Enhanced Lycopene Extraction from Tomato Peels by Optimized Mixed-Polarity Solvent Mixtures
Abstract
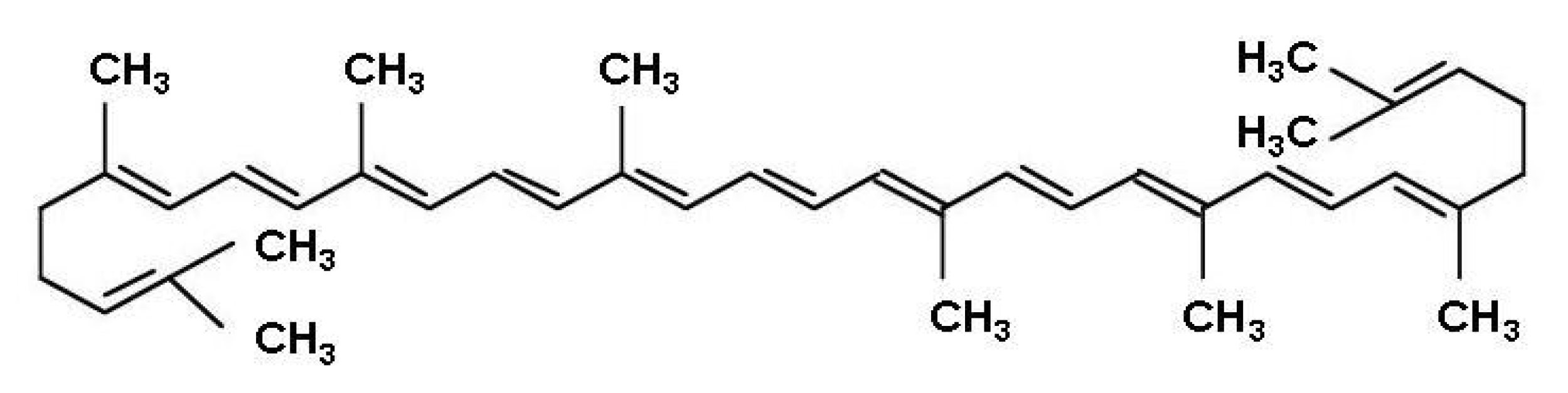
1. Introduction
2. Results and Discussion
2.1. Solvent Effects on Lycopene Extraction
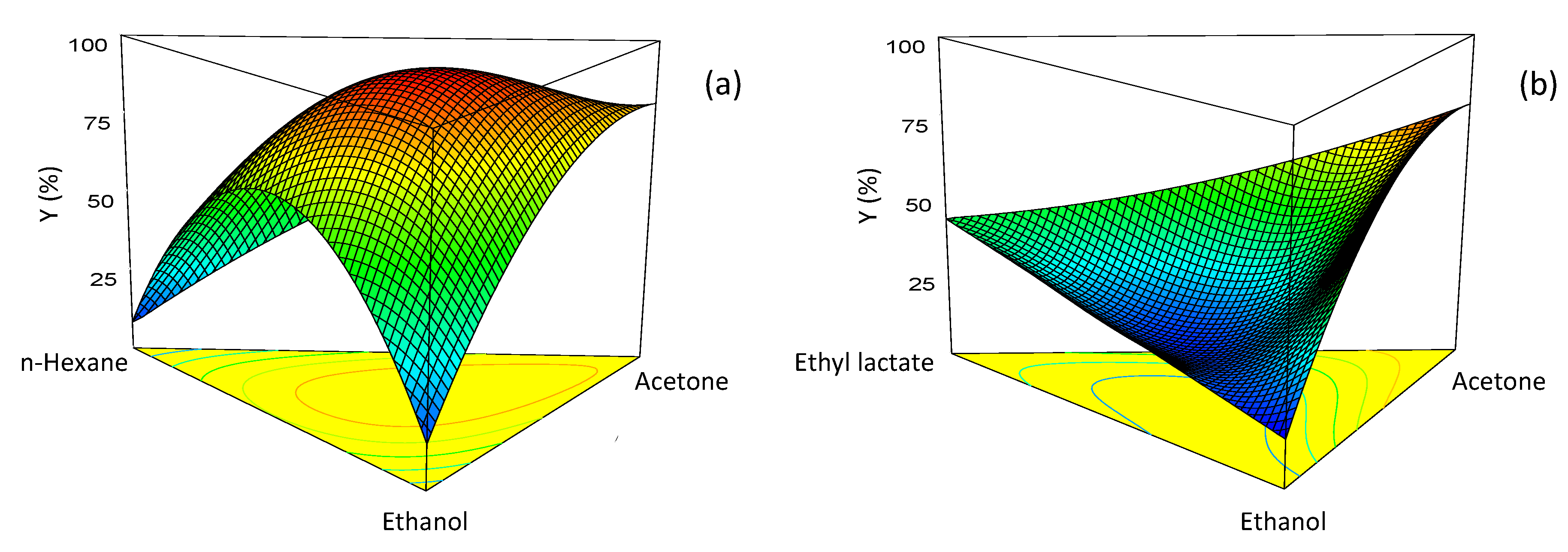
2.2. Optimization of Mixture Composition
2.3. Production of Tomato Oleoresin
3. Materials and Methods
3.1. Chemicals and Plant Material
3.2. Analytical Methods
3.3. Lycopene Extraction Experiments
3.4. Oleoresin Production
3.5. Mixture Design
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.W.; Siervo, M.; Lara, J. Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 141–158. [Google Scholar] [PubMed]
- Senkus, K.E.; Tan, L.; Crowe-White, K.M. Lycopene and metabolic syndrome: A systematic review of the literature. Adv. Nutr. 2019, 10, 19–29. [Google Scholar] [PubMed]
- Trejo-Solís, C.; Pedraza-Chaverrí, J.; Torres-Ramos, M.; Jiménez-Farfán, D.; Cruz Salgado, A.; Serrano-García, N.; Osorio-Rico, L.; Sotelo, J. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid.-Based Complement. Altern. Med. 2013, 2013, 705121. [Google Scholar]
- Wang, Y.; Cui, R.; Xiao, Y.; Fang, J.; Xu, Q. Effect of carotene and lycopene on the risk of prostate cancer: A systematic review and dose-response meta-analysis of observational studies. PLoS ONE 2015, 10, e0137427. [Google Scholar]
- Rowles, J.L.; Ranard, K.M.; Smith, J.W.; An, R.; Erdman, J.W. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017, 20, 361–377. [Google Scholar]
- Cho, K.S.; Shin, M.; Kim, S.; Lee, S.B. Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxidative Med. Cell. Longev. 2018, 2018, 4120458. [Google Scholar]
- Chen, D.; Huang, C.; Chen, Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed. Pharmacother. 2019, 111, 791–801. [Google Scholar]
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and its antioxidant role in the prevention of cardiovascular diseases—A critical review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1868–1879. [Google Scholar]
- Holzapfel, N.P.; Holzapfel, B.M.; Champ, S.; Feldthusen, J.; Clements, J.; Hutmacher, D.W. The potential role of lycopene for the prevention and therapy of prostate cancer: From molecular mechanisms to clinical evidence. Int. J. Mol. Sci. 2013, 14, 14620–14646. [Google Scholar]
- Carini, F.; David, S.; Tomasello, G.; Mazzola, M.; Damiani, P.; Rappa, F.; Battaglia, L.; Cappello, F.; Jurjus, A.; Gerges Geagea, A.; et al. Colorectal cancer: An update on the effects of lycopene on tumor progression and cell proliferation. J. Biol. Regul. Homeost. Agents 2017, 31, 769–774. [Google Scholar]
- Zefferino, R.; Piccoli, C.; Gioia, S.D.; Capitanio, N.; Conese, M. Gap junction intercellular communication in the carcinogenesis hallmarks: Is this a phenomenon or epiphenomenon? Cells 2019, 8, 896. [Google Scholar]
- Hantz, H.L.; Young, L.F.; Martin, K.R. Physiologically attainable concentrations of lycopene induce mitochondrial apoptosis in LNCaP human prostate cancer cells. Exp. Biol. Med. 2005, 230, 171–179. [Google Scholar]
- Przybylska, S. Lycopene—A bioactive carotenoid offering multiple health benefits: A review. Int. J. Food Sci. Technol. 2019, in press. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [PubMed]
- Cervantes Gracia, K.; Llanas-Cornejo, D.; Husi, H. CVD and Oxidative Stress. J. Clin. Med. 2017, 6, 22. [Google Scholar]
- Ciriminna, R.; Fidalgo, A.; Meneguzzo, F.; Ilharco, L.M.; Pagliaro, M. Lycopene: Emerging production methods and applications of a valued carotenoid. ACS Sustain. Chem. Eng. 2016, 4, 643–650. [Google Scholar]
- Papaioannou, E.H.; Liakopoulou-Kyriakides, M.; Karabelas, A.J. Natural origin lycopene and its “green” downstream processing. Crit. Rev. Food Sci. Nutr. 2016, 56, 686–709. [Google Scholar]
- Zuorro, A.; Lavecchia, R. Optimization of enzyme-assisted lycopene extraction from tomato processing waste. Adv. Mat. Res. 2013, 800, 173–176. [Google Scholar]
- Li, A.-N.; Li, S.; Xu, D.-P.; Xu, X.-R.; Chen, Y.-M.; Ling, W.-H.; Chen, F.; Li, H.-B. Optimization of ultrasound-assisted extraction of lycopene from papaya processing waste by response surface methodology. Food Anal. Methods 2015, 8, 1207–1214. [Google Scholar]
- Anarjan, N.; Jouyban, A. Preparation of lycopene nanodispersions from tomato processing waste: Effects of organic phase composition. Food Bioprod. Process. 2017, 103, 104–113. [Google Scholar]
- Kumcuoglu, S.; Yilmaz, T.; Tavman, S. Ultrasound assisted extraction of lycopene from tomato processing wastes. J. Food Sci. Technol. 2014, 51, 4102–4107. [Google Scholar] [PubMed]
- Ho, K.K.H.Y.; Ferruzzi, M.G.; Liceaga, A.M.; San Martín-González, M.F. Microwave-assisted extraction of lycopene in tomato peels: Effect of extraction conditions on all-trans and cis-isomer yields. LWT-Food Sci. Technol. 2015, 62, 160–168. [Google Scholar]
- Zuorro, A.; Fidaleo, M.; Lavecchia, R. Enzyme-assisted extraction of lycopene from tomato processing waste. Enzym. Microb. Technol. 2011, 49, 567–573. [Google Scholar]
- Fidale, L.C.; Ruiz, N.; Heinze, T.; El Seoud, O.A. Cellulose swelling by aprotic and protic solvents: What are the similarities and differences? Macromol. Chem. Phys. 2008, 209, 1240–1254. [Google Scholar]
- El Seoud, O.A. Understanding solvation. Pure Appl. Chem. 2009, 81, 697–707. [Google Scholar]
- Sadali, N.M.; Sowden, R.G.; Ling, Q.; Jarvis, R.P. Differentiation of chromoplasts and other plastids in plants. Plant Cell Rep. 2019, 38, 803–818. [Google Scholar]
- Egea, I.; Barsan, C.; Bian, W.; Purgatto, E.; Latché, A.; Chervin, C.; Bouzayen, M.; Pech, J.-C. Chromoplast differentiation: Current status and perspectives. Plant Cell Physiol. 2010, 51, 1601–1611. [Google Scholar]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar]
- Strati, I.F.; Oreopoulou, V. Effect of extraction parameters on the carotenoid recovery from tomato waste. Int. J. Food Sci. Technol. 2011, 46, 23–29. [Google Scholar]
- Fish, W.W.; Perkins-Veazie, P.; Collins, J.K. A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J. Food Compos. Anal. 2002, 15, 309–317. [Google Scholar]
- Sadler, G.; Davis, J.; Dezman, D. Rapid extraction of lycopene and β-carotene from reconstituted tomato paste and pink grapefruit homogenates. J. Food Sci. 1990, 55, 1460–1461. [Google Scholar]
- Periago, M.J.; Rincón, F.; Agüera, M.D.; Ros, G. Mixture approach for optimizing lycopene extraction from tomato and tomato products. J. Agric. Food Chem. 2004, 52, 5796–5802. [Google Scholar] [PubMed]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658–2671. [Google Scholar]
- Ishida, B.K.; Chapman, M.H. Carotenoid extraction from plants using a novel, environmentally friendly solvent. J. Agric. Food Chem. 2009, 57, 1051–1059. [Google Scholar]
- Lavecchia, R.; Zuorro, A. Improved lycopene extraction from tomato peels using cell-wall degrading enzymes. Eur. Food Res. Technol. 2008, 228, 153–158. [Google Scholar]
- Zuorro, A.; Lavecchia, R.; Medici, F.; Piga, L. Enzyme-assisted production of tomato seed oil enriched with lycopene from tomato pomace. Food Bioprocess Technol. 2013, 6, 3499–3509. [Google Scholar]
- Javanmardi, J.; Kubota, C. Variation of lycopene, antioxidant activity, total soluble solids and weight loss of tomato during postharvest storage. Postharvest Biol. Technol. 2006, 41, 151–155. [Google Scholar]
- Taoukis, D.; Assimakopoulos, J. Effect of growth season and slow-release fertilizers on quality characteristics of tomatoes. Commun. Soil Sci. Plant. Anal. 2010, 41, 945–955. [Google Scholar]
- Majidi Nezhad, M.; Groppi, D.; Laneve, G.; Marzialetti, P.; Piras, G. Oil spill detection analyzing “Sentinel 2” satellite images: A Persian Gulf case study. In Proceedings of the 3rd World Congress on Civil, Structural, and Environmental Engineering (CSEE’18), Budapest, Hungary, 8–10 April 2018; Volume 134, pp. 1–8. [Google Scholar]
- Hildebrand, J.H.; Scott, R.L. Solutions of nonelectrolytes. Annu. Rev. Phys. Chem. 1950, 1, 75–92. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 27–43. [Google Scholar]
- Lavecchia, R.; Zuorro, A. Cellulase Applications in Pigment and Bioactive Compound Extraction. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Cellulase System Properties and Applications; Gupta, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 209–222. [Google Scholar]
- Grunin, L.Y.; Grunin, Y.B.; Talantsev, V.I.; Nikolskaya, E.A.; Masas, D.S. Features of the structural organization and sorption properties of cellulose. Polym. Sci. Ser. A 2015, 57, 43–51. [Google Scholar]
- Prusov, A.N.; Prusova, S.M.; Zakharov, A.G. Interaction of cellulose and lignocellulosic polymers with water and aqueous systems. Russ. Chem. Bull. 2014, 63, 1926–1945. [Google Scholar]
- Mantanis, G.I.; Young, R.A.; Rowell, R.M. Swelling of wood. Part 1. Swelling in water. Wood Sci. Technol. 1994, 28, 119–134. [Google Scholar]
- Meier, P.; Kaps, T.; Kallavus, U. Swelling of pinewood (Pinus sylvestris) in binary aqueous solutions of organic substances. Mater. Sci.-Medzg. 2005, 11, 140–145. [Google Scholar]
- Zehentbauer, F.M.; Kiefer, J. Molecular solution behaviour of an intermediate biofuel feedstock: Acetone-Butanol-Ethanol (ABE). Chem. Phys. Chem. 2015, 16, 3846–3858. [Google Scholar] [PubMed]
- Ferris, T.D.; Zeidler, M.D.; Farrar, T.C. The concentration dependence of the proton chemical shift and the deuterium quadrupole coupling parameter for binary solutions of ethanol. Mol. Phys. 2000, 98, 737–744. [Google Scholar]
- Jadhav, D.L.; Karthick, N.K.; Kannan, P.P.; Shanmugam, R.; Elangovan, A.; Arivazhagan, G. Molecular interaction forces in acetone+ethanol binary liquid solutions: FTIR and theoretical studies. J. Mol. Struct. 2017, 1130, 497–502. [Google Scholar]
- Fernandez-Maestre, R.; Velasco, A.R.; Hill, H.H. Explaining the drift behavior of caffeine and glucosamine after addition of ethyl lactate in the buffer gas of an ion mobility spectrometer. Bull. Korean Chem. Soc. 2014, 35, 1023–1028. [Google Scholar]
- Aparicio, S.; Halajian, S.; Alcalde, R.; García, B.; Leal, J.M. Liquid structure of ethyl lactate, pure and water mixed, as seen by dielectric spectroscopy, solvatochromic and thermophysical studies. Chem. Phys. Lett. 2008, 454, 49–55. [Google Scholar]
- Vicente, G.; Paiva, A.; Fornari, T.; Najdanovic-Visak, V. Liquid-liquid equilibria for separation of tocopherol from olive oil using ethyl lactate. Chem. Eng. J. 2011, 172, 879–884. [Google Scholar]
- Qiao, H.; Zhang, S.; Wang, W. Fluorescence spectroscopic and viscosity studies of hydrogen bonding in Chinese fenjiu. J. Biosci. Bioeng. 2013, 115, 405–411. [Google Scholar]
- Zuorro, A.; Lavecchia, R. Mild enzymatic method for the extraction of lycopene from tomato paste. Biotechnol. Biotechnol. Equip. 2010, 24, 1854–1857. [Google Scholar]
- Conde, E.; Cara, C.; Moure, A.; Ruiz, E.; Castro, E.; Domínguez, H. Antioxidant activity of the phenolic compounds released by hydrothermal treatments of olive tree pruning. Food Chem. 2009, 114, 806–812. [Google Scholar]
- Zhou, C.-H.; Li, X.; Xu, C.-J.; Sun, C.-D.; Chen, K.-S. Hydrophilic and lipophilic antioxidant activity of loquat fruits. J. Food Biochem. 2012, 36, 621–626. [Google Scholar]
- Maina, S.; Kachrimanidou, V.; Koutinas, A. A roadmap towards a circular and sustainable bioeconomy through waste valorization. Curr. Opin. Green Sustain. Chem. 2018, 8, 18–23. [Google Scholar]
- de Santoli, L.; Lo Basso, G.; Astiaso Garcia, D.; Piras, G.; Spiridigliozzi, G. Dynamic simulation model of trans-critical carbon dioxide heat pump application for boosting low temperature distribution networks in dwellings. Energies 2019, 12, 484. [Google Scholar]
- Fritsch, C.; Staebler, A.; Happel, A.; Cubero Márquez, M.A.; Aguiló-Aguayo, I.; Abadias, M.; Gallur, M.; Cigognini, I.M.; Montanari, A.; López, M.J.; et al. Processing, valorization and application of bio-waste derived compounds from potato, tomato, olive and cereals: A review. Sustainability 2017, 9, 1492. [Google Scholar]
Sample Availability: Samples of the tomato oleoresin are available from the author. |
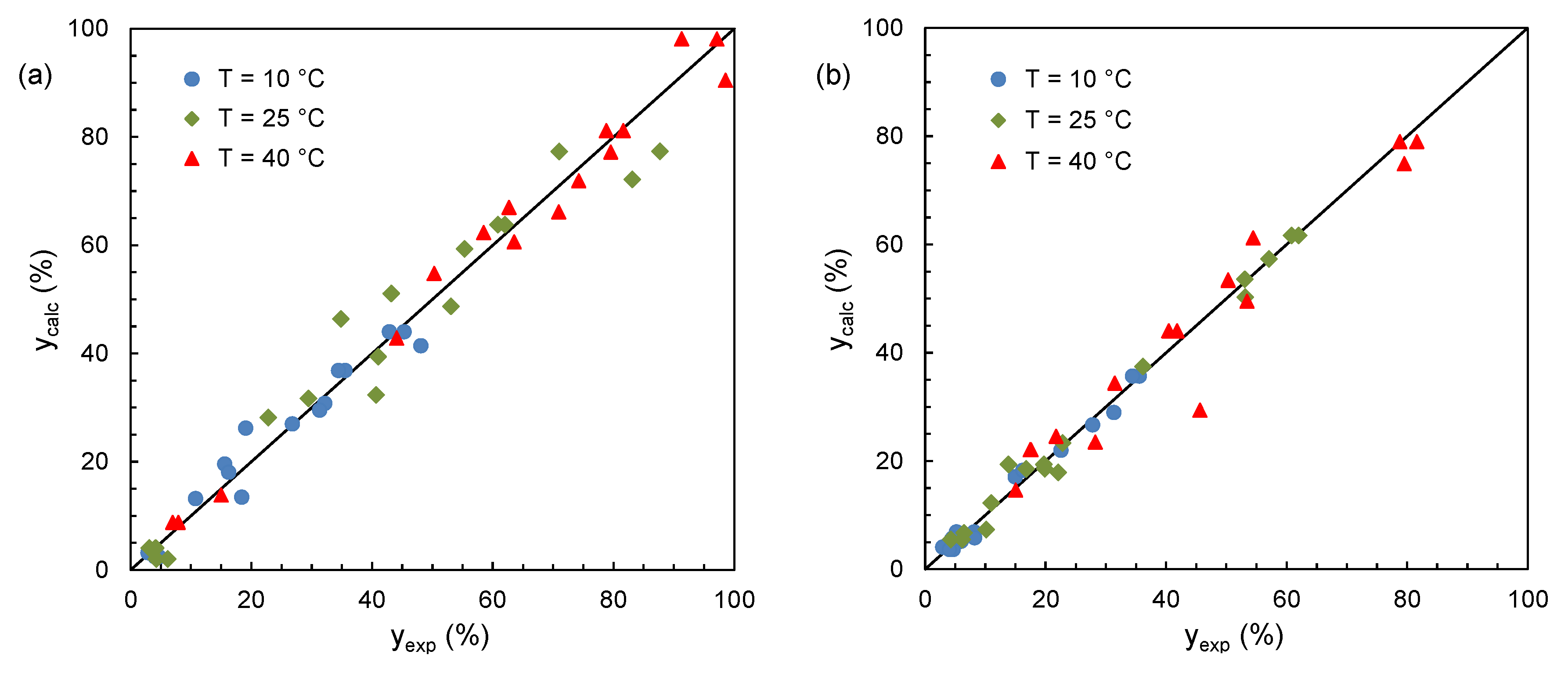

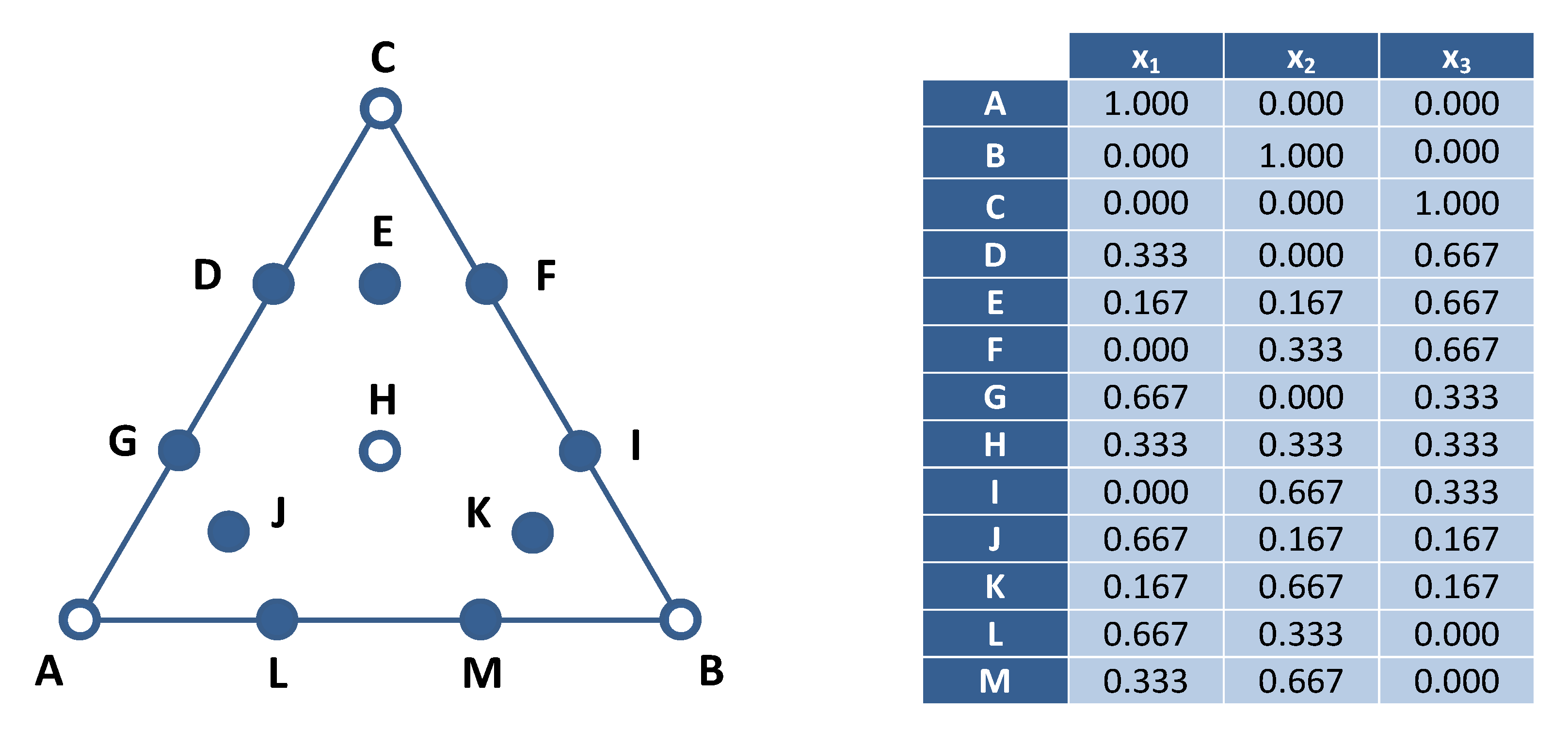
| SO | x1 | x2 | x3 | y (%) | ||
|---|---|---|---|---|---|---|
| 10 °C | 25 °C | 40 °C | ||||
| 1 | 1.000 | 0.000 | 0.000 | 3.09 | 4.15 | 6.97 |
| 2 | 0.667 | 0.333 | 0.000 | 18.41 | 40.69 | 63.57 |
| 3 | 0.667 | 0.000 | 0.333 | 15.59 | 41.04 | 44.08 |
| 4 | 0.333 | 0.667 | 0.000 | 10.79 | 29.46 | 58.49 |
| 5 | 0.333 | 0.333 | 0.333 | 42.87 | 87.71 | 97.14 |
| 6 | 0.333 | 0.000 | 0.667 | 32.18 | 55.32 | 62.72 |
| 7 | 0.000 | 1.000 | 0.000 | 4.69 | 4.28 | 15.04 |
| 8 | 0.000 | 0.667 | 0.333 | 16.26 | 22.82 | 50.32 |
| 9 | 0.000 | 0.333 | 0.667 | 31.34 | 53.08 | 79.55 |
| 10 | 0.000 | 0.000 | 1.000 | 35.55 | 60.84 | 78.81 |
| 11 | 0.667 | 0.167 | 0.167 | 26.82 | 43.19 | 70.92 |
| 12 | 0.167 | 0.667 | 0.167 | 19.07 | 34.83 | 74.25 |
| 13 | 0.167 | 0.167 | 0.667 | 48.09 | 83.13 | 98.55 |
| 14 | 1.000 | 0.000 | 0.000 | 2.91 | 3.10 | 7.93 |
| 15 | 0.000 | 1.000 | 0.000 | 4.07 | 6.17 | 15.04 |
| 16 | 0.000 | 0.000 | 1.000 | 34.45 | 62.00 | 81.65 |
| 17 | 0.333 | 0.333 | 0.333 | 45.31 | 71.01 | 91.32 |
| SO | x1 | x2 | x3 | y (%) | ||
|---|---|---|---|---|---|---|
| 10 °C | 25 °C | 40 °C | ||||
| 1 | 1.000 | 0.000 | 0.000 | 8.14 | 16.78 | 40.46 |
| 2 | 0.667 | 0.333 | 0.000 | 5.99 | 10.99 | 31.50 |
| 3 | 0.667 | 0.000 | 0.333 | 14.96 | 36.19 | 53.46 |
| 4 | 0.333 | 0.667 | 0.000 | 2.93 | 6.50 | 21.74 |
| 5 | 0.333 | 0.333 | 0.333 | 5.92 | 13.83 | 17.43 |
| 6 | 0.333 | 0.000 | 0.667 | 27.83 | 57.09 | 54.46 |
| 7 | 0.000 | 1.000 | 0.000 | 4.69 | 4.28 | 15.04 |
| 8 | 0.000 | 0.667 | 0.333 | 16.26 | 22.82 | 50.32 |
| 9 | 0.000 | 0.333 | 0.667 | 31.34 | 53.08 | 79.55 |
| 10 | 0.000 | 0.000 | 1.000 | 35.55 | 60.84 | 78.81 |
| 11 | 0.667 | 0.167 | 0.167 | 8.22 | 22.14 | 45.62 |
| 12 | 0.167 | 0.667 | 0.167 | 4.39 | 10.17 | 28.24 |
| 13 | 0.167 | 0.167 | 0.667 | 22.55 | 53.13 | 50.30 |
| 14 | 1.000 | 0.000 | 0.000 | 5.20 | 19.88 | 41.84 |
| 15 | 0.000 | 1.000 | 0.000 | 4.07 | 6.17 | 15.04 |
| 16 | 0.000 | 0.000 | 1.000 | 34.45 | 62.00 | 81.65 |
| 17 | 0.333 | 0.333 | 0.333 | 4.61 | 19.73 | 17.59 |
| Compound | MW (Da) | vm (cm3/mol) | δD (MPa0.5) | δP (MPa0.5) | δH (MPa0.5) | δ (MPa0.5) | D (MPa0.5) |
|---|---|---|---|---|---|---|---|
| Acetone | 58.08 | 74.0 | 15.5 | 10.4 | 7.0 | 19.9 | 12.5 |
| Ethanol | 46.07 | 58.5 | 15.8 | 8.8 | 19.4 | 26.5 | 21.3 |
| Ethyl lactate | 118.13 | 115.0 | 16.0 | 7.6 | 12.5 | 21.7 | 14.6 |
| n-Hexane | 86.18 | 131.6 | 14.9 | 0.0 | 0.0 | 14.9 | 0.7 |
| Lycopene | 536.87 | 604.2 | 15.6 | 0.0 | 0.0 | 15.6 | – |
| Mixture | x1 | x2 | x3 | y (%) | δmix (MPa0.5) | D (MPa0.5) |
|---|---|---|---|---|---|---|
| n-Hexane–ethanol–acetone | 0.167 | 0.167 | 0.667 | 98.55 | 19.3 | 11.5 |
| 0.333 | 0.333 | 0.333 | 94.23 | 18.7 | 10.8 | |
| Ethyl lactate–ethanol–acetone | 0.000 | 0.000 | 1.000 | 80.23 | 19.9 | 12.5 |
| 0.000 | 0.333 | 0.667 | 79.55 | 21.6 | 14.9 |
| T (°C) | x1 | x2 | x3 | ymod (%) | yexp (%) | ε (%) |
|---|---|---|---|---|---|---|
| 10 | 0.287 | 0.277 | 0.436 | 45.18 | 43.85 | 2.94 |
| 25 | 0.310 | 0.265 | 0.425 | 78.91 | 79.64 | –0.93 |
| 40 | 0.306 | 0.328 | 0.366 | 98.56 | 96.52 | 2.07 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuorro, A. Enhanced Lycopene Extraction from Tomato Peels by Optimized Mixed-Polarity Solvent Mixtures. Molecules 2020, 25, 2038. https://doi.org/10.3390/molecules25092038
Zuorro A. Enhanced Lycopene Extraction from Tomato Peels by Optimized Mixed-Polarity Solvent Mixtures. Molecules. 2020; 25(9):2038. https://doi.org/10.3390/molecules25092038
Chicago/Turabian StyleZuorro, Antonio. 2020. "Enhanced Lycopene Extraction from Tomato Peels by Optimized Mixed-Polarity Solvent Mixtures" Molecules 25, no. 9: 2038. https://doi.org/10.3390/molecules25092038
APA StyleZuorro, A. (2020). Enhanced Lycopene Extraction from Tomato Peels by Optimized Mixed-Polarity Solvent Mixtures. Molecules, 25(9), 2038. https://doi.org/10.3390/molecules25092038





