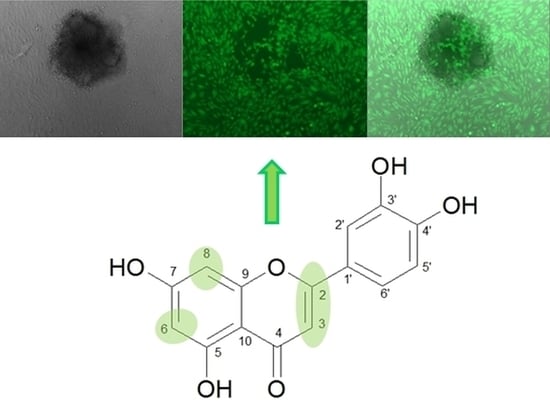
Flavonoids Distinctly Stabilize Lymph Endothelial- or Blood Endothelial Disintegration Induced by Colon Cancer Spheroids SW620
Abstract
1. Introduction
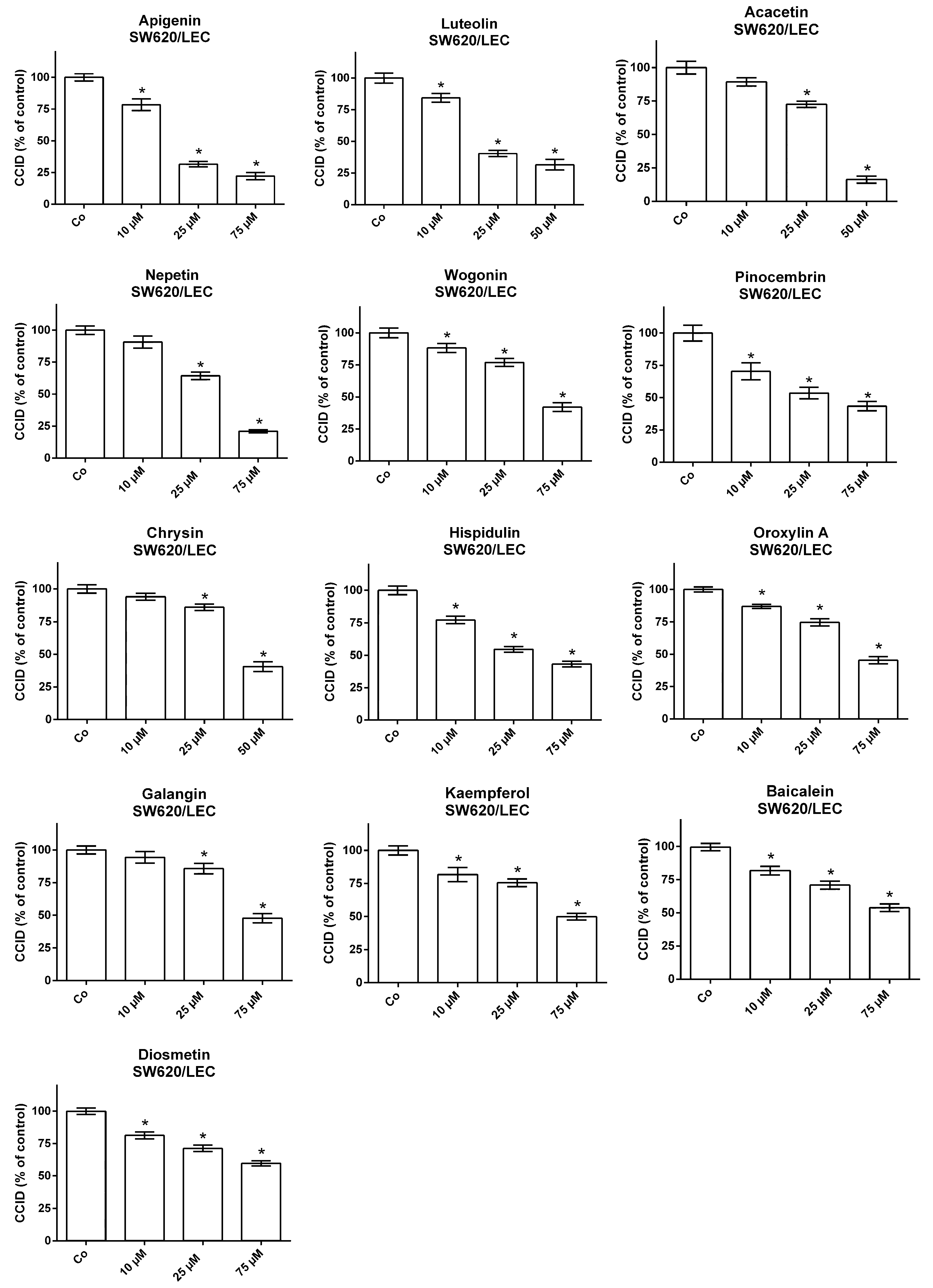
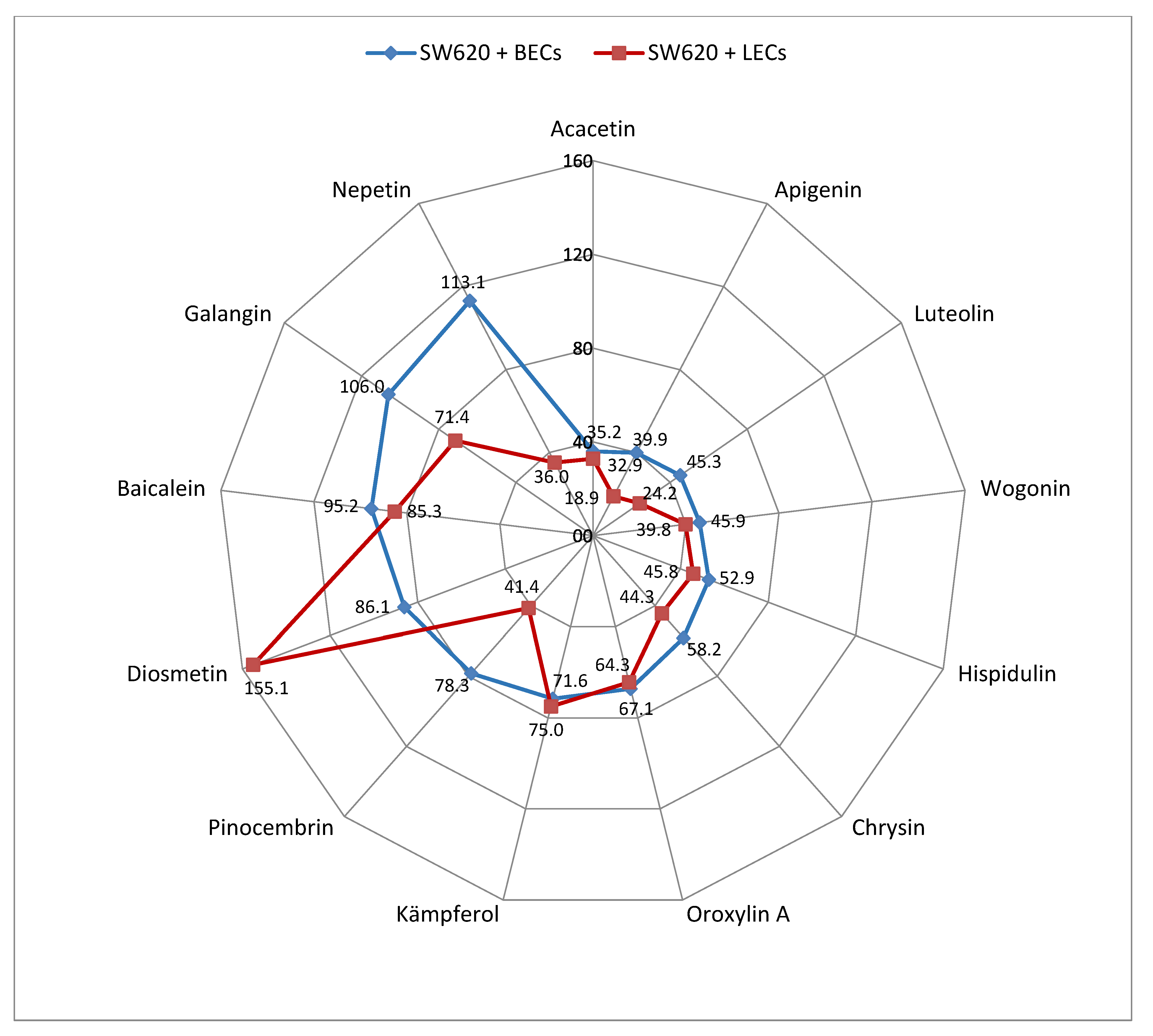
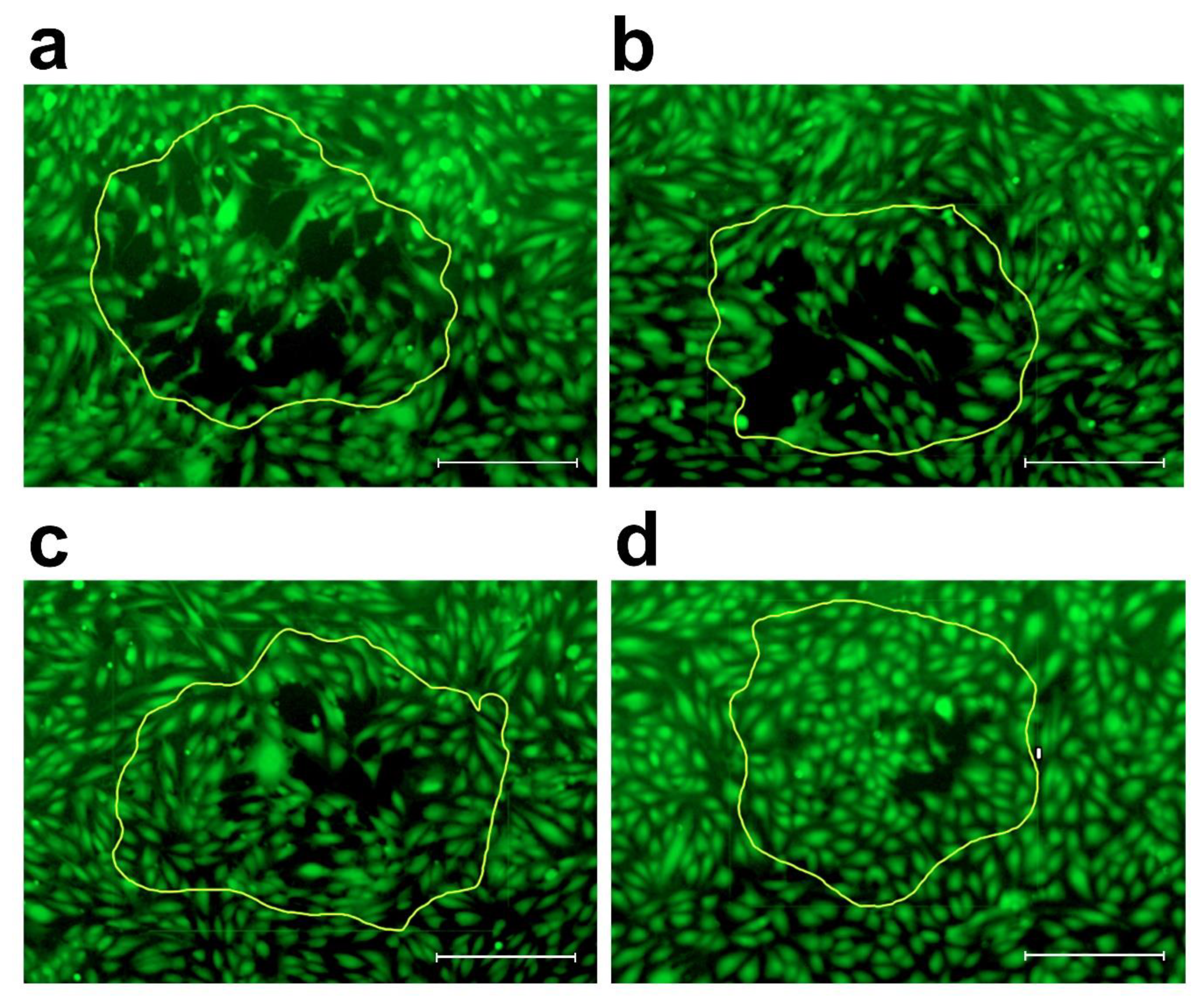
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.2.1. SW620 Spheroid Generation
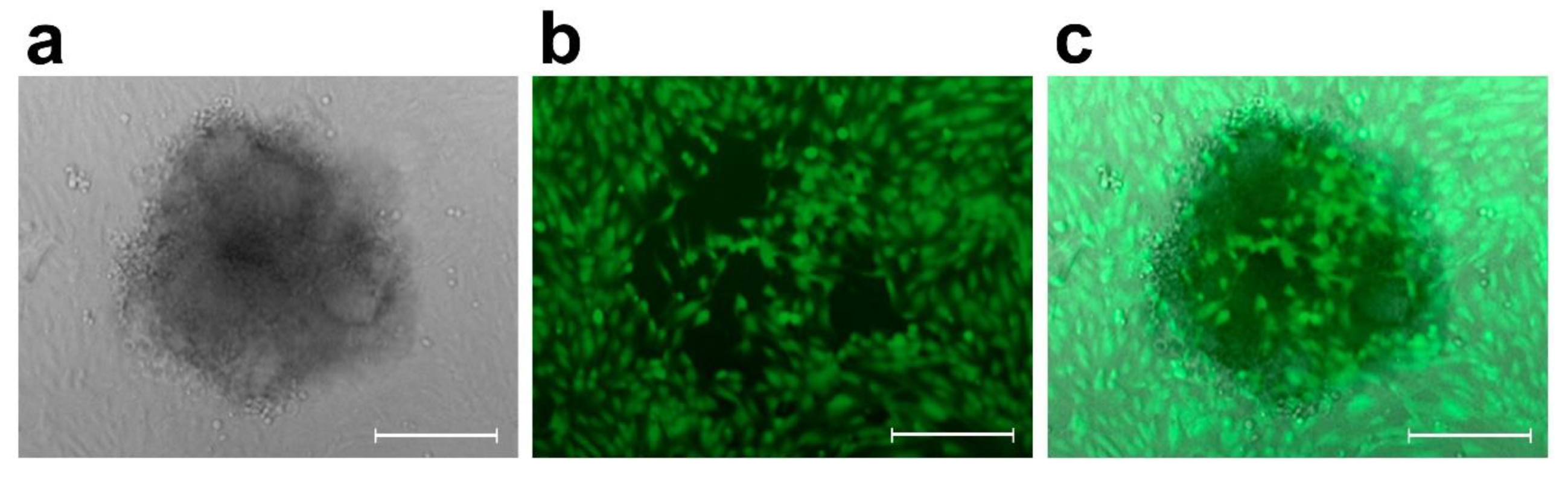
4.2.2. 3-D Co-Cultivation of SW620 Colon Cancer Cells with LECs and BECs and Circular Chemorepellent Induced Defect (CCID) Assay
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Enquist, I.B.; Good, Z.; Jubb, A.M.; Fuh, G.; Wang, X.; Junttila, M.R.; Jackson, E.L.; Leong, K.G. Lymph node-independent liver metastasis in a model of metastatic colorectal cancer. Nat. Commun. 2014, 5, 3530. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ding, J.; Ma, Z.; Sun, R.; Seoane, J.A.; Scott Shaffer, J.; Suarez, C.J.; Berghoff, A.S.; Cremolini, C.; Falcone, A.; et al. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat. Genet. 2019, 51, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Giessrigl, B.; Yazici, G.; Teichmann, M.; Kopf, S.; Ghassemi, S.; Atanasov, A.G.; Dirsch, V.M.; Grusch, M.; Jäger, W.; Özmen, A.; et al. Effects of Scrophularia extracts on tumor cell proliferation, death and intravasation through lymphoendothelial cell barriers. Int. J. Oncol. 2012, 40, 2063–2074. [Google Scholar] [CrossRef]
- Lewenhofer, V.; Schweighofer, L.; Ledermüller, T.; Eichsteininger, J.; Kählig, H.; Zehl, M.; Nguyen, C.H.; Krupitza, G.; Özmen, A.; Krenn, L. Chemical Composition of Scrophularia lucida and the Effects on Tumor Invasiveness in Vitro. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Eichsteininger, J.; Kirisits, K.; Smöch, C.; Stadlbauer, C.; Nguyen, C.H.; Jäger, W.; Özmen, A.; Ecker, G.; Krupitza, G.; Krenn, L. Structural Insight into the In Vitro Anti-Intravasative Properties of Flavonoids. Sci. Pharm. 2019, 87, 23. [Google Scholar] [CrossRef]
- Riihimaki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 2016, 6, 29765. [Google Scholar] [CrossRef]
- Senfter, D.; Holzner, S.; Kalipciyan, M.; Staribacher, A.; Walzl, A.; Huttary, N.; Krieger, S.; Brenner, S.; Jager, W.; Krupitza, G.; et al. Loss of miR-200 family in 5-fluorouracil resistant colon cancer drives lymphendothelial invasiveness in vitro. Hum. Mol. Genet. 2015, 24, 3689–3698. [Google Scholar] [CrossRef]
- Holzner, S.; Senfter, D.; Stadler, S.; Staribacher, A.; Nguyen, C.H.; Gaggl, A.; Geleff, S.; Huttary, N.; Krieger, S.; Jager, W.; et al. Colorectal cancer cell-derived microRNA200 modulates the resistance of adjacent blood endothelial barriers in vitro. Oncol. Rep. 2016, 36, 3065–3071. [Google Scholar] [CrossRef]
- Holzner, S.; Brenner, S.; Atanasov, A.G.; Senfter, D.; Stadler, S.; Nguyen, C.H.; Fristiohady, A.; Milovanovic, D.; Huttary, N.; Krieger, S.; et al. Intravasation of SW620 colon cancer cell spheroids through the blood endothelial barrier is inhibited by clinical drugs and flavonoids in vitro. Food Chem. Toxicol. 2017, 111, 114–124. [Google Scholar] [CrossRef]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Airoldi, C.; La Ferla, B.; D´Orazio, G.; Ciaramelli, C.; Palmioli, A. Flavonoids in the Treatment of Alzheimer’s and Other Neurodegenerative Diseases. Curr. Med. Chem. 2018, 25, 3228–3246. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H.Y. Flavonoids, Dairy Foods, and Cardiovascular and Metabolic Health: A Review of Emerging Biologic Pathways. Circ. Res. 2018, 122, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Honn, K.V.; Tang, D.G.; Grossi, I.; Duniec, Z.M.; Timar, J.; Renaud, C.; Leithauser, M.; Blair, I.; Johnson, C.R.; Diglio, C.A.; et al. Tumor Cell-derived 12(5)-Hydroxyeicosatetraenoic Acid Induces Microvascular Endothelial Cell Retraction. Cancer Res. 1994, 54, 565–574. [Google Scholar] [PubMed]
- Karlsson, M.C.; Gonzalez, S.F.; Welin, J.; Fuxe, J. Epithelial-mesenchymal transition in cancer metastasis through the lymphatic system. Mol. Oncol. 2017, 11, 781–791. [Google Scholar] [CrossRef]
- Lorusso, G.; Ruegg, C. New insights into the mechanisms of organ-specific breast cancer metastasis. Semin. Cancer Biol. 2012, 22, 226–233. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, C.; Sanchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef]
- Bisol, A.; de Campos, P.S.; Lamers, M.L. Flavonoids as anticancer therapies: A systematic review of clinical trials. Phytother. Res. 2019. [Google Scholar] [CrossRef]
- Choy, K.W.; Murugan, D.; Leong, X.F.; Abas, R.; Alias, A.; Mustafa, M.R. Flavonoids as Natural Anti-Inflammatory Agents Targeting Nuclear Factor-Kappa B (NFkappaB) Signaling in Cardiovascular Diseases: A Mini Review. Front. Pharmacol. 2019, 10, 1295. [Google Scholar] [CrossRef]
- Woo, H.D.; Kim, J. Dietary flavonoid intake and risk of stomach and colorectal cancer. World J. Gastroenterol. 2013, 19, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.M.; Zhang, Z.H.; Song, J.; Cheng, X.D.; Jiang, J.; Jia, X.B. Enhanced bioavailability of apigenin via preparation of a carbon nanopowder solid dispersion. Int. J. Nanomed. 2014, 9, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, L.P.; Luo, S.Q.; Jiang, H.D.; Zeng, S. Intestinal absorption of luteolin from peanut hull extract is more efficient than that from individual pure luteolin. J. Agric. Food Chem. 2008, 56, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Lirdprapamongkol, K.; Sakurai, H.; Abdelhamed, S.; Yokoyama, S.; Maruyama, T.; Athikomkulchai, S.; Viriyaroj, A.; Awale, S.; Yagita, H.; Ruchirawat, S.; et al. A flavonoid chrysin suppresses hypoxic survival and metastatic growth of mouse breast cancer cells. Oncol. Rep. 2013, 30, 2357–2364. [Google Scholar] [CrossRef]
- Ren, H.; Ma, J.; Si, L.; Ren, B.; Chen, X.; Wang, D.; Hao, W.; Tang, X.; Li, D.; Zheng, Q. Low dose of acacetin promotes breast cancer MCF-7 cells proliferation through the activation of ERK/PI3K/AKT and cyclin signaling pathway. Recent Pat. Anti Cancer 2018, 13, 368–377. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, M.K.; Li, K.; Hu, C.; Lu, M.H.; Situ, J. Eupafolin nanoparticle improves acute renal injury induced by LPS through inhibiting ROS and inflammation. Biomed. Pharmacother. 2017, 85, 704–711. [Google Scholar] [CrossRef]
- Fan, X.; Tao, J.; Fredimoses, M.; Wu, J.; Jiang, Z.; Zhang, K.; Zhang, X.; Li, S. Eupafolin Suppresses Esophagus Cancer Growth by Targeting T-LAK Cell-Originated Protein Kinase. Front. Pharmacol. 2019, 10, 1248. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, D.; Xu, D.; Yu, H.; Zhao, Z.; Ma, D.; Jin, J. Eupafolin Exhibits Potent Anti-Angiogenic and Antitumor Activity in Hepatocellular Carcinoma. Int. J. Biol. Sci. 2017, 13, 701–711. [Google Scholar] [CrossRef]
- Sung, H.C.; Liang, C.J.; Lee, C.W.; Yen, F.L.; Hsiao, C.Y.; Wang, S.H.; Jiang-Shieh, Y.F.; Tsai, J.S.; Chen, Y.L. The protective effect of eupafolin against TNF-alpha-induced lung inflammation via the reduction of intercellular cell adhesion molecule-1 expression. J. Ethnopharmacol. 2015, 170, 136–147. [Google Scholar] [CrossRef]
- Engleitner, S.; Milovanovic, D.; Kirisits, K.; Brenner, S.; Hong, J.; Ropek, N.; Huttary, N.; Rehak, J.; Nguyen, C.H.; Bago-Horvath, Z.; et al. Feed-back loops integrating RELA, SOX18 and FAK mediate the break-down of the lymph-endothelial barrier that is triggered by 12(S)-HETE. Int. J. Cancer 2020. [Google Scholar] [CrossRef]
- Viola, K.; Kopf, S.; Huttary, N.; Vonach, C.; Kretschy, N.; Teichmann, M.; Giessrigl, B.; Raab, I.; Stary, S.; Krieger, S.; et al. Bay11-7082 inhibits the disintegration of the lymphendothelial barrier triggered by MCF-7 breast cancer spheroids; the role of ICAM-1 and adhesion. Br. J. Cancer 2013, 108, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.H.; Senfter, D.; Basilio, J.; Holzner, S.; Stadler, S.; Krieger, S.; Huttary, N.; Milovanovic, D.; Viola, K.; Simonitsch-Klupp, I.; et al. NF-κB contributes to MMP1 expression in breast cancer spheroids causing paracrine PAR1 activation and disintegrations in the lymph endothelial barrier in vitro. Oncotarget 2015, 6, 39262–39275. [Google Scholar] [CrossRef] [PubMed]
- Ci, Y.; Qiao, J.; Han, M. Molecular Mechanisms and Metabolomics of Natural Polyphenols Interfering with Breast Cancer Metastasis. Molecules 2016, 21, 1634. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yuan, M.; Li, S.; Thuan, U.T.; Nguyen, T.T.; Kang, T.W.; Liao, W.; Lian, S.; Jung, Y.D. Apigenin Suppresses the IL-1β-Induced Expression of the Urokinase-Type Plasminogen Activator Receptor by Inhibiting MAPK-Mediated AP-1 and NF-κB Signaling in Human Bladder Cancer T24 Cells. J. Agric. Food Chem. 2018, 66, 7663–7673. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Park, C.G.; Jung, J.Y. Acacetin (5,7-dihydroxy-4’-methoxyflavone) exhibits in vitro and in vivo anticancer activity through the suppression of NF-κB/Akt signaling in prostate cancer cells. Int. J. Mol. Med. 2014, 33, 317–324. [Google Scholar] [CrossRef]
- Li, L.; Luo, W.; Qian, Y.; Zhu, W.; Qian, J.; Li, J.; Jin, Y.; Xu, X.; Liang, G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-κB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 2019, 59, 152774. [Google Scholar] [CrossRef]
- Chen, H.Y.; Jiang, Y.W.; Kuo, C.L.; Way, T.D.; Chou, Y.C.; Chang, Y.S.; Chung, J.G. Chrysin inhibit human melanoma A375.S2 cell migration and invasion via affecting MAPK signaling and NF-κB signaling pathway in vitro. Environ. Toxicol. 2019, 34, 434–442. [Google Scholar] [CrossRef]
- Nepal, M.; Choi, H.J.; Choi, B.Y.; Yang, M.S.; Chae, J.I.; Li, L.; Soh, Y. Hispidulin attenuates bone resorption and osteoclastogenesis via the RANKL-induced NF-κB and NFATc1 pathways. Eur. J. Pharmacol. 2013, 715, 96–104. [Google Scholar] [CrossRef]
- Jiao, D.; Jiang, Q.; Liu, Y.; Ji, L. Nephroprotective effect of wogonin against cadmium-induced nephrotoxicity via inhibition of oxidative stress-induced MAPK and NF-kB pathway in Sprague Dawley rats. Hum. Exp. Toxicol. 2019, 38, 1082–1091. [Google Scholar] [CrossRef]
- Chen, X.; Han, R.; Hao, P.; Wang, L.; Liu, M.; Jin, M.; Kong, D.; Li, X. Nepetin inhibits IL-1β induced inflammation via NF-κB and MAPKs signaling pathways in ARPE-19 cells. Biomed. Pharmacother. 2018, 101, 87–93. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.Z.; Song, J.K.; Sun, J.L.; Li, Y.J.; Zhou, S.B.; Zhang, T.T.; Du, G.H. Pinocembrin protects human brain microvascular endothelial cells against fibrillar amyloid-β(1-40) injury by suppressing the MAPK/NF-κB inflammatory pathways. Biomed. Res. Int. 2014, 2014, 470393. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Simard, M.J.; Huot, J. Endothelial microRNAs regulating the NF-κB pathway and cell adhesion molecules during inflammation. FASEB J. 2018, 32, 4070–4084. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, Y.; Wang, N. New paradigms in inflammatory signaling in vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H317–H325. [Google Scholar] [CrossRef]
- Schoppmann, S.F.; Soleiman, A.; Kalt, R.; Okubo, Y.; Benisch, C.; Nagavarapu, U.; Herron, G.S.; Geleff, S. Telomerase-immortalized lymphatic and blood vessel endothelial cells are functionally stable and retain their lineage specificity. Microcirculation 2004, 11, 261–269. [Google Scholar] [CrossRef]
- Madlener, S.; Saiko, P.; Vonach, C.; Viola, K.; Huttary, N.; Stark, N.; Popescu, R.; Gridling, M.; Vo, N.T.; Herbacek, I.; et al. Multifactorial anticancer effects of digalloyl-resveratrol encompass apoptosis, cell-cycle arrest, and inhibition of lymphendothelial gap formation in vitro. Br. J. Cancer 2010, 102, 1361–1370. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berenda, J.; Smöch, C.; Stadlbauer, C.; Mittermair, E.; Taxauer, K.; Huttary, N.; Krupitza, G.; Krenn, L. Flavonoids Distinctly Stabilize Lymph Endothelial- or Blood Endothelial Disintegration Induced by Colon Cancer Spheroids SW620. Molecules 2020, 25, 2066. https://doi.org/10.3390/molecules25092066
Berenda J, Smöch C, Stadlbauer C, Mittermair E, Taxauer K, Huttary N, Krupitza G, Krenn L. Flavonoids Distinctly Stabilize Lymph Endothelial- or Blood Endothelial Disintegration Induced by Colon Cancer Spheroids SW620. Molecules. 2020; 25(9):2066. https://doi.org/10.3390/molecules25092066
Chicago/Turabian StyleBerenda, Julia, Claudia Smöch, Christa Stadlbauer, Eva Mittermair, Karin Taxauer, Nicole Huttary, Georg Krupitza, and Liselotte Krenn. 2020. "Flavonoids Distinctly Stabilize Lymph Endothelial- or Blood Endothelial Disintegration Induced by Colon Cancer Spheroids SW620" Molecules 25, no. 9: 2066. https://doi.org/10.3390/molecules25092066
APA StyleBerenda, J., Smöch, C., Stadlbauer, C., Mittermair, E., Taxauer, K., Huttary, N., Krupitza, G., & Krenn, L. (2020). Flavonoids Distinctly Stabilize Lymph Endothelial- or Blood Endothelial Disintegration Induced by Colon Cancer Spheroids SW620. Molecules, 25(9), 2066. https://doi.org/10.3390/molecules25092066