Abstract
In search of unprecedented tri and/or tetrapod pharmacophoric conjugates, a series of 32 new 4-ethyl-1H-benzo[b][1,4]diazepin-2(3H)-ones were synthesized and properly elucidated using MS, IR, NMR, and elemental analysis. In vitro investigation of 11 compounds of this series, using a panel of two human tumor cell lines namely; human breast adenocarcinoma (MCF-7), and human colorectal carcinoma (HCT-116), revealed promising cytotoxic activities. Among all synthesized compounds, analogue 9 displayed maximum cytotoxicity with IC50 values of 16.19 ± 1.35 and 17.16 ± 1.54 μM against HCT-116 and MCF-7, respectively, compared to standard drug doxorubicin.
1. Introduction
Benzodiazepines (BDZ’s) are privileged heteroaromatic molecules and were considered to be the core of an essential class of pharmaceutically active analogues, so, their synthesis is of high value in the subject of medicinal and pharmaceutical chemistry [1,2,3,4]. 1,4-Benzodiazepines (Figure 1) are used as anti-microbial [5], in alcohol withdrawal syndrome (AWS) [6], as endothelin antagonist [7], hypnotics [8], anxiolytics [9], anticonvulsants [10], muscle relaxants [10], anticancer drugs [11,12], sedatives [13,14], and antipsychotics [15]. Recently, benzodiazepines were also recognized to have anti-proliferative [16], antimicrobial [17], anti-inflammatory [18], anti-plateletanti-ulcer [19], and analgesic [20].
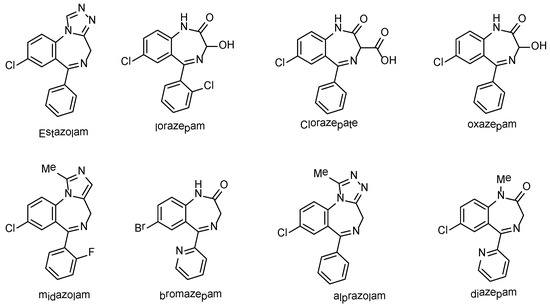
Figure 1.
Representative examples of biologically active 1,4-benzodiazepines.
Due to our admiration with the synthesis, modification, and studying the biological activities of benzodiazepines, herein, we freshened our sustained efforts [21,22,23,24,25,26,27], through the synthesis and utility of 5-methyl-4-(methylthio)-2-oxo-2,11-dihydrobenzo[b]pyrano[2,3-e][1,4]diazepine-3-carbonitrile (5) as a reactive precursor, for the annulation of benzopyranodiazepines of potential biological activity.
2. Results and Discussion
2.1. Chemistry
In our sustained efforts to synthesize various functionalized heterocyclic analogues, and studying their biological activities [21,22,23,24,25,26,27], we desired to report a new efficient and simple technique for the synthesis of benzo[b]pyrano[2,3-e][1,4]diazepines. Compound 1 [28] was selected as a substrate to condense with compound 2 [29] in stirred DMSO, containing catalytic amounts of NaOH at rt to furnish pyrano[1,4]diazepine derivative 5 with an 85% yield (Scheme 1). The existence of the nitrile group was assured using its IR absorption band at 2209 cm−1 and its 13C NMR as a singlet at 115.9 ppm, while the occurrence of the methylthio moiety was supported using 1H NMR as a singlet at 2.73 ppm. Further, evidence for compound 5 was obtained from its noted mass at m/z 297.06 matches with the formula C15H11N3O2S.
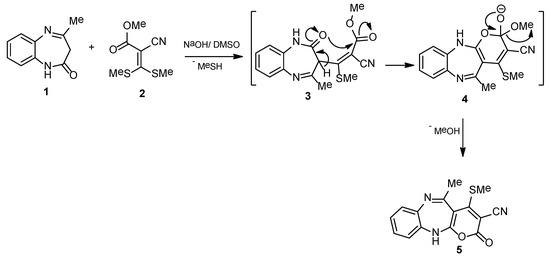
Scheme 1.
Synthesis of benzo[b]pyrano[2,3-e][1,4]diazepine derivative 5.
The good replaceable active methylthio moiety in compound 5 was cyclized with NH2NH2∙H2O, afforded the amino pyrazole derivative 6 with a 65% yield (Scheme 2). Similarly, it was smoothly condensed with hydroxylamine, phenyl hydrazine, thiosemicarbazide, urea, thiourea, and guanidine hydrochloride to provide the target compounds (7–12). The gesture of the methylthio protons initially observed in compound 5 (1H NMR) at 2.73 ppm was vanished, whereas the NH2 protons were observed around δ ~ 7.00 ppm.
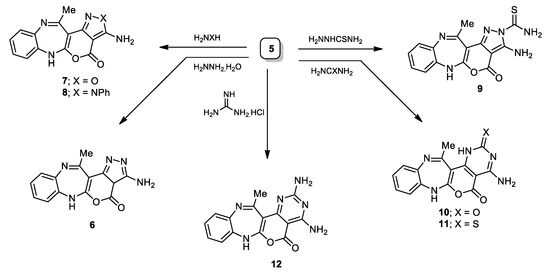
Scheme 2.
Synthetic pathways of compounds 6–12.
Moreover, compound 5 was condensed with 2-aminophenol or 2-aminothiophenol, and benzo[b][1,4]oxa(thia)zepine analogues (13 and 14) were obtained (Scheme 3). The construction of compound 13 was presumed to advance via the preliminary condensation of the hydroxyl proton of 2-aminophenol and the methylthio moiety of compound 5, through removal of the methanethiol fragment, followed by further, internal cyclization to oxazepine derivative 13, through nucleophilic attack of the amino moiety onto the nitrile. The noted mass at m/z 358.11 matching with the formula C20H14N4O3, as well the NH2 band (IR) at ~3325 cm−1, and its broad singlet (1H NMR) at 8.61 ppm, all confirmed structure 13. Upon treatment of compound 5 with tris(hydroxymethyl)aminomethane or PhNH2, the secondary amine analogues (15 and 16) were obtained. Compound 15 (IR) showed interest bands centered at 2217 and 3406 cm−1, owing to the hydroxyl and the nitrile groups, correspondingly, while, its 1H NMR scope exhibited three new singlets at 3.02, 3.81, and 5.74 ppm, owing to the hydroxyl, methylene, and the secondary amine protons, respectively. In a similar manner, compound 5 was subjected to some selected secondary amines namely; diethylamine, morpholine, N-methylpiperazine and/or piperazinyl to furnish the corresponding tertiary amines (17–20). Strong absorption sign centered around 2210 cm−1 owing to the nitrile group, supported these structures. Compound 17 (1H NMR) demonstrated a triplet and quartet signs allocated to the two equivalent ethyl moieties at 1.16 and 3.35 ppm, respectively. Furthermore, compound 5 was cyclocondensed with cyanothioacetamide, afforded 1,3-thiazine analogue 21 with an 80% yield (Scheme 3). The cyanomethyl and imino protons in compound 21 appeared as two singlets (1H NMR) at 4.15 and 8.91 ppm, respectively.
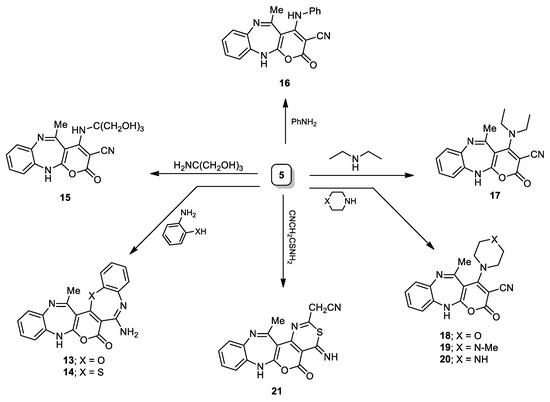
Scheme 3.
Synthetic pathways of compounds 13–21.
Similarly, upon smooth cyclocondensation of compound 5 with some heterocyclic amines, a series of ploy fused heterocyclic systems (22–28) were acquired (Scheme 4).
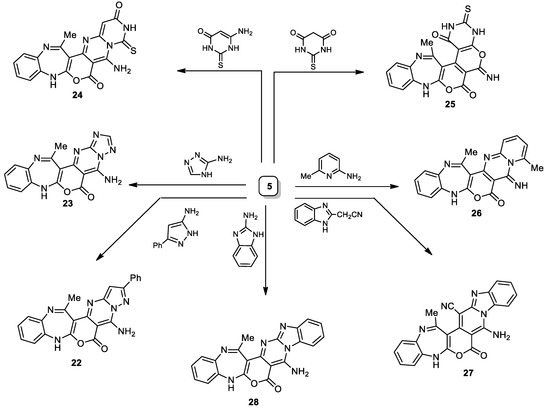
Scheme 4.
Synthetic pathways of compounds 22–28.
The adaptability of our heteroaromatic construction policy was spare, as demonstrated by the annulation of pyrano[c]pyran analogue. Thus, cyclocondensation of intermediate 5 with cyclopentanone or acetylacetone, afforded the pyrano[c]pyran derivatives (32 and 33), respectively (Scheme 5). The formation of compound 31 could be supposed to advance through the preliminary condensation of the energetic methylene in the cyclopentanone with the easily removable methylthio moiety in compound 5, to afford the non-isolable intermediates 29 and 30, which underwent internal cyclization through a nucleophilic attack of the OH to the C≡N fragment, to furnish the final product 31. The exocyclic imino moiety in compound 31, was easily converted to carbonyl via treatment with HCl in boiling EtOH, to afford cyclopenta[b]pyran-2-one derivative 32 (Scheme 5). Compound 32 (IR) showed intense bands at 1705, 3256 cm−1 owing to the carbonyl and imino moieties, correspondingly, whereas its mass spectrum presented a molecular ion peak (C19H14N2O4) at m/z 334.10.
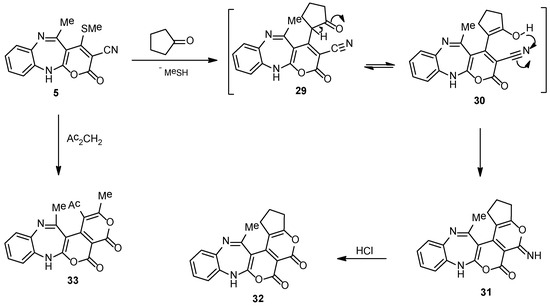
Scheme 5.
Synthetic pathways of compounds 31–33.
Finally, α-aminocarbothiamide analogue 9 was applied as a facile point to construct the target pyrazolo[1,5-a][1,3,5]triazine-4-thiones (34–38) (Scheme 6), through smooth condensation with triethyl orthoformate, acetyl chloride, ethyl cyanoacetate, chloroacetyl chloride, and carbon disulfide. The mass spectrum of compound 36 presented an ion peak at m/z 389.05 (C19H14N2O4), whereas its IR declared two distinctive bands owing to the C=S and C≡N moieties, at 1325 and 2215 cm−1 correspondingly.
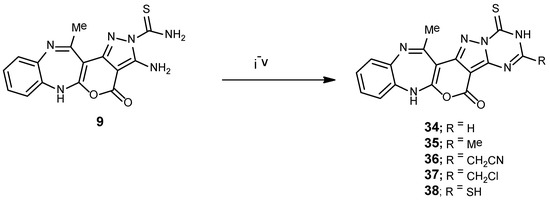
Scheme 6.
Reagents and conditions for synthesis of compounds 34–38. Note: i = HC(OEt)3; ii = AcCl; iii = CNCH2CO2Et; and iv = ClCOCH2Cl; v = CS2/EtOH.
2.2. Pharmacological Evaluation
Cytotoxic Impact
According to the Sulforhodamine B (SRB) method [30,31], new eleven conjugates were in vitro examined for their cytotoxic impact towards human colorectal carcinoma (HCT-116) and human breast adenocarcinoma (MCF-7). Doxorubicin was the positive drug, while DMSO was the negative control. The cytotoxic impacts of the investigated compounds are presented in Table 1.

Table 1.
Preliminary in vitro cytotoxicity values of some new synthesized compounds.
Analyses of the IC50 data as given in Table 1 declare that, majority of the investigated compounds own noteworthy cytotoxic activities versus these cell lines. Where, compound 9 was the most effective among the screened series with IC50 = 16.19 ± 1.35 and 17.16 ± 1.54 μM against HCT-116 and MCF-7, respectively. Moreover, the test cell lines were generally susceptible to conjugates 7, 6, 8, 15, 16, 18, 19, and 20, in a downward order, with IC50 < 60.00 μM. Extra reading of the obtained results stated that, compounds 5 and 17 were less robust towards the two tumor cell lines with IC50 > 60.00 μM.
In conclusion, a series of 32 new benzo[b][1,4]diazepines was synthesized as a bipod or tripod pharmacophoric architectures that could reinforce the cytotoxic impact. The presence of the parent skeleton benzo-pyrano-diazepine was vital for the extensive spectrum of cytotoxic action against the screened cell lines. Moreover, introducing the pyrazole ring bearing carbothioamide fragment to the parent skeleton enhanced the anti-tumor ability of compound 9 to become close to the doxorubicin. The cytotoxic activity of compounds 7, 6, and 8 was attributed to the presence of the amino oxazole as well as amino pyrazole moieties, in conjugation with the parent benzo-pyrano-diazepine skeleton. Whereas introduction of 1,1,1-tri hydroxymethyl methyl amine moiety in compound 15, enforced it to show an excellent potency; moreover, the existence of a phenylamino fragment in compound 16 improved the molecule to show a strong cytotoxicity. On the other hand, introducing the piperazinyl or morpholinyl moiety in compounds 17–20 minified the potency of these molecules, compared to the other tested compounds.
3. Materials and Methods
3.1. General Information
Reagents were purchased from Sigma Aldrich (Bayouni Trading Co. Ltd., Al-Khobar, Saudi Arabia) and used without further purification. The reaction progress was monitored by TLC on silica gel pre-coated F254 Merck plates (Merck, Darmstadt, Germany). Spots were visualized by ultraviolet irradiation. Melting points were determined on a digital Gallen-Kamp MFB-595 instrument (Gallenkamp, London, UK) using open capillary tubes and were uncorrected. IR spectra were recorded as potassium bromide discs using Bruker-Vector 22 FTIR spectrophotometer (Bruker, Manasquan, NJ, USA). The NMR spectra were recorded with a Varian Mercury VXR-300 NMR spectrometer (Bruker, Marietta, GA, USA) at 300 and 75 MHz for 1H and 13C NMR spectra, respectively, using DMSO-d6 as the solvent. Mass spectra were recorded on a Hewlett Packard MS-5988 spectrometer (Hewlett Packard, Palo Alto, CA, USA) at 70 eV. Elemental analyses were conducted at the Micro-Analytical Center of Taif University, Taif, KSA. The pharmaceutical activity assays were carried out at the applied research sector, Egyptian company for vaccine and serum (VACSERA, Cairo, Egypt).
5-Methyl-4-(methylthio)-2-oxo-2,11-dihydrobenzo[b]pyrano[2,3-e][1,4]diazepine-3-carbonitrile (5). Compound 1 [28] (0.17 g, 1 mmol), methyl 2-cyano-3,3-dimethylthioacrylate [29] (0.20 mg, 1 mmol) and powdered KOH (0.08 g, 1.5 mmol) in DMSO (25 mL), were stirred at rt for 3 h. The reaction mixture was transferred onto mashed ice under energetic stirring for 1 h. The isolated product was collected, dried, and recrystallized using EtOH to furnish 5 as a pale yellow solid, with an 85% yield; mp 149–151 °C; IR (KBr): (cm−1) 1612 (C=Nstr.), 1705 (C=Ostr.), 2209 (C≡Nstr.), 3206 (N-Hstr.); 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, Me), 2.73 (s, 3H, -SMe), 6.80–7.21 (m, 5H, Ar-H and N-HDiazep.); 13C NMR (75 MHz, DMSO-d6): 15.3, 25.6 (2Me), 115.9 (C≡N), 76.8, 86.4, 113.6, 123.5, 124.1, 126.6, 128.5, 137.3, 138.1 (9 C=C), 149.5 (C=O), 164.5 (C=N), 179.8 (=C-S-); MS (m/z, %): 297.06 (M+, 35); Anal. Calcd. for C15H11N3O2S (297.33): C, 60.59; H, 3.73; N, 14.13%. Found: C, 60.21; H, 3.35; N, 14.01%.
General procedure of the synthesis of compounds (6–28). Compound 5 (0.29 g, 1 mmol) and some selected amino compounds namely; ethylenediamine, hydrazine hydrate, hydroxylamine hydrochloride, phenyl hydrazine, thiosemicarbazide, urea, thiourea, guanidine hydrochloride, 2-aminophenol, 2-aminothiophenol, 2-amino-2-(hydroxymethyl)propane-1,3-diol, aniline, secondary amines, 2-cyanothioacetamide, 5-amino-3-phenyl-1H-pyrazole, 6-amino-2-thioxo-2,3-dihydropyrimidin-4(1H)-one, 3-amino-1,2,4-triazole, 2-thioxodihydropyrimidine-4,6(1H,5H)-dione, 6-methylpyridin-2-amine, 2-cyanomethyl benzimidazole, and 2-amino benzimidazole in 25 mL of EtOH has Et3N (0.3 mL), was refluxed for 7–10 h and the reaction advance was checked by TLC. After evaporation of EtOH, the crude product was triturated with acetone, and the isolated solid was filtered and purified by crystallization, using the proper solvent to furnish compounds (6–28) in fair yields.
3-Amino-12-methyl-1H-benzo[b]pyrazolo[3′,4′:4,5]pyrano[2,3-e][1,4]diazepin-4(6H)-one (6). Yellow powder (EtOH) with a 65% yield; mp 205–207 °C; IR (KBr): (cm−1) 1610–1623 (3C=Nstr.), 1705 (C=Ostr.), 3206–3325 (N-Hstr., N-H2str.); 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, Me), 2.51 (s, 1H, Pyran(C3)-H), 6.80–7.21 (m, 5H, Ar-H and N-HDiazep.), 8.43 (brs, 2H, NH2, D2O-exchangeable); MS (m/z, %): 281.09 (M+, 15); Anal. Calcd. for C14H11N5O2 (281.27): C, 59.78; H, 3.94; N, 24.90%. Found: C, 59.47; H, 3.63; N, 24.59%.
3-Amino-12-methylbenzo[b]isoxazolo[3′,4′:4,5]pyrano[2,3-e][1,4]diazepin-4(6H)-one (7). Yellow crystal (EtOH) with a 71% yield; mp 241–243 °C; IR (KBr): (cm−1) 1610–1623 (2C=Nstr.), 1705 (C=Ostr.), 3206–3325 (N-Hstr., N-H2str.); 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, Me), 6.80–7.21 (m, 7H, Ar-H, N-HDiazep. and NH2); MS (m/z, %): 282.07 (M+, 50); Anal. Calcd. for C14H10N4O3 (282.25): C, 59.57; H, 3.57; N, 19.85%. Found: C, 59.31; H, 3.20; N, 19.52%.
3-Amino-12-methyl-2-phenyl-2H-benzo[b]pyrazolo[3′,4′:4,5]pyrano[2,3-e][1,4]diazepin-4(6H)-one (8). Yellow solid (MeOH) with a 65% yield; mp 223–225 °C; IR (KBr): (cm−1) 1610–1623 (2C=Nstr.), 1705 (C=Ostr.), 3206–3325 (N-Hstr., N-H2str.); 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, Me), 6.80–7.21 (m, 13H, Ar-H, N-HDiazep. and NH2); MS (m/z, %): 357.12 (M+, 15); Anal. Calcd. for C20H15N5O2 (357.37): C, 67.22; H, 4.23; N, 19.60%. Found: C, 67.12; H, 4.10; N, 19.42%.
3-Amino-12-methyl-4-oxo-4,6-dihydro-2H-benzo[b]pyrazolo[3′,4′:4,5]pyrano[2,3-e][1,4]diazepine-2-carbothioamide (9). Pale-yellow powder (EtOH) with a 75% yield; mp 143–145 °C; IR (KBr): (cm−1) 1325 (C=Sstr.), 1610–1623 (2C=Nstr.), 1705 (C=Ostr.), 3206–3325 (N-Hstr., N-H2str.); 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, Me), 6.80–7.21 (m, 7H, Ar-H, N-HDiazep. and NH2), 8.53 (brs, 2H, CSNH2, D2O-exchangeable); MS (m/z, %): 340.07 (M+, 25); Anal. Calcd. for C15H12N6O2S (340.36): C, 52.93; H, 3.55; N, 24.69%. Found: C, 52.71; H, 3.26; N, 24.35%.
4-Amino-13-methylbenzo[b]pyrimido[4′,5′:4,5]pyrano[2,3-e][1,4]diazepine-2,5(1H,7H)-dione (10). Yellowish-brown solid (EtOH) with an 82% yield; mp 165–167 °C; IR (KBr): (cm−1) 1327 (C=Sstr.), 1610–1623 (2C=Nstr.), 1705–1715 (2C=Ostr.), 3206–3325 (N-Hstr., N-H2str.); 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, Me), 6.80–7.21 (m, 5H, Ar-H and N-HDiazep.), 8.61 (brs, 2H, NH2, D2O-exchangeable), 12.51 (brs, H, NHPyrim., D2O-exchangeable); 13C NMR (75 MHz, DMSO-d6): 25.6 (Me), 76.8, 91.5, 113.6, 123.5, 124.1, 126.6, 128.5, 137.3, 138.1, 152.5 (9 C=C), 156.2, 162.7 (2C=O), 164.5, 167.1 (2C=N); MS (m/z, %): 310.01 (M++1, 40); Anal. Calcd. for C15H11N5O3 (309.28): C, 58.25; H, 3.58; N, 22.64%. Found: C, 58.04; H, 3.21; N, 22.34%.
4-Amino-13-methyl-2-thioxo-1,2-dihydrobenzo[b]pyrimido[4′,5′:4,5]pyrano[2,3-e][1,4]diazepin-5(7H)-one (11). Yellow crystals (MeOH) with an 80% yield; mp 205–207 °C; IR (KBr): (cm−1) 1321 (C=Sstr.), 1610–1623 (2C=Nstr.), 1705 (C=Ostr.), 3206-3325 (N-Hstr., N-H2str.); 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, Me), 6.80–7.21 (m, 5H, Ar-H and N-HDiazep.), 8.61 (brs, 2H, NH2, D2O-exchangeable), 10.32 (brs, H, NHPyrim., D2O-exchangeable); MS (m/z, %): 325.06 (M+, 55); Anal. Calcd. for C15H11N5O2S (325.35): C, 55.38; H, 3.41; N, 21.53%. Found: C, 55.02; H, 3.15; N, 21.24%.
2,4-Diamino-13-methylbenzo[b]pyrimido[4′,5′:4,5]pyrano[2,3-e][1,4]diazepin-5(7H)-one (12). Yellow crystals (EtOH) with a 75% yield; mp 221–223 °C; IR (KBr): (cm−1) 1610–1623 (2C=Nstr.), 1705 (C=Ostr.), 3206–3325 (N-Hstr., 2N-H2str.); 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, Me), 6.80–7.21 (m, 9H, Ar-H, N-HDiazep. and 2 NH2.); MS (m/z, %): 308.10 (M+, 30); Anal. Calcd. for C15H12N6O2 (308.29): C, 58.44; H, 3.92; N, 27.26%. Found: C, 58.21; H, 3.65; N, 27.11%.
8-Amino-15-methylbenzo[b]benzo[2′,3′][1,4]diazepino[6′,5′:5,6]pyrano[3,4-f][1,4]oxazepin-7(5H)-one (13). Off-white powder (EtOH) with a 65% yield; mp 241–243 °C; IR (KBr): (cm−1) 1610–1623 (2C=Nstr.), 1705 (C=Ostr.), 3206–3325 (N-Hstr., N-H2str.); 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, Me), 6.80–7.21 (m, 9H, Ar-H and N-HDiazep.), 8.61 (brs, 2H, NH2, D2O-exchangeable); MS (m/z, %): 358.11 (M+, 43); Anal. Calcd. for C20H14N4O3 (358.35): C, 67.03; H, 3.94; N, 15.63%. Found: C, 66.86; H, 3.67; N, 15.42%.
8-Amino-15-methylbenzo[b]benzo[2′,3′][1,4]diazepino[6′,5′:5,6]pyrano[3,4-f][1,4]thiazepin-7(5H)-one (14). Yellow crystals (MeOH/dioxane (2:1)) with a 75% yield; mp 176–178 °C; IR (KBr): (cm−1) 1610–1623 (2C=Nstr.), 1705 (C=Ostr.), 3206–3325 (N-Hstr., N-H2str.); 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, Me), 6.80–7.21 (m, 9H, Ar-H and N-HDiazep.), 8.60 (brs, 2H, NH2, D2O-exchangeable); MS (m/z, %): 374.08 (M+, 15); Anal. Calcd. for C20H14N4O2S (374.42): C, 64.16; H, 3.77; N, 14.96%. Found: C, 64.01; H, 3.51; N, 14.58%.
4-((1,3-Dihydroxy-2-(hydroxymethyl)propan-2-yl)amino)-5-methyl-2-oxo-2,11-dihydrobenzo[b]pyrano[2,3-e][1,4]diazepine-3-carbonitrile (15). Yellowish-brown solid (MeOH) with a 75% yield; mp 215–217 °C; IR (KBr): (cm−1) 1615 (C=Nstr.), 1705 (C=Ostr.), 2217 (C≡Nstr.), 3226 (2N-Hstr.), 3406 (3 O-Hstr); 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, Me), 3.20 (s, 6H, 3CH2), 3.61 (brs, 3H, 3 OH, D2O-exchangeable), 4.02 (brs, 1H, NH, D2O-exchangeable), 6.80–7.21 (m, 5H, Ar-H and N-HDiazep.); 13C NMR (75 MHz, DMSO-d6): 26.3 (Me), 63.4 (3CH2), 114.6 (C≡N), 63.5, 74.9 (4C-C), 63.3, 76.8, 113.5, 123.5, 124.1, 126.6, 128.5, 137.3, 138.1, 181.9 (10 C=C), 162.3 (C=O), 164.5 (C=N); MS (m/z, %): 370.13 (M+, 25); Anal. Calcd. for C18H18N4O5 (370.36): C, 58.37; H, 4.90; N, 15.13%. Found: C, 58.12; H, 4.74; N, 15.02%.
5-Methyl-2-oxo-4-(phenylamino)-2,11-dihydrobenzo[b]pyrano[2,3-e][1,4]diazepine-3-carbonitrile (16). White powder (EtOH) with an 80% yield; mp 232–234 °C; IR (KBr): (cm−1) 1612 (C=Nstr.), 1705 (C=Ostr.), 2209 (C≡Nstr.), 3206 (2N-Hstr.); 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, Me), 6.80–7.21 (m, 10H, Ar-H and N-HDiazep.), 8.71 (brs, 1H, NH, D2O-exchangeable); MS (m/z, %): 342.10 (M+, 10); Anal. Calcd. for C20H14N4O2 (342.35): C, 70.17; H, 4.12; N, 16.37%. Found: C, 70.01; H, 4.02; N, 16.13%.
4-(Diethylamino)-5-methyl-2-oxo-2,11-dihydrobenzo[b]pyrano[2,3-e][1,4]diazepine-3-carbonitrile (17). Light brown solid (EtOH) with a 65% yield; mp 182–184 °C; IR (KBr): (cm−1) 1612 (C=Nstr.), 1705 (C=Ostr.), 2209 (C≡Nstr.), 3206 (N-Hstr.); 1H NMR (300 MHz, DMSO-d6): δ 1.16 (t, 6H, 2CH2CH3), 2.01 (s, 3H, Me), 3.35 (q, 4H, 2CH2CH3), 6.80-7.21 (m, 5H, Ar-H and N-HDiazep.); MS (m/z, %): 322.14 (M+, 25); Anal. Calcd. for C18H18N4O2 (322.36): C, 67.07; H, 5.63; N, 17.38%. Found: C, 66.86; H, 5.35; N, 17.14%.
5-Methyl-4-morpholino-2-oxo-2,11-dihydrobenzo[b]pyrano[2,3-e][1,4]diazepine-3-carbonitrile (18). Buff solid (EtOH) with a 70% yield; mp 142–144 °C; IR (KBr): (cm−1) 1612 (C=Nstr.), 1705 (C=Ostr.), 2209 (C≡Nstr.), 3206 (N-Hstr.); 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, Me), 3.64 (t, 4H, Morpho.(C2),(C6)-H4), 3.83 (m, 4H, Morpho.(C3),(C5)-H4), 6.80–7.21 (m, 5H, Ar-H and N-HDiazep.); 13C NMR (75 MHz, DMSO-d6): 26.1 (Me), 52.4, 67.3 (4CH2), 114.6 (C≡N), 59.3, 76.7, 113.5, 123.5, 124.1, 126.6, 128.5, 137.3, 138.1, 184.9 (10 C=C), 162.3 (C=O), 164.5 (C=N); MS (m/z, %): 336.12 (M+, 9); Anal. Calcd. for C18H16N4O3 (336.34): C, 64.28; H, 4.79; N, 16.66%. Found C, 64.10; H, 4.43; N, 16.28%.
5-Methyl-4-(4-methylpiperazin-1-yl)-2-oxo-2,11-dihydrobenzo[b]pyrano[2,3-e][1,4]diazepine-3-carbonitrile (19). Buff solid (EtOH) with a 75% yield; mp 193–195 °C; IR (KBr): (cm−1) 1612 (C=Nstr.), 1705 (C=Ostr.), 2209 (C≡Nstr.), 3206 (N-Hstr.); 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, Me), 2.21 (s, 3H, Me), 2.32 (t, 4H, Piperaz.(C2),(C6)-H4), 2.76 (t, 4H, Piperaz.(C3),(C5)-H4), 6.80–7.21 (m, 5H, Ar-H and N-HDiazep.); MS (m/z, %): 349.15 (M+, 15); Anal. Calcd. for C19H19N5O2 (349.39): C, 65.32; H, 5.48; N, 20.04%. Found C, 65.11; H, 5.23; N, 19.81%.
5-Methyl-2-oxo-4-(piperazin-1-yl)-2,11-dihydrobenzo[b]pyrano[2,3-e][1,4]diazepine-3-carbonitrile (20). Off-white powder (EtOH) with an 80% yield; mp 219–221 °C; IR (KBr): (cm−1) 1612 (C=Nstr.), 1705 (C=Ostr.), 2209 (C≡Nstr.), 3206–3211 (2N-Hstr.); 1H NMR (300 MHz, DMSO-d6): δ 2.32 (t, 4H, Piperaz.(C2),(C6)-H4), 2.76 (t, 4H, Piperaz.(C3),(C5)-H4), 3.01 (s, 1H, N-HPiperaz.), 6.80–7.21 (m, 5H, Ar-H and N-HDiazep.); MS (m/z, %): 335.10 (M+, 30); Anal. Calcd. for C18H17N5O2 (335.36): C, 64.47; H, 5.11; N, 20.88%. Found C, 64.25; H, 5.01; N, 20.52%.
2-(4-Imino-13-methyl-5-oxo-5,7-dihydro-4H-[1,3]thiazino[4′,5′:4,5]pyrano[2,3-e]benzo[b][1,4]diazepin-2-yl) acetonitrile (21). Yellow solid (MeOH/dioxane (2:1)) with an 80% yield; mp 206–208 °C; IR (KBr): (cm−1) 1610–1623 (2C=Nstr.), 1705 (C=Ostr.), 2209 (C≡Nstr.), 3206–3325 (2N-Hstr.); 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, Me), 4.15 (s, 2H, CH2), 6.80–7.21 (m, 9H, Ar-H and N-HDiazep.), 8.91 (brs, H, =NH, D2O-exchangeable); MS (m/z, %): 349.06 (M+, 45); Anal. Calcd. for C17H11N5O2S (349.37): C, 58.44; H, 3.17; N, 20.05%. Found: C, 58.20; H, 3.01; N, 19.76%.
5-Amino-14-methyl-2-phenylbenzo[b]pyrazolo[1″,5″:1′,2′]pyrimido[4′,5′:4,5]pyrano[2,3-e][1,4]diazepin-6(8H)-one (22). Pale yellow crystalline solid (EtOH) with an 85% yield; mp 228–230 °C; IR (KBr): (cm−1) 1610–1623 (3C=Nstr.), 1705 (C=Ostr.), 3206–3325 (N-Hstr., N-H2str.); 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, Me), 6.54 (s, 1H, Pyraz.(C4)-H), 6.80–8.03 (m, 12H, Ar-H, N-HDiazep. and NH2.); MS (m/z, %): 408.13 (M+, 10); Anal. Calcd. for C23H16N6O2 (408.41): C, 67.64; H, 3.95; N, 20.58%. Found: C, 67.32; H, 3.68; N, 20.26%.
5-Amino-14-methyl-[1,2,4]triazolo[1″,5″:1′,2′]pyrimido[4′,5′:4,5]pyrano[2,3-e]benzo[b][1,4]diazepin-6(8H)-one (23). Pale yellow powder solid (EtOH) with a 72% yield; mp 241–243 °C; IR (KBr): (cm−1) 1610–1623 (4C=Nstr.), 1705 (C=Ostr.), 3206–3325 (N-Hstr., N-H2str.); 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, Me), 6.80–7.23 (m, 5H, Ar-H and N-HDiazep.), 7.81 (brs, 2H, NH2, D2O-exchangeable), 8.65 (s, 1H, Triaz.(C3)-H); 13C NMR (75 MHz, DMSO-d6): 19.8 (Me), 83.9, 107.3, 113.5, 123.5, 124.1, 126.5, 128.5, 138.2, 139.1, 168.6 (9 C=C), 150.3 (C=O), 155.2, 156.1, 164.5, 166.1 (4C=N); MS (m/z, %): 333.09 (M+, 55); Anal. Calcd. for C16H11N7O2 (333.30): C, 57.66; H, 3.33; N, 29.42%. Found: C, 57.39; H, 3.13; N, 29.19%.
6-Amino-15-methyl-4-thioxo-3,4-dihydro-2H-benzo[b]pyrimido[1″,6″:1′,2′]pyrimido[4′,5′:4,5]pyrano[2,3-e][1,4]diazepine-2,7(9H)-dione (24). Light brown crystalline solid (EtOH) with an 80% yield; mp 195–197 °C; IR (KBr): (cm−1) 1321 (C=Sstr.), 1610–1623 (2C=Nstr.), 1705–1715 (2C=Ostr.), 3206–3325 (2N-Hstr., N-H2str.); 1H NMR (300 MHz, DMSO-d6): δ 2.06 (s, 3H, Me), 6.80–7.21 (m, 5H, Ar-H and N-HDiazep.), 8.52 (brs, 2H, NH2, D2O-exchangeable), 9.64 (brs, H, NHPyrim., D2O-exchangeable); MS (m/z, %): 392.08 (M+, 20); Anal. Calcd. for C18H12N6O3S (392.39): C, 55.10; H, 3.08; N, 21.42%. Found: C, 55.02; H, 3.10; N, 21.19%.
6-Imino-15-methyl-3-thioxo-3,4,6,9-tetrahydro-1H-benzo[b]pyrimido[5″,4″:5′,6′]pyrano[4′,3′:4,5]pyrano [2,3-e][1,4]diazepine-1,7(2H)-dione (25). Brown solid (EtOH) with a 75% yield; mp 165–167 °C; IR (KBr): (cm−1) 1321 (C=Sstr.), 1610–1623 (2C=Nstr.), 1705–1715 (2C=Ostr.), 3206-3325 (4N-Hstr.); 1H NMR (300 MHz, DMSO-d6): δ 2.06 (s, 3H, Me), 6.80–7.21 (m, 5H, Ar-H and N-HDiazep.), 8.68 (brs, H, =NH, D2O-exchangeable), 9.64, 13.21 (brs, 2H, 2NHPyrim., D2O-exchangeable); MS (m/z, %): 393.05 (M+, 60); Anal. Calcd. for C18H11N5O4S (393.38): C, 54.96; H, 2.82; N, 17.80%. Found: C, 54.65; H, 2.42; N, 17.58%.
8-Imino-10,15-dimethyl-5H-benzo[b]pyrido[1″,2″:1′,2′]pyrimido[4′,5′:4,5]pyrano[2,3-e][1,4]diazepin-7(8H)-one (26). Orange powder (MeOH/DMF (3:1)) with a 75% yield; mp > 300 °C; IR (KBr): (cm−1) 1610–1623 (3C=Nstr.), 1705 (C=Ostr.), 3206–3325 (2N-Hstr.); 1H NMR (300 MHz, DMSO-d6): δ 2.06, 2.75 (s, 6H, 2Me), 6.80–7.21 (m, 8H, Ar-H, N-HDiazep. and Pyrid.(C3,4,5)-3H), 8.92 (brs, H, =NH, D2O-exchangeable); 13C NMR (75 MHz, DMSO-d6): 25.2, 26.1 (2Me), 76.6, 108.2, 113.5, 115.5, 123.5, 124.1, 125.1, 126.5, 128.5, 134.1, 137.2, 138.2, 156.8, 159.6 (14 C=C), 162.7 (C=O), 150.5, 158.9, 164.5 (3C=N); MS (m/z, %): 357.12 (M+, 50); Anal. Calcd. for C20H15N5O2 (357.37): C, 67.22; H, 4.23; N, 19.60%. Found: C, 67.10; H, 4.11; N, 19.34%.
8-Amino-16-methyl-7-oxo-5,7-dihydrobenzo[b]benzo[4″,5″]imidazo[1″,2″:1′,6′]pyrido[4′,3′: 4,5]pyrano[2,3-e][1,4]diazepine-15-carbonitrile (27). Yellowish-brown powder (EtOH) with an 80% yield; mp 215–217 °C; IR (KBr): (cm−1) 1610–1623 (2C=Nstr.), 1705 (C=Ostr.), 2210 (C≡Nstr.), 3206–3325 (N-Hstr., N-H2str.); 1H NMR (300 MHz, DMSO-d6): δ 2.07 (s, 3H, Me), 6.51 (brs, 2H, NH2, D2O-exchangeable), 6.80–8.03 (m, 9H, Ar-H and N-HDiazep.); MS (m/z, %): 406.12 (M+, 15); Anal. Calcd. for C23H14N6O2 (406.40): C, 67.97; H, 3.47; N, 20.68%. Found: C, 67.61; H, 3.27; N, 20.38%.
8-Amino-16-methylbenzo[b]benzo[4″,5″]imidazo[1″,2″:1′,2′]pyrimido[4′,5′:4,5]pyrano[2,3-e][1,4]diazepin-7(5H)-one (28). Yellow powder (MeOH/DMF (3:1)) with a 75% yield; mp 175–177 °C; IR (KBr): (cm−1) 1610–1623 (3C=Nstr.), 1705 (C=Ostr.), 3206–3325 (N-Hstr., N-H2str.); 1H NMR (300 MHz, DMSO-d6): δ 2.07 (s, 3H, Me), 6.54 (brs, 2H, NH2, D2O-exchangeable), 6.80–8.45 (m, 9H, Ar-H and N-HDiazep.); MS (m/z, %): 382.13 (M+, 60); Anal. Calcd. for C21H14N6O2 (382.37): C, 65.96; H, 3.69; N, 21.98%. Found: C, 65.68; H, 3.38; N, 21.67%.
5-Imino-14-methyl-2,3,5,8-tetrahydrobenzo[b]cyclopenta[5′,6′]pyrano[4′,3′:4,5]pyrano[2,3-e][1,4]diazepin-6(1H)-one (31). A solution of 5 (0.29 g, 1 mmol) and cyclopentanone (0.08 g, 1 mmol) in ethanolic piperidine solution (30 mL) was refluxed for 7 h, afterwards the reaction mixture was left to cool to rt, the obtained precipitate was filtered, dried, and recrystallized using EtOH to afford 31 as a brown solid with a 65% yield, mp 268–270 °C; IR (KBr): (cm−1) 1610 (C=Nstr.), 1705 (C=Ostr.), 3206–3325 (2N-Hstr.); 1H NMR (300 MHz, DMSO-d6): δ 1.83 (m, 2H, CH2), 2.06 (s, 3H, Me), 2.43 (t, 2H, CH2), 2.81 (t, 2H, CH2), 6.80–7.21 (m, 5H, Ar-H and N-HDiazep.), 8.95 (brs, H, =NH, D2O-exchangeable); MS (m/z, %): 333.10 (M+, 25); Anal. Calcd. for C19H15N3O3 (333.34): C, 68.46; H, 4.54; N, 12.61%. Found: C, 68.18; H, 4.24; N, 12.31%.
14-Methyl-2,3-dihydrobenzo[b]cyclopenta[5′,6′]pyrano[4′,3′:4,5]pyrano[2,3-e][1,4]diazepine-5,6(1H,8H)-dione (32). A mixture of imino analogue 31 (0.33 g, 1 mmol), HClconc. (2 mL) in EtOH (30 mL) was refluxed for 1 h. After cooling the mixture was poured onto mashed ice. A yellow precipitate was collected, washed with H2O carefully, dried, and recrystallized using EtOH to furnish 32 with an 85% yield, mp 238–240 °C; IR (KBr): (cm−1) 1610 (C=Nstr.), 1705 (2 C=Ostr.), 3256 (N-Hstr.); 1H NMR (300 MHz, DMSO-d6): δ 1.83 (m, 2H, CH2), 2.06 (s, 3H, Me), 2.43 (t, 2H, CH2), 2.81 (t, 2H, CH2), 6.80–7.21 (m, 5H, Ar-H and N-HDiazep.); 13C NMR (75 MHz, DMSO-d6): 26.6 (2Me), 18.6, 36.1, 36.6 (3CH2), 76.6, 113.5, 116.1, 118.5, 123.5, 124.1, 126.5, 128.5, 137.2, 138.1, 140.6, 170.1 (12 C=C), 162.7, 162.9 (2 C=O), 164.6 (C=N); MS (m/z, %): 334.10 (M+, 30); Anal. Calcd. for C19H14N2O4 (334.33): C, 68.26; H, 4.22; N, 8.38%. Found: C, 68.12; H, 4.01; N, 8.15%.
1-Acetyl-2,13-dimethyl-4H-benzo[b]pyrano[4′,3′:4,5]pyrano[2,3-e][1,4]diazepine-4,5(7H)-dione (33). A mixture of compound 5 (0.29 g, 1 mmol) and acetylacetone (0.10 mL, 1 mmol) was refluxed in ethanolic-piperidine solution (25 mL) for 6 h. Upon cooling the reaction mixture an off-white precipitate was collected, and recrystallized using EtOH to give 33 with an 80% yield, mp 216–218 °C; IR (KBr): (cm−1) 1610 (C=Nstr.), 1705 (2 C=Ostr.), 3206 (N-Hstr.); 1H NMR (300 MHz, DMSO-d6): δ 2.06, 2.53 (m, 6H, 2Me), 2.36 (s, 3H, COMe), 6.80–7.21 (m, 5H, Ar-H and N-HDiazep.); MS (m/z, %): 350.09 (M+, 10); Anal. Calcd. for C19H14N2O5 (350.32): C, 65.14; H, 4.03; N, 8.00%. Found: C, 65.01; H, 3.86; N, 7.78%.
13-Methyl-1-thioxo-1,2-dihydro-[1,3,5]triazino[1″,2″:1′,5′]pyrazolo[3′,4′:4,5]pyrano[2,3-e]benzo[b][1,4] diazepin-5(7H)-one (34). A solution of α-aminocarbothiamide 9 (0.34 g, 1 mmol) and HC(OEt)3 (10 mL) was refluxed for 6 h. The mixture was cooled, a yellow solid was obtained, then collected by filtration, dried, and recrystallized using EtOH to give 34 with a 76% yield; mp 202–204 °C; IR (KBr): (cm−1) 1326 (C=Sstr.), 1610–1623 (3C=Nstr.), 1705 (C=Ostr.), 3206–3325 (2N-Hstr.); 1H NMR (300 MHz, DMSO-d6): δ 2.07 (s, 3H, Me), 6.80–7.95 (m, 6H, Ar-H, Triazi.(C6)-1H, and N-HDiazep.), 13.56 (brs, H, NHTriazi., D2O-exchangeable); MS (m/z, %): 350.05 (M+, 42); Anal. Calcd. for C16H10N6O2S (350.35): C, 54.85; H, 2.88; N, 23.99%. Found: C, 54.51; H, 2.49; N, 23.64%.
3,13-Dimethyl-1-thioxo-1,2-dihydro-[1,3,5]triazino[1″,2″:1′,5′]pyrazolo[3′,4′:4,5]pyrano[2,3-e]benzo[b][1,4] diazepin-5(7H)-one (35). A mixture of α-aminocarbothiamide 9 (0.68 g, 2 mmol) and acetyl chloride (2 mmol) was refluxed in benzene (30 mL) for 6 h. Upon cooling the isolated product was collected and recrystallized using benzene to afford 35 as an off-white powder, with a 70% yield; mp 182–184 °C; IR (KBr): (cm−1) 1326 (C=Sstr.), 1610–1623 (3C=Nstr.), 1705 (C=Ostr.), 3206–3325 (2N-Hstr.); 1H NMR (300 MHz, DMSO-d6): δ 2.07, 2.45 (s, 6H, 2Me), 6.80–7.95 (m, 5H, Ar-H and N-HDiazep.), 13.32 (brs, H, NHTriazi., D2O-exchangeable); 13C NMR (75 MHz, DMSO-d6): 19.8, 21.2 (2Me), 83.9, 113.5, 114.4, 123.5, 124.1, 126.5, 128.5, 138.1, 139.2, 147.1 (10 C=C), 150.4 (C=O), 133.8, 154.3, 164.6 (3 C=N), 189.5 (C=S); MS (m/z, %): 364.07 (M+, 15); Anal. Calcd. for C17H12N6O2S (364.38): C, 56.04; H, 3.32; N, 23.06%. Found: C, 55.85; H, 3.15; N, 22.79%.
2-(13-Methyl-5-oxo-1-thioxo-1,2,5,7-tetrahydro-[1,3,5]triazino[1″,2″:1′,5′]pyrazolo[3′,4′:4,5]pyrano[2,3-e] benzo[b][1,4]diazepin-3-yl)acetonitrile (36). It was synthesized under the same conditions as those described for the synthesis of compound 33. Yellow crystals (EtOH) with a 70% yield; mp 198–200 °C; IR (KBr): (cm−1) 1325 (C=Sstr.), 1610–1623 (3C=Nstr.), 1705 (C=Ostr.), 2215 (C≡Nstr.), 3206–3325 (2N-Hstr.); 1H NMR (300 MHz, DMSO-d6): δ 2.07 (s, 3H, Me), 4.17 (s, 2H, CH2), 6.80–7.95 (m, 5H, Ar-H and N-HDiazep.), 13.61 (brs, H, NHTriazi., D2O-exchangeable); 13C NMR (75 MHz, DMSO-d6): 19.8 (Me), 22.2 (CH2), 116.2 (C≡N), 83.9, 113.5, 114.4, 123.5, 124.1, 126.5, 128.5, 138.1, 139.2, 147.1 (10 C=C), 150.4 (C=O), 133.8, 154.3, 164.6 (3 C=N), 189.5 (C=S); MS (m/z, %): 389.05 (M+, 35); Anal. Calcd. for C18H11N7O2S (389.39): C, 55.52; H, 2.85; N, 25.18%. Found: C, 55.25; H, 2.63; N, 25.02%.
3-(Chloromethyl)-13-methyl-1-thioxo-1,2-dihydro-[1,3,5]triazino[1″,2″:1′,5′]pyrazolo[3′,4′:4,5] pyrano[2,3-e]benzo[b][1,4]diazepin-5(7H)-one (37). To a well-stirred solution of α-aminocarbothiamide 9 (0.68 g, 2 mmol) and Et3N (0.3 mL) in absolute EtOH (30 mL), ClCH2COCl (0.24 mL, 1 mmol) was added dropwise for 1 h at rt, and afterwards the mixture was warmed for 6 h at 60 °C. After cooling the reaction mixture and pouring onto mashed ice, a light brown solid was collected by filtration, dried, and recrystallized using EtOH, to furnish 37 with an 80% yield; mp 198–200 °C; IR (KBr): (cm−1) 1326 (C=Sstr.), 1610–1623 (3C=Nstr.), 1705 (C=Ostr.), 3206–3325 (2N-Hstr.); 1H NMR (300 MHz, DMSO-d6): δ 2.07 (s, 3H, Me), 3.29 (s, 2H, CH2), 6.80–7.95 (m, 5H, Ar-H and N-HDiazep.), 13.63 (brs, H, NHTriazi., D2O-exchangeable); MS (m/z, %): 400.05 (M++2, 10), 398.04 (M+, 32); Anal. Calcd. for C17H11ClN6O2S (398.83): C, 51.20; H, 2.78; Cl, 8.89; N, 21.07%. Found: C, 51.10; H, 2.56; Cl, 8.58; N, 20.85%.
3-Mercapto-13-methyl-1-thioxo-1,2-dihydro-[1,3,5]triazino[1″,2″:1′,5′]pyrazolo[3′,4′:4,5]pyrano[2,3-e] benzo[b][1,4]diazepin-5(7H)-one (38). CS2 (4 mmol) was added to solution of α-aminocarbothiamide 9 (0.68 g, 2 mmol) in EtOH (50 mL), afterwards the mixture was heated in water bath at 80 °C for 6 h. After evaporation of the solvent to one fourth of its volume, the mixture was poured into mashed ice. The formed product was collected, washed carefully with H2O, and recrystallized using MeOH to give 38 as a yellowish-brown powder, with a 65% yield; mp 213–215 °C; IR (KBr): (cm−1) 1326 (C=Sstr.), 1610–1623 (3C=Nstr.), 1705 (C=Ostr.), 2453 (S-Hstr.), 3206–3325 (2N-Hstr.); 1H NMR (300 MHz, DMSO-d6): δ 2.07 (s, 3H, Me), 6.80–7.95 (m, 5H, Ar-H and N-HDiazep.), 12.01 (s, 1H, S-H, D2O exchangeable), 13.41 (brs, H, NHTriazi., D2O-exchangeable); MS (m/z, %): 382.42 (M+, 35); Anal. Calcd. for C16H10N6O2S2 (382.42): C, 50.25; H, 2.64; N, 21.98%. Found: C, 50.14; H, 2.32; N, 21.69%.
3.2. Cytotoxic Assessment
Methodology
The preliminary cytotoxic impacts were achieved using the SRB method as previously reported [30,31].
4. Conclusions
The purpose of this study was to synthesize and evaluate the cytotoxic impact of some new benzo[b][1,4]diazepines. We synthesized 5-methyl-4-(methylthio)-2-oxo-2,11-dihydrobenzo[b] pyrano[2,3-e][1,4]diazepine-3-carbonitrile (5), and 3-amino-12-methyl-4-oxo-4,6-dihydro-2H-benzo[b]pyrazolo[3′,4′:4,5]pyrano[2,3-e][1,4]diazepine-2-carbothioamide (9), as a new bipod and tripod pharmacophoric architectures. The vitality of the terminal o-methylthionitrile as well as α-aminocarbothiamide tags were inspected in a sequence of treatments, including cyclocondensation at the annulation of new tri and/or tetrapod pharmacophoric analogues. The preliminary cytotoxicity declared that, majority of the examined compounds own momentous cytotoxic activities. Compound 9 was the most effective among the screened series with IC50 = 16.19 ± 1.35 and 17.16 ± 1.54 μM against HCT-116 and MCF-7, respectively. Moreover, the tested tumor cell lines were generally susceptible to conjugates 7, 6, 8, 15, 16, 18, 19, and 20, in a downward order, with IC50 < 60.00 μM.
Author Contributions
Conceptualization: I.H.E.A.; performing the research: I.H.E.A. and N.A.A.E.; writing—original draft: N.A.A.E.; and review, editing, and supervision: I.H.E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work is dedicated to my father (Helmy El Azab).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Verma, S.; Kumar, S.; Kumar, S. Design, synthesis, computational and biological evaluation of new benzodiazepines as CNS agents. Arab. J. Chem. 2020, 13, 863–874. [Google Scholar] [CrossRef]
- Sigel, E.; Ernst, M. The Benzodiazepine Binding Sites of GABAA Receptors. Trends Pharmacol Sci. 2018, 39, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.F.; Barthel, A.L.; Hofmann, S.G. Comparing the efficacy of benzodiazepines and serotonergic anti-depressants for adults with generalized anxiety disorder: A meta-analytic review. Expert Opin Pharmacother. 2018, 19, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, S.; Lindström, V.; Ingelsson, M.; Hammarlund-Udenaes, M.; Syvänen, S. Intact blood-brain barrier transport of small molecular drugs in animal models of amyloid beta and alpha-synuclein pathology. Neuropharmacology 2018, 128, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Chadha, S.; Paul, S.; Kapoor, K.K. Synthesis and biological screening of 4-(5-alkyl-2-isoxazolin-3-yl)-2-aryl-2,3-dihydro-1H-1,5-benzodiazepines. J. Chem. Pharm. Res. 2011, 3, 331–340. [Google Scholar]
- Sachdeva, A.; Choudhary, M.; Chandra, M. Alcohol withdrawal syndrome: Benzodiazepines and beyond. J. Clin. Diagn. Res. 2015, 9, VE01–VE07. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Li, J.; Sun, H. Insights into ETA subtype selectivity of benzodiazepine endothelin receptor antagonists by 3D-QSAR approaches. J. Mol. Model. 2012, 18, 1299–1311. [Google Scholar] [CrossRef]
- Soyka, M. Treatment of benzodiazepine dependence. N. Engl. J. Med. 2017, 376, 1147–1157. [Google Scholar] [CrossRef]
- Guina, J.; Merrill, B. Benzodiazepines I: Upping the care on downers: The evidence of risks, benefits and alternatives. J. Clin. Med. 2018, 7, 17. [Google Scholar] [CrossRef]
- Manconi, M.; Ferri, R.; Miano, S.; Maestri, M.; Bottasini, V.; Zucconi, M.; Ferini-Strambi, L. Sleep architecture in insomniacs with severe benzodiazepine abuse. J. Clin. Neurophysiol. 2017, 128, 875–881. [Google Scholar] [CrossRef]
- Lawrence, P.; Tardibono, Jr.; Marvin, J.M. Synthesis and Anti-Cancer Activity of New Hydroxamic Acid Containing 1,4-Benzodiazepines. Org. Lett. 2009, 11, 1575–1578. [Google Scholar]
- Menghani, S.S.; Chikhale, R.; Pant, A.; Mathew, B.; Khedekar, P. Molecular Docking, Synthesis and CNS Activity of Some Novel 1,4-Benzodiazepine Derivatives. Lett. Drug Des. Discovery 2017, 14, 690–698. [Google Scholar] [CrossRef]
- Griffin, C.E.; Kaye, A.M.; Bueno, F.R.; Kaye, A.D. Benzodiazepine pharmacology and central nervous system–mediated effects. Ochsner J. 2013, 13, 214–223. [Google Scholar] [PubMed]
- Kumaraswamy, S.; Chien-Shu, C.; Chi-Fen, C.; Srinivas, P.; Khagga, M.; Chi-Rei, W.; Ta-Hsien, C. Synthesis, Anticonvulsant, Sedative and Anxiolytic Activities of Novel Annulated Pyrrolo[1,4]benzodiazepines. Int. J. Mol. Sci. 2014, 15, 16500–16510. [Google Scholar]
- Włodarczyk, A.; Szarmach, J.; Cubała, W.J.; Wiglusz, M.S. Benzodiazepines in combination with antipsychotic drugs for schizophrenia: GABA-ergic targeted therapy. Psychiatr Danub. 2017, 29, 345–348. [Google Scholar]
- Mahmoud, A.A.; El-Sayed, W.M. The Anti-Proliferative Activity of Anisosciadone: A New Guaiane Sesquiterpene from Anisosciadium lanatum. Anti-Cancer Agents Med. Chem. 2019, 19, 1114–1119. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, Q.; Gao, L.; Tong, L.; Li, J.; Chen, Y.; Lu, W. Pyrazolo[4,3-b]pyrimido[4,5-e] [1,4]diazepine derivatives as new multi-targeted inhibitors of Aurora A/B and KDR. Eur. J. Med. Chem. 2018, 5, 428–441. [Google Scholar] [CrossRef]
- Bader, A.A.S.; Omar, A.H.; El-Odemi, M.H. Anti-inflammatory Effects of Diazepam on Different Models of Inflammation: Roles of Peripheral Benzodiazepine Receptors and Genes for Corticosterone, Nitric Oxide and Cytokines Biosynthesis. J. Clin. Epigenet. 2017, 3, 1–8. [Google Scholar]
- Jing-Jing, L.; Xin-Ying, W.; Jing-Lou, C.; Guan-Rong, C.; Jun, X.; Ye, G.; Hong-Ping, S. Antiplatelet drug ticagrelor delays gastric ulcer healing in rats. Exp. Ther. Med. 2017, 14, 3774–3779. [Google Scholar]
- Pavlovsky, V.I.; Tsymbalyuk, O.V.; Martynyuk, V.S.; Kabanova, T.A.; Semenishyna, E.A.; Khalimova, E.I.; Andronati, S.A. Analgesic Effects of 3-Substituted Derivatives of 1,4-Benzodiazepines and their Possible Mechanisms. Neurophysiology 2013, 45, 427–432. [Google Scholar] [CrossRef]
- El Azab, I.H.; Elkanzi, N.A.A. Synthesis and anti-tumour activity of some new sebacoyl chloride-based heterocycles. Curr. Org. Synth. 2017, 14, 309–320. [Google Scholar] [CrossRef]
- El Azab, I.H.; Elkanzi, N.A.A. Design, Synthesis, and Antimicrobial Evaluation of New Annelated Pyrimido[2,1-c][1,2,4]triazolo[3,4-f][1,2,4]triazines. Molecules 2020, 25, 1339. [Google Scholar] [CrossRef] [PubMed]
- El Azab, I.H.; Elkanzi, N.A.A.; Gobouri, A.A. Design and Synthesis of Some New Quinoxaline-Based Heterocycles. J. Heterocycl. Chem. 2018, 55, 65–76. [Google Scholar] [CrossRef]
- El Azab, I.H.; Gobouri, A.A.; Altalhi, T.A. 4-Chlorothiazole-5-carbaldehydes as Potent Precursors for Synthesis of Some New Pendant N-heterocyces Endowed with Anti-Tumor Activity. J. Heterocycl. Chem. 2019, 56, 281–295. [Google Scholar] [CrossRef]
- El Azab, I.H.; Elkanzi, N.A.A.; Gobouri, A.A.; Altalhi, T.A. Convenient Synthesis of Novel Nitrogen Bridgehead Heterocycles Utilizing 3-Mercapto-6H-[1,2,4,5]oxatriazino[3,2-a] isoindol-6-one as a New Synthon. J. Heterocycl. Chem. 2019, 56, 60–72. [Google Scholar] [CrossRef]
- El Azab, I.H.; Abu Ali, O.A.; El-Zahrani, A.H.; Gobouri, A.A.; Altalhi, T.A. Pyrazole-1-carbothioamide as a Potent Precursor for Synthesis of Some New N-heterocycles of Potential Biological Activity. J. Heterocycl. Chem. 2019, 56, 18–31. [Google Scholar] [CrossRef]
- El Azab, I.H.; Khalifa, M.E.; Gobouri, A.A.; Altalhi, T.A. Synthesis, Characterization, and Pharmacological Evaluation of Some New Pteridine-Based Heterocycles as Antimicrobial Agents. J. Heterocycl. Chem. 2019, 56, 1352–1361. [Google Scholar] [CrossRef]
- Israel, M.; Jones, L.C.; Modest, E.J. Thermal rearrangement of condensed dihydrodiazepinones. Tetrahedron Lett. 1968, 46, 4811–4814. [Google Scholar] [CrossRef]
- Maurya, H.K.; Vasudev, A.; Gupta, A. A regioselective synthesis of 2,6-diarylpyridines. RSC Adv. 2013, 3, 12955–12962. [Google Scholar] [CrossRef]
- Youns, M.; Fu, Y.J.; Zu, Y.G.; Kramer, A.; Konkimalla, V.B.; Radlwimmer, B.; Sultmann, H.; Efferth, T. Sensitivity and resistance towards isoliquiritigenin, doxorubicin and methotrexate in T cell acute lymphoblastic leukaemia cell lines by pharmacogenomics. Arch. Pharmacol. 2010, 382, 221–234. [Google Scholar] [CrossRef]
- Kuete, V.; Krusche, B.; Youns, M.; Voukeng, I.; Fankam, A.G.; Tankeo, S.; Lacmata, S.; Efferth, T. Cytotoxicity of some Cameroonian spices and selected medicinal plant extracts. J. Ethnopharmacol. 2011, 134, 803–812. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 6, 7, 8, 9, 15, 16, 18, 19 and 20 are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).