
| 8-hydroxy-2′-deoxyguanosine, 3-hydroxyphenanthrene, 1-hydroxypyrene | Human urine | Graphene oxide, poly(3,4-ethylenedioxythiophene), poly- pyrrole | In-valve | MS | 0.004–0.041/0.016–0.135 ng mL−1 | 2019 | [149] |
| 8-hydroxy-2′-deoxyguanosine | Human urine | Carboxen 1006 PLOT capillary column | Draw-inject | MS/MS | 8.3 pg mL−1/NM | 2016 | [49] |
| Perphenazine, chlorpromazine | Human urine, plasma | nanostructured Cu-Cr-Al ternary layered double hydroxide/polythiophene coating | In-valve | UV | 0.2–0.8 μg L−1/NM | 2018 | [119] |
| Nornicotine, anatabine, anabasine, nicotine, cotinine | Human urine, saliva | CP-Pora PLOT amine capillary column | Draw-inject | MS | 115–40 pg mL−1/NM | 2009 | [48] |
| Cortisol, dehydroepiandrosterone | Saliva | Supel-Q PLOT capillary column | Draw-inject | MS/MS | 0.9–12 pg mL−1/NM | 2012 | [47] |
| Testosterone, cortisol, dehydroepiandrosterone | Saliva | Supel-Q PLOT capillary column | Draw-inject | MS/MS | NM/0.01–0.29 ng mL−1 | 2013 | [55] |
| Oxytocin | Saliva | Supel-Q PLOT capillary column | Draw-inject | MS/MS | 4 pg mL−1/NM | 2015 | [150] |
| Butanal, pentanal, hexanal, heptanal, octanal and nonanal | Exhaled breath condensate | Graphene/polyaniline (G/PANI) electrodeposited coating | In-valve | UV | 0.02–0.04/0.07–0.13 nmol L−1 | 2015 | [102] |
| Butanal, pentanal, hexanal, heptanal, octanal and nonanal | Exhaled breath condensate | Polypyrrole/graphene (PPy/G) composite coating | In-valve | UV | 2.3–3.3/7.7–12.3 nmol L−1 | 2015 | [151] |
| Polycyclic aromatic hydrocarbons | Hair | CP-Sil 19CB (14% cyanopropyl phenyl methylsilicone) | In-valve | FLD | 0.5–20.4 pg mL−1/NM | 2015 | [152] |
| Heterocyclic amines | Hair | Supel-Q PLOT capillary column | Draw-inject | MS/MS | 0.10–0.78 pg mL−1/NM | 2013 | [36] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manousi, N.; Tzanavaras, P.D.; Zacharis, C.K. Bioanalytical HPLC Applications of In-Tube Solid Phase Microextraction: A Two-Decade Overview. Molecules 2020, 25, 2096. https://doi.org/10.3390/molecules25092096
Manousi N, Tzanavaras PD, Zacharis CK. Bioanalytical HPLC Applications of In-Tube Solid Phase Microextraction: A Two-Decade Overview. Molecules. 2020; 25(9):2096. https://doi.org/10.3390/molecules25092096
Chicago/Turabian StyleManousi, Natalia, Paraskevas D. Tzanavaras, and Constantinos K. Zacharis. 2020. "Bioanalytical HPLC Applications of In-Tube Solid Phase Microextraction: A Two-Decade Overview" Molecules 25, no. 9: 2096. https://doi.org/10.3390/molecules25092096
APA StyleManousi, N., Tzanavaras, P. D., & Zacharis, C. K. (2020). Bioanalytical HPLC Applications of In-Tube Solid Phase Microextraction: A Two-Decade Overview. Molecules, 25(9), 2096. https://doi.org/10.3390/molecules25092096








