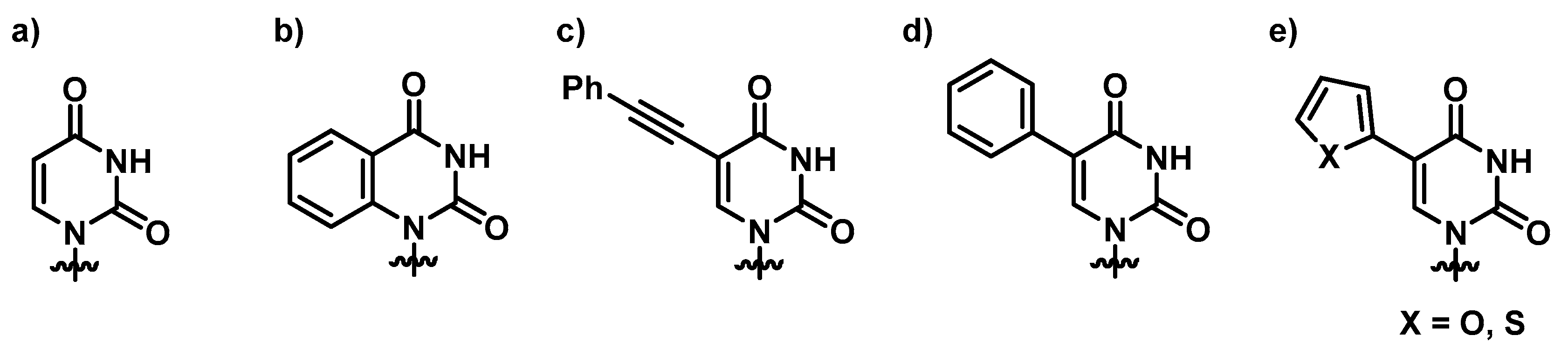
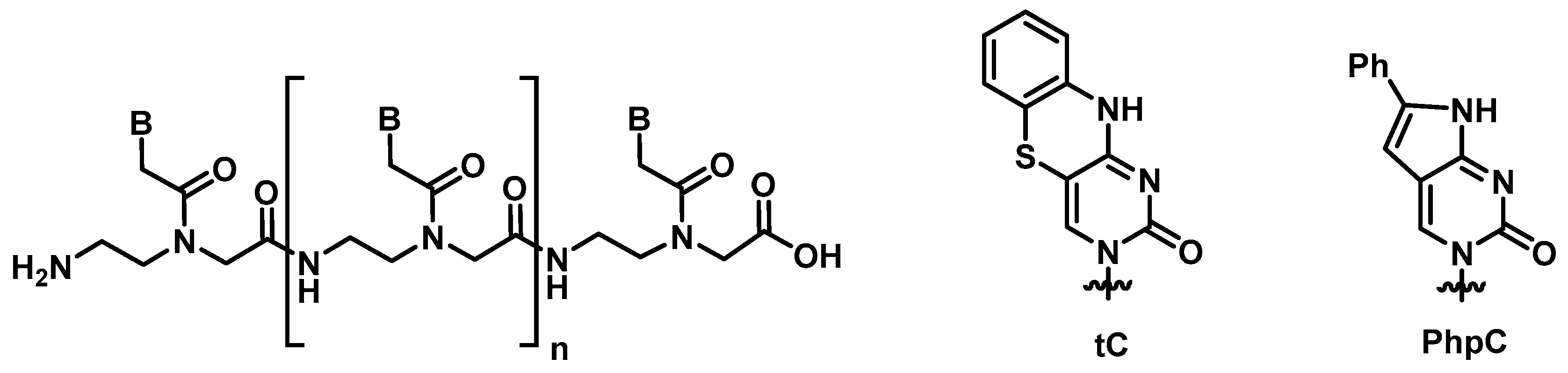
3.1. Synthesis and Characterization
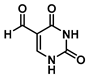
5-Formyluracil (1)
5-Hydroxymethyluracil (4.0 g, 28 mmol), was dissolved in 100 mL water by heating to 75 °C. The solution was allowed to cool to 40 °C, and then K
2S
2O
8 (13.6 g, 49.6 mmol) and AgNO
3 (0.14 g, 0.8 mmol) were added and the product began to slowly precipitate. The reaction was stirred for 15 min at 35 °C and then for 10 min at room temperature. The mixture was held at 4 °C for 1 h, after which time the product was collected by filtration, washed with cold water and dried to yield
1 (3.2 g, 22.7 mmol, 84%) as a white solid. The spectra matched the literature values [
14].
1H NMR (400 MHz, DMSO-
d6) δ 11.96 (s, 1H), 11.50 (s, 1H), 9.74 (s, 1H), 8.13 (s, 1H).
13C}
1H} NMR (101 MHz, DMSO-
d6) δ 186.9, 162.9, 150.9, 149.7, 110.6.
tert-Butyl (5-formyluracil-1-yl)acetate (2)
![Molecules 25 01995 i002 Molecules 25 01995 i002]()
5-Formyluracil
1 (2.0 g, 14.3 mmol) was added to 50 mL dry dimethylformamide (DMF), heated to 40 °C to dissolve and then allowed to cool to room temperature. Triethylamine (NEt
3, 2.0 mL, 14.3 mmol) was added to the solution in one portion.
tert-Butyl bromoacetate (2.11 mL, 14.3 mmol) was added dropwise to the stirred mixture over 30 min and then stirring was continued for 24 h under an N
2 atmosphere. The solvent was removed in vacuo and the residue was extracted with ethyl acetate and water. The organic layer was washed with brine, dried by Na
2SO
4 and concentrated in vacuo to give
2 as a pure, white solid product (3.16 g, 12.43 mmol, 87%). Spectroscopic analysis conformed to previous reports [
21].
1H NMR (400 MHz, DMSO-
d6) δ 11.92 (s, 1H), 9.79 (s, 1H), 8.51 (s, 1H), 4.56 (s, 2H), 4.02 (q,
J = 7.1 Hz, 1H), 1.98 (s, 1H), 1.42 (s, 12H).
13C}
1H} NMR (101 MHz, DMSO-
d6) δ 186.8, 167.0, 164.1, 163.7, 162.7, 154.1, 152.4, 150.4, 110.7, 102.6, 82.8, 60.3, 50.5, 50.4, 28.0, 27.9, 14.5.
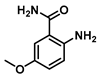
2-Amino-5-methoxybenzamide (3)
![Molecules 25 01995 i003 Molecules 25 01995 i003]()
2-Amino-5-methoxybenzoic acid (0.35 g, 2.5 mmol) was dissolved in 4 mL tetrahydrofuran (THF) and heated to 60 °C, and then triphosgene (0.25 g, 0.84 mmol) was added and the reaction was stirred for 10 h. The reaction progress was monitored by TLC analysis. The solution cooled down to room temperature, and the isatoic anhydride intermediate was filtered and collected. Cold 1N ammonia was added to the collected intermediate, and the mixture was stirred for 24 h at 4 °C. To the crude solution were added 10 mL of EtOAc/Hexanes (1:1) mixture, and the resulting precipitate was filtered and washed sequentially with water and diethyl ether to give 0.240 g (1.4 mmol, 58%) of Compound 3 as a brown solid. Spectra matched those reported by the Integrated Spectral Database System of Organic Compounds (AIST, Tokio, Japan). 1H NMR (400 MHz, DMSO-d6) δ 7.75 (s, 1H), 7.10 (d, J = 2.9 Hz, 1H), 7.08 (s, 1H), 6.84 (dd, J = 8.9, 2.9 Hz, 1H), 6.64 (d, J = 8.9 Hz, 1H), 6.12 (s, 2H), 3.68 (s, 3H). 13C}1H} NMR (101 MHz, DMSO-d6) δ 171.4, 149.6, 144.9, 120.3, 118.2, 114.4, 112.9, 56.0.
2-Amino-5-nitrobenzamide (4)
2-Amino-5-nitrobenzoic acid (0.785 g, 4.5 mmol) was dissolved in 10 mL of THF and heated to 60 °C, triphosgene (0.45 g, 1.5 mmol) was added and the reaction was stirred for 10 h. The reaction progress was monitored by TLC analysis. The solution was cooled to room temperature, and the isatoic anhydride intermediate was filtered and collected. Cold 1N ammonia was added to the collected intermediate, and the mixture was stirred for 24 h at 4 °C. The turbid solution was filtered and washed with water and ether to give 0.430 g (2.3 mmol, 48%) of Compound 4 as a yellow solid. Spectra matched those reported by the Integrated Spectral Database System of Organic Compounds (AIST, Japan). 1H NMR (400 MHz, DMSO-d6) δ 8.56 (d, J = 2.6 Hz, 1H), 8.21 (s, 1H), 8.02 (dd, J = 9.2, 2.6 Hz, 1H), 7.90 (s, 2H), 7.41 (s, 1H), 6.80 (d, J = 9.2 Hz, 1H). 13C}1H} NMR (101 MHz, DMSO-d6) δ 170.1, 156.2, 135.2, 128.0, 126.9, 116.4, 112.5.
2-Amino-4-methoxybenzamide (5)
2-Amino-4-methoxybenzoic acid (0.501 g, 3.0 mmol) was dissolved in 6 mL THF and heated to 60 °C, triphosgene (0.3 g, 1 mmol) was added and the reaction was stirred for 10 h. The reaction progress was monitored by TLC analysis. Cold 1N ammonia was added to the collected intermediate, and the mixture was stirred for 24 h at 4 °C. The turbid solution was filtered and washed with water and ether to give 0.25 g (1.5 mmol, 50%) of Compound 5 as a white solid. Spectra matched those reported by the Integrated Spectral Database System of Organic Compounds (AIST, Japan). 1H NMR (400 MHz, DMSO-d6) δ 7.49 (d, J = 8.8 Hz, 1H), 6.74 (s, 2H), 6.20 (d, J = 2.6 Hz, 1H), 6.07 (dd, J = 8.8, 2.6 Hz, 1H), 3.70 (s, 3H).
2-Amino-4-nitrobenzamide (6)
2-Amino-4-nitrobenzoic acid (0.549 g, 3.0 mmol) was dissolved in 6 mL THF and, heated to 60 °C, triphosgene (0.3 g, 1 mmol) was added, the reaction was stirred for 5 h and the reaction progress was monitored by TLC. The solution was cooled to room temperature, and the isatoic anhydride intermediate was filtered and collected. Cold 1N ammonia was added to the collected intermediate, and the mixture was stirred for 24 h at 4 °C. The turbid solution was filtered and washed with water and diethyl ether to give 0.430 g (2.3 mmol, 80%) of Compound 6 as an orange solid. Spectra matched those reported by the Integrated Spectral Database System of Organic Compounds (AIST, Japan). 1H NMR (400 MHz, DMSO-d6) δ 8.03 (s, 1H), 7.74 (d, J = 8.6 Hz, 1H), 7.57 (d, J = 2.4 Hz, 1H), 7.46 (s, 1H), 7.25 (dd, J = 8.6, 2.4 Hz, 1H), 7.02 (s, 2H).
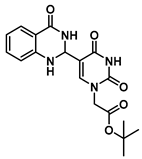
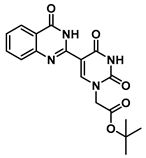
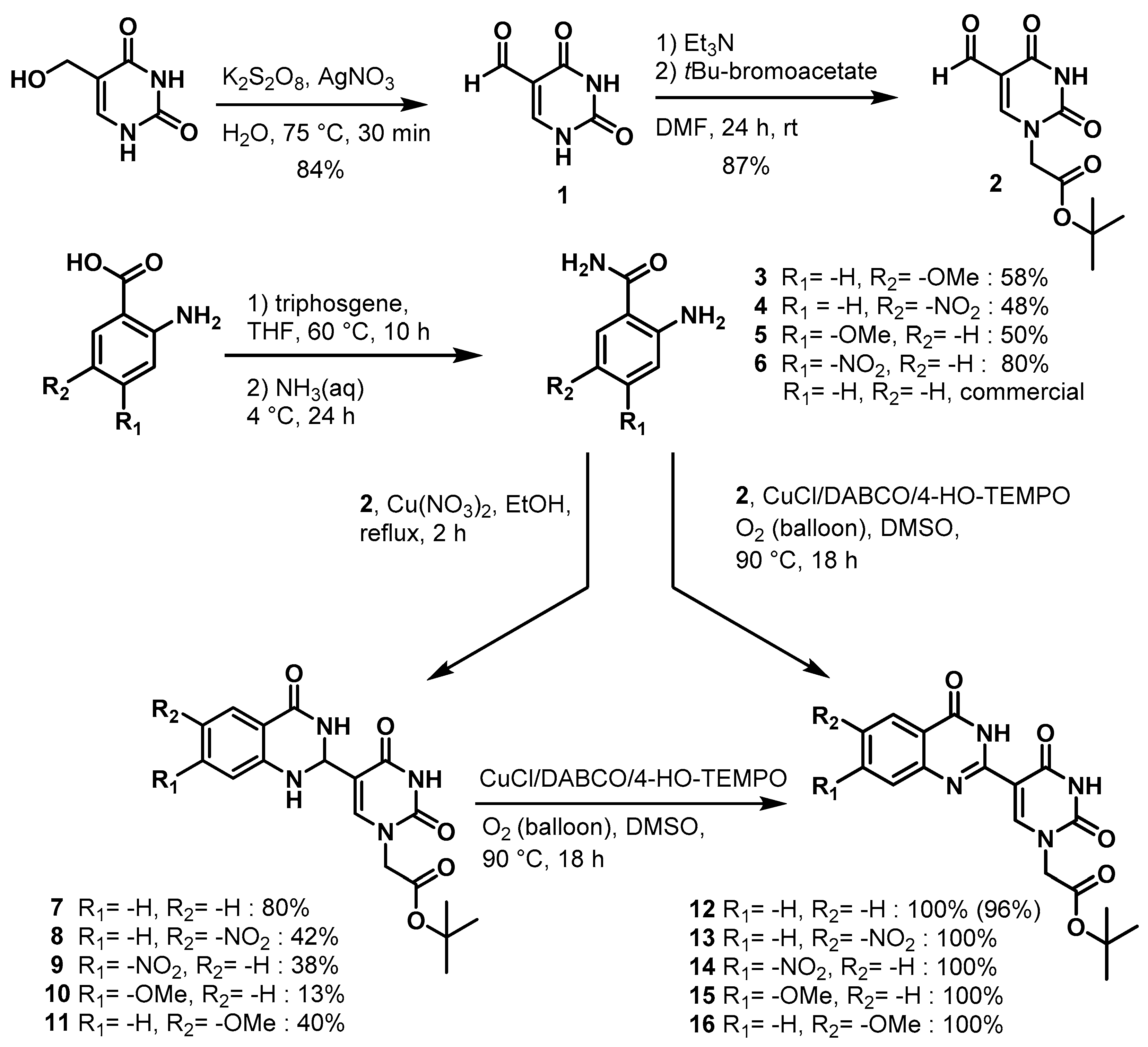
tert-Butyl 2-(5-(4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)uracil-1-yl)acetate (7)
To a solution of 0.50 g (2.0 mmol) of 2 dissolved in 10 mL of reflux ethanol were added 36 mg (0.13 mmol) of Cu(NO3)2·3H2O and 0.14 g (1.0 mmol) of 2-aminobenzamide. The reaction mixture was stirred for 2 h and analyzed by TLC. The solution was cooled to room temperature, and the resulting precipitate was filtered and washed sequentially with EtOH and MeOH to give 7 (0.298 g, 0.8 mmol, 80%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 11.62 (s, 1H), 7.95 (d, J = 1.8 Hz, 1H), 7.82 (s, 1H), 7.62 (dd, J = 7.8, 1.6 Hz, 1H), 7.25 (ddd, J = 8.5, 7.2, 1.7 Hz, 1H), 6.78 (d, J = 8.1 Hz, 1H), 6.75–6.66 (m, 2H), 5.68 (d, J = 2.0 Hz, 1H), 4.48 (s, 2H), 1.40 (s, 9H). 13C}1H} NMR (101 MHz, DMSO-d6) δ 167.5, 163.9, 163.3, 150.9, 148.2, 144.5, 133.7, 127.9, 117.9, 115.3, 112.9, 82.2, 60.4, 50.1, 28.1. HRMS (ESI/Q-TOF) m/z: [M]+ calculated for C18H20N4O5, 372.1434; found, 372.1428.
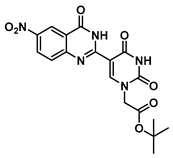
tert-Butyl 2-(5-(6-nitro-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)uracil-1-yl) acetate (8)
To a solution of 0.50 g (2.0 mmol) of 2 dissolved in 10 mL of refluxing ethanol, 36.2 mg (0.15 mmol) of Cu(NO3)2·3H2O and 0.18 g (1 mmol) of 2-amino-5-nitrobenzamide were added, and the reaction mixture was stirred for 2 h and monitored by TLC. The solution was cooled to room temperature, and the resulting precipitate was filtered and washed with EtOH and MeOH to give 8 (176 mg, 0.42 mmol, 42%) as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ 11.67 (s, 1H), 8.41 (dd, J = 8.3, 2.4 Hz, 2H), 8.30 (s, 1H), 8.09 (dd, J = 9.1, 2.8 Hz, 1H), 7.81 (s, 1H), 6.84 (d, J = 9.1 Hz, 1H), 5.85 (t, J = 2.1 Hz, 1H), 4.47 (s, 2H), 1.39 (s, 9H). 13C}1H} NMR (101 MHz, DMSO-d6) δ 167.7, 162.9, 161.6, 152.4, 150.9, 144.4, 137.5, 129.2, 124.5, 114.9, 113.0, 112.9, 82.3, 61.0, 49.9, 28.1. HRMS (ESI/Q-TOF) m/z: [M]+ calculated for C18H19N5O7, 417.1284; found, 417.1271.
tert-Butyl 2-(5-(7-nitro-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)uracil-1-yl)acetate (9)
To a solution of 0.50 g (2.0 mmol) of 2 dissolved in 10 mL of refluxing ethanol, 36.2 mg (0.15 mmol) of Cu(NO3)2·3H2O and 0.18 g (1 mmol) of 2-amino-4-nitrobenzamide were added, and the reaction mixture was stirred for 2 h and monitored by TLC. The solution was cooled down to room temperature, and the resulting precipitate was filtered and washed with EtOH and MeOH to give 9 (160 mg, 0.38 mmol, 38%) as a yellow solid. 1H NMR (600 MHz, DMSO-d6) δ 11.62 (s, 1H), 8.34 (s, 1H), 7.79 (d, J = 8.5 Hz, 1H), 7.74 (s, 1H), 7.59 (d, J = 2.3 Hz, 1H), 7.43–7.37 (m, 2H), 5.73 (s, 1H), 4.43 (s, 2H), 1.34 (s, 9H). 13C}1H} NMR (101 MHz, DMSO-d6) δ 166.5, 162.9, 161.6, 152.4, 150.9, 144.4, 137.5, 129.2, 124.5, 114.8, 113.0, 112.9, 82.3, 61.0, 49.9, 27.3. HRMS (ESI/Q-TOF) m/z: [M]+ calculated for C18H19N5O7, 417.1284; found, 417.1270.
tert-Butyl 2-(5-(7-methoxy-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)uracil-1-yl)acetate (10)
To a solution of 0.25 g (1.0 mmol) of 2 dissolved in 10 mL of refluxing ethanol, 18.1 mg (0.075 mmol) of Cu(NO3)2·3H2O and 83 mg (0.5 mmol) of 2-amino-4-methoxybenzamide were added, and the reaction mixture was stirred for 10 h and monitored by TLC. The solution was cooled to room temperature, and the resulting precipitate was filtered and washed with EtOH and MeOH to give 10 (51 mg, 0.13 mmol, 13%) as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ 12.37 (s, 1H), 12.03 (s, 1H), 9.02 (s, 1H), 8.02 (d, J = 8.7 Hz, 1H), 7.12–7.01 (m, 2H), 4.70 (s, 2H), 3.89 (s, 3H), 1.45 (s, 9H). 13C}1H} NMR (101 MHz, DMSO-d6) δ 167.2, 164.6, 164.5, 160.1, 151.3, 150.7, 150.1, 149.6, 128.1, 116.1, 114.8, 108.2, 103.2, 82.7, 56.1, 50.7, 28.1. HRMS (ESI/Q-TOF) m/z: [M]+ calculated for C19H22N4O6, 402.1539; found, 402.1536.
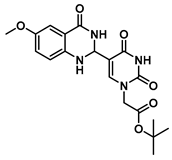
tert-Butyl 2-(5-(6-methoxy-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)uracil-1-yl)acetate (11)
To a solution of 0.25 g (1 mmol) of 2 dissolved in 10 mL of refluxing ethanol, 18.1 mg (0.075 mmol) of Cu(NO3)2·3H2O and 83 mg (0.5 mmol) of 2-amino-5-methoxybenzamide were added, and the reaction mixture was stirred for 4 h and monitored by TLC. The solution was cooled to room temperature, and the resulting precipitate was filtered and washed with EtOH and MeOH to give 11 (155 mg, 0.40 mmol, 40%) as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ 11.61 (s, 1H), 8.00 (t, J = 1.8 Hz, 1H), 7.82 (s, 1H), 7.16 (d, J = 3.0 Hz, 1H), 6.93 (dd, J = 8.7, 3.0 Hz, 1H), 6.77 (d, J = 8.8 Hz, 1H), 6.38 (d, J = 1.7 Hz, 1H), 5.62 (t, J = 1.7 Hz, 1H), 4.48 (s, 2H), 3.69 (s, 3H), 1.40 (s, 9H). 13C}1H} NMR (101 MHz, DMSO-d6) δ 167.5, 163.9, 163.3, 152.2, 150.9, 144.5, 142.5, 121.8, 116.9, 116.0, 112.7, 110.4, 82.2, 60.6, 55.7, 50.0, 28.1. HRMS (ESI/Q-TOF) m/z: [M]+ calculated for C19H22N4O6, 402.1539; found, 402.1537.
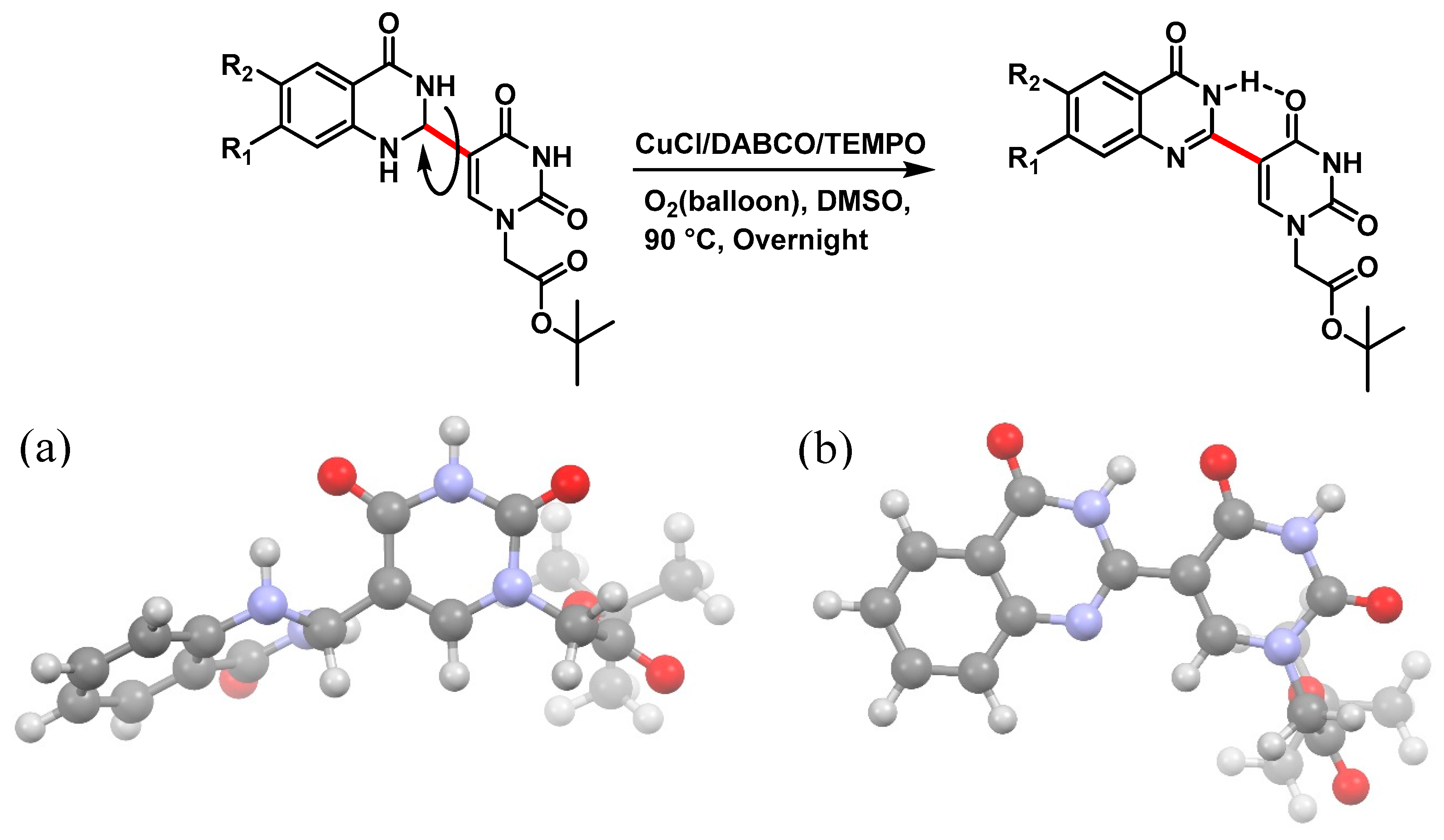
tert-Butyl 2-(5-(4-oxo-3,4-dihydroquinazolin-2-yl)uracil-1-yl)acetate (12)
![Molecules 25 01995 i012 Molecules 25 01995 i012]()
To a solution of 0.15 g, (0.4 mmol) of 7 fully dissolved in 1 mL of DMSO at 60 °C were added CuCl (1.2 mg, 0.01 mmol), 4-HO-TEMPO (1 mg, 0.005 mmol) and DABCO (1 mg, 0.009 mmol). The mixture was stirred at 90 °C for 18 h under an atmosphere of O2 (balloon). After the completion of the reaction (monitored by TLC), the reaction was cooled to room temperature and the resulting precipitate was washed with water and ethanol to give the product 12 (0.15 g, 0.4 mmol, 100%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 12.46 (s, 1H), 9.27 (s, 1H), 8.38 (d, J = 7.9 Hz, 1H), 8.08 (t, J = 7.7 Hz, 1H), 7.90 (d, J = 8.2 Hz, 1H), 7.74 (t, J = 7.5 Hz, 1H), 4.96 (s, 2H), 1.71 (s, 9H). 13C}1H} NMR (151 MHz, DMSO-d6) δ 166.8, 164.3, 150.5, 150.0, 149.1, 134.8, 126.4, 126.3, 121.5, 110.0, 103.5, 82.8, 50.8, 40.8, 40.7, 40.6, 40.4, 40.3, 40.2, 40.0, 28.2. HRMS (ESI/Q-TOF) m/z: [M]+ calculated for C18H18N4O5, 370.1277; found, 370.1279.
tert-Butyl 2-(5-(6-nitro-4-oxo-1,4-dihydroquinazolin-2-yl)uracil-1-yl)acetate (13)
To a solution of 0.13 g (0.31 mmol) of 8 in 1 mL of DMSO dissolved at 60 °C were added CuCl (2.9 mg, 0.02 mmol), 4-HO-TEMPO (2 mg, 0.012 mmol) and DABCO (2 mg, 0.022 mmol). The mixture was stirred at 90 °C overnight under an atmosphere of O2 (balloon). After the completion of the reaction (monitored by TLC), the reaction was cooled to room temperature and the resulting precipitate was washed with water and ethanol to give the product 13 as a yellow solid (0.13 g, 0.31 mmol, 100%). 1H NMR (400 MHz, DMSO-d6) δ 12.51 (s, 1H), 9.15 (s, 1H), 8.80 (d, J = 2.7 Hz, 1H), 8.55 (dd, J = 9.0, 2.8 Hz, 1H), 7.77 (d, J = 9.0 Hz, 1H), 4.74 (s, 2H), 1.46 (s, 9H). 13C}1H} NMR (101 MHz, DMSO-d6) δ 167.1, 159.9, 152.2, 144.6, 129.2, 128.7, 122.6, 121.2, 82.8, 50.9, 28.1. HRMS (ESI/Q-TOF) m/z: [M]+ calculated for C18H17N5O7, 415.1128; found, 415.1130.
tert-Butyl 2-(5-(7-nitro-4-oxo-1,4-dihydroquinazolin-2-yl)uracil-1-yl)acetate (14)
![Molecules 25 01995 i014 Molecules 25 01995 i014]()
To 1 mL of of DMSO was added 0.12 g (0.29 mmol) of 9, and the temperature was increased to 60 °C until a clear solution was obtained. To the same solution were added CuCl (2.9 mg, 0.02 mmol), 4-HO-TEMPO (2 mg, 0.012 mmol) and DABCO (2 mg, 0.022 mmol), and the mixture was stirred at 90 °C overnight under O2 atmosphere (balloon). After the completion of the reaction (monitored by TLC), the reaction was cooled down to room temperature and the resulting precipitate was washed with water and ethanol to give the product 14 as a yellow solid (0.12 g, 0.29 mmol, 100%).1H NMR (400 MHz, DMSO-d6) δ 12.50–12.39 (m, 2H), 9.12 (s, 1H), 8.36–8.27 (m, 2H), 8.18 (dd, J = 8.7, 2.2 Hz, 1H), 4.72 (s, 2H), 1.46 (s, 9H). 13C}1H} NMR (101 MHz, DMSO-d6) δ 167.1, 159.7, 151.8, 151.6, 151.4, 150.0, 149.6, 128.8, 125.7, 121.9, 119.8, 102.7, 82.8, 50.9, 28.1. HRMS (ESI/Q-TOF) m/z: [M]+ calculated for C18H17N5O7, 415.1128; found, 415.1133.
tert-Butyl 2-(5-(7-methoxy-4-oxo-1,4-dihydroquinazolin-2-yl)uracil-1-yl)acetate (15)
![Molecules 25 01995 i015 Molecules 25 01995 i015]()
To 1 mL of of DMSO was added 0.14 g (0.35 mmol) of 10, and the temperature was increased to 60 °C until a clear solution was obtained. To the same solution were added CuCl (2.9 mg, 0.02 mmol), 4-HO-TEMPO (2 mg, 0.012 mmol) and DABCO (2 mg, 0.022 mmol), and the mixture was stirred at 90 °C overnight under an O2 atmosphere (balloon). After the completion of the reaction (monitored by TLC), the reaction was cooled down to room temperature and the resulting precipitate was washed with water and ethanol to give the product 15 as a yellow solid (0.14 g, 0.35 mmol, 100%). 1H NMR (400 MHz, DMSO-d6) δ 12.37 (s, 1H), 12.03 (s, 1H), 9.02 (s, 1H), 8.02 (d, J = 8.7 Hz, 1H), 7.12–7.01 (m, 2H), 4.70 (s, 2H), 3.89 (s, 3H), 1.45 (s, 9H). 13C }1H} NMR (101 MHz, DMSO-d6) δ 167.2, 164.7, 164.5, 160.1, 151.3, 150.7, 150.1, 149.7, 128.1, 116.1, 114.8, 108.2, 103.2, 82.7, 56.1, 50.7, 28.1. HRMS (ESI/Q-TOF) m/z: [M]+ calculated for C19H20N4O6, 400.1383; found, 400.1379.
tert-Butyl 2-(5-(6-methoxy-4-oxo-3,4-dihydroquinazolin-2-yl)uracil-1-yl)acetate (16)
![Molecules 25 01995 i016 Molecules 25 01995 i016]()
To 1 mL of of DMSO was added 0.14 g (0.35 mmol) of 11, and the temperature was increased to 60 °C until a clear solution was obtained. To the same solution were added CuCl (2.9 mg, 0.02 mmol), 4-HO-TEMPO (2 mg, 0.012 mmol) and DABCO (2 mg, 0.022 mmol), and the mixture was stirred at 90 °C overnight under an O2 atmosphere (balloon). After the completion of the reaction (monitored by TLC), the reaction was cooled down to room temperature and the resulting precipitate was washed with water and ethanol to give the product 16 as a yellow solid (0.14 g, 0.35 mmol, 100%). 1H NMR (400 MHz, DMSO-d6) δ 12.37 (s, 1H), 12.03 (s, 1H), 9.02 (s, 1H), 8.02 (d, J = 8.7 Hz, 1H), 7.12–7.01 (m, 2H), 4.70 (s, 2H), 3.89 (s, 3H), 1.45 (s, 9H). 13C}1H} NMR (101 MHz, DMSO-d6) δ 167.2, 164.7, 164.5, 160.1, 151.3, 150.7, 150.1, 149.7, 128.1, 116.1, 114.8, 108.2, 103.2, 82.7, 56.1, 50.7, 28.1. HRMS (ESI/Q-TOF) m/z: [M]+ calculated for C19H20N4O6, 400.1383; found, 400.1382.
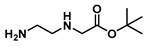
tert-Butyl (2-aminoethyl)glycinate (17)
tert-Butyl bromoacetate (6.9 mL, 47 mmol) was mixed with 37.5 mL of dichloromethane and added dropwise to a mixture of 25 mL (0.37 mol) of ethylenediamine (dissolved in 175 mL of dichloromethane) in an ice bath. The reaction continued for 19 h. Then, it was extracted with water (90 mL × 2) and dichloromethane (60 mL × 3), dried with sodium sulphate and concentrated in vacuo to give 15.8 g (90 mmol, 51%) of ester 17 as a colorless liquid. 1H NMR (400 MHz, Chloroform-d) δ 3.22 (s, 2H), 2.74–2.66 (m, 2H), 2.64–2.52 (m, 2H), 1.39 (s, 9H).
tert-Butyl (2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)ethyl)glycinate (18)
![Molecules 25 01995 i018 Molecules 25 01995 i018]()
Ester
17 (15.0 g, 88 mmol) and 15.2 mL of diisopropylethylamine (90 mmol) were dissolved in 680 mL of dichloromethane. To this solution was added, dropwise over the course of 70 min, a solution of 26.3 g (77 mmol) of Fmoc-OSu dissolved in 192 mL of dichloromethane. The reaction mixture was stirred overnight and washed with 1 M HCl (3 × 100 mL) and brine (100 mL). The organic layer was dried with sodium sulphate and filtered. The solution was partially concentrated to 50 mL in vacuo and cooled in a freezer (−20 °C) overnight. The white precipitate was filtered the next day and washed with dichloromethane and mother liquor and then returned to the freezer to generate more of product
18 (8.0 g, 19 mmol, 20%) as a white precipitate.
1H NMR (400 MHz, DMSO-
d6) δ 7.91–7.80 (m, 3H), 7.68 (dd,
J = 16.3, 7.9 Hz, 2H), 7.46–7.23 (m, 5H), 4.36–4.27 (m, 2H), 4.22 (q,
J = 7.3 Hz, 1H), 3.20 (s, 1H), 3.07 (q,
J = 6.2 Hz, 2H), 2.58 (t,
J = 6.4 Hz, 1H), 1.41 (s, 9H).
13C}
1H} NMR (101 MHz, DMSO-
d6) δ 171.9, 156.7, 144.4, 141.2, 129.4, 128.0, 127.7, 127.5, 125.6, 121.8, 120.6, 120.5, 110.1, 80.5, 65.8, 51.3, 48.7, 47.2, 40.8, 28.2. This corresponded to the NMR spectra previously reported in the literature [
22].
2-(2,4-dioxo-5-(4-oxo-3,4-dihydroquinazolin-2-yl)uracil-1-yl)acetic acid (19)
To 0.1 g (0.27 mmol) of ester 12 were added TFA (1 mL) and triethylsilane (three drops). The reaction was stirred for 2 h at room temperature, then the excess acid was evaporated under a nitrogen stream, and the product was washed with hexanes and ethyl ether to give acid 19 as a yellow solid (67 mg, 0.21 mmol, 80%). 1H NMR (400 MHz, DMSO-d6) 12.66 (s, 1H), 9.27 (s, 1H), 8.38 (d, J = 7.9 Hz, 1H), 8.08 (t, J = 7.7 Hz, 1H), 7.92 (d, J = 8.2 Hz, 1H), 7.64 (t, J = 7.5 Hz, 1H), 4.86 (s, 2H). 13C}1H} NMR (101 MHz, DMSO-d6) δ 169.4, 166.8, 164.3, 150.5, 150.0, 149.1, 134.8, 126.4, 126.3, 121.5, 110.0, 103.5, 82.8, 50.8. HRMS (ESI/Q-TOF) m/z: [M]+ calculated for C14H10N4O5, 314.0651; found, 314.0658.
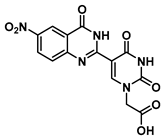
2-(5-(6-nitro-4-oxo-3,4-dihydroquinazolin-2-yl)uracil-1-yl)acetic acid (20)
To 0.1 g (0.24 mmol) of ester 13 were added TFA (1 mL) and triethylsilane (three drops). The reaction was stirred for 2 h at room temperature, then the excess acid was evaporated under a nitrogen stream, and the product was washed with hexane and ethyl ether to give acid 20 as a yellow solid (70 mg, 0.19 mmol, 80%). 1H NMR (400 MHz, DMSO-d6) δ 13.39 (s, 1H), 12.49 (s, 1H), 9.16 (d, J = 1.4 Hz, 1H), 8.78 (q, J = 2.6 Hz, 1H), 8.54 (dq, J = 9.1, 2.6 Hz, 1H), 7.80–7.72 (m, 2H), 4.76 (s, 2H). 13C}1H} NMR (101 MHz, DMSO-d6) δ 169.4, 164.5, 160.0, 152.6, 152.3, 149.9, 144.5, 129.2, 128.7, 122.6, 121.2, 102.4, 56.5, 50.3, 19.0. HRMS (ESI/Q-TOF) m/z: [M]+ calculated for C14H9N5O7, 359.0502; found, 359.0509.
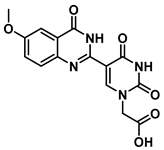
2-(5-(6-methoxy-4-oxo-3,4-dihydroquinazolin-2-yl)uracil-1-yl)acetic acid (21)
To 0.1 g (0.25 mmol) of ester 16 were added TFA (1 mL) and triethylsilane (three drops). The reaction was stirred for 2 h at room temperature, then the excess acid was evaporated under a nitrogen stream, and the product was washed with hexane and ethyl ether to give acid 21 as a light green solid (65 mg, 0.19 mmol, 75%). 1H NMR (400 MHz, DMSO-d6) δ 12.32 (s, 1H), 12.13 (s, 1H), 8.95 (s, 1H), 7.59 (d, J = 8.9 Hz, 1H), 7.51 (d, J = 3.0 Hz, 1H), 7.43 (dd, J = 8.9, 3.0 Hz, 1H), 4.72 (s, 2H), 3.88 (s, 3H). 13C}1H} NMR (101 MHz, DMSO-d6) δ 169.6, 164.5, 160.4, 157.8, 150.2, 150.1, 146.9, 143.6, 128.9, 124.8, 122.1, 106.4, 103.5, 56.1, 50.0. HRMS (ESI/Q-TOF) m/z: [M]+ calculated for C15H12N4O6, 344.0757; found, 344.0759.
tert-Butyl N-(2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)ethyl)-N-(2-(2,4-dioxo-5-(4-oxo-3,4-dihydroquinazolin-2-yl)uracil-1-yl)acetyl)glycinate (22)
![Molecules 25 01995 i022 Molecules 25 01995 i022]()
In 2 mL of DMF at 0 °C were dissolved 60 mg (0.19 mmol) of 19, HOBt (24 mg, 0.18 mmol) and DCC (67 mg, 0.32 mmol). This mixture was stirred until the formation of precipitation was observed, then 70 mg (0.15 mmol) of 6 as a free base with DMAP (1.8 mg, 0.015 mmol) were added, and the reaction continued for 3 h and was checked by TLC. The mixture was diluted with saturated aqueous NaHCO3, extracted with dichloromethane (DCM), washed with brine, concentrated in vacuo and purified by column chromatography (1:1 to 4:1 ethyl acetate/hexanes) to yield 22 as a white solid (81 mg, 0.1 mmol, 62%). 1H NMR (400 MHz, DMF-d7) δ 8.38 (dd, J = 7.7, 3.6 Hz, 3H), 8.09 (dd, J = 10.0, 7.5 Hz, 3H), 7.84 (dd, J = 8.6, 6.2 Hz, 3H), 7.79–7.71 (m, 3H), 4.85–4.57 (m, 4H), 4.43 (d, J = 4.0 Hz, 2H), 4.02 (d, J = 2.4 Hz, 1H), 3.38–3.27 (m, 3H), 2.76 (q, J = 7.3 Hz, 1H), 1.84 (s, 9H). 13C{1H} NMR (101 MHz, DMF-d7) δ 169.99, 153.96, 144.68, 141.65, 129.81, 128.57, 128.17, 128.01, 128.00, 125.97, 122.27, 121.03, 110.63, 81.40, 68.20, 67.64, 67.23, 55.58, 55.30, 48.40, 47.59, 33.9, 31.2, 28.1. HRMS (ESI/Q-TOF) m/z: [M + Na]+ calculated for C37H36N6O8Na, 715.2492; found, 715.2487.
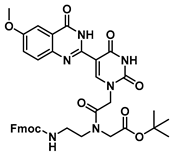
tert-Butyl N-(2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)ethyl)-N-(2-(5-(6-methoxy-4-oxo-3,4-dihydroquinazolin-2-yl)uracil-1-yl)acetyl)glycinate (23)
![Molecules 25 01995 i023 Molecules 25 01995 i023]()
To 0.1 g (0.29 mmol) of 21 dissolved in 2 mL of DMF at 0 °C were added HOBt (26 mg, 0.20 mmol) and DCC (70 mg, 0.34 mmol). This reaction was stirred until the formation of precipitation was observed, then 70 mg (0.15 mmol) of 6 as a free base with DMAP (1.8 mg, 0.015 mmol) were added, and the reaction continued for 3 h and was checked by TLC. The mixture was diluted with saturated aqueous NaHCO3, extracted with DCM, washed with brine, concentrated in vacuo and purified by column chromatography (1:1 to 4:1 ethyl acetate/hexanes) to yield 23 as a white solid (77 mg, 0.1 mmol, 66%). 1H NMR (400 MHz, DMF-d7) δ 8.31 (dd, J = 7.7, 3.6 Hz, 3H), 8.07 (dd, J = 10.0, 7.5 Hz, 3H), 7.84 (dd, J = 8.6, 6.2 Hz, 3H), 7.79–7.71 (m, 3H), 4.83–4.59 (m, 4H), 4.43 (d, J = 4.0 Hz, 2H), 4.01 (d, J = 2.4 Hz, 1H), 3.75 (s, 3H), 3.36–3.26 (m, 3H), 2.76 (q, J = 7.3 Hz, 1H), 1.84 (s, 9H). 13C{1H} NMR (101 MHz, DMF-d7) δ 169.99, 153.96, 144.68, 141.65, 129.81, 128.57, 128.17, 128.01, 128.00, 125.97, 122.27, 121.03, 110.63, 81.40, 68.20, 67.64, 67.23, 55.58, 55.30, 52.05, 48.40, 47.59, 32.9, 30.2, 28.1. HRMS (ESI/Q-TOF) m/z: [M + Na]+ calculated for C38H38N6O9Na, 745.2598; found, 745.2590.
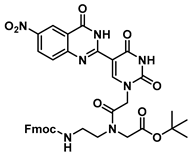
tert-Butyl N-(2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)ethyl)-N-(2-(5-(6-nitro-4-oxo-3,4-dihydroquinazolin-2-yl)uracil-1-yl)acetyl)glycinate (24)
![Molecules 25 01995 i024 Molecules 25 01995 i024]()
To 0.1 g (0.24 mmol) of 20 dissolved in 2 mL of DMF at 0 °C were added HOBt (23 mg, 0.19 mmol) and DCC (70 mg, 0.34 mmol), and the reaction was stirred until the formation of precipitation was observed. Then, 70 mg (0.15 mmol) of 6 as a free base with DMAP (1.8 mg, 0.015 mmol) were added, and the reaction continued for 3 h and was checked by TLC. The mixture was diluted with saturated aqueous NaHCO3, extracted with DCM, washed with brine, concentrated in vacuo and purified by column chromatography (1:1 to 4:1 ethyl acetate/hexanes) to yield 24 as a yellow solid (66 mg, 0.09 mmol, 60%). 1H NMR (400 MHz, DMSO-d6) δ 8.85–8.70 (m, 1H), 7.91–7.82 (m, 3H), 7.69 (dd, J = 10.0, 7.0 Hz, 2H), 7.47–7.18 (m, 5H), 4.01 (ddd, J = 15.7, 14.1, 5.9 Hz, 1H), 3.19 (d, J = 3.2 Hz, 4H), 2.90 (s, 1H), 2.75–2.56 (m, 7H), 1.42 (s, 9H). 13C{1H} NMR (101 MHz, Chloroform-d) δ 171.9, 146.0, 141.0, 128.7, 127.4, 127.2, 127.1, 126.9, 126.9, 125.2, 124.7, 121.0, 119.9, 119.8, 119.7, 107.7, 81.3, 57.3, 53.0, 51.4, 48.8, 47.9, 44.7, 33.9, 31.2, 28.1. HRMS (ESI/Q-TOF) m/z: [M + Na]+ calculated for C37H35N7O10Na, 760.2343; found, 760.2350.
N-(2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)ethyl)-N-(2-(5-(4-oxo-3,4-dihydroquinazolin-2-yl)uracil-1-yl)acetyl)glycine (25)
![Molecules 25 01995 i025 Molecules 25 01995 i025]()
To 75 mg (0.10 mmol) of ester 22 were added TFA (1 mL) and triethylsilane (three drops). The reaction was stirred for 2 h at room temperature, then the excess acid was evaporated under a nitrogen stream, and the product was washed with hexane and ethyl ether to give acid 25 as a white solid (55 mg, 0.08 mmol, 80%). 1H NMR (400 MHz, DMSO-d6) δ 12.34 (s, 3H), 8.76 (s, 2H), 8.71 (s, 1H), 7.81 (dd, J = 7.5, 5.1 Hz, 6H), 7.68 (t, J = 6.8 Hz, 6H), 7.49 (ddd, J = 8.4, 5.2, 1.6 Hz, 8H), 7.38 (dt, J = 7.9, 2.1 Hz, 6H), 7.35–7.29 (m, 6H), 5.57 (s, 1H), 5.03 (s, 3H), 4.84 (s, 2H), 4.36 (d, J = 6.9 Hz, 4H), 4.23 (d, J = 7.0 Hz, 2H), 4.25 (t, J = 3.4 Hz, 4H), 4.02 (s, 3H), 1.25–1.03 (m, 9H).13C{1H} NMR (101 MHz, DMSO) δ 171.9, 170.5, 165.2, 164.6, 160.6, 157.7, 156.8, 156.6, 150.4, 150.1, 144.3, 141.2, 141.1, 134.3, 128.1, 128.0, 127.8, 127.5, 125.6, 125.6, 120.6, 106.4, 103.1, 49.4, 47.9, 47.2, 40.6, 40.4, 40.2, 39.9, 39.78, 39.6, 39.4, 36.7, 33.8, 25.8, 24.9. HRMS (ESI/Q-TOF) m/z: [M + Na]+ calculated for C33H28N6O8Na, 659.1866; found, 659.1859.
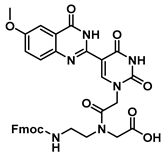
N-(2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)ethyl)-N-(2-(5-(6-methoxy-4-oxo-3,4-dihydroquinazolin-2-yl)uracil-1-yl)acetyl)glycine (26)
![Molecules 25 01995 i026 Molecules 25 01995 i026]()
To 66 mg (0.09 mmol) of ester 23 were added TFA (1 mL) and triethylsilane (three drops). The reaction was stirred for 2 h at room temperature, then the excess acid was evaporated under a nitrogen stream, and the product was washed with hexane and ethyl ether to give acid 26 as a white solid (47 mg, 0.07 mmol, 75%). 1H NMR (400 MHz, DMSO-d6) δ 12.30 (s, 3H), 8.78 (s, 2H), 8.72 (s, 1H), 7.86 (dd, J = 7.5, 5.1 Hz, 6H), 7.68 (t, J = 6.8 Hz, 6H), 7.49 (ddd, J = 8.4, 5.2, 1.6 Hz, 8H), 7.38 (dt, J = 7.9, 2.1 Hz, 6H), 7.35–7.29 (m, 6H), 5.57 (s, 1H), 5.03 (s, 3H), 4.84 (s, 2H), 4.36 (d, J = 6.9 Hz, 4H), 4.29 (d, J = 7.0 Hz, 2H), 4.25 (t, J = 3.4 Hz, 4H), 4.02 (s, 3H), 3.88 (s, 3H), 1.27–1.03 (m, 9H).13C{1H} NMR (101 MHz, DMSO) δ 172.8, 170.8, 167.2, 164.6, 160.6, 157.7, 156.8, 156.6, 150.4, 150.1, 144.3, 141.2, 141.1, 134.3, 128.1, 128.0, 127.8, 127.5, 125.6, 125.6, 120.6, 106.4, 103.1, 56.1, 49.4, 47.9, 47.2, 40.6, 40.4, 40.2, 39.9, 39.78, 39.6, 39.4, 36.7, 33.8, 25.8, 24.9. HRMS (ESI/Q-TOF) m/z: [M + Na]+ calculated for C34H36N6O9Na, 695.2441; found, 695.2458.
N-(2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)ethyl)-N-(2-(5-(6-nitro-4-oxo-3,4-dihydroquinazolin-2-yl)uracil-1-yl)acetyl)glycine (27)
![Molecules 25 01995 i027 Molecules 25 01995 i027]()
To ester 24 (58 mg, 0.07 mmol) were added TFA (1 mL) and triethylsilane (three drops). The reaction was stirred for 2 h at room temperature, then the excess acid was evaporated under a nitrogen stream, and the product was washed with hexane and ethyl ether to give acid 27 as a light yellow solid (47 mg, 0.05 mmol, 83%). 1H NMR (400 MHz, DMSO-d6) δ 12.57 (s, 1H), 9.05 (d, J = 3.4 Hz, 2H), 8.81 (s, 3H), 8.55 (d, J = 8.9 Hz, 4H), 8.49 (s, 2H), 7.91 (d, J = 7.2 Hz, 3H), 7.86 (t, J = 9.8 Hz, 5H), 7.76 (dd, J = 14.8, 8.1 Hz, 6H), 7.69 (s, 3H), 7.46–7.29 (m, 11H), 5.07 (s, 2H), 4.87 (s, 2H), 4.68 (s, 3H), 4.13 (s, 2H), 4.03 (s, 2H), 3.56 (s, 4H), 3.28 (s, 8H), 2.92 (d, J = 21.0 Hz, 11H). 13C{1H} NMR (101 MHz, DMSO-d6) δ 171.7, 169.7, 167.1, 163.5, 160.6, 157.8, 156.8, 156.6, 150.4, 150.1, 144.3, 141.2, 141.1, 134.3, 128.1, 128.0, 127.8, 127.5, 125.6, 125.6, 120.5, 106.4, 103.1, 49.4, 47.9, 47.2, 40.6, 40.4, 40.2, 39.9, 39.8, 39.6, 39.4, 36.7, 33.8, 25.8, 24.9. HRMS (ESI/Q-TOF) m/z: [M + Na]+ calculated for C33H27N7O10Na, 704.1717; found, 704.1739.